This review describes the role of intracellular signaling pathways and the estrogen receptor in breast cancer, the role of anti‐estrogens in the treatment of HR1 advanced breast cancer, the development of resistance to anti‐estrogen therapy, and the use of endocrine and endocrine‐based combination therapy in breast cancer.
Keywords: Endocrine therapy, Estrogen receptor degrader, CDK4/6 inhibitor, Aromatase inhibitor, mTOR inhibitor
Abstract
Advancements in molecular profiling and endocrine therapy (ET) have led to more focused clinical attention on precision medicine. These advances have expanded our understanding of breast cancer (BC) pathogenesis and hold promising implications for the future of therapy. The estrogen receptor‐α is a predominant endocrine regulatory protein in the breast and in estrogen‐induced BC. Successful targeting of proteins and genes within estrogen receptor (ER) nuclear and nonnuclear pathways remains a clinical goal. Several classes of antiestrogenic agents are available for patients with early, advanced, or metastatic BC, including selective ER modulators, aromatase inhibitors, and a selective ER degrader. Clinical development is focused upon characterizing the efficacy and tolerability of inhibitors that target the phosphatidylinositol 3 kinase (PI3K)/akt murine thymoma viral oncogene (AKT)/mammalian target of rapamycin inhibitor (mTOR) signaling pathway or the cyclin‐dependent kinase 4/6 (CDK4/6) cell cycle pathway in women with hormone receptor‐positive, human epidermal growth receptor 2‐negative BC who have demonstrated disease recurrence or progression. De novo and acquired resistance remain a major challenge for women with BC receiving antiestrogenic therapy. Therefore, sequential combination of targeted ET is preferred in these patients, and the ever‐increasing understanding of resistance mechanisms may better inform the selection of future therapy. This review describes the intricate roles of the PI3K/AKT/mTOR and CDK4/6 pathways in intracellular signaling and the use of endocrine and endocrine‐based combination therapy in BC.
Implications for Practice.
The foundational strategy for treating hormone receptor‐positive, human epidermal growth receptor 2‐negative, advanced breast cancer includes the use of endocrine therapy either alone or in combination with targeted agents. The use of combination therapy aims to downregulate cell‐signaling pathways with the intent of minimizing cellular “crosstalk,” which can otherwise result in continued tumorigenesis or progression through redundant pathways. This review provides the clinician with the molecular rationale and clinical evidence for these treatments and refers to evidence‐based guidelines to inform the decision‐making process.
摘要
分子表达谱和内分泌疗法 (ET) 的进步使得精准医疗获得更为集中的临床关注。这些进步拓展了我们对乳腺癌 (BC) 发病机制的了解, 并预示未来疗法的良好前景。雌激素受体 α 是乳腺及雌激素诱导 BC 中的主要内分泌调节蛋白。对雌激素受体 (ER) 核途径和非核途径中的蛋白质和基因进行成功靶向仍是临床目标。早期、晚期和转移性 BC 患者现可使用多种抗雌激素药物, 包括选择性 ER 调节剂、芳香化酶抑制剂和选择性 ER 降解剂。临床开发专注于表征以磷脂酰肌醇 3 激酶 (PI3K)/akt 鼠胸腺瘤病毒致癌基因 (AKT)/哺乳动物雷帕霉素靶蛋白 (mTOR) 抑制剂信号传导通路或细胞周期依赖性激酶 4/6 (CDK4/6) 细胞周期通路为靶标的抑制剂在显现疾病复发或进展的激素受体阳性、人表皮生长受体 2 阴性 BC 女性中的疗效和耐受性。在接受抗雌激素治疗的 BC 女性中, 原发性及获得性耐药仍是主要挑战。因此, 靶向 ET 的序贯联合治疗是这些患者的首选, 对耐药性机制的理解不断增加可更好地指导未来疗法的选择。本综述介绍了 PI3K/AKT/mTOR 和 CDK4/6 通路在细胞内信号传导中的复杂作用以及内分泌和以内分泌为基础的联合疗法在 BC 中的使用。
对临床实践的启示:激素受体阳性、人表皮生长受体 2 阴性晚期乳腺癌的基本治疗策略包括单独使用内分泌疗法或与靶向药物联用。使用联合疗法旨在下调细胞信号传导通路, 目的最大程度地减少细胞“串扰”, 否则可能会通过冗余途径导致持续肿瘤发生或进展。本综述为临床医生提供了该类治疗的分子依据和临床证据, 并援引循证指南指导决策过程
Introduction
Approximately 70% of all breast cancers (BC) express the estrogen receptor (ER), progesterone receptor (PgR), or both, and such tumors are considered hormone receptor‐positive (HR+) [1]. In addition to testing for the presence of ER and PgR, testing for human epidermal growth receptor 2 (HER2) protein overexpression and/or HER2 gene amplification is also performed at the time of diagnosis, and these test results aid in informing treatment decisions [2]. Molecular profiling has uncovered intrinsic subtypes in BC, including luminal A, luminal B, HER2‐enriched, basal‐like, and normal‐like, which are associated with specific morphological and molecular features of BC [3]. Over the last decade we have also improved our understanding of intracellular signaling pathways and the cancer cell cycle, and these advances have identified promising targets for cancer therapy.
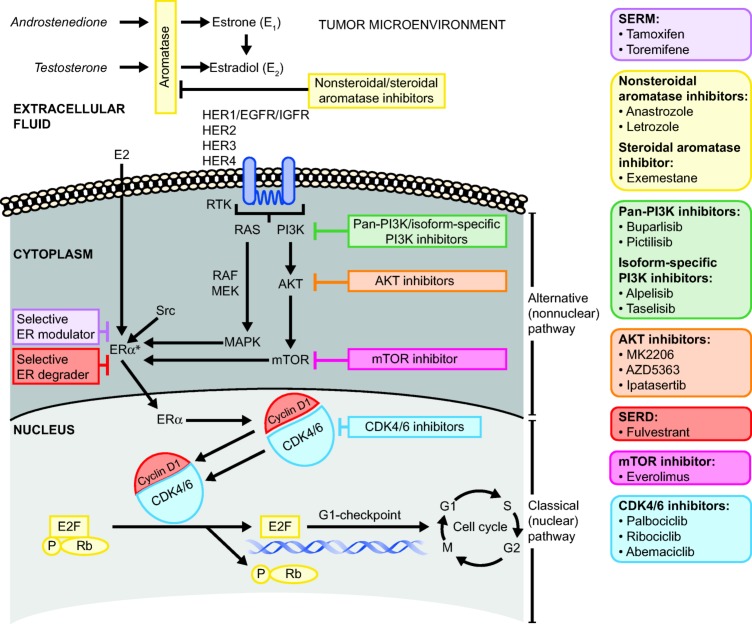
A number of classes of antiestrogenic agents are approved for patients with early, advanced, or metastatic BC, which include selective estrogen receptor modulators (SERMs), aromatase inhibitors (AIs), and a selective estrogen receptor degrader (SERD; Fig. 1) [4], [5]. However, the clinical development of combinations of antiestrogenic therapy with targeted agents that inhibit the phosphatidylinositol 3 kinase (PI3K)/akt murine thymoma viral oncogene (AKT)/mammalian target of rapamycin inhibitor (mTOR) signaling pathway or the cyclin‐dependent kinase 4/6 (CDK4/6) pathway at the G1/S checkpoint of the cell cycle is currently a key focus of clinical research in patients with HR+ BC who have demonstrated disease recurrence or progression [6], [7], [8]. This review describes the role of these signaling pathways and the ER in BC, the role of antiestrogens in the treatment of HR+ advanced BC, the development of resistance to antiestrogen therapy, and the use of endocrine and endocrine‐based combination therapy in BC. Supporting evidence for their benefits is provided by completed phase III studies (Table 1) [9], [10], [11], [12], [13], [14], [15], [16], [17] and ongoing phase III studies (Table 2) [18], [19].
Figure 1.
Critical nuclear (classical) and nonnuclear (alternative) signaling pathways implicated in endocrine resistance and targets for drugs in development. Note: Upon ligand binding, the estrogen‐ER complex dimerizes and interacts with coregulator proteins and specific sequences of DNA and the estrogen response elements to promote the transcription of a wide range of genes that participate in the regulation of the cell cycle, DNA replication, cellular differentiation, apoptosis, and angiogenesis.
