Abstract
In the last decade, numerous growth factors and biomaterials have been explored for the treatment of myocardial infarction (MI). While pre-clinical studies have demonstrated promising results, clinical trials have been disappointing and inconsistent, likely due to poor translatability. In the present study, we investigate a potential myocardial regenerative therapy consisting of a protein-engineered dimeric fragment of hepatocyte growth factor (HGFdf) encapsulated in a shear-thinning, self-healing, bioengineered hydrogel (SHIELD). We hypothesized that SHIELD would facilitate targeted, sustained intramyocardial delivery of HGFdf thereby attenuating myocardial injury and post-infarction remodeling. Adult male Wistar rats (n=45) underwent sham surgery or induction of MI followed by injection of phosphate buffered saline (PBS), 10 μg HGFdf alone, SHIELD alone, or SHIELD encapsulating 10 μg HGFdf. Ventricular function, infarct size, and angiogenic response were assessed 4-weeks post-infarction. Treatment with SHIELD+HGFdf significantly reduced infarct size and increased both ejection fraction and borderzone arteriole density compared to the controls. Thus, sustained delivery of HGFdf via SHIELD limits post-infarction adverse ventricular remodeling by increasing angiogenesis and reducing fibrosis. Encapsulation of HGFdf in SHIELD improves clinical translatability by enabling minimally-invasive delivery and subsequent retention and sustained delivery of this novel, potent angiogenic protein analog.
Keywords: Hydrogel, Angiogenesis, Growth factor, Myocardial Regeneration, Myocardial Infarction
Introduction
Ischemic heart disease remains the leading cause of global morbidity and mortality (World Health Statistics 2014). Revascularization via percutaneous catheter intervention or coronary artery bypass grafting effectively restores macrovascular perfusion following MI, but does not treat coexistent microvascular dysfunction (Heidenreich et al. 2011, Boodhwani et al. 2006). The resulting microvascular perfusion deficit leads to adverse left ventricular (LV) remodeling and subsequent heart failure, which many survivors of MI will eventually succumb to without ventricular assist device implantation or heart transplantation (World Health Statistics 2014, Mozaffarian et al. 2016). The discovery of alternative revascularization therapies to restore microvascular perfusion and limit subsequent maladaptive LV remodeling is critically needed.
Significant progress has been made in the field of regenerative medicine and biomaterials for the treatment of ischemic cardiomyopathy (Rane et al. 2011, Segers et al. 2011). Cardiac regenerative medicine approaches utilize cell and cytokine delivery to enhance microvascular angiogenesis, ultimately aiming to restore functional heart tissue. Hepatocyte growth factor (HGF) has been studied pervasively as a potential therapy for MI, demonstrating anti-apoptotic, pro-angiogenic, and cardioprotective properties both in vitro and in vivo (Jin et al. 2004, Sala et al. 2011). As a potent agonist of the tyrosine kinase surface receptor c-MET, HGF activates downstream angiogenic canonical pathways, including Akt and MAPK/ERK (Jin et al. 2004, Sala et al. 2011, Jones et al. 2011). Despite its promise, clinical translation of recombinant human HGF (rh-HGF) is limited primarily due to its prohibitively costly and difficult manufacture, as well as its instability, exhibiting a short in vivo half-life. Recently, a novel dimeric fragment of HGF (HGFdf) was engineered with similar potency to rh-HGF, but with increased thermal stability and expression yield (Jones et al. 2011, Liu et al. 2014). A small pilot study in a rat ischemia-reperfusion model demonstrated HGFdf released via an extracellular matrix hydrogel to have beneficial functional effects after ischemia (Sonnenberg 2015). This study also highlighted the importance of a drug vehicle when delivering HGFdf to the heart.
While, direct intramyocardial injection of cytokines and growth factors can stimulate endogenous regenerative pathways, this method has limitations. Proteins delivered to the tissue are subject to rapid diffusion away from the injection site and degradation, lowering the in vivo half-life and rendering the therapy ineffective (Tous et al. 2011, Jones et al. 2011). Encapsulation of cytokines within injectable hydrogels offers an attractive delivery platform for extending delivery in the region of interest and sustaining therapeutic efficacy. Many hydrogels have been investigated for cytokine delivery, but few are suitable for minimally-invasive myocardial delivery (Rane et al. 2011, Segers et al. 2011, Tous et al. 2011). To address this issue, Mulyasasmita et al. engineered an injectable hydrogel that undergoes a reversible physical crosslinking mechanism to enable shear-thinning during injection and rapid self-healing post-injection (Mulyasamita et al. 2014). The crosslinking occurs between an engineered protein and a peptide-conjugated polymer that associate at specific molecular recognition motifs to form a gel state. When subjected to shear force (i.e. injection), the hydrogel flows as a solution and then rapidly recovers back into a gel state once the force is removed. This hydrogel is unique because the heterodimeric molecular-recognition between peptide motifs eliminates the need for non-physiologic crosslinking mechanisms, which can damage encapsulated growth factors, and allows for precise control over hydrogel mechanics (Cai et al. 2015, Mulyasamita et al. 2014, Cai et al. 2016). Due to their potential use in clinically translatable delivery strategies, these materials were termed Shear-thinning Hydrogels for Injectable Encapsulation and Long-term Delivery, or SHIELD (Cai et al. 2015).
In this study, we addressed the limitations of naked growth factor delivery by encapsulating HGFdf into SHIELD for precise and prolonged HGFdf delivery. We hypothesized that SHIELD would facilitate targeted, sustained intramyocardial delivery of HGFdf and attenuate myocardial injury and post-infarction remodeling through pro-angiogenic and anti-fibrotic effects.
Methods
HGFdf Synthesis
HGFdf is a dimeric form of the eNK1 monomer linked by disulfide bonds. The eNK1 monomer contains the N domain and first kringle domain of HGF, engineered to have eight point mutations resulting in increased stability and expression yield. HGFdf was synthesized and characterized as previously reported (Liu et al. 2014). Briefly, the DNA encoding HGFdf was cloned into the pPIC9K plasmid (Life Technologies, Grand Island, NY) and transformed into the Pichia pastoris vector strain GS115. Colonies that survived geneticin selection were inoculated and induced with methanol for 3 days. Yeast cells were pelleted by centrifugation, and the supernatant collected for Ni-nitrilotriacetic acid affinity chromatography. The elution fractions containing HGFdf were buffer-exchanged into 1×PBS + 500 mM NaCl (PBS500) and further purified with size exclusion chromatography using a Superdex 75 10/300 GL (GE Healthcare, Pittsburgh, PA). Protein purity was analyzed using 12% Tris-Glycine SDS-PAGE (Life Technologies, Grand Island, NY). Protein was flash-frozen in 0.01% Tween80 in PBS500 and stored at −80°C. Thawed protein was kept at 4°C and used within 3 weeks.
