Abstract
Anxiety and stress disorders occur at a higher rate in women compared to men as well as in smokers in comparison to non-smoker population. Nicotine is known to impair fear extinction, which is altered in anxiety disorders. However, nicotine differentially affects fear learning in men and women, which may mean that sex and nicotine-product use can interact to also alter fear extinction. For this study, we examined sex differences in the effects of acute and chronic nicotine administration on fear memory extinction in male and female C57BL/6J mice. To study the acute effects of nicotine, animals trained in a background contextual fear conditioning paradigm were administered nicotine (0.09, 0.18 or 0.36 mg/kg) prior to extinction sessions. For chronic nicotine, animals continuously receiving nicotine (12.6, 18, or 24 mg/kg/day) were trained in a background contextual fear conditioning paradigm followed by fear extinction sessions. Males exhibited contextual fear extinction deficits following acute and chronic nicotine exposure. Females also exhibited extinction deficits, but only at the highest doses of acute nicotine (0.36 mg/kg) while chronic nicotine did not result in extinction deficits in female mice. These results suggest that sex mediates sensitivity to nicotine’s effects on contextual fear memory extinction.
Keywords: nicotine, extinction, hippocampus, anxiety, sex differences
Introduction
Anxiety and stress disorders are a family of mental illnesses that include post-traumatic stress disorder (PTSD; American Psychiatric Association, 2013). Common among anxiety and stress disorder symptomology is an exaggerated fear response to stimuli associated with an aversive experience (Parsons & Ressler, 2013; Rau et al., 2005). These fear-inducing associations can be weakened through exposure therapy in humans and with fear extinction in animals, which involves repeated exposure to the fear-inducing stimuli in the absence of danger (Milad & Quirk, 2012; Rauch et al., 2012). Importantly, nicotine use is bi-directionally associated with anxiety and stress disorders (Koenen et al., 2005; Morissette et al., 2007; Thorndike et al., 2006). Although nicotine product usage and anxiety disorders co-occur in both men and women, the strength of these associations differs between sexes (Breslau, 1995; Thorndike et al., 2006). PTSD and nicotine use are significantly associated only in men, but generalized anxiety and nicotine use are associated to the same extent in men and women (Breslau, 1995; Thorndike et al., 2006). Thus, sex may modulate the relationship between nicotine use and anxiety and stress disorders.
Conditioned fear extinction, which involves repeated exposures to a previously fear-associated cue until the fear response diminishes, is used as a model of exposure therapy in both animal and human studies. It has been shown that acute nicotine enhances contextual fear learning in male and female mice (Gould, 2003; Gould et al., 2004; Gould & Wehner, 1999). In male mice, acute nicotine has been shown to impair contextual fear extinction, stunting the normal decline of the fear response across extinction trials without affecting general freezing behavior (Kutlu & Gould, 2014; Kutlu et al., 2016a; 2017a,b) and enhance spontaneous recovery of extinguished contextual fear (Kutlu et al., 2016b). Chronic nicotine has similar effects on contextual fear learning process. Results from our lab previously demonstrated that chronic nicotine delayed contextual fear extinction in male mice and this effect was sustained during withdrawal parallel to the period of nicotine-induced hippocampal nicotinic acetylcholine receptor upregulation (Kutlu et al., 2016c). However, sex differences in acute and chronic nicotine’s effects on extinction of fear memory have not been explored. Therefore, in the present study, we examined sex differences in the effects of acute and chronic nicotine exposure on fear extinction.
Methods
Subjects were male and female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME), 8–12 weeks of age. Animals were group-housed in a colony room maintained on a 12 h light/dark cycle (lights on at 9:00 am and lights off at 9:00 pm) with ad libitum access to food and water. Behavioral procedures occurred between 9:00 a.m. and 6:00 p.m. Experiments were conducted by individuals blind to conditions. All behavioral procedures were approved by the Temple University and Penn State University Institutional Animal Care and Use Committees. All behavioral procedures were performed at Temple University.