Abbreviations: AKT, akt murine thymoma viral oncogene; CDK4/6, cyclin‐dependent kinase 4/6; E2, estradiol; E2F, E2F transcription factors; EGFR, epidermal growth factor receptor; ER, estrogen receptor; ERα, estrogen receptor‐α; HER1, human epidermal growth receptor 1; HER2, human epidermal growth receptor 2; HER3, human epidermal growth receptor 3; HER4, human epidermal growth receptor 4; IGFR, insulin‐like growth factor receptor; MAPK, mitogen‐activated protein kinase; MEK, mitogen‐activated ERK‐activating kinase; mTOR, mammalian target of rapamycin; P, phosphate; PI3K, phosphoinositide‐3‐kinase; RAF, rapidly accelerated fibrosarcoma; RAS, rat sarcoma; Rb, retinoblastoma protein; RTK, receptor tyrosine kinase; SERD, selective estrogen receptor downregulator; SERM, selective estrogen receptor modulator.
*Upon ligand binding, the estrogen‐ER complex dimerizes and interacts with coregulator proteins and specific sequences of DNA and the estrogen response elements (EREs) to promote the transcription of a wide range of genes that participate in the regulation of the cell cycle, DNA replication, cellular differentiation, apoptosis, and angiogenesis.
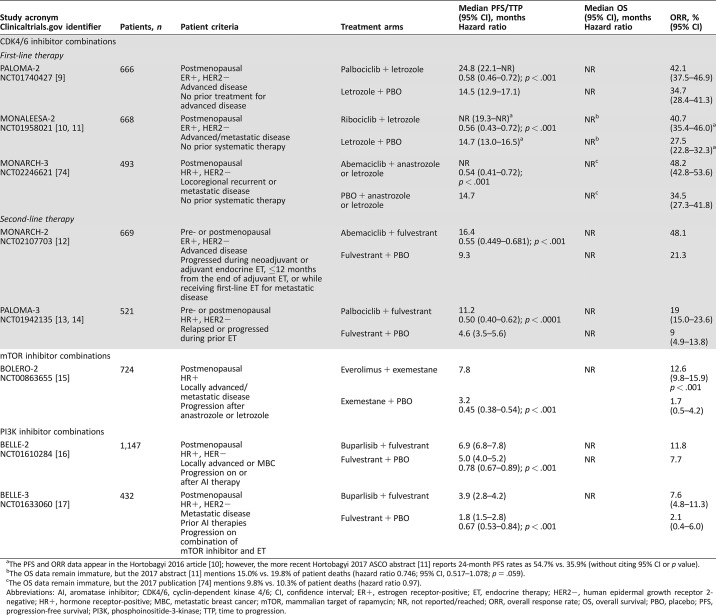
Table 1. Completed phase III studies of endocrine‐based combination therapies for advanced or metastatic breast cancer.
The PFS and ORR data appear in the Hortobagyi 2016 article [10]; however, the more recent Hortobagyi 2017 ASCO abstract [11] reports 24‐month PFS rates as 54.7% vs. 35.9% (without citing 95% CI or p value).
The OS data remain immature, but the 2017 abstract [11] mentions 15.0% vs. 19.8% of patient deaths (hazard ratio 0.746; 95% CI, 0.517–1.078; p = .059).
The OS data remain immature, but the 2017 publication [74] mentions 9.8% vs. 10.3% of patient deaths (hazard ratio 0.97).
Abbreviations: AI, aromatase inhibitor; CDK4/6, cyclin‐dependent kinase 4/6; CI, confidence interval; ER+, estrogen receptor‐positive; ET, endocrine therapy; HER2−, human epidermal growth receptor 2‐negative; HR+, hormone receptor‐positive; MBC, metastatic breast cancer; mTOR, mammalian target of rapamycin; NR, not reported/reached; ORR, overall response rate; OS, overall survival; PBO, placebo; PFS, progression‐free survival; PI3K, phosphoinositide‐3‐kinase; TTP, time to progression.
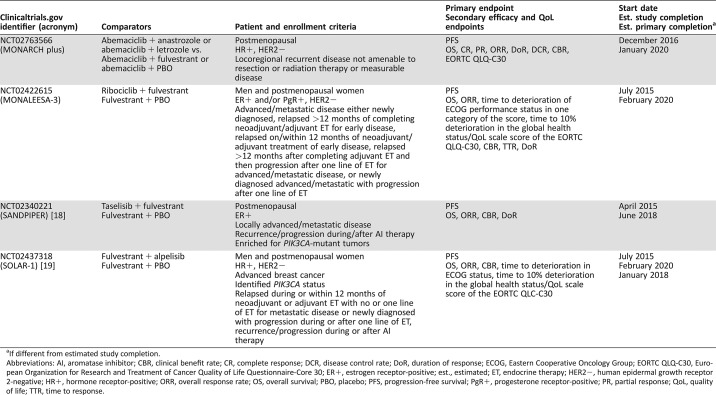
Table 2. In‐progress phase III studies of endocrine‐based combination therapies for advanced/metastatic breast cancer.
If different from estimated study completion.
Abbreviations: AI, aromatase inhibitor; CBR, clinical benefit rate; CR, complete response; DCR, disease control rate; DoR, duration of response; ECOG, Eastern Cooperative Oncology Group; EORTC QLQ‐C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 30; ER+, estrogen receptor‐positive; est., estimated; ET, endocrine therapy; HER2−, human epidermal growth receptor 2‐negative; HR+, hormone receptor‐positive; ORR, overall response rate; OS, overall survival; PBO, placebo; PFS, progression‐free survival; PgR+, progesterone receptor‐positive; PR, partial response; QoL, quality of life; TTR, time to response.
The Estrogen Receptor and Crosstalk
There are two functionally distinct ERs, ER‐alpha (ERα, ESR1 gene) and ER‐beta (ESR2 gene). ERα is a predominant endocrine regulatory protein in the breast and in estrogen‐induced BC [20]. In this review, references to the ER pertain to ERα/ESR1. Estrogen binds to the ER with high affinity and specificity and functions through two main types of pathways, the classical (or nuclear) pathway and the alternative (nonnuclear) pathway (Fig. 1) [21]. Successful targeting of genes within these nuclear and nonnuclear pathways remains an important clinical goal. Along the classical pathway, the estrogen‐ER complex dimerizes upon ligand binding and interacts with coregulator proteins and specific sequences of DNA called estrogen responsive elements. These interactions promote the transcription of a wide range of genes that participate in the regulation of the cell cycle, DNA replication, cellular differentiation, apoptosis, and angiogenesis [7], [21].
The engagement of the ER with estrogen through nonnuclear pathways originates in the cytoplasm to trigger coregulator growth factor and G‐protein coupled signaling (Fig. 1). Coregulators in the nonnuclear pathways include receptors (e.g., insulin‐like growth factor‐1 receptor, fibroblast growth factor receptor [FGFR], HER2), and kinases (e.g., mitogen‐activated protein kinases, receptor tyrosine kinase, PI3K, AKT, mTOR, Src, and CDK). Because the ER can also be activated through ligand‐independent mechanisms, multiple opportunities exist for crosstalk between the ER, growth factors, and protein kinases, which can activate or modulate ER activity [7], [21].
Estrogen‐Mediated Effects on the Cell Cycle
Progression through the cell cycle is controlled by the binding of cyclins and the ensuing dimerization of CDKs, which are synthesized and degraded at specific points throughout the cell cycle (Fig. 1). Estrogen is instrumental to this process by facilitating the G1 to S phase transition by means of the activation and binding of cyclin D1 to dimerized CDK4/6 [6, 22, 23]. Dysregulation of the cyclin D1/CDK4/6 pathway is reported to be an early and essential gateway for breast tumorigenesis because the overexpression of cyclin D1 has been implicated in the development of BC [24], [25], [26], several other solid tumors, and hematologic malignancies [26], [27]. Amplification of the gene for cyclin D1, CCND1, occurs in many human BC tumors, including 29% of luminal A cancers, 58% of luminal B cancers, and 38% of the HER2‐expressing molecular subtypes; overexpression of CDK4 occurs in 14%, 25%, and 24% of these subtypes, respectively [28].