In Vitro Receptor Activation
The activation of c-MET and ERK receptors in response to HGFdf was assessed using Wes™ western blot technology per the manufacturer’s protocol (ProteinSimple, San Jose, CA). Neonatal rat cardiomyocytes were isolated from 0–3 day old Sprague-Dawley rats using the Pierce™ Primary Cardiomyocyte Isolation Kit (ThermoFisher Scientific, Waltham, MA). The cells were cultured in DMEM, 10% fetal bovine serum, and 1% Penicillin/Streptomycin (Sigma Aldrich, St. Louis, MO). The cells were pre-plated for 2 hours at 37°C and 5% CO2 to remove fibroblasts. The remaining cell suspension was collected and plated in a 12-well tissue culture plate at 1×106 cells per well (Corning™ Costar™, Fisher Scientific, Waltham, MA). After 24 hours, the media was switched to serum-free DMEM and 1% Pencillin/Stretomycin for another 24 hours. Next, the experimental groups (n=4/group): untreated, 10 nM HGFdf, or 10 nM rh-HGF (R&D Systems, Minneapolis, MN) were added to the wells, and cells were then placed in hypoxia (1% O2) for 4 hours. Cells were then lysed and the sample concentration was adjusted to 0.2 mg/mL using a Bradford assay (BioRad, Hercules, CA). Samples were combined 1 part with 4 parts 5X Fluorescent Master Mix, denatured, and loaded into the Wes Separation 12–20 kDa Capillary Cartridges with antibodies per the manufacturer’s instructions. Primary antibodies include total MET (tMET) (1:25, ab51067 [Abcam, Cambridge, UK]), phospho-MET (pMET) (1:25, sc-34086 [Santa Cruz Biotechnology, Inc., Santa Cruz, CA]), total ERK (tERK) (1:150, ab115799 [Abcam]), and phoshpo-ERK (pERK) (1:50, cs4376 [Cell Signaling Technology, Danvers, MA]). The digital images were analyzed with Compass software (ProteinSimple) on Wes and band density differences reported as width of band.
In Vitro Cell Experiments
For the tetrazolium salt WST-1 cell viability assay (Abcam), neonatal cardiomyocytes were isolated from 0–3 day old Sprague-Dawley rats and cultured as above. After pre-plating, the remaining cell suspension was collected and plated at 400,000 cells per well of a 24-well tissue culture well (Fisher Scientific, Waltham, MA). After 24 hours of serum-starvation, the same experimental groups (n=4/group) were added to the wells and cells were placed in hypoxia (1% O2) for 4 hours, after which the WST-1 cell viability assay was performed and absorbance was analyzed in a Synergy 2 Biotek Microplate plate-reader at 440 nm.
For ApoLive-Glo™ Multiplex Assay to assess caspase-3/7 activation, cardiomyocytes were cultured as above and plated at 30,000 cells per well of a 96-well plate. After the 24-hour serum starvation, the same experimental groups were added to the wells (n=8/group). After 24 hours, the ApoLive-Glo™ assay (Promega Life Sciences, Madison, WI) was performed and luminescence was analyzed via microplate reader.
For the analysis of in vitro angiogenesis, human umbilical vein endothelial cells (HUVECs) were resuspended and cultured in basal medium (Lonza), basal medium plus 10 nM rh-HGF, or basal medium plus 10 nM HGFdf. The cells were seeded (75,000 cells/well) on top of Matrigel in a 24-well plate and allowed to adhere and grow for 48 hours, after which the cells were fixed in 4% paraformaldehyde and permeabilized with 0.25% Triton X-100 solution in PBS. The cells were stained with rhodamine phalloidin (1:300, Life Technologies, Carlsbad, CA) and DAPI. The extent of tubule formation was analyzed with microscopy (DMi8, Leica Microsystems, Wetzlar, Germany). The total network length, number of junctions, number of branches, and loop perimeter were quantified for each image using ImageJ software.
SHIELD Synthesis and Preparation
SHIELD was synthesized as previously reported (Cai et al. 2015). Briefly, 8-arm polyethylene glycol sulfone (PEG-VS) (MW 20 kDa) was purchased from JenKem Technology (Plano, TX) and the custom peptide P1 (sequence: EYPPYPPPPYPSGC) was purchased from Genscript Corp (Piscataway, NJ, USA). The PEG-P1 copolymer was synthesized by reacting 8-arm PEG-VS in excess P1 in the presence of tris(2-carboxyethyl)phosphine. Unbound P1 is removed via dialysis. The DNA encoding the C7 linear protein block copolymer was cloned into the pET-15b vector (Novagen) and transformed into the BL21(DE3)pLysS Escherichia coli host strain (Life Technologies). The protein was expressed following isopropyl β-D-1-thiogalactopyranoside induction, purified by affinity chromatography via the specific binding of N-terminal polyhistidine tag to Ni-nitrilotriacetic acid resin (Qiagen), dialyzed against PBS, and concentrated by diafiltration across Amicon Ultracel filter units (Millipore). Each of the seven WW domains in C7 was treated as one C unit, and each pendant peptide group in PEG-P1 was treated as one P unit. SHIELD was formed by mixing C7 and PEG-P1 copolymer to achieve a C:P ratio of 1:1 and a final concentration of 10% w/v in PBS.
HGFdf Conjugation to SHIELD
HGFdf was immobilized to SHIELD via reaction between malemide and sulfhydryl groups, respectively. To add sulfhydryl groups onto C7, the concentration of C7 was adjusted to 5 mg/mL in PBS containing 2 mM EDTA. Traut’s reagent (2 mg/mL) (ThermoFisher Scientific, Waltham, MA) was added. After 1 hour at room temperature, Traut’s reagent was removed using a desalting column (Fisher Scientific). HGFdf (1.25 μg/μL) was prepared with maleimide groups by addition of 50 μL of Sulfo-SMCC (5 mg/mL) (ThermoFisher) to react with HGFdf amines for 2 hours at 4°C. Excess Sulfo-SMCC was removed using a desalting column. The sulfhydryl-modified C7 and malemide-modified HGFdf were mixed in 1 mL of triethanolamine buffer with 5 mM TCEP for 2 hours at 4°C to induce conjugation of HGF-C7. The mixture was dialyzed against PBS using a 10,000 MWCO cassette overnight at 4°C. HGF-C7 was mixed with standard C7 to achieve desired concentrations before use.
