This article summarizes the scientific review of panobinostat that led to regulatory approval in the European Union.
Keywords: Panobinostat, Multiple myeloma, European Medicines Agency
Abstract
On August 28, 2015, a marketing authorization valid through the European Union was issued for panobinostat, in combination with bortezomib and dexamethasone, for the treatment of adult patients with relapsed and/or refractory multiple myeloma who have received at least two prior regimens including bortezomib and an immunomodulatory agent (IMiD).
Panobinostat is an orally available histone deacetylase (HDAC) inhibitor that inhibits the enzymatic activity of HDAC proteins at nanomolar concentrations. HDAC proteins catalyze the removal of acetyl groups from the lysine residues of histones and some nonhistone proteins. Inhibition of HDAC activity results in increased acetylation of histone proteins, an epigenetic alteration that results in a relaxing of chromatin, leading to transcriptional activation. The recommended starting dose of panobinostat is 20 mg, taken orally in a cyclical manner for up to 48 weeks.
The use of panobinostat in combination with bortezomib and dexamethasone was studied in a randomized, double‐blind, placebo‐controlled, multicenter phase III study (PANORAMA I) in 768 patients with relapsed or relapsed and refractory multiple myeloma who had received one to three prior lines of therapies. In the subgroup of patients who have received at least two prior regimens including bortezomib and an IMiD, there was a difference of 7.8 months in the progression‐free survival in favor of the experimental arm (12.5 months for panobinostat + bortezomib + dexamethasone vs. 4.7 months for placebo + bortezomib + dexamethasone; hazard ratio = 0.47, 95% confidence interal 0.31–0.72; log‐rank p value = .0003). The incidence of grade 3–4 adverse events suspected to be related to study drug was 76.9% vs. 51.2%, for the panobinostat and the placebo group, respectively. The most common side effects (grade 3–4) associated with panobinostat included diarrhea (18.9%), fatigue (14.7%), nausea (4.5%), vomiting (5.5%), thrombocytopenia (43.6%), anemia (7.9%), neutropenia (16.5%) and lymphopenia (8.1%).
This article summarizes the scientific review of the application leading to regulatory approval in the European Union. The full scientific assessment report and product information, including the Summary of Product Characteristics, are available on the European Medicines Agency website (http://www.ema.europa.eu/ema/index.jsp?curl=pages/includes/medicines/medicines_landing_page.jsp&mid=).
Implications for Practice.
Farydak was approved in the European Union in combination with bortezomib and dexamethasone, for the treatment of adult patients with relapsed and/or refractory multiple myeloma who have received at least two prior regimens including bortezomib and an immunomodulatory agent (IMiD). The addition of panobinostat to bortezomib and dexamethasone resulted in a clinically meaningful and statistically significant improvement of progression‐free survival compared with bortezomib and dexamethasone, and an additional therapeutic option with a new mechanism of action was considered valuable. Although the toxicity associated with panobinostat combination was significant, at the time of the marketing authorization of panobinostat, it was considered that it was acceptable and that it should be left to the clinician and the patient to decide whether the panobinostat combination is the preferred treatment option or not.
Background
Multiple myeloma (MM) is a neoplastic plasma‐cell disorder that is characterized by clonal proliferation of malignant plasma cells in the bone marrow microenvironment, monoclonal protein in the blood or urine, and associated organ dysfunction [1] due to the accumulation of the monoclonal protein to the organs. The estimated incidence of MM was 35,309 cases in the European Union in 2015 [2]. The median age at diagnosis is 71 years; only 10% and 2% of patients are younger than 50 and 40 years, respectively [3], [4], [5], and the median survival in the present era is 5–7 years from the diagnosis of the disease [6].
At the time of the marketing authorization of panobinostat, treatment options for refractory/relapsed multiple myeloma of the following six main classes of agents: proteasome inhibitors (bortezomib), immunomodulatory drugs (thalidomide, lenalidomide, pomalidomide), corticosteroids, alkylators, anthracyclines, nitrosoureas (to a lesser extent), plus high‐dose chemotherapy and autologous or allogeneic hematopoietic stem cell transplantation for those who are eligible [7].