Endocrine Therapy Action on Estrogen Receptor and Tumor Microenvironment
Antiestrogenic therapies used to treat HR+ BC may target either estrogen production or the estrogen receptor (Fig. 1) [20]. In existing treatment guidelines, the use of monotherapy or combination approaches may vary based on prior adjuvant endocrine therapy (ET) exposure status and whether relapse or recurrence occurred before or after 12 months since adjuvant treatment. Endocrine therapies currently utilized in the first‐ or second‐line for estrogen‐positive BC include the AIs, anastrozole, letrozole, and exemestane; the SERD, fulvestrant [2], [4], [5]; and the SERMs, tamoxifen and the chlorinated derivative toremifene [29]. High‐dose estrogen (ethinyl estradiol), progestin (megestrol acetate), and the androgen, fluoxymesterone, are recommended as third‐ and later‐line therapy [5]. In addition, several targeted therapies have become available for use either as monotherapy or in combination with ET, including other CDK4/6 inhibitors (e.g., abemaciclib, palbociclib, and ribociclib) and the mTOR inhibitor, everolimus.
The downstream effects of SERMs binding the ER are tissue‐specific and may differ among the various agents, for example, agents that act as antagonists in BC tissue and, alternatively, as partial agonists in other tissues [30]. These disparate properties are also demonstrated by other SERMs, such as raloxifene, which is used to reduce the risk of BC in postmenopausal patients with osteoporosis or who are otherwise at risk of invasive breast cancer and also as a treatment for osteoporosis [31].
Aromatase inhibitors reduce the production of estrogen by inhibiting the aromatase enzyme activity in peripheral tissues and within the tumor microenvironment (Fig. 1). Exemestane is a steroidal molecule that irreversibly and covalently binds to aromatase [32]. The nonsteroidal agents, anastrozole and letrozole, reversibly bind aromatase [33]. Preclinical studies suggested that letrozole alone was superior to tamoxifen, and no additional benefits were evident for the combination treatment; studies with anastrozole in combination with tamoxifen reported findings similar to those with letrozole [34], [35]. Lastly, no advantage of the atamestane (a steroidal AI) plus toremifene combination over letrozole monotherapy was observed in a phase III study of postmenopausal women with advanced receptor‐positive BC [36]. Consequently, there has not been a strong rationale to further explore tamoxifen or toremifene in combination with AIs as a first‐line doublet.
The SERD fulvestrant is a 7α‐alkylsulphinyl analogue of 17β‐estradiol [37] that competitively inhibits the binding of estradiol to the ER and binds with similar affinity as estradiol (50% inhibitory concentration [IC50], 0.89), and a much higher affinity than does tamoxifen (IC50, 0.19–0.25) [38], [39]. The binding of fulvestrant to ER monomers exerts several effects: inhibition of ER dimerization, inactivation of subunit transcription activating factor 1 and activating factor 2, attenuation of the translocation of ER to the nucleus, accelerated ER degradation, and ER downregulation (Fig. 1) [40]. The activity of fulvestrant is characterized by pure ER antagonism with exclusively antiestrogenic effects on breast tissue, resulting in the inhibition of estrogen‐dependent breast tumor cell proliferation [38], [40], [41]. Fulvestrant is known for its effects on the cell during the G1/S phase transition, by increasing the proportion of cells in G0/G1 and decreasing the proportion of cells undergoing continued DNA synthesis [38].
The activity of fulvestrant is characterized by pure ER antagonism with exclusively antiestrogenic effects on breast tissue, resulting in the inhibition of estrogen‐dependent breast tumor cell proliferation
The initial U.S. Food and Drug Administration (FDA) approval of fulvestrant was for a monthly 250‐mg dose administered by intramuscular injection [42], [43]. Some of the early fulvestrant studies also used a 500‐mg loading dose. Subsequently, however, the CONFIRM study provided the basis for approval of the 500‐mg dose by comparing fulvestrant 250 mg with 500 mg and demonstrating improved efficacy and a similar safety profile for the higher dose [44], [45]. The FALCON study conducted in postmenopausal ET‐naïve patients with HR+, HER2‐negative (HER2−) BC found that a 500‐mg fulvestrant loading schedule was associated with a significantly longer progression‐free survival (PFS; 16.6 vs. 13.8 months; p = .049; primary endpoint) and provided significantly longer duration of response (11.4 vs. 7.5 months; p = .037) compared with anastrozole [4]. Updated analysis of this trial indicates that fulvestrant is also associated with maintenance of health‐related quality of life in this population [46]. In August 2017, the indication of fulvestrant was expanded to include treatment of HR+, HER2−, advanced BC in postmenopausal women not previously treated with endocrine therapy [47].
Antiestrogenic Effects on Signaling Pathways and Development of Resistance to Antiestrogenic Therapy in Breast Tumor Cells
Development of endocrine resistance has been linked to overexpression and/or amplification of a number of genes in growth factor pathways, including those mediated by HER2, human epidermal growth receptor 3, epidermal growth factor receptor, FGFR‐1, and insulin‐like growth factor receptor‐1 (Fig. 1) [48], [49], [50], [51]. These proteins signal along the PI3K/AKT/mTOR pathway [1]. Overexpression of HER2, which occurs in approximately 15% to 20% of all BC [52], mediates cell growth and survival through activation of its downstream mediators, PI3K/AKT/mTOR and rat sarcoma/rapidly accelerated fibrosarcoma/mitogen‐activated ERK‐activating kinase/mitogen‐activated protein kinase. Aberrant activation of the PI3K/AKT/mTOR pathway, a key pathway axis in the signaling network, is associated with ligand‐independent ER activation and subsequent activation of downstream pathways without traditional binding or regulation of estrogen [52].
The PIK3CA gene is mutated in as many as half of breast tumors [53], [54], [55], with mutation status being highly concordant between primary and metastatic tumors [56], [57]. Alterations in the PI3K/AKT pathway, including AKT mutations and loss of phosphatase and tensin homolog (PTEN), occur in more than 70% of breast tumors [58]. The mTOR protein complex is a major downstream target of AKT [59].
A number of studies have implicated cyclin D1 and cyclin‐dependent kinases in endocrine resistance. In vitro studies demonstrated that HR+ BC cells induced to overexpress cyclin D1 continued to grow in the presence of tamoxifen [60], and cyclin D1 has been shown to be essential for the proliferation of tamoxifen‐resistant cells [61]. Breast tumor cells that develop resistance to ET maintain activation of cyclin D1 and the subsequent phosphorylation of retinoblastoma protein (Fig. 1) [62]. Higher levels of cyclin D1 mRNA in HR+ primary tumors among patients receiving tamoxifen have been associated with worse outcomes, including significantly shorter time to recurrence (p = .025), time to metastasis (p = .019), and overall survival (OS; p = .025) [63].
Adjuvant AI therapy appears to select for ESR1 mutations under the stress of estrogen deprivation, in which there is genetic intratumoral heterogeneity and clonal diversity. In contrast, treatment with fulvestrant does not select for ESR1 mutations conferring constitutive activation of ERα
Mutations in ESR1 have also been linked to acquired endocrine resistance, with the most common ESR1 mutations affecting one of two residues in the ER ligand‐binding domain [1]. These ESR1 mutations confer constitutive or estrogen‐independent activation of the ER and resistance to AI therapy [64], [65]. ESR1 mutations are rarely found in treatment‐naïve patients and are hardly ever the cause of primary resistance [1]. Instead, ESR1 mutations associated with endocrine resistance are found in patients with metastatic disease who have been treated with AIs [65], [66], [67]. Adjuvant AI therapy appears to select for ESR1 mutations under the stress of estrogen deprivation, in which there is genetic intratumoral heterogeneity and clonal diversity [68], [69]. In contrast, treatment with fulvestrant does not select for ESR1 mutations conferring constitutive activation of ERα [67]. More work is ongoing to clarify the impact of ESR1 mutations on response to therapy, with future trials incorporating serial cell‐free DNA sampling to quantify and follow ESR1 mutational burden and correlate with response to therapy.
Endocrine Therapy Options to Manage Resistance
Although ETs are a common first‐line treatment in advanced or metastatic breast cancer (MBC), resistance inevitably develops [5]. A deeper understanding of the fundamental mechanisms of ET resistance has led to the development of treatment options and strategies, as well as a greater awareness of how to better utilize existing agents.