In Vitro HGFdf Release and Activity
Cyanine5 NHS ester (Lumiprobe Corporation, Hallandale Beach, FL) was used to fluorescently label HGFdf following the manufacturer’s protocol. Briefly, HGFdf was dissolved in a 9:10 reaction volume with pH 8.3 using a 0.1 M bicarbonate buffer solution. The Cyanine5 NHS ester was dissolved in 1:10 reaction volume of DMSO and added to the HGFdf solution to yield a labeled product. Excess Cyanine5 was removed via Amicon Ultracel filter units. Subsequently, labeled HGFdf was then conjugated to the C7 component of SHIELD as described above. After conjugation, labeled HGFdf-C7 was mixed with additional C7 to the desired volume (30 μL) and HGFdf concentration (10 μg/30 μL) and then mixed with PEG-P1 (30 μL) to create a gel with a C:P ratio of 1:1. Gels (60 μL) were mixed and formed in a 1.5 mL Eppendorf tube (n=4) and allowed to undergo sol-gel phase transition for 15 min at 37°C in a humidified incubator. 1 mL of PBS was added to each tube and volumes of 100 μL were sampled from the PBS supernatant for testing and replenished with 100 μL of fresh PBS over a period of 14 days. Release of HGFdf from SHIELD at each time point was measured with a SpectraMax M Series Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, CA).
To determine HGFdf activity after release, rat neonatal cardiomyocytes were cultured at 400,000 cells/well in a 24-well plate. Hydrogels (n=4, volume=60 μL) containing 10 μg of HGFdf were prepared as described above. After 24-hour serum starvation, cylindrical hydrogels were formed in the barrel of a 1 mL insulin syringe. The formed gel was deposited into a transwell suspended over the cardiomyocytes and submerged into the cell media. The cardiomyocytes were then subjected to 4 hours of hypoxia, after which the WST-1 cell viability assay (Abcam) was performed and absorbance was analyzed via microplate reader at 440 nm.
Animal Care and Biosafety
Rats weighing 250–300 g were obtained from Charles River Laboratories (Wilmington, MA). All experiments conformed to the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health. This protocol was approved by the Administrative Panel on Laboratory Animal Care of Stanford University (protocol #28921).
Animal Model
Adult male Wistar rats (n=45) underwent sham surgery or permanent ligation of the left anterior descending artery (LAD) to induce an MI using an established and highly reproducible model (MacArthur et al. 2013, Shudo et al. 2015). Briefly, anesthesia was induced with 2% isoflurane. Animals were endotracheally intubated with a 16-gauge angiocatheter and mechanically ventilated (Hallowell EMC, Pittsfield, MA) with 1.5% isoflurane. The heart was exposed via left thoracotomy and a 6-0 polypropylene suture was used to ligate the LAD 1–2 mm below the left atrial appendage to produce an anterolateral MI. Immediately following ligation, animals received a 60 μL injection of PBS (n=10), 10 μg HGFdf alone (n=10), SHIELD (n=10), or SHIELD+10 μg of HGFdf (n=10). Five animals underwent sham surgery without coronary ligation. The thoracotomy was closed with 4-0 polypropylene suture; buprenorphine (0.5 mg/kg) and carprofen (5 mg/kg) were given for analgesia, and animals were recovered.
Echocardiographic Functional Assessment
Left ventricular function was evaluated at 4 weeks using a VisualSonics Vevo 2100 (FUJIFILM Visual Sonics Inc, Ontario, Canada) digital imaging system with a MS250 transducer (13 MHz). LV parasternal long-axis 2-dimensional and M-mode images were acquired at two levels within the LV (mid-papillary and between the papillary muscles and apex). Image acquisition and all analyses were performed by a single, blinded investigator.
Histological Analysis and Immunohistochemistry
To assess infarct size angiogenesis, hearts were arrested with KCl, explanted, flushed with PBS, filled retrograde with Tissue Tek optimum cutting temperature (OCT) compound (Sekura, The Netherlands), frozen, and stored at −80°C. Fifteen 10-μm-thick sections were prepared from each heart at five different levels throughout the infarct.
In a subset of animals (n=8/group), two sections at the level of the mid-papillary were stained with Masson’s trichrome. Standard digital photographs were taken with an Epson V550 Color Scanner (Epson, Long Beach, CA). Photographs were uploaded to ImageJ, and the size of the infarct was calculated as the percent of total circumference of the LV. Two 10-μm-thick sections from each animal were stained with antibodies directed against α-smooth muscle actinin (SMA) (1:200) and troponin (1:200) (Abcam) to quantify vessel density in the infarct and borderzone. Sections were fixed with 4% paraformaldehyde, blocked with 10% fetal bovine serum, incubated with the primary antibodies, and then stained with fluorescently labeled secondary antibodies at a 1:200 dilution (Alexa Fluor 488 and Alexa Fluor 647, respectively; Invitrogen, Carlsbad, CA). Images were acquired with a Zeiss confocal microscope with a 10× objective. Arterioles were defined and quantified by the following criteria: (1) a visible lumen, (2) lumen diameter in the range of 10–100 μm and (3) positive α-SMA staining.
Statistical Analysis
All analyzed variables approximated a normal distribution and are reported as mean ± standard error of the mean (SEM). Pairwise Student t tests were used to assess differences between variables with a Bonferroni correction used for multiple comparisons. Analyses were performed with RStudio (RStudio Inc.). The threshold for statistical significance was P<0.05.
Results
HGFdf activates the c-Met receptor and downstream signaling pathway in cardiomyocytes
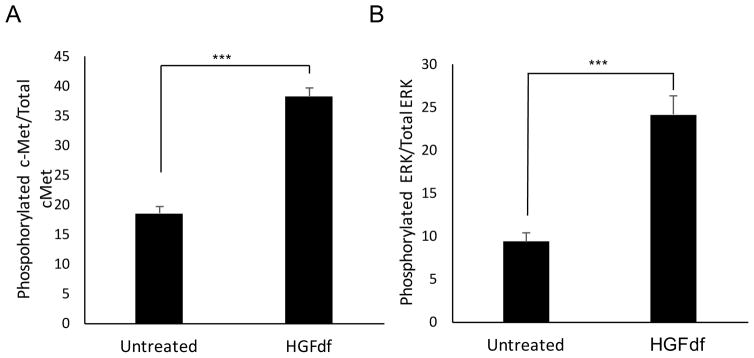
To determine the effect of HGFdf on cardiomyocytes, we measured in vitro receptor activation of c-Met, the cognate receptor of full-length HGF. In rat cardiomyocytes that were serum and oxygen starved, treatment with HGFdf resulted in significant phosphorylation of the c-Met receptor compared to the untreated control (Figure 1A). We investigated activation of a downstream receptor in the c-Met signaling pathway, namely ERK, which inhibits apoptosis (Jones et al. 2011). HGFdf treatment led to significant cardiomyocyte activation of ERK compared to controls (Figure 1B).
