This article summarizes the scientific review of daratumumab that led to regulatory approval in the European Union.
Keywords: Daratumumab, Multiple myeloma, European Medicines Agency
Abstract
On May 20, 2016, a conditional marketing authorization valid through the European Union (EU) was issued for daratumumab as monotherapy for the treatment of adult patients with relapsed and refractory multiple myeloma, whose prior therapy included a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD) and who had demonstrated disease progression on the last therapy. The review of daratumumab was conducted under the EMA's accelerated assessment program for drugs that are of major interest for public health, especially from the point of view of therapeutic innovation.
Daratumumab monotherapy achieved an overall response rate of 29.2% (95% confidence interval [CI] 20.8 to 38.9) in patients with multiple myeloma who had received at least three prior lines of therapy (including a PI and IMiD) or were double refractory to a PI and an IMiD (Study MMY2002). In patients with multiple myeloma relapsed from or refractory to two or more different prior therapies, including IMiDs (e.g., thalidomide, lenalidomide) and PI, an overall response was observed in 15 patients (35.7%, 95% CI: 21.6 to 52.0) (Study GEN501).
On April 28, 2017, the therapeutic indication was extended to include the use of daratumumab in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of adult patients with multiple myeloma who have received at least one prior therapy. This was based on two subsequent phase III studies of daratumumab in combination with lenalidomide/low‐dose dexamethasone (MMY3003) and bortezomib/low dose dexamethasone (MMY3004).
The most common side effects (grade 3–4) associated with daratumumab included neutropenia (37%), thrombocytopenia (23%), anemia (16%), pneumonia (10%), lymphopenia (8%), infusion‐related reactions (6%), upper respiratory tract infection (5%), and fatigue (5%).
The objective of this study was to summarize the scientific review done by the CHMP of the application leading to regulatory approval in the EU. The full scientific assessment report and product information, including the Summary of Product Characteristics (SmPC), are available on the EMA website (www.ema.europa.eu).
Implications for Practice.
A conditional Marketing authorization was issued in the European Union for daratumamb as monotherapy for the treatment of adult patients with relapsed and refractory multiple myeloma, based on the response rate data from two single‐agent studies. Darzalex, a novel monoclonal antibody targeted against CD38, demonstrated a durable response rate in a heavily pre‐treated population with limited treatment options based on the response rate data from two single‐agent studies. The addition of daratumumab to lenalidomide and dexamethasone (study MMY3003), or bortezomib and dexamethasone (MMY3004), demonstrated a positive effect on progression‐free survival in patients with multiple myeloma who had received at least one prior therapy. Following submission of the controlled data of the MMY3003 and MMY3004 studies, the efficacy and safety of daratumumab was confirmed and the approval of daratumumab was converted to standard approval.
Background
Multiple myeloma (MM) is characterized by the proliferative disorder of plasma cells in the bone marrow with excessive monoclonal protein production [1]. Multiple myeloma is a disease of older adults, with a median age at diagnosis of 72 years [1]. The estimated incidence of MM was 35,309 cases in the European Union (EU) in 2015 [2].
Overall survival (OS) of patients with newly diagnosed MM has increased from approximately 3 years during the years 1985–1998 to 6–10 years today [3], [4]. Despite these advances, MM remains incurable, and all patients eventually relapse. Patients who are heavily pretreated and/or refractory to both a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD) have a dismal prognosis and are difficult to get back into a durable remission, and median overall survival is only 8–9 months [5].
At the time of the initial marketing authorization of daratumumab (HuMax‐CD38 or Darzalex) in the EU, treatment options for patients with relapsed and/or refractory MM included salvage therapy (if possible, this could include autologous or allogeneic hematopoietic stem cell transplantation) until relapse or toxicity and then continuing with the next salvage option. In this setting, for patients who have received at least two prior therapies, including bortezomib and an IMiD, and have shown relapsed or refractory disease, pomalidomide (in combination with dexamethasone) and panobinostat (in combination with bortezomib and dexamethasone) were approved agents in the EU. The proteasome inhibitor carfilzomib and the monoclonal antibody (mAb) elotuzumab, both in combination with lenalidomide and dexamethasone, were approved for the treatment of adult patients with MM who have received at least one prior therapy.
Daratumumab is an IgG1κ human mAb that binds to the CD38 protein, a surface protein that is overexpressed on MM cells and inhibits the in vivo growth of CD38‐expressing tumor cells.
The recommended dose of daratumumab monotherapy and of the combination with lenalidomide is 16 mg/kg, administered as an intravenous infusion weekly during weeks 1–8, every 2 weeks during weeks 9–24, and then every 4 weeks from week 25 onwards until disease progression. The recommended dose of daratumumab in combination with bortezomib is 16 mg/kg, administered as an intravenous infusion weekly during weeks 1–9, every 3 weeks during weeks 10–24, and then every 4 weeks from week 25 onwards until disease progression.
Nonclinical Aspects and Clinical Pharmacology
Daratumumab is an IgG1κ human mAb that binds to the CD38 protein expressed at a high level on the surface of MM tumor cells, as well as other cell types and tissues at various levels. Daratumumab inhibits the in vivo growth of CD38‐expressing tumor cells and has the potential to bind to all Fcg receptors (FcγRs), inducing antibody‐dependent cell‐mediated cytotoxicity, complement‐dependent cytotoxicity, and antibody‐dependent cellular phagocytosis, resulting in high tumor‐cell lysis. Binding of daratumumab to CD38 also mediated cell apoptosis and modulated enzymatic activities of CD38, inhibiting cyclase enzyme activity and stimulating hydrolase activity.