Apparatus
All behavioral procedures took place in one of four identical chambers (26.5 × 20.4 × 20.8 cm) housed in sound attenuated boxes. Chamber walls and ceilings were composed of clear Plexiglas and the floor was a grid of metal bars (0.20 cm diameter) spaced 1.0 cm apart and connected to a shock generator and scrambler (Med Associate, St. Albans, VT, model ENV-414). A speaker for CS presentation was mounted directly above the Plexiglas chamber in each box, and a ventilation fan attached to the side of the box provided background noise and air exchange. Each box was illuminated by a 4-watt light bulb mounted beside the speaker. Conditioned and unconditioned stimuli were controlled by a PC running LabView software (National Instruments, Austin, TX).
Drug Administration
Nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) was dissolved in 0.9% saline. For acute nicotine experiments, saline or nicotine (0.09, 0.18, and 0.36 mg/kg; freebase weights) was administered via intraperitoneal injection 2–4 min prior to behavioral procedures. The acute nicotine doses are chosen based on previous studies from our laboratory examining the effects of acute nicotine on fear extinction (Kutlu & Gould, 2014). For chronic nicotine experiments, nicotine was delivered via subcutaneous osmotic minipump (Alzet, Model 1002, Durect, Cupertino, CA), which allows continuous nicotine administration up to 14 days. Minipumps were surgically inserted through an incision made on the upper back of the mouse while mice were under 2–5% isoflurane anesthesia. Surgeries were conducted under aseptic conditions. Mice in the chronic nicotine experiment received continuous nicotine (12.6, 18, or 24 mg/kg/day; freebase weights). Control animals received sham surgeries, for which identical surgical procedures aside from pump implantation were performed. It should be noted that true blinding to drug conditions could not be achieved, as pumps were present during behavioral training for nicotine mice. As training began on the 7th day following minipump implantation or sham surgery, animals were allowed 6 full days to recover prior to behavioral training. All mini-pumps were validated following removal to ensure adequate amount of nicotine administration. Chronic nicotine doses are based on previous studies from our laboratory showing the impairing effects of chronic nicotine on contextual fear extinction in male mice (Kutlu et al., 2016c).
Behavioral Procedures
Fear extinction procedures were based on previous reports examining the effects of acute nicotine on contextual fear extinction (Kutlu & Gould, 2014). Animals first underwent contextual fear conditioning training, wherein mice received two white noise-footshock (0.57 mA) pairings. All behavioral sessions were approximately 5 minutes in length. All freezing behavior was scored using a time sampling method, for which the experimenter live-scored mice (freezing: yes/no) every 10 seconds. Training sessions consisted of 2 minutes of free movement within the chamber to measure baseline freezing followed by 30 seconds of white noise. 0.57 mA shocks were 2 seconds in length, and co-terminated with white noise. Following termination of the first white noise-shock pairing, animals were scored for immediate freezing to shock for 2 minutes. After this, another 30 seconds of white noise with a co-terminating 2 second 0.57 mA shock. 24 hours later, mice were returned to the training context for retention testing to determine initial freezing to the context. For contextual freezing, mice were placed back in the training context for five minutes in the absence of foot-shocks, and conditioned freezing was measured. Specifically, mice were simply allowed to move freely within the behavioral chambers for 5 minutes, and freezing behaviors were scored throughout. Beginning 24 hours after the retention testing, all mice received single daily extinction sessions for 5 days. For each extinction session, mice were placed back in the training context for five minutes in the absence of foot-shocks and conditioned freezing was measured in the same manner as described above. For acute nicotine experiments, mice received drug 2–4 mins before each extinction session. For the chronic nicotine experiments, training began on the 7th day following minipump implantation or sham surgery. Sample sizes for the acute nicotine were as follows: males n=7–17, females n=9–25. Disproportionate sample sizes between experimental and control groups resulted from the collapsing of saline animals run alongside all dose groups into one control group. Sample sizes for chronic nicotine were as follows: males n=8–10, females n=9–10.