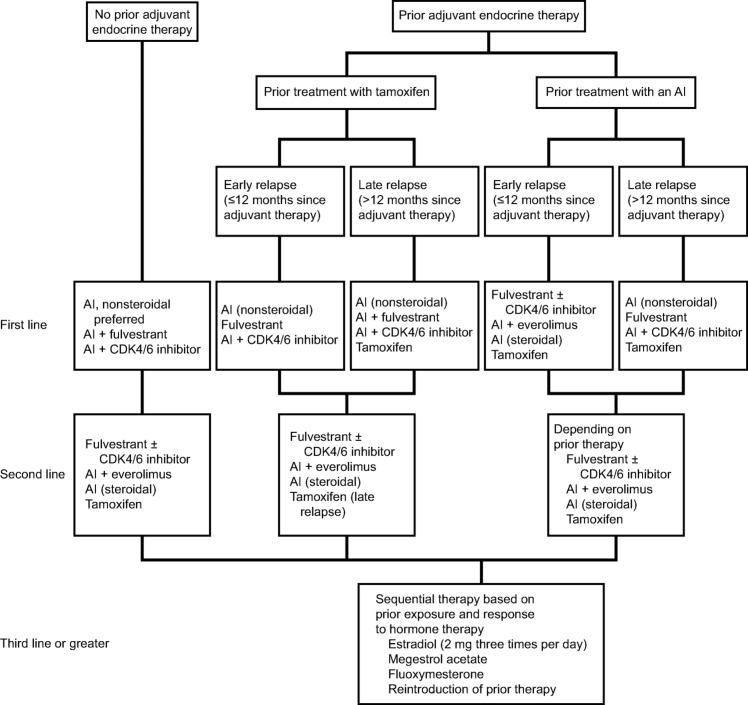
Whereas some patients may develop resistance to ET with one agent class, a response to treatment may occur with exposure to another class. Sequential ET is preferred in postmenopausal women with HR+, HER2− MBC [2], [5]. Guidelines currently recommend AIs with the CDK4/6 inhibitors, palbociclib or ribociclib, or fulvestrant (either as monotherapy or in combination with anastrozole) as a first‐line ET option. As a second‐line ET option, fulvestrant in combination with palbociclib or abemaciclib is recommended for patients with prior adjuvant ET exposure or patients who received ET in the metastatic setting. Everolimus may also be administered with exemestane upon disease progression in women who are refractory to nonsteroidal AIs. The ET combination studies described below have been designed with impactful clinical outcomes in mind, which may circumvent resistance challenges (Fig. 2, Table 1).
Figure 2.
Treatment algorithm for endocrine therapy for hormone receptor‐positive metastatic breast cancer. Treatment recommendations for premenopausal patients include ovarian suppression. In the setting of patients with no prior adjuvant endocrine therapy, tamoxifen may be considered for premenopausal women. (Adapted from Rugo et al. [5] with permission from the American Society of Clinical Oncology.) Note: Treatment alternatives include an AI with or without a CDK4/6 inhibitor or fulvestrant with or without a CKD4/6 inhibitor.
Abbreviations: AI, aromatase inhibitor; CDK4/6, cyclin‐dependent kinase 4/6.
Targeted CDK4/6 Inhibitors
First‐Line Regimens.
Palbociclib (PD0332991) is a highly selective and potent inhibitor of CDK4/6 (Fig. 1) [70]. This oral agent is now indicated in combination with any AI as first‐line endocrine‐based treatment of patients with HR+, HER2−, locally advanced or metastatic BC and in combination with fulvestrant in women with disease progression after ET, based on data indicating that it improves PFS compared with ET alone.
PALOMA‐1 evaluated palbociclib plus letrozole in patients with HR+, HER2−, advanced BC. A second cohort required cancer to have cyclin D1 amplification and/or loss of p16 [71]. Treatment with palbociclib and letrozole was associated with a significantly longer PFS versus letrozole monotherapy (20.2 vs. 10.2; hazard ratio 0.49, p < .001) [71]; however, the difference in OS was not significant (37.5 vs. 34.5 months; hazard ratio 0.84, p = .28) [72]. In the confirmatory phase III PALOMA‐2 study, conducted in postmenopausal patients without prior systemic therapy for advanced BC, palbociclib plus letrozole was superior to letrozole alone in terms of median PFS (24.8 vs. 14.5 months; hazard ratio 0.58, p < .001), objective response rate (ORR; 42.1% vs. 34.7%; p = .06), and confirmed ORR in patients with measurable disease (55.3% vs. 44.4%; p = .03; Table 1). The most common adverse events (AEs) with the combination were neutropenia, leukopenia, fatigue, nausea, arthralgia, and alopecia [9].
Ribociclib (LEE011) became the second CDK4/6 inhibitor to receive FDA approval as a first‐line treatment for HR+, HER2−, advanced BC in combination with any AI in postmenopausal women [73], based on the results of the MONALEESA‐2 study (Table 1) [10], [11]. After a median follow‐up of 26.4 months, PFS (the primary endpoint) significantly favored the ribociclib plus letrozole group over the letrozole‐only group, with 24‐month PFS rates of 54.7% and 35.9%, respectively. The OS data remain immature, with 15% of patient deaths in the combination arm versus 19.8% in the letrozole‐only arm (hazard ratio 0.746; p = .059) [11]. The most common AEs with ribociclib were neutropenia, nausea, infections, fatigue, and diarrhea [10]. Furthermore, a preplanned interim analysis of the ongoing MONARCH‐3 trial has found that abemaciclib in combination with an AI met its primary endpoint of improved PFS versus AI plus placebo (hazard ratio 0.54; 95% confidence interval [CI], 0.41–0.72; p = .000021) in women with HR+, HER2−, advanced BC [74].
Second‐Line Regimens.
Palbociclib in combination with fulvestrant in CDK4/6 inhibitor‐naïve women with disease progression after ET represents a second‐line option in HR+ advanced or metastatic BC [5], [75]. Studies compared the combination of palbociclib plus fulvestrant versus fulvestrant alone in patients with HR+, HER2− metastatic disease with progression after prior ET (including tamoxifen and AIs) [13]. In the PALOMA‐3 study, patients receiving palbociclib plus fulvestrant demonstrated significantly longer PFS compared with fulvestrant plus placebo (11.2 vs. 4.6 months; hazard ratio 0.50, p < .001) [14]. All subgroup analyses of PFS favored palbociclib plus fulvestrant over fulvestrant alone. Findings from the PALOMA‐3 study (Table 1) were the basis of FDA and European Union approval of the combination of palbociclib with fulvestrant as a second‐line treatment option.
Studies are ongoing with the CDK4/6 inhibitor, abemaciclib (LY2835219), either alone or in combination with ET, for patients with HR+, HER2− advanced or metastatic BC who have relapsed after ET [76], including studies to treat brain metastases [12], [77], [78]. The efficacy of abemaciclib monotherapy was demonstrated in the phase II MONARCH‐1 study in heavily pretreated women with HR+, HER2− metastatic disease with progression during or after ET and one or two prior chemotherapy regimens administered for advanced‐stage disease [79]. At the 12‐month analysis, ORR was 19.7%, the clinical benefit rate (CBR) was 42.4%, median PFS was 6 months, and OS was 17.7 months [79]. In MONARCH‐2, a phase III study in patients with HR+, HER2− MBC who experienced relapse or progression after ET, the addition of abemaciclib to fulvestrant demonstrated a significant improvement in PFS (16.4 months) compared with fulvestrant alone (9.3 months). The ORR among patients treated with abemaciclib plus fulvestrant was 48.1% compared with 21.3% in the control arm (Table 1) [12]. Based on these results, abemaciclib was recently approved in combination with fulvestrant for women with HR+/HER2− advanced or metastatic BC with disease progression after ET and as monotherapy after ET and prior chemotherapy in the metastatic setting [80].
mTOR Inhibitors
Everolimus is an analog of rapamycin that inhibits the mTOR complex and leads to a variety of downstream effects, including blocking cell growth, angiogenesis, and dysregulation of cellular metabolism (Fig. 1) [81]. Everolimus is approved in the U.S. for use in combination with exemestane in postmenopausal women with HR+, HER2− advanced BC who demonstrated progression after failure of treatment with anastrozole or letrozole [82]. Approval was based on the findings of the BOLERO‐2 study, during which patients received everolimus plus exemestane or exemestane plus placebo. The majority of patients (80%) had received prior therapy, including tamoxifen (48%), fulvestrant (17%), or chemotherapy (26%) [15]. In the final analysis, the PFS for everolimus plus exemestane was significantly greater than for everolimus plus placebo (7.8 months vs. 3.2 months; hazard ratio 0.45, p < .001; Table 1) [15]. In this study, the most common AEs in the combination arm were stomatitis, rash, fatigue, diarrhea, nausea, decreased appetite, weight loss, and cough; however, with everolimus alone, patients experienced mainly nausea and fatigue [15].