No treatment‐related adverse effects on cardiovascular, respiratory, or central nervous system parameters were observed in the 6‐ and 2‐week intravenous repeat‐dose toxicology studies in chimpanzees and Cynomolgus monkeys administered daratumumab or HuMab‐CD38 (a human IgG1 mAb that specifically binds human and Cynomolgus monkey CD38), respectively. The two primary toxicities observed in the 6‐week repeat‐dose chimpanzee study were infusion‐related reactions (IRRs) and thrombocytopenia. Histopathological findings reported in the 2‐week repeat‐dose study in Cynomolgus monkeys consisted of atrophy in thymus and lymphoid depletion in the mandibular and mesenteric lymph nodes, Peyer's patch, and spleen.
The pharmacokinetic (PK) properties of daratumumab were evaluated in 232 subjects treated with daratumumab monotherapy in the MMY1002, MMY2002, and GEN501 studies [6], [7], [8]. Pharmacokinetic data showed the half‐life of daratumumab is both concentration‐ and time‐dependent; the model‐derived mean half‐life associated with the linear elimination was approximately 18 ±9 days.
The effect of daratumumab on the corrected QT (QTc) interval was evaluated in an open‐label study for 83 patients (GEN501) with relapsed and refractory MM following daratumumab infusions. Single and triplicate electrocardiograms were evaluated for the final 11 patients dosed during Part 1 (4–24 mg/kg). A separate analysis was performed for 72 patients (8 mg/kg n = 30; 16 mg/kg n = 42) in Part 2. Linear mixed PK‐pharmacodynamic analyses indicated no large increase in mean QTcF interval (i.e., greater than 20 msec) at daratumumab maximum serum concentration (Cmax).
At the time of the initial marketing authorization, no dedicated PK studies had been completed in patients with hepatic and renal impairment; however, the results of additional pharmacokinetic data from the MMY3003 and MMY3004 studies have been submitted since approval, confirming that there are no clinically important differences in exposure to daratumumab between patients with hepatic and renal impairment and those with normal hepatic and renal function, respectively.
No interaction studies have been conducted with daratumumab; however, because it is an IgG1κ mAb, renal excretion and hepatic enzyme‐mediated metabolism of intact daratumumab are unlikely to represent major elimination routes. Therefore, variations in drug‐metabolizing enzymes are not expected to affect the elimination of daratumumab. Because of its high affinity to a unique epitope on CD38, daratumumab is not anticipated to alter drug‐metabolizing enzymes.
Patients were evaluated for antitherapeutic antibody responses to daratumumab at multiple time points during treatment and up to 8 weeks following the end of treatment. However, because the employed assay had limitations in detecting anti‐daratumumab antibodies in the presence of high concentrations of daratumumab, the applicant committed to develop a new method for detecting antidrug antibodies.
Daratumumab may be detected on serum protein electrophoresis (SPE) and immunofixation (IFE) assays used for monitoring disease monoclonal immunoglobulins, which could lead to false‐positive SPE and IFE assay results for patients with IgG kappa myeloma protein, impacting the initial assessment of complete responses by International Myeloma Working Group (IMWG) criteria. Therefore, in patients with a persistently very good partial response, other methods to evaluate the depth of response should be considered.
Daratumumab Monotherapy
Clinical Efficacy
One phase II study (MMY2002) was submitted in support of the use of daratumumab in the initially claimed indication, that is, as monotherapy for the treatment of adult patients with relapsed and refractory MM whose prior therapy included a PI and an IMiD and who demonstrated disease progression on the last therapy.
MMY2002 was a phase II, single‐arm study designed to investigate the efficacy and safety of daratumumab in patients with MM who had received at least three prior lines of therapy (including a proteasome inhibitor and IMiD) or who were double refractory to a proteasome inhibitor and an IMiD.
The main criteria for inclusion selected patients who had an Eastern Cooperative Oncology Group performance status score ≤2 and had received at least three prior lines of therapy, including a proteasome inhibitor and an IMiD, in any order during the course of treatment, or patients whose disease was double refractory to a PI and an IMiD. Patients who had previously received daratumumab or other anti‐CD38 therapies or had previously received an allogeneic stem cell transplant (ASCT) were not eligible.
Daratumumab was administered as an intravenous infusion in 28‐day cycles until disease progression, unacceptable toxicity, or other reasons, such as concurrent (nonprotocol) treatment for MM. The dose selection of daratumumab was based on a phase I/II study (GEN501) and on Part 1 of the MMY2002 study, in which two dose cohorts were evaluated by use of Simon's two‐stage design in patients with MM whose disease was relapsed or refractory to at least two prior lines of therapies.
The primary endpoint was overall response rate (ORR); main secondary endpoints included duration of response and OS.
In Part 1 of MMY2002 study, a total of 34 patients were randomly assigned to one of two treatment groups (daratumumab dosed at 8 mg/kg or 16 mg/kg), and in Part 2 of the study, 106 patients in total received 16 mg/kg. Patients were stratified by International Staging System (ISS; I, II, or III) and refractory status (none, refractory to either a PI or an IMiD, or refractory to both a PI and an IMiD).
An overall response was observed in 31 patients (29.2%, 95% confidence interval [CI] 20.8%–38.9%), and 3 (2.8%, 95% CI 0.6%–8.0%) had a stringent complete remission. The duration of response was 7.4 months (95% CI 5.5 months–not estimable). The median OS was 17.5 months (95% CI 13.7 months–not estimable) at a subsequent analysis (cutoff date June 30, 2015). A summary of key favorable effects is displayed in Table 1.
Table 1. Key favorable and unfavorable effects of daratumumab for the treatment of adult patients with relapsed and refractory multiple myeloma, whose prior therapy included a proteasome inhibitor and an immunomodulatory agent and who demonstrated disease progression on the last therapy.
The data cutoff for the MMY2002 study was January 9, 2015, and the data cutoff for the GEN501 study was February 6, 2015.