Statistical Analyses
For acute and chronic nicotine extinction, a 2-way ANOVA (drug × extinction day) was conducted on normalized freezing levels (freezing × 100/initial freezing), wherein extinction day was treated as a repeated measure. Freezing was normalized to ensure differences in baseline freezing did not affect subsequent freezing curves (Tian et al., 2008; Kutlu et al., 2016b). Planned comparisons of extinction across doses were made using Bonferroni-corrected t-tests.
Results
Acute Nicotine Extinction
No significant Sex × Drug × Trial interaction (F(15, 465)=0.862, p=0.60) was found. Thus, Drug × Trial interaction was evaluated separately for each sex. For the acute nicotine experiment, animals underwent contextual fear conditioning and testing followed by 5 consecutive days of fear extinction. No baseline sex differences in initial freezing (Sex main effect: F(1, 40)=0.157, p=0.694) or in extinction (Sex × Trial interaction: F(5, 200)=1.135, p=0.343) were observed in saline treated animals. When administered before extinction sessions, acute nicotine impaired extinction in both males (Figure 1a) and females (Figure 1b). Separate 2-way ANOVAs yielded a significant interaction between Extinction Day and Drug in males (F(15, 200)=3.438, p<0.001) and in females (F(15, 265)=2.456, p=0.002). In addition, the Drug main effect was also significant for both males (F(3, 40)=6.654, p=0.001) and females (F(3, 53)=7.436, p<0.001). These results suggest that both males and females were affected by the nicotine treatment. However, Bonferroni-corrected t-tests showed that all doses of nicotine effectively impaired fear extinction in males (0.09 mg/kg: 4th day p=0.05; 0.18 mg/kg: 3rd, 4th, and 5th days p<0.05; 0.36 mg/kg: 3rd, 4th, and 5th days p<0.05) whereas in females, only the 0.36 mg/kg dose impaired extinction (0.36 mg/kg: 2nd, 3rd, 5th days p<0.05). Therefore, males may be more sensitive to the impairing effects of acute nicotine on fear extinction than females (see Figure 1).
Figure 1. Males are more sensitive than females to the impairing effects of acute nicotine on fear extinction.
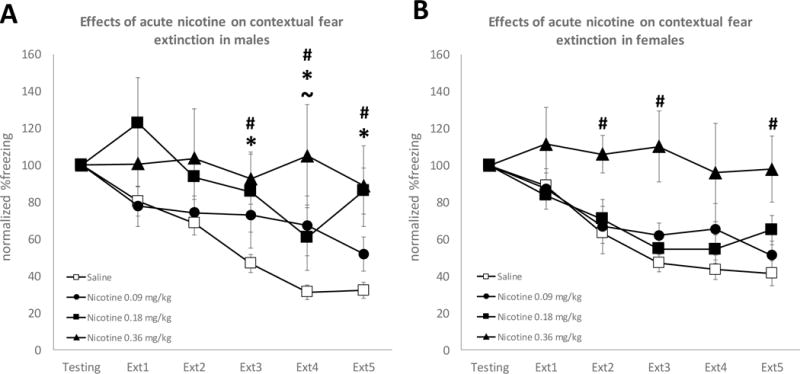
Panel A: Acute nicotine impairs extinction at all doses on extinction days 3–5 in male mice (n=7–17 per group). Panel B: Only the highest dose of nicotine impairs extinction in female mice (n=9–25 per group). ~ p =0.05 on t-tests comparing the 0.09 mg/kg to saline. * p < 0.05 on t-tests comparing the 0.18 mg/kg to saline. # p < 0.05 on t-test comparing 0.36 mg/kg nicotine to saline.