Figure 1.
c-Met receptor activation by HGFdf. Cardiomyocytes were serum starved for 24 hours before administration of HGFdf, at which point the cells were placed into hypoxia for 4 hours. Cells that were treated with HGFdf exhibited significant activation (phosphorylation) of the (A) c-Met receptor and (B) ERK (***p<0.001).
HGFdf significantly enhances cardiomyocyte viability and reduces apoptosis after serum starvation and hypoxia
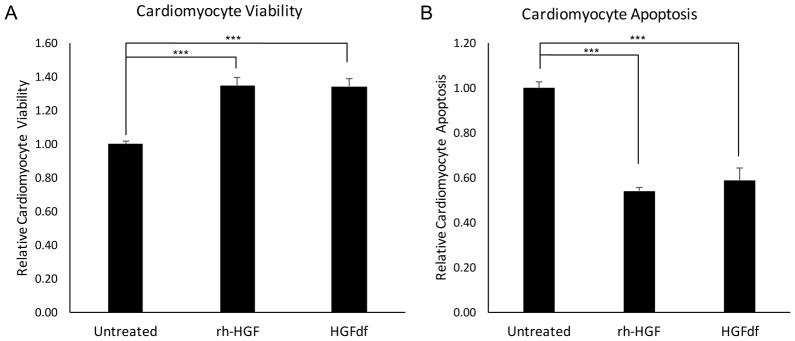
After 24 hours of serum starvation and 4 hours of hypoxia, rat neonatal cardiomyocytes treated with HGFdf exhibited a 34±5% increase in cellular viability and a 41±6% decrease in apoptosis compared to the untreated control. Similarly, cells treated with rh-HGF demonstrated a 34±4% increase in cellular viability and 46±2% decrease in apoptosis (Figure 2). Cell viability and extent of apoptosis did not differ between cells treated with rh-HGF and HGFdf (p=.94, p=.45, respectively), thereby suggesting that HGFdf and rh-HGF are similarly potent.
Figure 2.
In vitro efficacy of HGFdf on cardiomyocytes. (A) HGFdf significantly enhanced cardiomyocyte viability by 35% as measured by WST-1 metabolic activity assay. (B) HGFdf significantly reduced cardiomyocyte apoptosis by 41% compared to the untreated control as measured by caspase activity (***p<0.001).
HGFdf significantly enhances in vitro angiogenesis
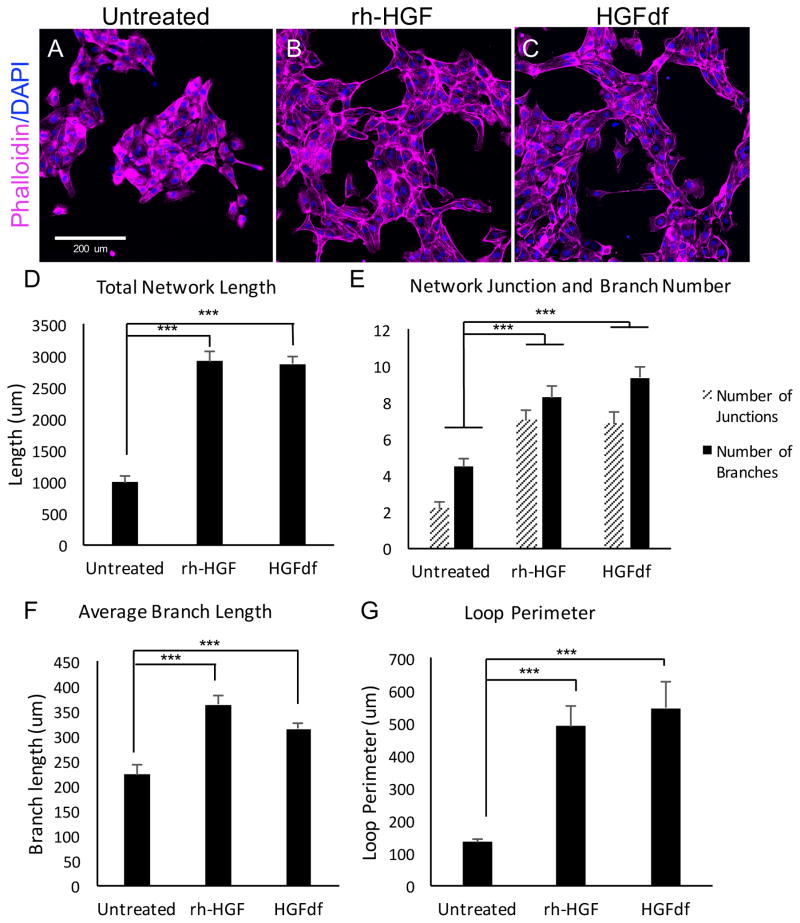
Measuring network tubule formation is an established in vitro method of quantifying angiogenesis (Cai et al. 2013, Staton et al. 2009, Khoo et al. 2011). We measured the angiogenic-like behavior of HUVECs cultured in basal media when treated with HGFdf or left untreated. Cells that were treated with HGFdf exhibited extensive network formation compared with untreated control, as HGFdf significantly increased total network length (2869±131μm; 990±103μm; p<0.001), number of junctions (6.8±0.6; 2.2±0.3; p<0.001), number of branches (9.4±0.5; 4.5±0.4; p<0.001), average branch length (315±11.1μm; 223±18.1μm; p<0.001) and loop perimeter (547±81.5μm; 134±7.5μm; p<0.001) (Figure 3). Network formation was not different between rh-HGF- and HGFdf-treated cells.
Figure 3.
In vitro angiogenesis in response to HGFdf treatment. Representative micrographs of human umbilical cord endothelial cells cultured in basal media and then (A) left untreated, treated with (B) rh-HGF or (C) HGFdf. Treatment with rh-HGF or HGFdf leads to (D) a significant increase in total network length, (E) a significant increase in the number of junctions and branches, (F) a significant increase in branch length, and finally, (G) increased loop perimeter (***p<0.001).