Refractory disease can sometimes be treated with a particular agent to which resistance has developed if the agent is used in conjunction with other compounds that produce a synergistic anti‐MM effect or at a different posology. At second or subsequent relapses when the patient has often been treated with bortezomib and at least one immunomodulatory agent, there are limited options. Once patients have become refractory to both agents (i.e., double refractory), median overall survival (OS) and event‐free survival have been reported to be 13 and 5 months, respectively, based on clinical study data [8].
Treatment of cells with panobinostat resulted in accumulation of acetylated histones and nonhistone proteins as well as cell death and cell cycle arrest including human multiple myeloma cells. HDACi single‐agent activity against multiple myeloma is modest, but synergistic with proteasome inhibitors. The molecular basis of this synergy is likely multifactorial and involves interference with protein degradation and the interaction of myeloma cells with microenvironment [9].
Nonclinical Aspects and Clinical Pharmacology
Panobinostat is a histone deacetylase (HDAC) inhibitor, inhibiting all HDAC proteins of HDAC families I, II and IV in nanomolar range in vitro (Figure 1). In cellular assays, it was shown that panobinostat enhances histone acetylation and affects processes known to be regulated by histone acetylation status (gene expression of 21 kilodalton cyclin dependent kinase inhibitor (p21), TPRM, Hep27, thymidine kinase). Inhibition of tumor cell proliferation of a variety of cancer cell lines in vitro was observed, with leukemic cell lines, including MM cell lines, among the most sensitive cells. The effect on histone acetylation was confirmed in vivo in tumor tissue harvested from subcutaneous human colon tumor cell line (HCT116) (colon) xenograft tumors. Antitumor activity of single‐agent panobinostat was shown in several experimental models including two MM xenograft models. The antitumorigenic potential of panobinostat was increased when combined with either bortezomib or dexamethasone, and most prominent in the triple therapy.
Safety pharmacology studies showed no effect of panobinostat on respiratory function in male rats through the highest doses tested (10 mg/kg, intravenous [IV]). A repeated oral dose telemetry study conducted in dogs at a dose of 1.5 mg/kg showed prolongation in QT interval corrected for heart rate (QTc) upwards of 25 milliseconds within some animals over the monitoring period. Concomitant administration of medicinal products (e.g., chloroquine, halofantrine, clarithromycin, methadone, moxifloxacin, bepridil, and pimozide) that are known to cause QTc prolongation should be done with caution.
Repeat‐dose toxicity studies conducted in rats and dogs, showed effects on the bone marrow, on cell proliferation in gastrointestinal tract and on the secretory function of different glands (among others, testis, epididymis, salivary gland, thyroid gland).
Panobinostat has demonstrated mutagenic potential. There is a high likelihood of panobinostat increasing the risk of fetal death and developmental skeletal abnormalities. In studies conducted in rats and rabbits, embryo fetal lethality and increases in skeletal anomalies were seen above exposures corresponding to 0.25 of the human clinical area under the curve. Effective methods of contraception should be used during treatment and for 3 months after the last dose of panobinostat.
Panobinostat should only be used during pregnancy if the expected benefits outweigh the potential risks to the fetus, and breastfeeding is contraindicated during panobinostat treatment.
The pharmacokinetics of panobinostat monotherapy were evaluated in 14 clinical studies and in in vitro studies with human biomaterials. The metabolism of panobinostat is through both non‐Cytochrome P450 (CYP)‐ and CYP‐mediated routes with approximately 40% of panobinostat metabolized through CYP3A4. Panobinostat is also a p‐glycoprotein (P‐gp) substrate. In patients taking strong CYP3A and/or P‐gp inhibitors, the dose of panobinostat should be reduced. In clinical studies in multiple myeloma, the exposure of panobinostat was decreased by approximately 20% upon the concomitant use of dexamethasone, which is a dose‐dependent mild/moderate CYP3A4 inducer. Strong inducers are expected to have greater effects, and may reduce the efficacy of panobinostat. It is currently unknown whether panobinostat may reduce the effectiveness of hormonal contraceptives.