Chronic Nicotine Extinction
No significant Sex × Drug × Trial interaction (F(15, 335)=1.286, p=0.23) was found. Thus, Drug × Trial interaction was evaluated separately for each sex. For the chronic nicotine experiment, animals were administered nicotine for 14 days. Fear conditioning took place on the 7th day of chronic nicotine exposure, and the last day of fear extinction took place on the 14th day of chronic nicotine exposure. No baseline sex differences in initial freezing (Sex main effect: F(1, 18)=0.724, p=0.406) or in extinction (Sex × Trial interaction: F(5, 90)=0.861, p=0.510) were observed in sham surgery animals. Chronic nicotine impaired extinction only in males (Figure 2). Separate 2-way ANOVAs revealed that Extinction Day × Drug interaction was not significant for males (F(15, 160)=1.310, p=0.288) or females (F(15, 175)=1.551, p=0.092). However, the Drug main effect was significant for males (F(3, 32)=5.329, p=0.004) but not for females (F(3, 35)=1.034, p=0.390). Therefore, our results suggest that similar to acute nicotine, chronic nicotine also differentially affects contextual fear extinction in males and females. That is, female mice were less sensitive to the impairing effects of nicotine on fear extinction compared to males (Figures 2).
Figure 2. Chronic nicotine impairs contextual fear extinction in male but not in female mice.
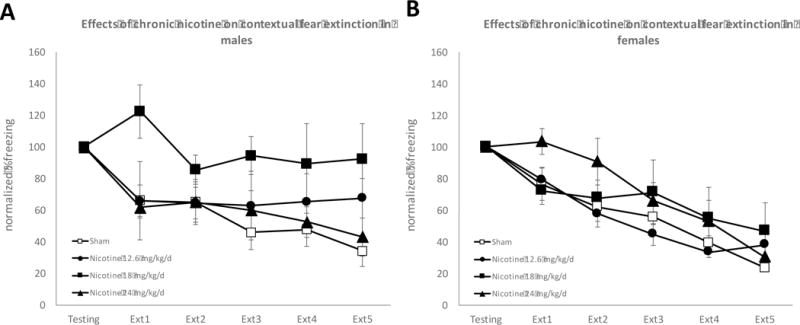
Panel A: Chronic nicotine impairs extinction at the 18 mg/kg/d dose on extinction days 2 and 4 in male mice (n=8–10 per group). Panel B: Chronic nicotine did not affect contextual fear extinction in female mice (n=9–10 per group).
Discussion
Our results indicate that male and female mice are differentially sensitive to the effects of acute and chronic nicotine exposure on the extinction of contextual fear memories. Specifically, when nicotine was administered before each fear extinction trial (acute exposure), males had impaired contextual fear extinction at a broader range of doses than females. When nicotine was continuously administered over an extended period of time (chronic exposure), male mice were again more sensitive than females to nicotine’s impairing effects on contextual fear extinction, with the effect of drug only emerging as significant for males. These results are consistent with previous work suggesting that sex moderates the effects of drugs of abuse on learning and memory (Cha et al., 2007; Kanit et al., 1998; Russo et al., 2003; Kelly et al., 1988; Yilmaz et al., 1997). We found no sex differences in baseline (saline treatment and sham surgery) extinction, consistent with findings that female gonadal hormones do not affect acquisition of extinction memories (Milad et al., 2009). We also found no significant sex differences in baseline fear conditioning (initial freezing), although previous studies (Chang et al., 2009; Gupta et al., 2001) have indicated different contextual fear conditioning between male and female rats, a discrepancy that may be explained by species differences or estrous cycle stage in female subjects (Markus & Zecevic, 1997).