OS cutoff date: June 30, 2015.
Abbreviations: —, not applicable; AE, adverse event; CI, confidence interval; CR, complete response; DOR, duration of response; IRRs, infusion‐related reactions; NE, not estimated; ORR, overall response rate; OS, overall survival; PR, partial response; pts, patients; QTc, corrected QT; sCR/CR, stringent complete remission ratio to complete remission.
GEN501 was a phase I/II safety study divided into two parts. Part 1 was a dose‐escalation phase; Part 2 was a single‐arm phase with multiple cohorts, based on the dose levels established in Part 1. Patients relapsed from or refractory to two or more different prior therapies, including IMiDs and PIs, chemotherapy‐based regimens, or ASCT, and without further established treatment options were included in the study. The primary efficacy endpoint was ORR, which was defined as the proportion of patients who achieved partial response (PR) or better according to the IMWG criteria [9], [10]. In Part 2 of the study, patients received daratumumab dosed at 8 mg/kg (30 patients) or 16 mg/kg (42 patients). The ORR was 10% (95% CI 2.1%–26.5%) in the cohort that received 8 mg/kg and 35.7% (95% CI 21.6%–52.0%) in the cohort that received 16 mg/kg. A summary of key favorable effects is displayed in Table 1.
Clinical Safety
The safety database included 237 patients with MM who were treated with daratumumab as monotherapy (156 of whom received 16 mg/kg) from the MMY2002, GEN501, and MMY1002 studies.
For all treated subjects, the median relative dose intensity was 100% of the target dose for both treatment groups (8 and 16 mg/kg). The most frequently reported adverse reactions were IRRs. Other frequently reported adverse reactions (occurring in >20% of patients) were fatigue, pyrexia, cough, nausea, back pain, upper respiratory tract infection, anemia, neutropenia, and thrombocytopenia.
Infusion‐related reactions were reported in approximately half of all patients treated with daratumumab. The majority (79%) of IRRs occurred at the first infusion. Five percent of all patients had an IRR at more than one infusion. Symptoms predominantly included nasal congestion, cough, throat irritation, chills, vomiting, and nausea. Severe IRRs included bronchospasm, dyspnea, laryngeal edema, pulmonary edema, hypoxia, and hypertension. Patients should be premedicated with antihistamines, antipyretics, and corticosteroids to reduce the risk of IRRs prior to treatment with daratumumab. Daratumumab infusion should be interrupted for IRRs of any severity. Medical management and supportive treatment for IRRs should be instituted as needed. The infusion rate should be reduced when restarting the infusion. For the prevention of delayed IRRs, oral corticosteroids should be administered to all patients on the first and second day after all infusions. Additionally, the use of postinfusion medications (e.g., inhaled corticosteroids, short‐ and long‐acting bronchodilators) should be considered for patients with a history of obstructive pulmonary disorder to manage respiratory complications, should they occur.
Subcutaneous (SC) delivery of daratumumab is being tested in combination with the recombinant human hyaluronidase enzyme (rHuPH20) to facilitate systemic absorption of daratumumab after SC infusion into the abdominal wall [11]. Interference with blood typing was another important identified risk reported with daratumumab because binding to CD38, found also at low levels on red blood cells (RBCs), may result in a positive indirect Coombs test. A daratumumab‐mediated positive indirect Coombs test may persist for up to 6 months after the last daratumumab infusion. It should be recognized that daratumumab bound to RBCs may mask detection of antibodies to minor antigens in the patient's serum. However, the determination of a patient's ABO blood group and Rhesus (Rh) blood types are not impacted. In the event of a planned transfusion, blood transfusion centers should be notified of this interference with indirect antiglobulin tests. Additionally, to make health‐care providers and blood banks aware of the risk associated with blood typing, educational materials will be distributed that include a health‐care provider and blood bank brochure and a patient alert card. Survey tools will be used to measure (and increase) the awareness of the risk of interference with blood typing associated with daratumumab.
A statistically significant QTc prolongation in Part 2 of the GEN501 study has been observed; however, no plausible cause for the findings has been identified. A thorough QTc study was not considered feasible because of the lack of a biologic mechanism for an effect on QTc and because it is not possible to study daratumumab in healthy, normal volunteers or to expose oncology subjects to supratherapeutic doses. However, the company committed to incorporate adequate data collection in a substudy of the ongoing SMM2001 study to evaluate further a relationship between daratumumab concentration and QTc. This study of subjects with smoldering myeloma allows assessment of QTc at an earlier stage of the disease state in relatively healthy subjects. The results are planned to be submitted by December 31, 2018.
The Risk Management Plan (RMP) submitted by the company was agreed upon. Missing information included safety in pregnancy and lactation, safety in patients aged ≥75 years, safety in patients with moderate or severe hepatic impairment, safety in long‐term use (>2 years), and reproductive and developmental toxicity. A summary of key unfavorable effects is displayed in Table 1.
Benefit‐Risk Assessment
The review of daratumumab was conducted under the accelerated assessment program of the European Medicines Agency (EMA). Accelerated assessment reduces the time frame for the EMA Committee for Medicinal Products for Human Use (CHMP) to review a marketing‐authorization application to less than 150 days, instead of 210 days using the standard timetable. Applications may be eligible for accelerated assessment if the CHMP decides the product is of major interest for public health and therapeutic innovation.
An ORR of 29.2% obtained with daratumumab 16 mg/kg in the pivotal MMY2002 study was considered clinically relevant in patients with relapsed and refractory MM, whose prior therapy included a PI and an IMiD and who demonstrated disease progression on the last therapy, despite the absence of confirmatory controlled data. The responses were rapid, with a median time to response of 1 month and a median duration of response of 7.4 months. The ORR was consistent across different clinically relevant subgroups, such as number of prior lines of therapy, type of myeloma (IgG, non‐IgG), and baseline renal function. Patients were heavily pretreated, and 79.8% and 69.4% had received more than three lines of prior therapy in the MMY2002 and GEN501 studies, respectively; furthermore, 93.5% and 95.8%, respectively, were refractory to both PIs and IMiDs.