In patients with impaired hepatic function, plasma exposure of panobinostat increased by 43% and 105% in patients with mild (total bilirubin > upper limit of normal [ULN] and ≤ 1.5 × ULN and aspartate aminotransferase [AST] = any value or total bilirubin ≤ ULN and AST > ULN) and moderate hepatic impairment (total bilirubin > 1.5 and ≤ 3.0 × ULN any AST value), respectively, and reduced starting doses with possible subsequent dose escalation need to be considered. Panobinostat should not be administered in patients with severe hepatic impairment due to lack of experience and safety data in this population.
Clinical Efficacy
The pivotal study D2308 (PANORAMA I) was a multicenter, randomized, double‐blind, phase III study of panobinostat in combination with bortezomib and dexamethasone (PAN + BTZ + Dex) compared with placebo plus bortezomib and dexamethasone (PBO + BTZ + Dex) in patients with multiple myeloma [10].
The main criteria for inclusion selected patients with relapsed or relapsed and refractory disease who had received one to three prior lines of therapy and had Eastern Cooperative Oncology Group performance status ≤2. Patients who had progressed under all prior lines of anti‐MM therapy (some of them received autologous bone marrow stem cell‐supported high‐dose therapy) and had been shown to be refractory to prior bortezomib were not eligible.
Relapsed MM was defined as recurrent disease in a patient who had responded to a prior therapy by achieving a minimal response (MR) or better, and had not progressed up to 60 days after the last dose of this therapy. Relapsed‐and‐refractory disease was defined as relapse to at least one prior line of therapy and being refractory to another line (except bortezomib), by either not achieving a MR, or having progressed while on this therapy or within 60 days of its last dose.
Patients received panobinostat (20 mg taken orally once a day, three times per week, on a 2 weeks on and 1 week off dosing regimen), in combination with bortezomib (1.3 mg/m2 injected intravenously) and dexamethasone (20 mg). Treatment was administered for a maximum of 16 cycles. This dosing regimen was based on study B2207, a phase Ib, multicenter, open‐label, dose‐escalation study of oral panobinostat and IV bortezomib in adult patients with multiple myeloma; the maximum tolerated dose was declared at 20 mg panobinostat three times per week IV [11].
Randomization in study D2308 was stratified by number of prior lines of antimyeloma therapy (1, 2, or 3) and by prior use of bortezomib (yes or no). The study enrolled 768 patients, of whom 387 received panobinostat and 381 received placebo.
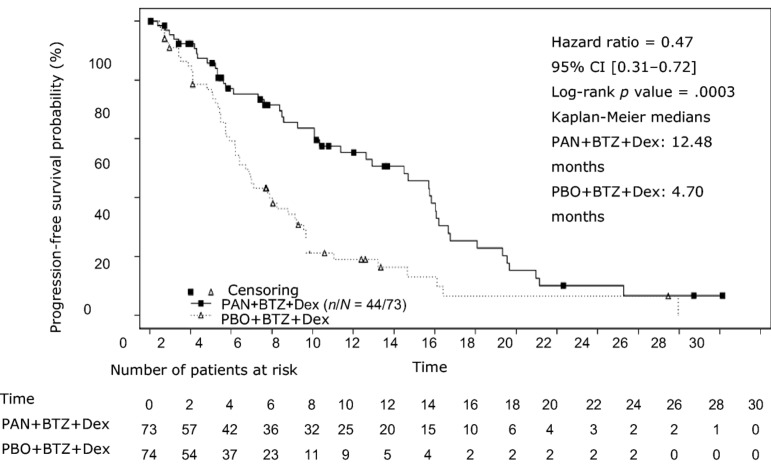
The primary endpoint was progression‐free survival (PFS) based on modified European Society for Blood and Marrow Transplantation (mEBMT) criteria assessed by the investigator. Based on the results in the full analysis set population (all randomized patients, according to randomized treatment), the median PFS was 12 months for the PAN + BTZ + Dex group compared with 8.1 months for PBO + BTZ + Dex (hazard ratio [HR] = 0.63, 95% confidence interval [CI]: 0.52–0.76; log‐rank p value < .0001). In the subgroup of patients who have received at least two prior regimens including bortezomib and an immunomodulatory agent (IMiD), the median PFS was 12.5 months for PAN + BTZ + Dex versus 4.7 months for PBO + BTZ + Dex (HR = 0.47, 95% CI: 0.31–0.72; log‐rank p value = .0003). A summary of efficacy results is shown in Table 1 and Figure 2. The median OS was 25.5 months in patients receiving PAN + BTZ + Dex treatment and 19.5 in the placebo group (HR = 1.01, 95% CI: 0.68–1.50; cutoff November 2015).