Our results indicated that, although acute nicotine impairs extinction in both sexes, either the threshold for sensitivity to the effects of nicotine on contextual fear extinction is higher in females or the magnitude of impairment is greater in males. This suggests females may have a potential protective mechanism against nicotine-induced impairment of fear extinction. Estrogen modulates behavioral and synaptic correlates of learning and memory (Li et al., 2004; Spencer et al., 2008; Wallace et al., 2006; Woolley & McEwen, 1992), and this association has been shown to be mediated by acetylcholinergic function (Daniel & Dohanich, 2001). Thus, differences in circulating estrogen levels between males and females is a potential underlying mechanism for the differences in the effects of nicotine on extinction between males and females. However, predictions about the contribution of estrogen to sex differences in nicotine’s effects on extinction are limited as we did not control for estrous cycle stage in female mice because the entire mouse estrous cycle is shorter than the extinction procedure (Byers et al., 2012). Additionally, it has been previously shown that higher acute doses of nicotine may impair locomotor activity in male mice; thus, true differences in freezing between male and female mice at the 0.36 mg/kg dose may have been masked by sex differences in locomotor sensitivity to nicotine (Kutlu et al., 2016d; Marks et al., 1989). Another possibility is that acute nicotine administered only during extinction may serve as an internal contextual cue (Bouton, 2002). However, we ruled out the possibility in our previous work where we showed that mice tested in a novel physical context following contextual fear conditioning did not show increased freezing behavior (Kutlu & Gould, 2014). This suggest that context switch following fear learning does not contribute to sustained freezing response seen in acute nicotine-administered group.
In sum, the present findings suggest that nicotine could differently impact anxiety and stress disorder development and prognosis in men and women. In the current study, female mice were, to a degree, protected from the impairing effects of nicotine on contextual fear extinction. This would appear to be in contrast with findings that women are more likely to be diagnosed with PTSD and other anxiety disorders; however, it has been found that comorbidity of anxiety disorders and substance abuse is more prevalent in men than in women (McLean et al., 2011). This may imply that men are more sensitive to the exacerbating effect of substance use on anxiety and stress disorder symptomology. These findings are the first to suggest that sex is a moderator of nicotine’s effects on contextual fear extinction.
Highlights.
Males exhibited contextual fear extinction deficits following acute and chronic nicotine exposure.
Females also exhibited extinction deficits, but only at the highest doses of acute nicotine (0.36 mg/kg).
Chronic nicotine did not result in extinction deficits in female mice.
Acknowledgments
This work was funded with grant support from the National Institute on Drug Abuse (T.J.G., DA017949; 1U01DA041632), Jean Phillips Shibley Endowment, and Penn State Biobehavioral Health Department.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We declare no potential conflict of interest.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behavior Genetics. 1995;25(2):95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Archives of general psychiatry. 2003;60(3):289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Archives of general psychiatry. 1991;48(12):1069–1074. doi: 10.1001/archpsyc.1991.01810360033005. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biological psychiatry. 2002;52(10):976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PloS one. 2012;7(4):e35538. doi: 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YM, Jones KH, Kuhn CM, Wilson WA, Swartzwelder HS. Sex differences in the effects of Δ9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behavioural pharmacology. 2007;18(5–6):563–569. doi: 10.1097/FBP.0b013e3282ee7b7e. [DOI] [PubMed] [Google Scholar]
- Chang YJ, Yang CH, Liang YC, Yeh CM, Huang CC, Hsu KS. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor β. Hippocampus. 2009;19(11):1142–1150. doi: 10.1002/hipo.20581. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. Journal of Neuroscience. 2001;21(17):6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ. Nicotine produces a within-subject enhancement of contextual fear conditioning in C57BL/6 mice independent of sex. Integrative physiological and behavioral science. 2003;38(2):124–132. doi: 10.1007/BF02688830. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Feiro O, Moore D. Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behavioural brain research. 2004;155(1):167–173. doi: 10.1016/j.bbr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behavioural brain research. 1999;102(1):31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats. Brain research. 2001;888(2):356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Kanit L, Taşkıran D, Furedy JJ, Kulali B, McDonald R, Pogun S. Nicotine interacts with sex in affecting rat choice between “look-out” and “navigational” cognitive styles in the Morris water maze place learning task. Brain Research Bulletin. 