There were no data regarding the efficacy of daratumumab in patients previously exposed to panobinostat (together with bortezomib and dexamethasone) or carfilzomib (in combination with lenalidomide). Based on the data from the clinical studies submitted, it seemed reasonable to assume that the response rate could be expected to be similar regardless of the previous treatment. However, no firm conclusions could be drawn about the expected response.
Clinical experience with daratumumab in patients >75 years of age is quite limited, although age was not a statistically significant covariate on the pharmacokinetics of daratumumab. Although the numbers are small, there were no clinically meaningful differences in the overall safety profile of subjects aged >75 years compared with other age groups. A consistent ORR was observed among the different age groups (30% in patients aged <65 years, 35% in patients aged 65 to <75 years, and 25% in patients aged ≥75 years). Thus, the generalization of observed effects to an older population was considered reasonable. The company will continue to evaluate older subjects in the ongoing phase III studies.
An updated OS analysis as of a December 31, 2015, cutoff date was requested for the 148 subjects treated with 16 mg/kg daratumumab monotherapy in the MMY2002 and GEN501 studies. After a median duration of follow‐up of 20.7 months, the median OS for MMY2002 was 18.6 months. After a median duration of follow‐up of 20.1 months for GEN501, the median OS was not reached. Combined, after a median duration of follow‐up of 20.7 months, the Kaplan‐Meier‐based median OS was 20.1 months. The new cutoff has provided approximately 10% and 12% more events for the MMY2002 and GEN501 studies, respectively. The medians of OS do not significantly change from the previous cutoff date. When contextualizing these updates with the published data in the important setting of relapsed and refractory MM after at least two previous treatments (the FOCUS study of carfilzomib reported 10.2 months OS [12], and the MM‐003 study of pomalidomide reported 12.7 months OS after adjusting for crossover [13]), it is worth highlighting the longer survival for those patients exposed to daratumumab, especially those refractory to either pomalidomide or carfilzomib in monotherapy.
Median progression‐free survival (PFS) was short, around 4 months, but survival after progression was long. Daratumumab is a novel mAb with a completely new mechanism of action. Whether it can change the response of subsequent therapies or the natural course of the disease remains to be elucidated.
However, the CHMP considered that the absence of a control arm and the small number of patients treated with daratumumab in the MMY2002 and GEN501 studies impacted the interpretation of the clinical benefit of daratumumab. Therefore, additional controlled data in a larger target population with the same condition were considered necessary to confirm the benefit of daratumumab. The company was therefore requested, as a specific obligation for approval, to provide the comprehensive clinical data from the phase III studies assessing lenalidomide and dexamethasone with or without daratumumab (MMY3003) and bortezomib and dexamethasone with or without daratumumab (MMY3004) in patients with relapsed or refractory MM.
Translational biomarker studies were performed in the GEN501 and MMY2002 studies, showing previously unknown immunomodulatory effects of daratumumab. The company is planning to use these findings and analyses of CD38 expression, subsets of CD38+ regulatory T cells and CD38+ regulatory B cells, enzymatic activity of C38, and T‐cell clonality in the ongoing and future MMY3003 and MMY3004 studies. The company has also planned a comprehensive translational research strategy for the MMY3003 and MMY3004 studies, including whole‐genome sequencing and mRNA sequencing of CD38+ MM cells to correlate these with clinical response and progression. The CHMP recommended that the company submit these data, which might identify potential biomarkers of response and resistance to daratumumab.
Daratumumab may mask the detection of antibodies to minor antigens, but ABO and Rh blood types are not impacted. Based on the literature, transfusion reactions caused by minor antigen incompatibility are rare (1:204,000). During clinical studies and postmarketing experience (>1,500 patients treated in clinical studies and more than 750 postmarketing), there have been no cases of transfusion reactions reported. Interference mitigation methods can be used if the blood bank is made aware. Thus, the risk of transfusion reactions can be considered low, even in the emergency setting. The company committed to monitor indirect antiglobulin testing interference and transfusion reactions and to use a targeted follow‐up questionnaire. Given the low risk and proposed monitoring activities, a postauthorization safety study can be dismissed at this stage. The proposed text to be included in the Summary of Product Characteristics (sections 4.4 and 4.5) is acceptable.
The overall safety profile of daratumumab 16 mg/kg dosing regimen was considered acceptable, and the adverse events appeared manageable. However, the safety data provided, based on a single‐arm, open‐label pivotal study, were quite limited. Additional safety data from the ongoing comparative phase III studies (MMY3003 and MMY3004) were requested to further establish the safety profile of daratumumab.
Based on the above, the CHMP recommended the granting of a “conditional” marketing authorization. A conditional approval is reserved for medicinal drugs that treat, prevent, or diagnose seriously debilitating diseases, life‐threatening diseases, or rare diseases (orphan medicinal products) or drugs to be used in emergency situations in response to threats. With this approval, the applicant company is obliged to submit additional data, with the aim of confirming that the benefit‐risk balance is positive. A conditional approval is only valid for 1 year but can be renewed. The renewal is given on the basis of the confirmation of the benefit‐risk balance, taking into account the specific obligations and the time frame for their fulfilment. Once it is judged that remaining data have been provided or are no longer required, the approval can be converted to a “standard” approval. If at any time the benefit‐risk balance is considered to be negative, the marketing authorization can be suspended or revoked.
Daratumumab in Combination with Lenalidomide and Dexamethasone or with Bortezomib and Dexamethasone
Clinical Efficacy and Safety
The extension of the approved indication in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of adult patients with MM who have received at least one prior therapy was supported by two phase III studies (MMY3003 and MMY3004).