Table 1. Summary of key favorable and unfavorable effects (Study D2308).
Subgroup of patients who received at least two prior regimens including bortezomib and an immunomodulating agent.
FAS population.
Abbreviations: BTZ, bortezomib; Dex, dexamethasone; FAS, full analysis set; HR, hazard ratio; OS, overall survival; PFS, progression‐free survival; PAN, panobinostat.
Figure 2.
Kaplan‐Meier plot of progression‐free survival in patients with multiple myeloma who received at least two prior regimens including bortezomib and an immunomodulatory agent—Study D2308.
Abbreviations: BTZ, bortezomib; CI, confidence interval; Dex, dexamethasone; PAN, panobinostat; PBO, placebo.
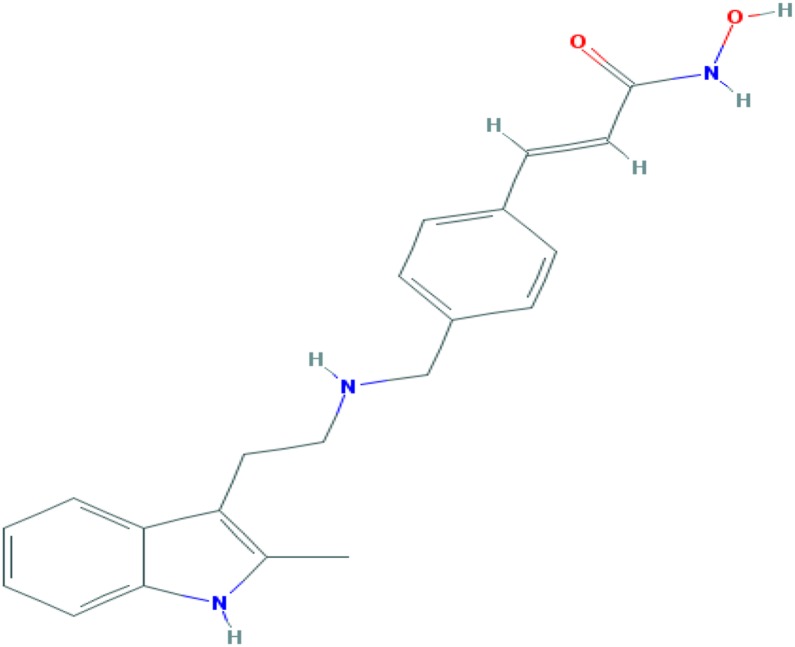
Figure 1.
Molecular structure of panobinostat. Molecular formula: C21H23N3O2. Relative molecular mass: 439.51 gmol−1. The chemical name of panobinostat is (2E)‐N‐hydroxy‐3‐[4‐({[2‐(2‐methyl‐1H‐indol‐3‐yl)ethyl]amino}methyl)phenyl]prop‐2‐enamide 2‐hydroxypropanoate (1:1).
Global health status/quality of life (QOL) scores of the European Organisation for Research and Treatment of Cancer Quality‐of‐life Questionnaire Core 30 (EORTC QLQ‐C30) initially declined in both treatment arms over the study treatment period, before returning to baseline levels after week 18 in both the PAN + BTZ + Dex and PBO + BTZ + Dex arms. The decline in mean change from baseline global health status/QOL scores (minimal important change = 5) at week 12, week 24, and week 48 were −9.853, −7.867, and −2.986 in the PAN + BTZ + Dex arm, and −4.044, −1.518, and 4.345 in the PBO + BTZ + Dex arm, respectively.