1998;46(5):441–445. doi: 10.1016/s0361-9230(98)00008-2. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Goodlett CR, Hulsether SA, West JR. Impaired spatial navigation in adult female but not adult male rats exposed to alcohol during the brain growth spurt. Behavioural brain research. 1988;27(3):247–257. doi: 10.1016/0166-4328(88)90121-0. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J, Tsuang M. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Archives of general psychiatry. 2005;62(11):1258–1265. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- Kutlu MG, Gould TJ. Acute nicotine delays extinction of contextual fear in mice. Behavioural brain research. 2014;263:133–137. doi: 10.1016/j.bbr.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Holliday E, Gould TJ. High-affinity α4β2 nicotinic receptors mediate the impairing effects of acute nicotine on contextual fear extinction. Neurobiology of Learning and Memory. 2016a;128:17–22. doi: 10.1016/j.nlm.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Oliver C, Huang P, Liu-Chen LY, Gould TJ. Impairment of contextual fear extinction by chronic nicotine and withdrawal from chronic nicotine is associated with hippocampal nAChR upregulation. Neuropharmacology. 2016b;109:341–348. doi: 10.1016/j.neuropharm.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Tumolo JM, Holliday E, Garrett B, Gould TJ. Acute nicotine enhances spontaneous recovery of contextual fear and changes c-fos early gene expression in infralimbic cortex, hippocampus, and amygdala. Learning & Memory. 2016c;23(8):405–414. doi: 10.1101/lm.042655.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Braak DC, Tumolo JM, Gould TJ. Adolescent mice are less sensitive to the effects of acute nicotine on context pre-exposure than adults. Brain research. 2016d;1642:445–451. doi: 10.1016/j.brainres.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Zeid D, Tumolo JM, Gould TJ. Adolescent mice are less sensitive to the impairing effects of acute nicotine on extinction. Brain Research Bulletin. 2017a doi: 10.1016/j.brainresbull.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Garrett B, Gadiwalla S, Tumolo JM, Gould TJ. Acute nicotine disrupts consolidation of contextual fear extinction and alters long-term memory-associated hippocampal kinase activity. Neurobiology of learning and memory. 2017b doi: 10.1016/j.nlm.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proceedings of the National Academy of Sciences. 2004;101(7):2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Genetic influences on nicotine responses. Pharmacology Biochemistry and Behavior. 1989;33(3):667–678. doi: 10.1016/0091-3057(89)90406-1. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Zecevic M. Sex differences and estrous cycle changes in hippocampus-dependent fear conditioning. Psychobiology. 1997;25(3):246–252. [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. Journal of psychiatric research. 2011;45(8):1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164(3):887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annual review of psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette SB, Tull MT, Gulliver SB, Kamholz BW, Zimering RT. Anxiety, anxiety disorders, tobacco use, and nicotine: a critical review of interrelationships. Psychological bulletin. 2007;133(2):245. doi: 10.1037/0033-2909.133.2.245. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Ressler KJ. Implications of memory modulation for post-traumatic stress and fear disorders. Nature neuroscience. 2013;16(2):146–153. doi: 10.1038/nn.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neuroscience & Biobehavioral Reviews. 2005;29(8):1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Rauch M, Sheila A, Eftekhari A, Ruzek JI. Review of exposure therapy: a gold standard for PTSD treatment. Journal of Rehabilitation Research & Development. 2012;49(5) doi: 10.1682/jrrd.2011.08.0152. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain research. 2003;970(1):214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Frontiers in neuroendocrinology. 2008;29(2):219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike FP, Wernicke R, Pearlman MY, Haaga DA. Nicotine dependence, PTSD symptoms, and depression proneness among male and female smokers. Addictive behaviors. 2006;31(2):223–231. doi: 10.1016/j.addbeh.2005.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Gao J, Han L, Fu J, Li C, Li Z. Prior chronic nicotine impairs cued fear extinction but enhances contextual fear conditioning in rats. Neuroscience. 2008;153(4):935–943. doi: 10.1016/j.neuroscience.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain research. 2006;1126(1):176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat [published erratum appears in J Neurosci 1992 Oct; 12 (10): following table of contents] Journal of Neuroscience. 1992;12(7):2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz Ö, Kanit L, Okur BE, Pogun S. Effects of nicotine on active avoidance learning in rats: sex differences. Behavioural pharmacology. 1997 [PubMed] [Google Scholar]