MMY3003 was a phase III, open‐label, multicenter study comparing the efficacy of daratumumab, combined with lenalidomide and low‐dose dexamethasone (DRd), with lenalidomide and low‐dose dexamethasone (Rd) in 569 patients with relapsed or refractory MM [14].
MMY3004 was a phase III, open‐label, multicenter study comparing the efficacy of daratumumab, combined with bortezomib and low‐dose dexamethasone (DVd), with bortezomib and low‐dose dexamethasone (Vd) in 498 patients with relapsed or refractory MM [15].
The eligibility criteria for both studies included patients with relapsed or refractory MM who had received at least one prior line of therapy and achieved at least a PR to one or more of their prior therapies for MM and had documented progressive disease according to the IMWG criteria for response on or after their last regimen. Patients refractory or intolerant to lenalidomide were excluded from the MMY3003 study. Patients refractory or intolerant to bortezomib or another PI, such as ixazomib or carfilzomib, were excluded from the MMY3004 study.
The primary efficacy endpoint was PFS, defined as the duration from the date of randomization to either progressive disease, according to the IMWG criteria, or death, whichever occurred first. Randomization was stratified by International Staging System (I, II, or III), number of prior lines of therapy (1 vs. 2 or 3 vs. >3), and prior lenalidomide/bortezomib treatment (no vs. yes).
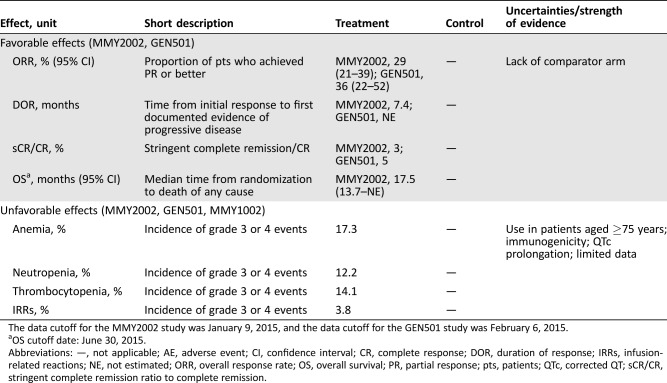
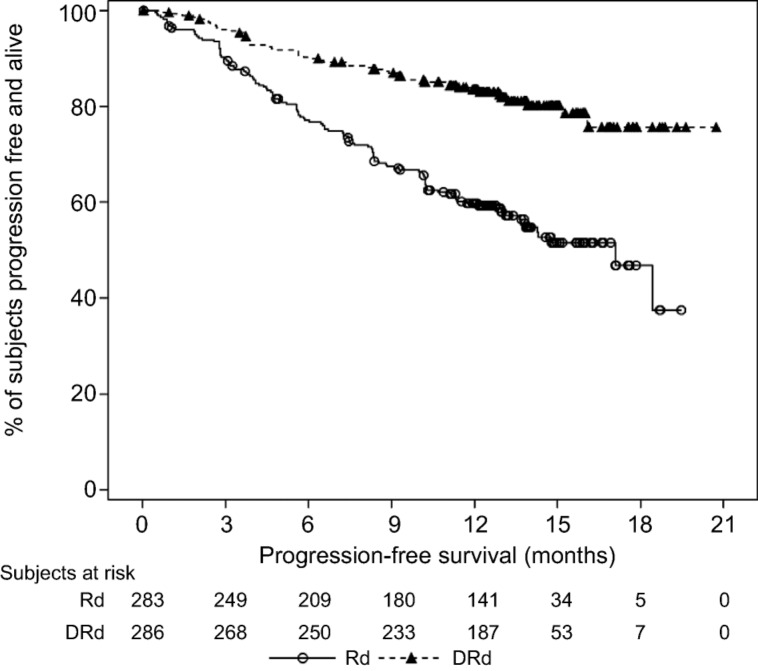
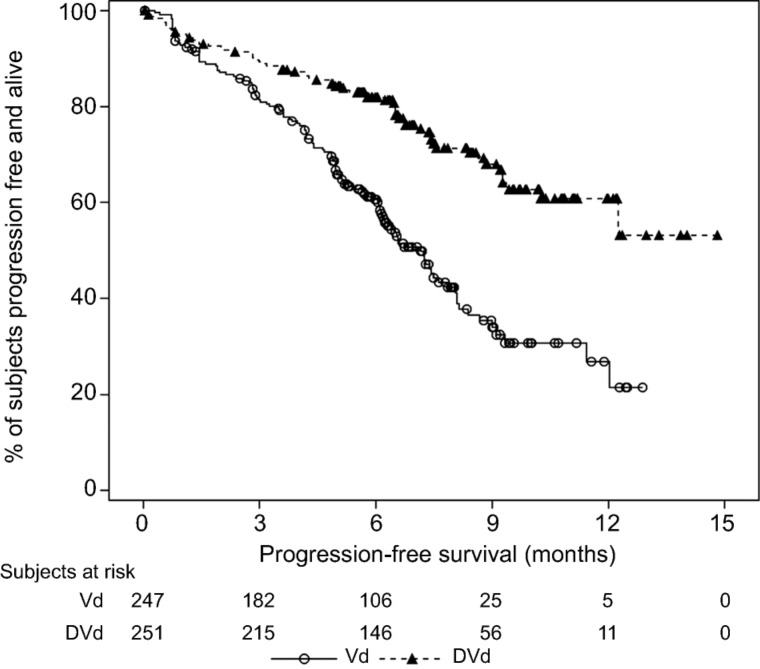
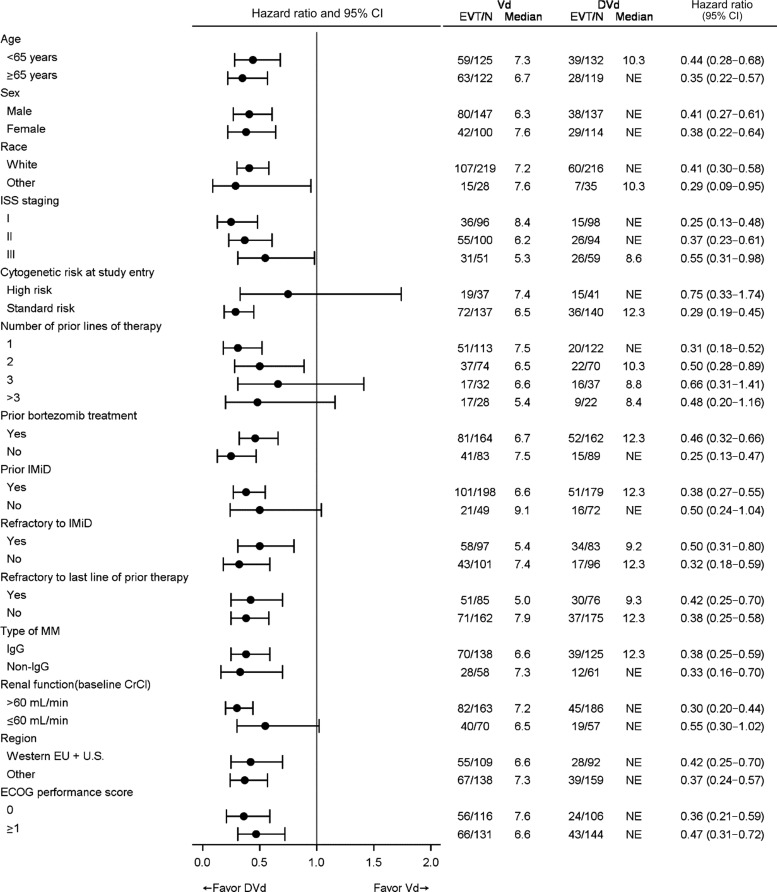
In the MMY3003 and MMY3004 studies, the median PFS was not reached for the daratumumab group and was 18.4 months for the Rd group (hazard ratio [HR] 0.37; 95% CI 0.27–0.52; p < .0001) and 7.2 months for the Vd group (HR 0.39; 95% CI 0.28–0.53; p < .0001), respectively (Figs. 1, 2). Sensitivity analyses of PFS were consistent across all prespecified subgroups in both studies and showed PFS improvement for patients in the daratumumab group versus patients in the Rd and Vd groups, including patients who had received three or more than three prior lines of therapy, those with advanced‐stage disease (ISS stage III), those with high‐risk cytogenetic abnormalities, those with prior lenalidomide treatment, and those refractory to their last line of prior therapy. Summaries of subgroup results for PFS are displayed in Figures 3 and 4.