The supportive study DUS71 (PANORAMA II) was a two‐stage, single‐arm, open‐label, multicenter, phase II study of oral panobinostat (20 mg) in combination with bortezomib (1.3 mg/m2) and dexamethasone (20 mg) in 55 patients with relapsed and refractory multiple myeloma, who were bortezomib refractory and had received at least two prior lines of therapy. Patients had to be exposed to an IMiD (lenalidomide or thalidomide). Refractoriness to bortezomib was defined as disease progression on or within 60 days of the last bortezomib‐containing line of therapy. The primary endpoint was overall response rate (ORR) after eight cycles of therapy as per mEBMT criteria. Patients achieved an ORR (≥partial response) of 34.5% and 52.7% (≥MR) [12].
Clinical Safety
The main safety data have been obtained from the study D2308. Additional safety data concerning the treatment of MM patients with PAN + BTZ + Dex became available from the expansion phase of the single‐arm dose‐escalation phase Ib study B2207 (n = 15) and from the single‐arm phase II study DUS71 (n = 55) in BTZ‐refractory patients.
The most common nonhematological adverse reactions were diarrhea, fatigue, nausea, and vomiting. Treatment‐emergent hematological toxicities included thrombocytopenia, anemia, neutropenia, and lymphopenia. The most important grade 3–4 treatment‐emergent toxicities in study D2308 are summarized in Table 1.
In study D2308, study treatment discontinuation due to adverse events (AEs) was 36.2% (138 patients) in the PAN + BTZ + Dex arm and 20.4% (77 patients) in the PBO + BTZ + Dex arm, and the single most frequent AEs leading to treatment discontinuations in the PAN + BTZ + Dex arm were diarrhea (4.5%), fatigue (2.9%), asthenia (2.9%), and peripheral neuropathy (3.7%). The overall incidence of AEs requiring dose adjustments was 88.7%. The most frequently reported all‐grade serious adverse events (with an incidence ≥5.0%) were pneumonia (14.7%), diarrhea (11.3%), and thrombocytopenia (7.3%). More patients required hospitalization due to AEs in the PAN + BTZ + Dex arm (55%) than in the PAN + BTZ + Dex arm (37%).
Increased rate of deaths on‐treatment was observed for panobinostat (30 deaths [7.9%] in the PAN + BTZ + Dex arm vs. 18 deaths [4.8%] in the PBO + BTZ + Dex arm). The main causes of on‐treatment deaths by system organ class (PAN + BTZ + Dex arm vs. PBO + BTZ + Dex arm) were infections and infestations (1.8% vs. 1.3%), respiratory, thoracic, and mediastinal disorders (1.6% vs. 0.5%), cardiac disorders (1.0% vs. 0.8%), and study indication (1.0% vs. 1.6%).
Important identified risks included QTc prolongation, myelosuppression, severe hemorrhage, severe infections (including sepsis and pneumonia), severe diarrhea, and increased toxicity in elderly patients (aged ≥65 years). Important potential risks included ischemic heart disease, venous thromboembolism, carcinogenicity and second primary malignancy, medication errors, use in patients with hepatic impairment, and use in patients with renal impairment. Missing information included safety in patients with cardiac diseases and renal impairment. A noninterventional observational study (LBH589D2408) of panobinostat is expected to further characterize some of these risks.
Discussion and Benefit‐Risk Assessment
Initially, the applicant company applied for an indication in MM patients who have received at least one prior therapy. During the initial review, a number of issues were raised concerning the benefit‐risk balance. The concerns related to the clinical importance of the observed difference in PFS, the lack of a significant benefit in OS (HR = 1.01, 95% CI: 0.68–1.50), versus decreased health‐related quality of life and substantial toxicity, including a high proportion of patients discontinuing therapy due to toxicity. At that time, it was not possible to conclude that the benefit‐risk balance was positive and further consideration was needed to determine if a subgroup of patients could be identified with a better balance. Indeed, a more convincing effect on PFS and similar toxicity was observed in patients who were treated earlier with an IMiD and BTZ, and possibly in the group of patients with relapsed/refractory disease, based on exploratory analyses. A Scientific Advisory Group (SAG) was convened to discuss whether efficacy in MM patients who had received at least one prior therapy was sufficient to justify exposing these patients to the severe adverse event profile of the drug, and whether a suitable (sub) population could be identified for whom the balance of benefits and risk could be considered positive.