SHIELD+HGFdf formation, release kinetics, and activity after release
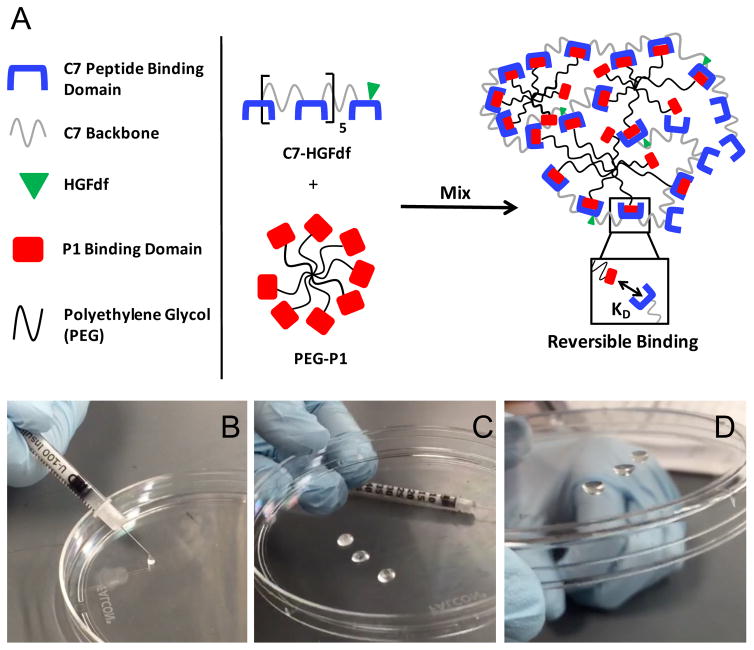
Upon mixing the C7 and PEG-P1 components inside the barrel of an insulin syringe, the SHIELD gel was formed within three seconds. The SHIELD gel was previously reported to have a shear storage modulus (G′) of ~10 Pa (Cai et al. 2015). This gel state was reversible upon hand-application of a shear force to easily eject the material from the syringe needle. Once ejected, the material self-heals to reform the gel state within 3 seconds (Figure 4).
Figure 4.
SHIELD formation and mechanics. (A) SHIELD is composed of an engineered C7 protein and an 8-arm PEG conjugated with P1 binding peptides. HGFdf is conjugated to the C7 component of SHIELD. SHIELD is formed upon mixing the two components under physiologic conditions. (B–D) Shear-thinning and self-healing capability of SHIELD. The two components were mixed in the barrel of the insulin syringe and allowed to gel. Upon hand-application of a shear force, the solution state reformed and injected through the needle. Almost immediately after, the gel state reformed, demonstrated by (D) flipping the dish upside down.
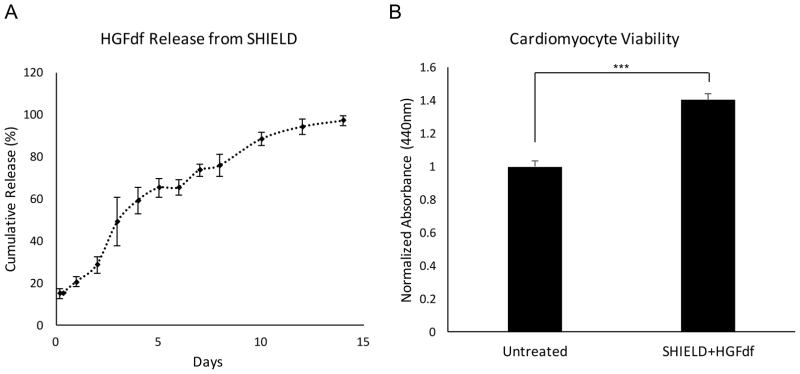
HGFdf release was sustained for approximately 14 days in vitro when conjugated to the C7 component of SHIELD (Figure 5A). A brief burst release on day 0 was observed, presumably due to the initial dilution of SHIELD components in PBS. After the initial burst period, HGFdf release was steady for approximately 14 days as the hydrogel eroded. Following chemical conjugation of HGFdf to SHIELD, we investigated fragment bioactivity on serum- and oxygen-starved neonatal cardiomyocytes after release to ensure preserved functionality. Compared to the untreated control, treatment with HGFdf released from SHIELD resulted in a 40±8% improvement in cell viability, indicating that the fragment remained functional after conjugation (Figure 5B).
Figure 5.
HGFdf release profile and activity after conjugation to SHIELD. (A) HGFdf exhibited an initial burst release from SHIELD, likely due to the dilution of SHIELD in PBS. After the burst period on day 0, HGFdf demonstrated a steady release for the subsequent 14 days. (B) The biofunctionality of HGFdf after release from SHIELD was quantified using serum- and oxygen-starved cardiomyocytes. After a 4-hour incubation with SHIELD+HGFdf, treated cardiomyocytes exhibited a 40% increase in viability (***p<0.001).
SHIELD+HGFdf enhances left ventricular function, reduces infarct size, and increases infarct arteriole formation
Echocardiographic assessment of cardiac function revealed significant functional benefits in the SHIELD+HGFdf treated animals compared to the other study groups (Table 1). At 4 weeks following the MI, ejection fraction (EF) was significantly enhanced compared with that of the PBS group (SHIELD+HGFdf, 40.0±0.9%; PBS, 27.4+1.6% p<0.001), the HGFdf alone group (29.0±2.9%, p=0.0022) and the SHIELD alone group (32.1±1.0%, p=0.046). Similarly, fractional area change (FAC) was significantly enhanced in SHIELD+HGFdf animals compared to the PBS and HGFdf groups (SHIELD+HGFdf, 27.7±1.1%; PBS, 17.7±1.6%, p=0.0012; HGFdf 20.0±2.5, p=0.039). In addition, stroke volume (SV) was preserved in the SHIELD+HGFdf group and was significantly greater compared with that of the PBS group (SHIELD+HGFdf, 318.3±13.7 μL; PBS, 216.1±53.8 μL, p<0.001), the HGFdf group (197.5±21.7 μL, p<0.001) and the SHIELD alone group (241.3±13.1, p=0.022). We also observed improvement in LV geometry with SHIELD+HGFdf treated animals having reduced LV end-systolic volume and LV inner diameter at end-systole (459.2±28.7 and 6.9±0.2) compared to the PBS control (628.9±38.8 and 8.1±0.3, p=0.0084 and p=0.053, respectively).
Table I.