Figure 1.
Kaplan‐Meier plot for progression‐free survival, intent‐to‐treat population (Study MMY3003).
Abbreviations: DRd, daratumumab + lenalidomide + low‐dose dexamethasone; Rd, lenalidomide + low‐dose dexamethasone.
Figure 2.
Kaplan‐Meier plot for progression‐free survival, intent‐to‐treat population (Study MMY3004).
Abbreviations: DVd, daratumumab + bortezomib + low‐dose dexamethasone; Vd, bortezomib + low‐dose dexamethasone.
Figure 3.
Forest plot of subgroup analyses of progression‐free survival based on computer algorithm, intent‐to‐treat population (Study MMY3003).
Abbreviations: CA, Canada; CI, confidence interval; CrCl, creatine clearance rate; DRd, daratumumab + lenalidomide + low‐dose dexamethasone; ECOG, Eastern Cooperative Oncology Group; EU, European Union; EVT/N, number of progression events or deaths/total number; ISS, International Staging System; MM, multiple myeloma; NE, not estimated; PI, proteasome inhibitor; Rd, lenalidomide + low‐dose dexamethasone.
Figure 4.
Forest plot of subgroup analyses of progression‐free survival based on computer algorithm, intent‐to‐treat population (Study MMY3004).
Abbreviations: CI, confidence interval; CrCl, creatine clearance rate; DVd, daratumumab + bortezomib + low‐dose dexamethasone; ECOG, Eastern Cooperative Oncology Group; EU, European Union; EVT/N, number of progression events or deaths/total number; IMiD, immunomodulatory drug; ISS, International Staging System; MM, multiple myeloma; NE, not estimated; Vd, bortezomib + low‐dose dexamethasone.
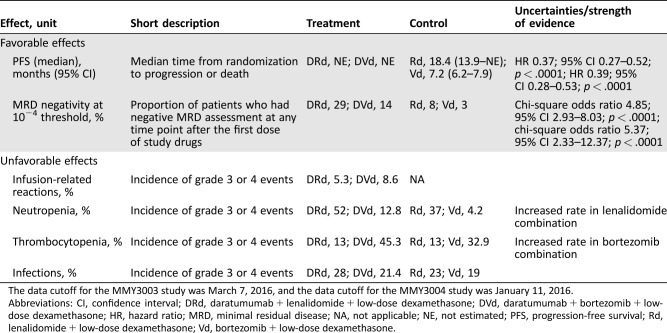
In the MMY3003 and MMY3004 studies, the daratumumab group showed a greater incidence of minimal residual disease (MRD) negativity compared with the Rd and Vd groups, respectively. Twenty‐nine percent (29%) of the patients in the DRd group achieved MRD negativity status by the threshold of 10−4 versus 8% in the Rd group (Mantel‐Haenszel odds ratio 4.88; 95% CI 2.94–8.08; p < .0001), and 14% of the DVd group achieved MRD negativity status compared with 3% of patients in the Vd group (Mantel‐Haenszel odds ratio 5.56; 95% CI 2.37–13.04; p < .0001).