According to some experts, albeit with some uncertainty, the clinical benefit (although moderate) was considered established. The observed effect in terms of PFS was considered clinically important. In terms of OS, it was possible to rule out a detrimental effect on OS based on the Kaplan‐Meier curves. The lack of a statistically significant difference in OS could actually be due to the relatively long postprogressive survival. A transient deterioration of QoL during treatment was expected in view of the toxicity profile. The high complete response/near complete response rate was also considered as important and potentially enabling stem cell transplant (SCT). Although pomalidomide and other agents that had been registered in the meantime could be used in this indication, relapsed/refractory MM was at the time of the pivotal trial a setting with very few therapeutic options and poor prospect of cure. Panobinostat was expected to provide an additional option with a new mechanism of action that can be of benefit when all other therapeutic options have failed or when it is preferable to reserve the few available options for later lines of treatment. Adequate toxicity management was considered paramount and expected to improve with further experience. Furthermore, future studies will aim to decrease toxicity of the combination by improving the BTZ schedule. Given the benefit observed, the small number of alternative treatment options and the high unmet medical need, the toxicity profile (although significant) was considered acceptable. According to some of the experts and patient representatives, the availability of a new treatment, even if associated with modest benefits and significant toxicity is of value for patients. The likelihood of experiencing unfavorable effects and the likelihood of benefit should be clearly described to allow informed treatment choice by physicians and patients, considering the available therapeutic options. Other experts disagreed and considered that the clinical benefit could not be regarded established in the absence of improved QoL, symptoms, or OS, or evidence of higher access to SCT, and that the toxicity, including a higher number of treatment‐related deaths associated with panobinostat, especially in elderly patients, was unacceptable. Further data were considered to be needed to establish that clinical benefits exist and that toxicity can be actively managed and improved without loss of activity. The SAG also identified the subpopulation of MM patients with relapsed/refractory disease who have received at least two lines of therapy in which the unmet need was higher, in view of the poor prognosis and few available treatment options, but without agreement on positive benefit/risk.
Finally, taking into account the expert advice and additional exploratory analyses, the Committee for Medicinal Products for Human Use (CHMP) considered that, in the subgroup of adult patients with relapsed and/or refractory multiple myeloma who have received at least two prior regimens including bortezomib and an immunomodulatory agent, the efficacy of panobinostat was considered established and an additional therapeutic option with a new mechanism of action was considered valuable. Although the toxicity associated with panobinostat combination was significant, given the limited treatment options and poor prognosis, it was considered at the time of registration that it was acceptable and that it should be left to the clinician and the patient to decide whether the panobinostat combination is the preferred treatment option or not. This should be considered in the context of the increasingly complex treatment landscape that is attributable to an improved understanding of the disease biology and an increase in available therapies. Both aspects contribute to further segmentation of the heterogeneous MM patient population, influencing treatment decisions. In this respect, the recently approved anti‐CD38 daratumumab may change some of the paradigms of treatment, as it can be combined with different agents, including chemotherapy, without significant increase of toxicity. Elotuzumab and ixazomib are similar examples of newly approved agents that can be combined with existing treatment and may be considered when treating patients with relapsed and/or refractory MM. Thus, treatment strategy is complex and further depends on the type of prior treatments, response to prior therapy, treatment tolerability, and patient characteristics. More isoform‐ and/or class‐selective HDAC inhibitors are also being developed to enhance tolerability without diminishing anti‐MM activity, but this will need further clinical testing [12], [13].