PI3K Inhibitors
The PI3K signaling pathway is an active regulator of cellular processes, including cell proliferation, growth, survival, migration, and metabolism. Hyperactivation of the PI3K/AKT pathway occurs frequently in human cancers, which makes it a therapeutic target of particular interest [58], [83], [84]. Oral PI3K inhibitors in development for advanced or metastatic BC in combination with antiestrogen therapies (Fig. 1) include the selective isoform‐specific PI3Kα inhibitors taselisib (GDC‐0032) [85] and alpelisib (BYL719) [86]. Two pan‐PI3K inhibitors (Fig. 1), buparlisib (BKM120) [16], [87] and pictilisib (GDC‐0941) [88], [89], were under investigation, but poor toxicity led to the discontinuation of further development.
AKT Inhibitors
The investigational drug MK‐2206 is a potent and specific allosteric inhibitor of the AKT family in vitro and displayed no activity against more than 250 protein kinases during in vivo testing (Fig. 1) [90]. A phase I study was conducted to determine the recommended phase II treatment dose and activity of MK‐2206 in combination with anastrozole and with fulvestrant in postmenopausal patients with HR+ MBC [91]. The CBR was 36.7%, the median time to progression was 5.8 months, and the ORR was 15.4%. The activity of these combinations was lower than observed with endocrine monotherapy. Possible reasons included the low dose used because of treatment‐associated rash, possible mismatch between the mechanism of action of MK‐2206 and tumor resistance mechanisms of the study participants because confirmation of AKT mutation was not performed, and differences in tumor cell behavior in the clinical and preclinical settings [91].
The investigational agent AZD5363 is a potent inhibitor of the AKT family that in combination with fulvestrant showed synergy in an HR+ patient‐derived xenograft model and delayed tumor progression after treatment ended [92]. Preclinical studies of AZD5363 oral dosing resulted in significant inhibition of estrogen‐responsive human breast xenografts [93]. The phase I study FAKTION (NCT01992952) will assess AZD5363 in combination with fulvestrant in a subgroup of AKT1 (E17K) mutation‐positive patients. Completed early clinical studies have reported responses in patients with and without AKT1 (E17K) mutations [94], [95].
Ipatasertib is an AKT inhibitor currently in phase II studies for triple‐negative breast cancer and phase III studies for prostate cancer. In the phase II LOTUS study, ipatasertib or placebo was added to paclitaxel for first‐line treatment of women (n = 124) with locally advanced or metastatic triple‐negative breast cancer [96]. After a median follow‐up of 10.4 months in the ipatasertib group and 10.2 months in the placebo group, median PFS was 6.2 months with ipatasertib and 4.9 months with placebo (hazard ratio 0.60, 95% CI, 0.37–0.98; p = .037). In patients with PTEN‐low tumors (n = 48), PFS was 6.2 months with ipatasertib versus 3.7 months with placebo (hazard ratio 0.59, 95% CI, 0.26–1.32; p = .18) [96]. These results suggest that further development is warranted.
Future Directions in Endocrine Therapy Research
In vitro and preclinical data for various solid tumors support combining CDK4/6 inhibition with PI3K inhibition [97]. In a preclinical mouse model of PI3K inhibitor‐resistant BC, ribociclib plus pictilisib or alpelisib showed synergistic activity. The most active combination to date in vivo consists of ribociclib plus buparlisib or alpelisib and letrozole or fulvestrant. Triplet combinations using CDK4/6 and PI3K inhibitors and endocrine therapies are being evaluated in a few early‐stage clinical studies. These include the phase I study comparisons of fulvestrant plus ribociclib with and without buparlisib (NCT02088684), a study of letrozole plus ribociclib plus alpelisib (NCT01872260), and a study of ribociclib plus everolimus plus exemestane (NCT01857193). In other advances, researchers have exploited similarities in the ATP‐binding sites of PI3K and mTOR to create dual inhibitors that target all isoforms of PI3K and also both mTOR complexes [98], [99]. It is hoped that this strategy may eliminate the potential for molecular crosstalk loops that activate AKT when mTOR is inhibited. For example, the PI3K inhibitor, gedatolisib, is currently being investigated in a phase I dose‐escalation study (NCT02626507) with palbociclib and fulvestrant in the neoadjuvant setting for previously untreated patients with ER‐positive, HER2− BC.
There are also new classes of agents that are being combined with ET in patients with HR+ disease. This includes FGFR inhibitors that can reverse endocrine resistance in BC cells. The FGFR inhibitor AZD4547 is currently being evaluated in combination with letrozole or anastrozole in patients with disease progression on these AIs [100]. Bromodomain and extraterminal (BET) proteins reduce ER expression and downregulate ER‐dependent gene expression. The BET inhibitor GSK626762 is being evaluated in combination with fulvestrant in patients with ER‐positive advanced or metastatic BC [101]. Other SERDs are also under investigation. Elacestrant (RAD1901) is a SERD that has shown antitumor activity in multiple ER‐positive BC patient‐derived xenograft models [102] and is under investigation both for metastatic BC and for menopausal vasomotor symptoms. GDC‐0810 is being studied as monotherapy and in combination with palbociclib and/or a luteinizing hormone‐releasing hormone agonist (NCT01823835), as well as in a phase II study versus fulvestrant (NCT02569801).
Several phase III studies investigating dual combinations with fulvestrant therapy are ongoing (Table 2). These studies include SANDPIPER, which is evaluating fulvestrant plus taselisib [18], and SOLAR‐1, which is assessing fulvestrant plus alpelisib versus fulvestrant plus placebo [19]. MONALEESA‐3 (fulvestrant plus ribociclib vs. ribociclib plus placebo) is an ongoing CDK4/6 study expected to complete in February 2020. Although all patients with MBC ultimately progress on fulvestrant, the exact mechanisms of progression are currently not well characterized. It is expected that these mechanisms will be further elucidated as our understanding of resistance grows. However, fulvestrant combinations may potentially provide a longer course to the emergence of ER resistance.
Adjuvant therapy is another area under intense investigation. There are several ongoing placebo‐controlled phase III studies evaluating the efficacy and safety of adding CDK4/6 inhibitors to standard adjuvant ET. These adjuvant studies include PALLAS, which is investigating the addition of palbociclib to standard adjuvant ET for patients with HR+, HER2−, early BC (NCT02513394); monarchE, which is evaluating abemaciclib plus adjuvant ET in patients with HR+, HER2−, high‐risk, node‐positive, early‐stage BC (NCT03155997); and earLEE‐1 (NCT03078751) and earLEE‐2 (NCT03081234), both of which will evaluate the efficacy and safety of ribociclib plus adjuvant ET in patients with high‐risk and intermediate‐risk early BC, respectively. The mTOR inhibitor everolimus is being evaluated as adjuvant therapy in a phase III study in combination with ET in patients with high‐risk, HR+, HER2− BC (NCT01674140).
Conclusion
Molecular profiling of breast tumors is providing a better understanding of ET resistance, which will assist in the development of agents with new targets in order to deliver precision medicine to patients with HR+, HER2− MBC. Despite this improved insight, resistance to ET is a hallmark of relapse and progression in MBC. Some patients may retain tumor cells with functional hormone receptors, and many breast tumor cells may develop resistance to ET.
Combination therapies that efficaciously inhibit tumor growth by affecting cell cycle modulators and regulators of key signaling pathways have recently been approved, and new agents with antiestrogenic effects on the intracellular pathways are being tested. Endocrine agents also continue to be explored as monotherapy. Endocrine therapy is generally well tolerated and associated with low toxicity; however, increased risk of toxicity may be noted in patients with advanced BC when therapy is combined with certain targeted therapy. Implementation of molecular profiling, histopathology, treatment modalities, and general patient well‐being in clinical practice all have a role in patient outcomes.