A summary of key favorable effects is displayed in Table 2.
Table 2. Key favorable and unfavorable effects for DRd versus Rd and for DVd versus Vd.
The data cutoff for the MMY3003 study was March 7, 2016, and the data cutoff for the MMY3004 study was January 11, 2016.
Abbreviations: CI, confidence interval; DRd, daratumumab + lenalidomide + low‐dose dexamethasone; DVd, daratumumab + bortezomib + low‐dose dexamethasone; HR, hazard ratio; MRD, minimal residual disease; NA, not applicable; NE, not estimated; PFS, progression‐free survival; Rd, lenalidomide + low‐dose dexamethasone; Vd, bortezomib + low‐dose dexamethasone.
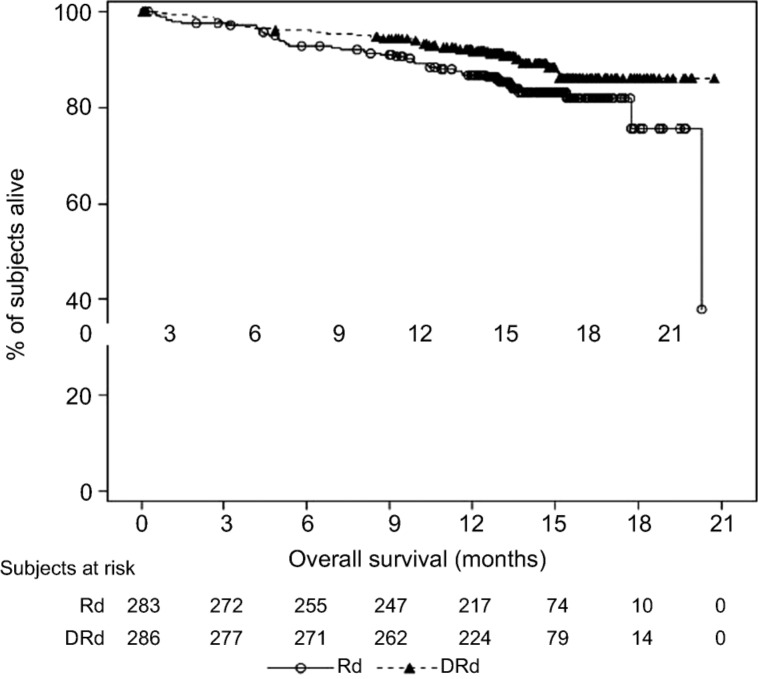
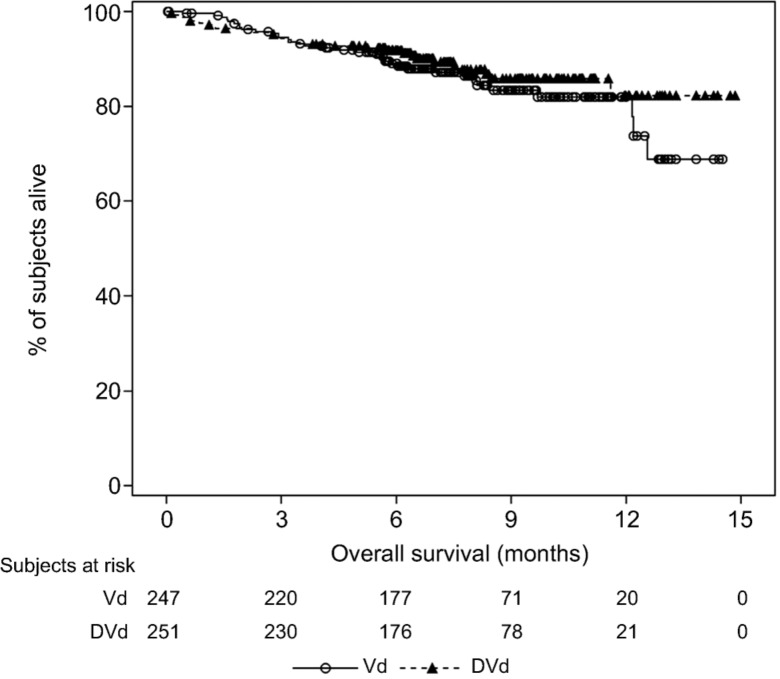
As of the clinical cutoff dates of the MMY3003 and MMY3004 studies, the median OS was not reached for either treatment group (Figs. 5, 6).
Figure 5.
Kaplan‐Meier plot for overall survival, intent‐to‐treat population (Study MMY3003).
Abbreviations: DRd, daratumumab + lenalidomide + low‐dose dexamethasone; Rd, lenalidomide + low‐dose dexamethasone.
Figure 6.
Kaplan‐Meier plot for overall survival, intent‐to‐treat population (Study MMY3004).
Abbreviations: DVd, daratumumab + bortezomib + low‐dose dexamethasone; Vd, bortezomib + low‐dose dexamethasone.
Updated data with longer follow‐up about the efficacy of daratumumab in combination with bortezomib or lenalidomide plus dexamethasone are now available [16], [17]; however, these data have not yet been assessed by the CHMP.
In the combination treatment, the safety profile was consistent with the known toxicities of the respective background therapies and daratumumab monotherapy. However, daratumumab may increase the rate of cytopenias known to be associated with each background therapy (neutropenia with lenalidomide and thrombocytopenia with bortezomib). Both neutropenia and thrombocytopenia have been classified as important identified risks in the RMP. A summary of key unfavorable effects is displayed in Table 2.