The impact of adverse events may be lessened by close monitoring and timely interventions, and the Summary of product characteristics (SmPC) contains recommendations for patient monitoring and for dose modifications, interruption, or discontinuation in case of adverse events. In addition to the routine pharmacovigilance activities, the applicant company will continue to monitor safety in a noninterventional, observational study (LBH589D2408A) of panobinostat use in relapsed and/or refractory multiple myeloma patients (in the real‐world setting).
In the U.S. Food and Drug Administration, the marketing approval of panobinostat was granted under accelerated approval for the same indication, and further survival data to confirm the beneficial effect on PFS were required. EMA took into consideration the clinically relevant effect size for PFS and the unmet medical need in this patient population, and considered the results clinically meaningful for a full marketing authorization.
Acknowledgments
The scientific assessment summarized in this report is based on important contributions from the CHMP members and additional experts following the application for a marketing authorization from the company.
Disclaimer
This publication is a summary of the European Public Assessment Report, the summary of product characteristics, and other product information as published on the EMA website (www.ema.europa.eu). For the most current information on this marketing authorization, please refer to the EMA website. The authors of this article remain solely responsible for the opinions expressed in this publication.
Footnotes
Editor's Note: See the related commentary, “Panobinostat and Multiple Myeloma in 2018,” by Andrew J. Yee and Noopur S. Raje, on page 516 of this issue.
Author Contributions
Interpretation of data: Paula van Hennik, Ita Walsh, Pieter De Graeff, Annika Folin, Jan Sjöberg
Manuscript writing: Kyriaki Tzogani, Paula van Hennik, Ita Walsh, Pieter De Graeff, Annika Folin, Jan Sjöberg, Francesco Pignatti
Final approval of manuscript: Kyriaki Tzogani, Paula van Hennik, Ita Walsh, Pieter De Graeff, Annika Folin, Jan Sjöberg, Tomas Salmonson, Jonas Bergh, Edward Laane, Heinz Ludwig, Christian Gisselbrecht, Francesco Pignatti
Disclosures
Heinz Ludwig: Amgen, Celgene, Janssen, Bristol‐Myers Squibb, Takeda (H), Takeda, Amgen (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med 2004;351:1860–1873. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–1403. [DOI] [PubMed] [Google Scholar]
- 3. Kyle RA, Gertz MA, Witzig TE et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003;78:21–33. [DOI] [PubMed] [Google Scholar]
- 4. Bladé J, Samson D, Reece D et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high‐dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol 1998;102:1115–1123. [DOI] [PubMed] [Google Scholar]
- 5. Costa LJ, Brill IK, Omel J et al. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood Adv 2017;1:282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rajkumar SV, Kumar S. Multiple myeloma: Diagnosis and treatment. Mayo Clin Proc 2016;91:101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network [NCCN] . Multiple Myeloma Guidelines Version 2. 2017. Available at https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf
- 8. Kumar SK, Lee JH, Lahuerta JJ et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia 2012;26:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cea M, Cagnetta A, Gobbi M et al. New insights into the treatment of multiple myeloma with histone deacetylase inhibitors. Curr Pharm Des 2013;19:734–744. [PMC free article] [PubMed] [Google Scholar]
- 10. San‐Miguel JF, Hungria VTM, Yoon S-S, Beksac M, Dimopoulos MA, Elghandour A et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. The Lancet Oncology 2014;15:1195–1206. [DOI] [PubMed] [Google Scholar]
- 11. San‐Miguel JF, Richardson PG, Günther A et al. Phase Ib study of panobinostat and bortezomib in relapsed or relapsed and refractory multiple myeloma. J Clin Oncol 2013;31:3696–3703. [DOI] [PubMed] [Google Scholar]
- 12. Richardson PG, Schlossman RL, Alsina M et al. PANORAMA 2: Panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib‐refractory myeloma. Blood 2013;122:2331–2337. [DOI] [PubMed] [Google Scholar]
- 13. Harada T, Hideshima T, Anderson KC. Histone deacetylase inhibitors in multiple myeloma: From bench to bedside. Int J Hematol 2016;104:300–309. [DOI] [PubMed] [Google Scholar]