Acknowledgments
We thank Emily Cullinan, Greg Tardie, and Joan Hudson of the Lockwood Group (Stamford, CT) for providing medical writing and editorial support, which was in accordance with Good Publication Practice (GPP3) guidelines and funded by AstraZeneca (Wilmington, DE). Memorial Sloan Kettering Cancer Center provided core grant P30 CA 008748 for Dr. Dickler. This manuscript was funded by AstraZeneca LP. The sponsor participated in manuscript review and the decision to submit the paper for publication.
Footnotes
For Further Reading: Kathleen I. Pritchard, Stephen K. Chia, Christine Simmons et al. Enhancing Endocrine Therapy Combination Strategies for the Treatment of Postmenopausal HR+/HER2 Advanced Breast Cancer. The Oncologist 2017;22:12–24; first published on November 18, 2016.
Implications for Practice: Emerging data show that new endocrine therapy (ET) combinations can improve progression–free and overall survival outcomes in patients with hormone receptor–positive, HER2–negative (HR+/HER−) advanced breast cancer. Level 1 evidence supports consideration of dual ET regimens, particularly in ET‐naïve patients, or palbociclib plus letrozole as first‐line therapy, as well as the addition of mTOR or CDK4/6 inhibitors to established ET in the second‐line setting and in select first‐line patients. Some combinations are associated with increased risk of class‐specific toxicities that will require individualized risk stratification, earlier and more rigorous agent‐specific monitoring, and patient education. Recent data on a noninvasive biomarker assay that predicts response to a phosphoinositide 3–kinase inhibitor demonstrates the feasibility of this minimally invasive technique as an alternative to traditional tissue analysis.
Author Contributions
Conception/design: Adam M. Brufsky, Maura N. Dickler
Collection and/or assembly of data: Adam M. Brufsky, Maura N. Dickler
Data analysis and interpretation: Adam M. Brufsky, Maura N. Dickler
Manuscript writing: Adam M. Brufsky, Maura N. Dickler
Final approval of manuscript: Adam M. Brufsky, Maura N. Dickler
Disclosures
Adam M. Brufsky: AstraZeneca, Eli Lilly, Pfizer, Novartis (C/A); Maura N. Dickler: Genentech/Roche, Novartis, Eli Lilly (RF); TapImmune, Puma, G1 Therapeutics, Genentech/Roche, Novartis, Pfizer, AstraZeneca, Syndax (C/A); Eli Lilly, Novartis (SAB).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Murphy CG, Dickler MN. Endocrine resistance in hormone‐responsive breast cancer: Mechanisms and therapeutic strategies. Endocr Relat Cancer 2016;23:R337–R352. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 3.2017. November 10, 2017. Available at https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed November 16, 2017.
- 3. Heng YJ, Lester SC, Tse GM et al. The molecular basis of breast cancer pathological phenotypes. J Pathol 2017;241:375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robertson JF, Bondarenko IM, Trishkina E et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor‐positive advanced breast cancer (FALCON): An international, randomised, double‐blind, phase 3 trial. Lancet 2017;388:2997–3005. [DOI] [PubMed] [Google Scholar]
- 5. Rugo HS, Rumble RB, Macrae E et al. Endocrine therapy for hormone receptor‐positive metastatic breast cancer: American Society of Clinical Oncology guideline . J Clin Oncol 2016;34:3069–3103. [DOI] [PubMed] [Google Scholar]
- 6. Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin‐dependent kinases (CDK) 4/6 in estrogen receptor‐positive breast cancers. Breast Cancer Res 2016;18:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glück S. Consequences of the convergence of multiple alternate pathways on the estrogen receptor in the treatment of metastatic breast cancer. Clin Breast Cancer 2017;17:79–90. [DOI] [PubMed] [Google Scholar]
- 8. Murphy CG, Dickler MN. The role of CDK4/6 inhibition in breast cancer. The Oncologist 2015;20:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Finn RS, Martin M, Rugo HS et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016;375:1925–1936. [DOI] [PubMed] [Google Scholar]
- 10. Hortobagyi GN, Stemmer SM, Burris HA et al. Ribociclib as first‐line therapy for HR‐positive, advanced breast cancer. N Engl J Med 2016;375:1738–1748. [DOI] [PubMed] [Google Scholar]
- 11. Hortobagyi GN, Stemmer SM, Burris HA et al. Updated results from MONALEESA‐2, a phase 3 trial of first‐line ribociclib + letrozole in hormone receptor‐positive (HR+), HER2‐negative (HER2−), advanced breast cancer (ABC) [abstract and poster presented at the 2017 ASCO Annual Meeting]. J Clin Oncol 2017;35(suppl 15):1038A. [Google Scholar]
- 12. Sledge GW Jr, Toi M, Neven P et al. MONARCH 2: Abemaciclib in combination with fulvestrant in patients with HR+/HER2− advanced breast cancer who progressed on endocrine therapy [abstract presented at the 2017 ASCO Annual Meeting]. J Clin Oncol 2017;35(suppl 15):1000A. [DOI] [PubMed] [Google Scholar]
- 13. Cristofanilli M, Turner NC, Bondarenko I et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone‐receptor‐positive, HER2‐negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA‐3): Final analysis of the multicentre, double‐blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–439. [DOI] [PubMed] [Google Scholar]
- 14. Turner NC, André F, Cristofanilli M et al. Treatment postprogression in women with endocrine‐resistant HR+/HER2− advanced breast cancer who received palbociclib plus fulvestrant in PALOMA‐3 [abstract presented at the 2016 San Antonio Breast Cancer Symposium]. Cancer Res 2017;77(suppl 4):P4–22‐06A. [Google Scholar]
- 15. Yardley DA, Noguchi S, Pritchard KI et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO‐2 final progression‐free survival analysis. Advances Ther 2013;30:870–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baselga J, Im SA, Iwata H et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor‐positive, HER2‐negative, advanced breast cancer (BELLE‐2): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2017;18:904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di Leo A, Seok Lee K, Ciruelos EM et al. BELLE‐3: A phase III study of buparlisib + fulvestrant in postmenopausal women with HR+, HER2−, aromatase inhibitor‐treated, locally advanced or metastatic breast cancer, who progressed on or after mTOR inhibitor‐based treatment [abstract and slides presented at the 2016 San Antonio Breast Cancer Symposium]. Cancer Res 2017;77(suppl 4):S4–07A. [Google Scholar]
- 18. Baselga J, Cortes J, DeLaurentiis M et al. SANDPIPER: Phase III study of the PI3‐kinase (PI3K) inhibitor taselisib (GDC‐0032) plus fulvestrant in patients (pts) with estrogen receptor (ER)‐positive, HER2‐negative locally advanced or metastatic breast cancer (BC) enriched for pts with PIK3CA‐mutant tumors [abstract and poster presented at the 2017 ASCO Annual Meeting]. J Clin Oncol 2017;35(suppl 15):TPS617A. [Google Scholar]
- 19. Rugo HS, Andre F, Rubovszky G et al. A phase 3 study of alpelisib (ALP) plus fulvestrant (FUL) in men and postmenopausal women with hormone receptor‐positive (HR+), human epidermal growth factor receptor 2‐negative (HER2−) ABC progressing on or after aromatase inhibitor (AI) therapy: SOLAR‐1 [abstract and poster presented at the 2017 ASCO Annual Meeting]. J Clin Oncol 2017;35(suppl 15): TPS1111A. [Google Scholar]
- 20. Rondón‐Lagos M, Villegas VE, Rangel N et al. Tamoxifen resistance: Emerging molecular targets. Int J Mol Sci 2016;17:E1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nardone A, De Angelis C, Trivedi MV et al. The changing role of ER in endocrine resistance. Breast 2015;24(suppl 2):S60–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Foster JS, Henley DC, Ahamed S et al. Estrogens and cell‐cycle regulation in breast cancer. Trends Endocrinol Metab 2001;12:320–327. [DOI] [PubMed] [Google Scholar]
- 23. Doisneau‐Sixou SF, Sergio CM, Carroll JS et al. Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. Endocr Relat Cancer 2003;10:179–186. [DOI] [PubMed] [Google Scholar]
- 24. Wang TC, Cardiff RD, Zukerberg L et al. Mammary hyperplasia and carcinoma in MMTV‐cyclin D1 transgenic mice. Nature 1994;369:669–671. [DOI] [PubMed] [Google Scholar]
- 25. Gillett C, Fantl V, Smith R et al. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res 1994;54:1812–1817. [PubMed] [Google Scholar]
- 26. Bartkova J, Lukas J, Strauss M et al. The PRAD‐1/cyclin D1 oncogene product accumulates aberrantly in a subset of colorectal carcinomas. Int J Cancer 1994;58:568–573. [DOI] [PubMed] [Google Scholar]
- 27. Bartkova J, Lukas J, Strauss M et al. Cyclin D1 oncoprotein aberrantly accumulates in malignancies of diverse histogenesis. Oncogene 1995;10:775–778. [PubMed] [Google Scholar]
- 28. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wiseman LR, Goa KL. Toremifene. A review of its pharmacological properties and clinical efficacy in the management of advanced breast cancer. Drugs 1997;54:141–160. [DOI] [PubMed] [Google Scholar]
- 30. McDonnell DP, Wardell SE. The molecular mechanisms underlying the pharmacological actions of ER modulators: Implications for new drug discovery in breast cancer. Curr Opin Pharmacol 2010;10:620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evista (raloxifene hydrochloride) tablets [prescribing information]. Indianapolis, IN: Eli Lilly LLC; 2016.