Benefit‐Risk Assessment
The two pivotal MMY3003 and MMY3004 studies demonstrated a positive effect on PFS, which was considered clinically significant when compared with background therapies alone. Overall, the effect observed on PFS was sufficient to establish the efficacy of the combination of daratumumab, lenalidomide, and dexamethasone, and of the combination of daratumumab, bortezomib, and dexamethasone for the proposed indication. Daratumumab may increase the rate of cytopenias (neutropenia and thrombocytopenia) known to be associated with each background therapy. However, they appeared to be manageable with supportive care and dose modifications, and did not result in an increase in discontinuations of study treatment or deaths. Therefore, the benefit‐risk balance was considered positive for the treatment of adult patients with multiple myeloma who have received at least one prior therapy.
Moreover, following submission of the controlled data of the MMY3003 and MMY3004 studies, the efficacy and safety of daratumumab was confirmed. The CHMP agreed on the fulfilment of the specific obligations and the approval of daratumumab was converted to a “standard” approval.
Acknowledgments
The scientific assessment summarized in this report is based on important contributions from the rapporteur and corapporteur assessment teams, CHMP members, and additional experts following the application for a marketing authorization from the company.
This publication is a summary of the European Public Assessment Report, the Summary of Product Characteristics, and other product information published on the EMA website (www.ema.europa.eu). For the most current information on this marketing authorization, please refer to the EMA website. The authors of this paper remain solely responsible for the opinions expressed in this publication.
Footnotes
Editor's Note: See the related commentary, “Monoclonal Antibodies and Multiple Myeloma: All in All It's Just Another Brick in the Wall?” by Pellegrino Musto, on page 511 of this issue.
Author Contributions
Data analysis and interpretation: Elisabeth Penninga, Marie Louise Schougaard Christiansen, Doris Hovgaard, Sinan B. Sarac, Jorge Camarero Jimenez, Isabel Garcia, Marta Lafuente, Arantxa Sancho‐López
Manuscript writing: Kyriaki Tzogani, Elisabeth Penninga, Marie Louise Schougaard Christiansen, Doris Hovgaard, Sinan B. Sarac, Jorge Camarero Jimenez, Isabel Garcia, Marta Lafuente, Arantxa Sancho‐López, Francesco Pignatti
Final approval of manuscript: Kyriaki Tzogani, Elisabeth Penninga, Marie Louise Schougaard Christiansen, Doris Hovgaard, Sinan B. Sarac, Jorge Camarero Jimenez, Isabel Garcia, Marta Lafuente, Arantxa Sancho‐López, Tomas Salmonson, Christian Gisselbrecht, Francesco Pignatti
Disclosures
The authors indicated no financial relationships.
References
- 1. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011;364:1046–1060. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–1403. [DOI] [PubMed] [Google Scholar]
- 3. Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood 2015;125:3076–3084. [DOI] [PubMed] [Google Scholar]
- 4. Kyle RA, Gertz MA, Witzig TE et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003;78:21–33. [DOI] [PubMed] [Google Scholar]
- 5. Kumar SK, Lee JH, Lahuerta JJ et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter International Myeloma Working Group study. Leukemia 2012;26:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iida S, Suzuki K, Kusumoto S et al. Safety and efficacy of daratumumab in Japanese patients with relapsed or refractory multiple myeloma: A multicenter, phase 1, dose‐escalation study. Int J Hematol 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7. Lonial S, Weiss BM, Usmani SZ et al. Daratumumab monotherapy in patients with treatment‐refractory multiple myeloma (SIRIUS): An open‐label, randomised, phase 2 trial. Lancet 387:1551–1560. [DOI] [PubMed] [Google Scholar]
- 8. Lokhorst HM, Plesner T, Laubach JP et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Eng J Med 2015;373:1207–1219. [DOI] [PubMed] [Google Scholar]
- 9. Durie BGM, Harousseau JL, Miguel JS et al. International uniform response criteria for multiple myeloma. Leukemia 2006;20:1467–1473. [DOI] [PubMed] [Google Scholar]
- 10. Rajkumar SV, Harousseau JL, Durie B et al. Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood 2011;117:4691–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Usmani SZ, Nahi H, Mateos MV et al. Open‐label, multicenter, dose escalation phase 1b study to assess the subcutaneous delivery of daratumumab in patients (pts) with relapsed or refractory multiple myeloma (PAVO). Blood 2016;128:1149a. [Google Scholar]
- 12. Hájek R, Masszi T, Petrucci MT et al. A randomized phase III study of carfilzomib vs low‐dose corticosteroids with optional cyclophosphamide in relapsed and refractory multiple myeloma (FOCUS). Leukemia 2017;31:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morgan G, Palumbo A, Dhanasiri S et al. Overall survival of relapsed and refractory multiple myeloma patients after adjusting for crossover in the MM‐003 trial for pomalidomide plus low‐dose dexamethasone. Br J Haematol 2015;168:820–823. [DOI] [PubMed] [Google Scholar]
- 14. Dimopoulos MA, Oriol A, Nahi H et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 2016;375:1319–1331. [DOI] [PubMed] [Google Scholar]
- 15. Palumbo A, Chanan‐Khan A, Weisel K et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 2016;375:754–766. [DOI] [PubMed] [Google Scholar]
- 16. Maria‐Victoria M, Estell J, Wolney B et al. Efficacy of daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory myeloma based on prior lines of therapy: Updated analysis of CASTOR. Abstract presented at American Society of Hematology 58th Annual Meeting; December 3–6, 2016; San Diego; 1150.
- 17. Usmani SZ, Dimopoulos MA, Belch A et al. Efficacy of daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma patients with 1 to 3 prior lines of therapy: Updated analysis of POLLUX. Abstract presented at American Society of Hematology 58th Annual Meeting; December 3–6, 2016; San Diego; 1151.