Left Ventricular Function and Geometry Four Weeks Post-MI
| PBS | HGFdf | SHIELD | SHIELD+HGFdf | Sham | |
|---|---|---|---|---|---|
|
| |||||
| FAC, % | 17.7±1.6 | 20.0±2.5 | 20.8±0.7 | 27.7±1.1 | 51.2±3.6 |
| p=0.0012 | p=0.039 | p=0.089 | p<0.001 | ||
|
| |||||
| EF, % | 27.4+1.6 | 29.0±2.9 | 32.1±1.0 | 40.0±0.9 | 69.5±3.9 |
| p=0.00013 | p=0.0022 | p=0.046 | p<0.001 | ||
|
| |||||
| SV, μL | 216.1±15.5 | 197.5±21.7 | 241.3±13.1 | 318.3±13.7 | 365.6±28.4 |
| p=0.00051 | p=0.00012 | p=0.022 | p=1.0 | ||
|
| |||||
| Vs, μL | 628.9±128.7 | 547.9±96.3 | 571.4±128.0 | 459.2±90.7 | 195.0±26.0 |
| p=0.0084 | p=0.91 | p=0.33 | p<0.001 | ||
|
| |||||
| LVIDs | 8.1±0.3 | 8.0±0.3 | 8.3±0.3 | 7.0±0.2 | 4.0±0.3 |
| p=0.053 | p=0.16 | p=0.034 | p<0.001 | ||
|
| |||||
| LVIDd | 9.0±0.2 | 9.3±0.2 | 9.6±0.4 | 8.6±0.2 | 7.4±0.3 |
| p=0.71 | p=0.14 | p=0.16 | p<0.001 | ||
P values refer to comparison between SHIELD+HGFdf group and the control group, PBS, SHIELD alone or HGFdf alone. Values reported at mean±standard error of the mean. EF indicates ejection fraction; FAC, fractional area change; HGFdf, engineered hepatocyte growth factor dimeric fragment; LVIDd, left ventricular inner diameter at end diastole; LVIDs, left ventricular inner diameter at end systole; PBS, phosphate buffered saline; SHIELD, shear-thinning hydrogel for injection encapsulation and long-term delivery; SV, stroke volume; and Vs, volume at end systole.
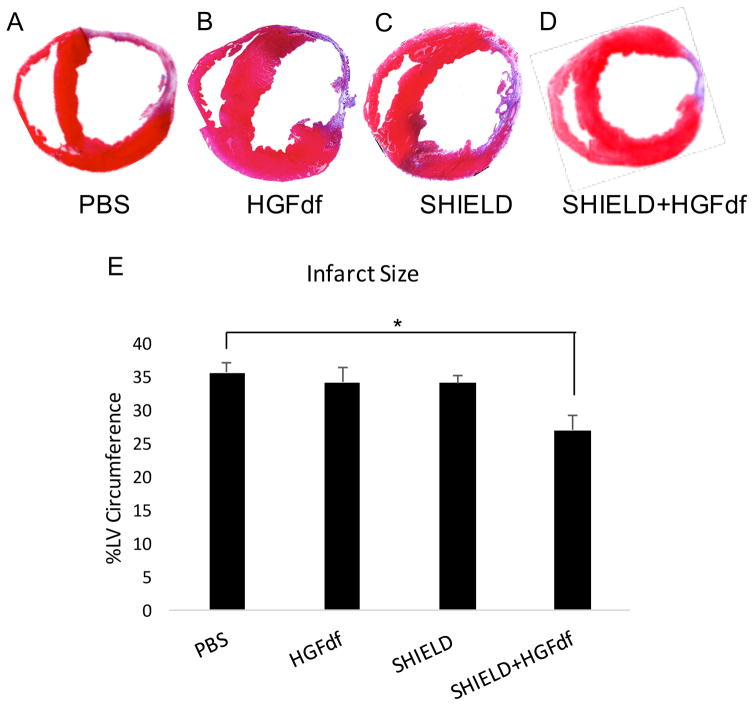
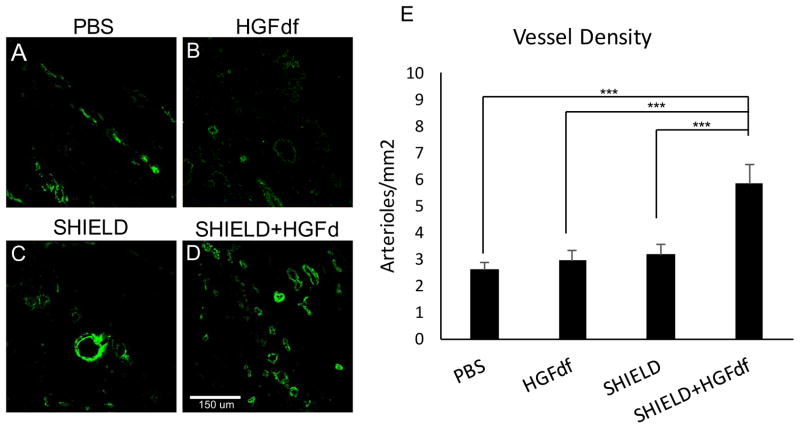
Masson’s trichrome analysis demonstrated significantly smaller infarct sizes in SHIELD+HGFdf animals compared with that of the PBS control (27.1±2.2%; 35.6±1.6%, p=0.017) and a trend in smaller infarcts compared to the other two study groups (HGFdf alone, 34.2±2.3%, p=0.065; SHIELD alone, 34.1±1.2%, p=0.069) (Figure 6). Treatment with SHIELD+HGFdf significantly increased myocardial arteriole density in the infarct and borderzone regions compared with that of the other study groups (Figure 7). SHIELD+HGFdf, 5.8±0.7 arterioles/mm2; PBS, 2.6±0.3 arterioles/mm2 (p<0.001); HGFdf alone, 3.0±0.3 arterioles/mm2 (p<0.001); SHIELD alone, 3.2±0.4 arterioles/mm2 (p<0.001).
Figure 6.
Quantification of infarct size via Masson’s Trichrome staining. Shown are representative sections of Masson’s trichrome-staining for groups treated with (A) PBS, (B) HGFdf, (C) SHIELD, and (D) SHIELD+HGFdf. (D) Infarct size was calculated by transmural infarct percent circumference of the left ventricle (*p<0.05).
Figure 7.
Histological characterization and quantification of arterial density (stained for alpha- SMA). Representative confocal images are shown for explanted MI tissue treated with (A) PBS, (B) HGFdf, (C) SHIELD, and (D) SHIELD+HGFdf. (E) Analysis of immunofluorescence expression of α-SMA revealed a significant increase in arteriole formation in the SHIELD+HGFdf group compared to all other study groups (***p<0.001).
Discussion
During the last decade, there has been a surge in the development of injectable biomaterials for the treatment of MI (Rane et al. 2011, Tous et al. 2011). Work thus far has shown promise by demonstrating that delivery of both loaded and unloaded biomaterials can limit infarct expansion by reducing abnormal stress distributions in the wall of the ventricle and enhancing angiogenesis in the relatively ischemic, yet initially viable border zone, as well as infarct regions (Tous et al. 2011, Ifkovits et al. 2010, Tous et al. 2011, Wall et al. 2006). Combining growth factors with injectable biomaterials permits targeting of specific regenerative pathways while increasing the effective half-life of the growth factor, yielding a prolonged therapeutic effect. Despite progress, drug-loaded hydrogels have not entered the clinic, and naked growth factor treatment has not yet yielded clinically meaningful results (Rane et al. 2011, Tous et al. 2011, Kische 2008). Therefore, it is necessary to improve upon both the delivery vehicle and the delivery agent in order to optimize myocardial repair and regeneration after an ischemic insult.