- 32.Aromasin (exemestane) tablets [prescribing information]. New York, NY: Pfizer USA; 2016. [Google Scholar]
- 33. Pietras RJ. Biologic basis of sequential and combination therapies for hormone‐responsive breast cancer. The Oncologist 2006;11:704–717. [DOI] [PubMed] [Google Scholar]
- 34. Lu Q, Liu Y, Long BJ et al. The effect of combining aromatase inhibitors with antiestrogens on tumor growth in a nude mouse model for breast cancer. Breast Cancer Res Treat 1999;57:183–192. [DOI] [PubMed] [Google Scholar]
- 35. Perrone F, Gallo C, De Laurentiis M et al. Phase 3 randomized study of adjuvant anastrozole (A), exemestane (E), or letrozole (L) with or without tamoxifen (T) in postmenopausal women with hormone‐responsive (HR) breast cancer: The FATA‐GIM3 trial [abstract and poster presented at the 2017 ASCO Annual Meeting]. J Clin Oncol 2017;35(suppl 15):515A. 27621388 [Google Scholar]
- 36. Goss P, Bondarenko IN, Manikhas GN et al. Phase III, double‐blind, controlled trial of atamestane plus toremifene compared with letrozole in postmenopausal women with advanced receptor‐positive breast cancer. J Clin Oncol 2007;25:4961–4966. [DOI] [PubMed] [Google Scholar]
- 37. Curran M, Wiseman L. Fulvestrant. Drugs 2001;61:807–813; discussion 814. [DOI] [PubMed] [Google Scholar]
- 38. Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res 1991;51:3867–3873. [PubMed] [Google Scholar]
- 39. Wakeling AE, Bowler J. Steroidal pure antioestrogens. J Endocrinol 1987;112:R7–R10. [DOI] [PubMed] [Google Scholar]
- 40. Carlson RW. The history and mechanism of action of fulvestrant. Clin Breast Cancer 2005;6(suppl 1):S5–S8. [DOI] [PubMed] [Google Scholar]
- 41. Wakeling AE. Similarities and distinctions in the mode of action of different classes of antioestrogens. Endocr Relat Cancer 2000;7:17–28. [DOI] [PubMed] [Google Scholar]
- 42. Bross PF, Baird A, Chen G et al. Fulvestrant in postmenopausal women with advanced breast cancer. Clin Cancer Res 2003;9:4309–4317. [PubMed] [Google Scholar]
- 43. Bross PF, Cohen MH, Williams GA et al. FDA drug approval summaries: Fulvestrant. The Oncologist 2002;7:477–480. [DOI] [PubMed] [Google Scholar]
- 44. Di Leo A, Jerusalem G, Petruzelka L et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor‐positive advanced breast cancer. J Clin Oncol 2010;28:4594–4600. [DOI] [PubMed] [Google Scholar]
- 45. Di Leo A, Jerusalem G, Petruzelka L et al. Final overall survival: Fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst 2014;106:djt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Robertson JFR, Cheung KL, Noguchi S et al. Health‐related quality of life from a phase 3 randomized trial of fulvestrant 500 mg vs anastrozole for hormone receptor‐positive advanced breast cancer (FALCON) [abstract and poster presented at the 2017 ASCO Annual Meeting]. J Clin Oncol 2017;35(suppl 15):1048A. [Google Scholar]
- 47.Faslodex (fulvestrant) injection, for intramuscular use [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2017.
- 48. Ellis MJ, Tao Y, Young O et al. Estrogen‐independent proliferation is present in estrogen‐receptor HER2‐positive primary breast cancer after neoadjuvant letrozole. J Clin Oncol 2006;24:3019–3025. [DOI] [PubMed] [Google Scholar]
- 49. Fox EM, Miller TW, Balko JM et al. A kinome‐wide screen identifies the insulin/IGF‐I receptor pathway as a mechanism of escape from hormone dependence in breast cancer. Cancer Res 2011;71:6773–6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Frogne T, Benjaminsen RV, Sonne‐Hansen K et al. Activation of ERBB3, EGFR and Erk is essential for growth of human breast cancer cell lines with acquired resistance to fulvestrant. Breast Cancer Res Treat 2009;114:263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Turner N, Pearson A, Sharpe R et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res 2010;70:2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Milani A, Geuna E, Mittica G et al. Overcoming endocrine resistance in metastatic breast cancer: Current evidence and future directions. World J Clin Oncol 2014;5:990–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Campbell IG, Russell SE, Choong DY et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res 2004;64:7678–7681. [DOI] [PubMed] [Google Scholar]
- 54. Levine DA, Bogomolniy F, Yee CJ et al. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res 2005;11:2875–2878. [DOI] [PubMed] [Google Scholar]
- 55. Saal LH, Holm K, Maurer M et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res 2005;65:2554–2559. [DOI] [PubMed] [Google Scholar]
- 56. Arthur LM, Turnbull AK, Renshaw L et al. Changes in PIK3CA mutation status are not associated with recurrence, metastatic disease or progression in endocrine‐treated breast cancer. Breast Cancer Res Treat 2014;147:211–219. [DOI] [PubMed] [Google Scholar]
- 57. Meric‐Bernstam F, Frampton GM, Ferrer‐Lozano J et al. Concordance of genomic alterations between primary and recurrent breast cancer. Mol Cancer Ther 2014;13:1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Miller TW, Rexer BN, Garrett JT et al. Mutations in the phosphatidylinositol 3‐kinase pathway: Role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res 2011;13:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lauring J, Park BH, Wolff AC. The phosphoinositide‐3‐kinase‐Akt‐mTOR pathway as a therapeutic target in breast cancer. J Natl Compr Canc Netw 2013;11:670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wilcken NR, Prall OW, Musgrove EA et al. Inducible overexpression of cyclin D1 in breast cancer cells reverses the growth‐inhibitory effects of antiestrogens. Clin Cancer Res 1997;3:849–854. [PubMed] [Google Scholar]
- 61. Kilker RL, Planas‐Silva MD. Cyclin D1 is necessary for tamoxifen‐induced cell cycle progression in human breast cancer cells. Cancer Res 2006;66:11478–11484. [DOI] [PubMed] [Google Scholar]
- 62. Thangavel C, Dean JL, Ertel A et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy‐resistant breast cancer. Endocr Relat Cancer 2011;18:333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kenny FS, Hui R, Musgrove EA et al. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor‐positive breast cancer. Clin Cancer Res 1999;5:2069–2076. [PubMed] [Google Scholar]
- 64. Robinson DR, Wu YM, Vats P et al. Activating ESR1 mutations in hormone‐resistant metastatic breast cancer. Nat Genet 2013;45:1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Toy W, Shen Y, Won H et al. ESR1 ligand‐binding domain mutations in hormone‐resistant breast cancer. Nat Genet 2013;45:1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chandarlapaty S, Chen D, He W et al. Prevalence of ESR1 mutations in cell‐free DNA and outcomes in metastatic breast cancer: A secondary analysis of the BOLERO‐2 clinical trial. JAMA Oncol 2016;2:1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Spoerke JM, Gendreau S, Walter K et al. Heterogeneity and clinical significance of ESR1 mutations in ER‐positive metastatic breast cancer patients receiving fulvestrant. Nat Commun 2016;7:11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jeselsohn R, Buchwalter G, De Angelis C et al. ESR1 mutations ‐ a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol 2015;12:573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schiavon G, Hrebien S, Garcia‐Murillas I et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med 2015;7:313ra182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fry DW, Harvey PJ, Keller PR et al. Specific inhibition of cyclin‐dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 2004;3:1427–1438. [PubMed] [Google Scholar]