In the present study, we utilized a novel shear-thinning hydrogel, SHIELD, to encapsulate a protein-engineered dimeric fragment of HGF for sustained release and targeted intramyocardial delivery. SHIELD is a bioengineered hydrogel, composed of two components that undergo a physical crosslinking mechanism to form a gel state upon mixing. This interaction is reversible so that when a shear force is applied to SHIELD, the two components dissociate from one another and flow as a liquid solution; however, when the shear force is removed, the two components rapidly re-associate to form the gel state. This crosslinking mechanism is advantageous compared to other hydrogel crosslinking mechanisms for two important reasons. First, the shear-thinning and self-healing behavior facilitates minimally-invasive delivery, significantly improving the translatability of hydrogel therapy for cardiovascular disease. Second, SHIELD can be formed under physiologic conditions, whereas other synthetic hydrogel formulations require subjecting the encapsulated material to non-physiologic conditions (e.g. high ionic strength or low pH), risking protein inactivation (Cai et al. 2015, Guvendiren et al. 2012, Bakota et al. 2011, Caplan et al. 2002). Combined, SHIELD is an important advancement in hydrogel technology for the treatment of MI.
To further increase the therapeutic potential of SHIELD, we encapsulated HGFdf, a protein-engineered fragment with enhanced stability and production yield over full-length HGF. While full-length HGF has been studied extensively for its pro-angiogenic and cardioprotective effects, clinical translation of this cytokine is limited due to its complex manufacturing process and short half-life in vivo (Jones et al. 2011, Liu et al. 2014). Stable agonists of the c-Met receptor, such as HGFdf, may therefore improve clinical utility and enable commercialization. Sonnenberg et al. demonstrated the ability of HGFdf to limit negative remodeling following a period of ischemia, and the importance of encapsulation in a biomaterial. The HGFdf was retained within an extracellular matrix-derived hydrogel, of which also exhibited a minor functional impact (Sonnen-berg et al. 2015). Encapsulation of HGFdf within SHIELD, however, is advantageous due to the tunability of this scaffold. Naturally-derived hydrogels are subject to batch-to-batch variation, and have limited mechanical properties (Rane et al. 2011, Cai et al. 2015). Thus, immobilization of HGFdf to SHIELD has significant advantages due to its shear-thinning and self-healing mechanisms, and tunable mechanical properties.
Our in vitro studies confirmed that HGFdf successfully retains the ability to activate the c-Met receptor and downstream targets in rat cardiomyocytes leading to significantly enhanced cardiomyocyte metabolism and reduced apoptosis during hypoxia and serum starvation. Furthermore, HGFdf had a robust effect on HUVEC network formation. Given the statistically similar potency between HGFdf and rh-HGF in our in vitro studies, we aimed to test the efficacy of HGFdf in a small animal model of acute MI. We conjugated the HGFdf to SHIELD for a prolonged and targeted release, and observed a steady release of HGFdf for a period of 14 days. Activity of the released HGFdf was confirmed by its ability to enhance viability of cardiomyocytes after 24 hours of serum starvation and 4 hours of hypoxia.
Finally, in vivo treatment with SHIELD+HGFdf resulted in smaller infarcts, stimulated angiogenesis in the infarct region, and enhanced LV function and geometry compared to the control groups. SHIELD is an attractive delivery vehicle for the treatment of myocardial infarction due to its tunability and shear-thinning behavior (Cai et al. 2015, Cai et al. 2016). The engineered components of SHIELD allow precise control over hydrogel stiffness, growth factor delivery profiles, and amenability for minimally-invasive delivery modalities. Future investigation of different SHIELD formulations is warranted to understand the full potential of SHIELD as a vehicle for regenerative therapies to the heart.
One limitation of this study is direct injection of the therapy immediately following coronary ligation. While this acute model provides a reliable and reproducible way to investigate the effect of SHIELD+HGFdf on the ischemic myocardium, it does not accurately recapitulate the clinical scenario. However, as a proof-of-concept study, we have demonstrated that sustained release of HGFdf from SHIELD is feasible in the heart and may prevent adverse changes during ischemia. Future studies are warranted to test SHIELD+HGFdf in a chronic, and more clinically-relevant model.
In this study, we demonstrated the synergistic effect of SHIELD+HGFdf on the ischemic heart, which resulted in improved ventricular function and enhanced angiogenesis. Based on our in vitro studies, it is likely that HGFdf exerted both anti-apoptotic and anti-fibrotic effects in vivo, leading to decreased myocardial fibrosis indicated by the Masson’s trichrome stain. We also observed a significant increase in mature vessel formation in the infarct region, demonstrating the potent pro-angiogenic activity of HGFdf when delivered in SHIELD. Together, the decrease in fibrosis and the increased vascularization resulted in a significant functional benefit in animals treated with SHIELD+HGFdf. The results of our study support the notion that SHIELD+HGFdf has the potential to limit damage after a MI. The anti-apoptotic effects of HGFdf and potential for minimally-invasive delivery via SHIELD encapsulation may make this therapy particularly well-suited for patients with acute MI. The injectability, and potential for catheter-based delivery enables treatment both before and after the onset of heart failure, but further studies must be performed to test its efficacy in these scenarios.
In summary, we have been able to develop a novel shear-thinning hydrogel system that sustains release of a stable, protein-engineered HGF that is effective both in vitro and in vivo. This dual treatment approach is a significant advancement over both current growth factor therapies and hydrogel technologies, due to its enhanced clinical translatability.
Acknowledgments
Sources of Funding. This study was supported, in part, by the National Institutes of Health (NIH) grant R01 HL089315-01 (YJW), 2015 Stanford Cardiovascular Institute Seed Grant (ANS), Stan-ford Bio-X Seed Grant IIP-7-75 (SCH), NIH R21 EB020235 (SCH), CIRM RT3-07948 (SCH), Stanford Neuroscience Institute Interdisciplinary Scholar Award (LC), NIH grant R01CA151706 (JRC), NIH NIGMS Training Grant in Biotechnology [5T32GM008412] (ACM), Stanford-Agilent Fellowship (ACM), Stanford NIST Predoctoral Fellowship (ACM), and Siebel Scholars Award (ACM).
References
- Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC. Improving Viability of Stem Cells During Syringe Needle Flow Through the Design of Hydrogel Cell Carriers. Tissue Eng Part A. 2012;18(7–8):806–815. doi: 10.1089/ten.tea.2011.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakota EL, Wang Y, Danesh FR, Hartgerink JD. Injectable Multidomain Peptide Nanofiber Hydrogel Agent for Stem Cell Secretome. Biomacromolecules. 2011;12:1651–1657. doi: 10.1021/bm200035r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boodhwani M, Sodha NR, Laham RJ, Selke FW. The future of therapeutic myocardial angiogenesis. Shock. 2006;26:331–341. doi: 10.1097/01.shk.0000225318.08681.a7. [DOI] [PubMed] [Google Scholar]
- Cai L, Dewi RE, Goldstone AB, Cohen JE, Steele AS, Woo YJ, Heilshorn SC. Regulating Stem Cell Secretome Using Injectable Hydrogels with In Situ Network Formation. Adv Healthc Mater. 2016;5(21):2758–2764. doi: 10.1002/adhm.201600497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Dewi RE, Heilshorn SC. Injectable Hydrogels with In Situ Double Network Formation Enhance Retention of Transplanted Stem Cells. Adv Funct Mater. 2015;25(9):1344–1351. doi: 10.1002/adfm.201403631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan MR, Schwartzfarb EM, Zhang S, Kamm RD, Lauffenburder DA. J Biomater Sci, Polym Ed. 2002;13:225–236. doi: 10.1163/156856202320176493. [DOI] [PubMed] [Google Scholar]
- Christman KL, Fok HH, Sievers RE, Fang QH, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Engineering. 2004;10(3–4):403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- Guvendiren M, Lu HD, Burdick JA. Shear-thinning hydrogels for biomedical applications. Soft Matter. 2012;8:260–272. [Google Scholar]
- Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the Future of Cardiovascular Disease in the United States. Circ. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- Ifkovits JL, Tous E, Minakawa M, Morita M, Robb JD, Koomalsingh KJ, Gorman JH, 3rd, Gorman RC, Burdick JA. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci USA. 2010;107(25):11507–12. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Wyss JM, Yang R, Schwall R. The therapeutic potential of hepatocyte growth factor for myocardial infarction and heart failure. Curr Pharm Des. 2004;10(20):2525–33. doi: 10.2174/1381612043383863. [DOI] [PubMed] [Google Scholar]
- Jones DS, 2nd, Tsai PC, Cochran JR. Engineering hepatocyte growth factor fragments with high stability and activity as Met receptor agonists and antagonists. Proc Natl Acad Sci USA. 2011;108(32):13035–40. doi: 10.1073/pnas.1102561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo CP, Micklem K, Watt SM. A Comparison of Methods for Quantifying Angiogenesis Assay In Vitro. Tissue Eng Part C Methods. 2011;17(9):895–906. doi: 10.1089/ten.tec.2011.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kische S, Nienaber CA, Ince H. Clinical view on experimental stem cell and cytokine research in cardiac disease. Eur Heart J Suppl. 2008;10(K):K2–K6. [Google Scholar]
- Liu Cassie J, Jones DS, 2nd, Tsai PC, Venkataramana A, Cochran JR. An engineered dimeric fragment of hepatocyte growth factor is a potent c-MET agonist. FEBS Lett. 2014;588(24):4831–7. doi: 10.1016/j.febslet.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur JW, Purcell BP, Shudo Y, et al. Sustained Release of Engineered Stromal Cell–Derived Factor 1-α From Injectable Hydrogels Effectively Recruits Endothelial Progenitor Cells and Preserves Ventricular Function After Myocardial Infarction. Circulation. 2013;128(11 0 1):S79–S86. doi: 10.1161/CIRCULATIONAHA.112.000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliaras K, Marban E. Cardiac cell therapy: where we’ve been, where we are, and where we should be headed. Br Med Bull. 2011;98(1):161–185. doi: 10.1093/bmb/ldr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, et al. Executive Summary: Heart Disease and Stroke Statistics-2016 Update. Circ. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- Mulyasasmita W, Cai L, Dewi RE, Jha A, Ullmann SD, Long RH, Huang NF, Heilshorn SC. Avidity-controlled hydrogels for injectable co-delivery of induced pluripotent stem cell-derived endothelial cells and growth factors. J Control Release. 2014;191:71–81. doi: 10.1016/j.jconrel.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane AA, Christman KL. Biomaterials for the Treatment of Myocardial Infarction: A 5-Year Update. J Am Coll Cardiol. 2011;58(25):2615–29. doi: 10.1016/j.jacc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Sala V, Crepaldi T. Novel therapy for myocardial infarction: can HGF/Met be beneficial? Cell Mol Life Sci. 2011;68(10):1703–17. doi: 10.1007/s00018-011-0633-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers VFM, Lee RT, Dimmeler S, Losordo D. Biomaterials to Enhance Stem Cell Function in the Heart. Circ Res. 2011;109:910–922. doi: 10.1161/CIRCRESAHA.111.249052. [DOI] [PubMed] [Google Scholar]
- Shudo Y, Cohen JE, MacArthur JW, Goldstone AB, Otsuru S, Trubelja A, Patel J, Edwards BB, Hung G, Fairman AS, Brusalis C, Hiesinger W, Atluri P, Hiraoka A, Miyagawa S, Sawa Y, Woo YJ. A Tissue-Engineered Chondrocyte Cell Sheet Induces Extracellular Matrix Modification to Enhance Ventricular Biomechanics and Attenuate Myocardial Stiffness in Ischemic Cardiomyopathy. Tissue Eng Part A. 2015;21(19–20):2515–25. doi: 10.1089/ten.tea.2014.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg S, Rane AA, Liu CJ, et al. Delivery of an engineered HGF fragment in an extracellular matrix-derived hydrogel prevents negative LV remodeling post-myocardial infarction. Biomaterials. 2015;45:56–63. doi: 10.1016/j.biomaterials.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton CA, Reed MW, Brown NJ. A critical analysis of current in vitro and in vivo angiogenesis assays. Int J Exp Pathol. 2009;90:195. doi: 10.1111/j.1365-2613.2008.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tous E, Ifkovits JL, Koomalsingh KJ, Shuto T, Soeda T, Kondo N, Gorman JH, 3rd, Gorman RC, Burdick JA. Influence of injectable hyaluronic acid hydrogel degradation behavior in infarction-induced ventricular remodeling. Biomacromolecules. 2011;12(11):4127–35. doi: 10.1021/bm201198x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tous E, Purcell B, Ifkovits, Burdick JA. Injectable acellular hydrogels for cardiac repair. J Cardiovasc Transl Res. 2011;4(5):528–42. doi: 10.1007/s12265-011-9291-1. [DOI] [PubMed] [Google Scholar]
- Tsur-Gang O, Ruvinov E, Landa N, Holbova R, Feinberg MS, Leor J, Cohen S. The effects of peptide-based modification of alginate on left ventricular remodeling and function after myocardial infarction. Biomaterials. 2009;30(2):5409–5416. doi: 10.1016/j.biomaterials.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: A finite element model simulation. Circulation. 2006;114(24):2627–2635. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- World Health Statistics 2014. Geneva: World Health Organization; 2014. [Google Scholar]