- 71. Finn RS, Crown JP, Lang I et al. The cyclin‐dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first‐line treatment of oestrogen receptor‐positive, HER2‐negative, advanced breast cancer (PALOMA‐1/TRIO‐18): A randomised phase 2 study. Lancet Oncol 2015;16:25–35. [DOI] [PubMed] [Google Scholar]
- 72. Finn RS, Crown J, Lang I et al. Overall survival results from the randomized phase II study of palbociclib (P) in combination with letrozole (L) vs letrozole alone for frontline treatment of ER+/HER2− advanced breast cancer (PALOMA‐1; TRIO‐18) [abstract presented at the 2017 ASCO Annual Meeting]. J Clin Oncol 2017;35(suppl 15):1001A. [Google Scholar]
- 73.Kisqali (ribociclib) tablets [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2017. [Google Scholar]
- 74. Goetz MP, Toi M, Campone M et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; 35:3638–3646. [DOI] [PubMed] [Google Scholar]
- 75.Ibrance (palbociclib) capsules [prescribing information]. New York, NY: Pfizer Inc; 2017. [Google Scholar]
- 76. Barroso‐Sousa R, Shapiro GI, Tolaney SM. Clinical development of the CDK4/6 inhibitors ribociclib and abemaciclib in breast cancer. Breast Care (Basel) 2016;11:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gelbert LM, Cai S, Lin X et al. Identification and characterization of LY2835219: A potent oral inhibitor of the cyclin‐dependent kinases 4 and 6 (CDK4/6) with broad in vivo antitumor activity [abstract presented at the 2011 AACR‐NCI‐EORTC International Conference on Molecular Targets and Cancer Therapeutics]. Mol Cancer Ther 2011;10(suppl 11):B233A. [Google Scholar]
- 78. Gelbert LM, Cai S, Lin X et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: In‐vivo cell cycle‐dependent/independent anti‐tumor activities alone/in combination with gemcitabine. Invest New Drugs 2014;32:825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dickler MN, Tolaney SM, Rugo HS et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2− metastatic breast cancer. Clin Cancer Res 2017;23:5218–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Verzenio (abemaciclib) tablets [prescribing information]. Indianapolis, IN: Eli Lilly and Company; 2017.
- 81. Lousberg L, Jerusalem G. Safety, efficacy, and patient acceptability of everolimus in the treatment of breast cancer. Breast Cancer (Auckl) 2017;10:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Afinitor (everolimus) tablets for oral administration [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. [Google Scholar]
- 83. Katso R, Okkenhaug K, Ahmadi K et al. Cellular function of phosphoinositide 3‐kinases: Implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol 2001;17:615–675. [DOI] [PubMed] [Google Scholar]
- 84. Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011;62:233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ndubaku CO, Heffron TP, Staben ST et al. Discovery of 2‐{3‐[2‐(1‐isopropyl‐3‐methyl‐1H‐1,2–4‐triazol‐5‐yl)‐5,6‐dihydrobenzo[f]imidazo[1, 2‐d][1,4]oxazepin‐9‐yl]‐1H‐pyrazol‐1‐yl}‐2‐methylpropanamide (GDC‐0032): A β‐sparing phosphoinositide 3‐kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J Med Chem 2013;56:4597–4610. [DOI] [PubMed] [Google Scholar]
- 86. Fritsch C, Huang A, Chatenay‐Rivauday C et al. Characterization of the novel and specific PI3Kα inhibitor NVP‐BYL719 and development of the patient stratification strategy for clinical trials. Mol Cancer Ther 2014;13:1117–1129. [DOI] [PubMed] [Google Scholar]
- 87. Maira SM, Pecchi S, Huang A et al. Identification and characterization of NVP‐BKM120, an orally available pan‐class I PI3‐kinase inhibitor. Mol Cancer Ther 2012;11:317–328. [DOI] [PubMed] [Google Scholar]
- 88. Folkes AJ, Ahmadi K, Alderton WK et al. The identification of 2‐(1H‐indazol‐4‐yl)‐6‐(4‐methanesulfonyl‐piperazin‐1‐ylmethyl)‐4‐morpholin‐4‐yl‐t hieno[3,2‐d]pyrimidine (GDC‐0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem 2008;51:5522–5532. [DOI] [PubMed] [Google Scholar]
- 89. Krop IE, Mayer IA, Ganju V et al. Pictilisib for oestrogen receptor‐positive, aromatase inhibitor‐resistant, advanced or metastatic breast cancer (FERGI): A randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Oncol 2016;17:811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yan L. MK‐2206: A potent oral allosteric AKT inhibitor [abstract presented at the 2009 American Association for Cancer Research Annual Meeting]. Cancer Res 2009;69(suppl 9):DDT01‐01A. [Google Scholar]
- 91. Ma CX, Sanchez C, Gao F et al. A phase I study of the AKT inhibitor MK‐2206 in combination with hormonal therapy in postmenopausal women with estrogen receptor‐positive metastatic breast cancer. Clin Cancer Res 2016;22:2650–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ribas R, Pancholi S, Guest SK et al. AKT antagonist AZD5363 influences estrogen receptor function in endocrine‐resistant breast cancer and synergizes with fulvestrant (ICI182780) in vivo. Mol Cancer Ther 2015;14:2035–2048. [DOI] [PubMed] [Google Scholar]
- 93. Davies BR, Greenwood H, Dudley P et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: Pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther 2012;11:873–887. [DOI] [PubMed] [Google Scholar]
- 94. Hyman DM, Smyth LM, Donoghue MTA et al. AKT inhibition in solid tumors with AKT1 mutations. J Clin Oncol 2017;35:2251–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tamura K, Hashimoto J, Tanabe Y et al. Safety and tolerability of AZD5363 in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 2016;77:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kim SB, Dent R, Im SA et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first‐line therapy for metastatic triple‐negative breast cancer (LOTUS): A multicentre, randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Oncol 2017;18:1360–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hamilton E, Infante JR. Targeting CDK4/6 in patients with cancer. Cancer Treat Rev 2016;45:129–138. [DOI] [PubMed] [Google Scholar]
- 98. Liu YN, Wan RZ, Liu ZP. Recent developments of small molecule PI3K/mTOR dual inhibitors. Mini Rev Med Chem 2013;13:2047–2059. [DOI] [PubMed] [Google Scholar]
- 99. Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer 2015;15:7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Seckl M, Badman PD, Liu X et al. RADICAL trial: A phase Ib/IIa study to assess the safety and efficacy of AZD4547 in combination with either anastrozole or letrozole in ER positive breast cancer patients progressing on these aromatase inhibitors (AIs) [abstract and poster presented at the 2017 ASCO Annual Meeting]. J Clin Oncol 2017;35(suppl 15):1059A. [Google Scholar]
- 101. Sparano JA, Cescon DW, Oliveira M et al. A phase I/II dose escalation and expansion study to investigate the safety, pharmacokinetics, pharmacodynamics and clinical activity of GSK525762 in combination with fulvestrant in subjects with ER+ breast cancer [abstract and poster presented at the 2017 ASCO Annual Meeting]. J Clin Oncol 2017;35(suppl 15):TPS1114A. [Google Scholar]
- 102. Bihani T, Patel HK, Arlt H et al. Elacestrant (RAD1901), a selective estrogen receptor degrader (SERD), has antitumor activity in multiple ER+ breast cancer patient‐derived xenograft models. Clin Cancer Res 2017;23:4793–4804. [DOI] [PubMed] [Google Scholar]