Abstract
Lessons Learned.
The combination of cisplatin, docetaxel, and erlotinib as frontline treatment for recurrent and/or metastatic head and neck squamous cell carcinomas led to a response rate of 62%.
This result exceeded the prespecified target response rate of 50% and represented an improvement compared with historical controls.
This regimen warrants further investigation.
Background.
The epidermal growth factor receptor (EGFR) plays a key role in the carcinogenesis of head and neck squamous cell carcinomas (HNSCC). We conducted this clinical study to test the hypothesis that the addition of erlotinib to first‐line cisplatin and docetaxel for patients with recurrent and/or metastatic HNSCC would yield a response rate of at least 50%, representing an improvement from historical controls.
Methods.
Patients with recurrent and/or metastatic HNSCC, with at least one measurable lesion, no prior chemotherapy for recurrent and/or metastatic disease, prior combined modality therapy completed >6 months before enrollment, and performance status ≤2 were treated with cisplatin, docetaxel, and erlotinib for up to six cycles, followed by maintenance erlotinib until disease progression. The primary endpoint was response rate.
Results.
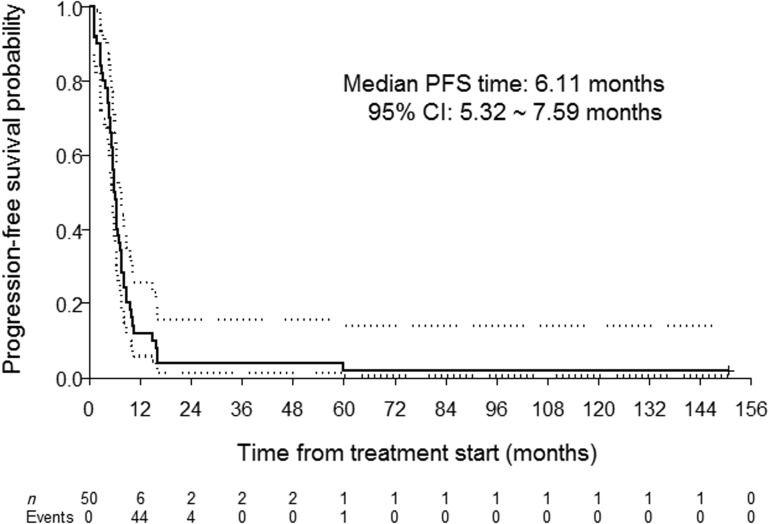
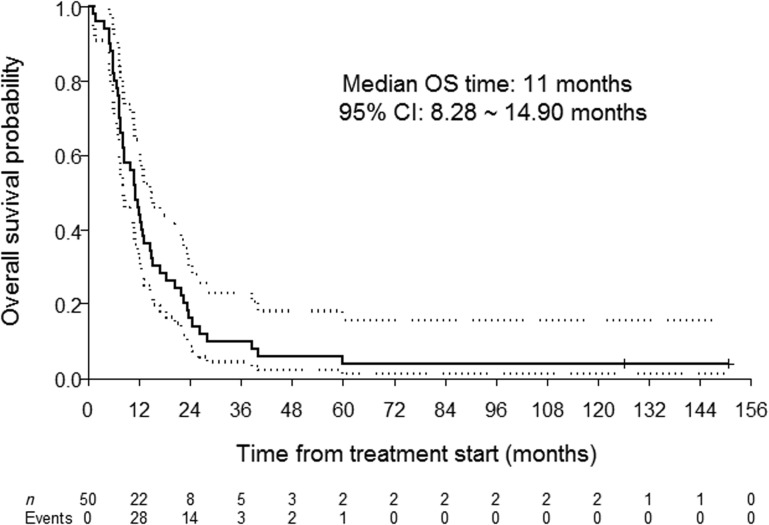
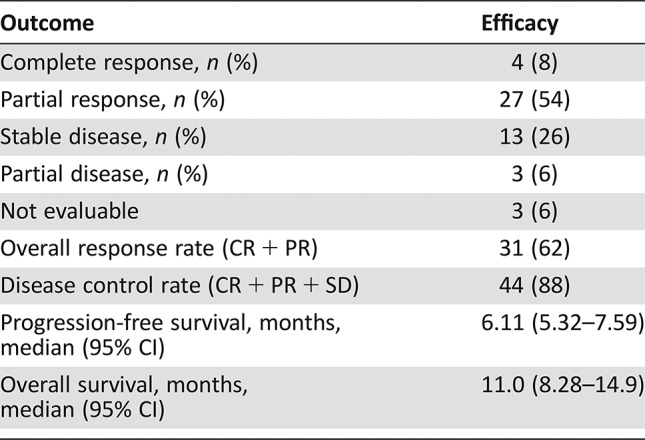
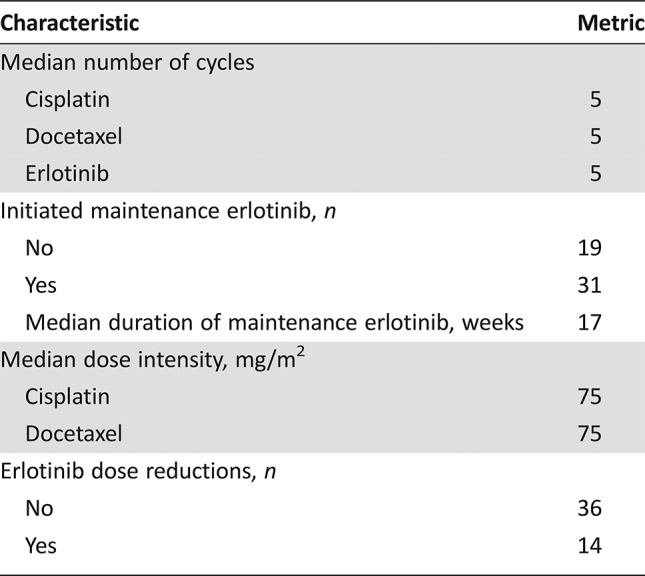
Fifty patients were enrolled (42 male, 12 never smokers, 19 with oropharynx cancer). The median number of cycles was five; 31 patients initiated maintenance erlotinib; 14 patients required erlotinib dose reductions. The objective response rate was 62%, and the median progression‐free and overall survival were 6.1 and 11.0 months, respectively. Toxicity profiles were consistent with the known side effects of the study drugs.
Conclusion.
The study met its primary endpoint and improved response rates compared with historical controls. The findings support further evaluation of the regimen for recurrent and/or metastatic HNSCCs.
Abstract
经验总结
将顺铂、多西他赛联合埃罗替尼作为复发性和/或转移性头颈部鳞状细胞癌的一线治疗, 缓解率为62%。
此结果高于预先设定的目标缓解率50%, 表示与历史对照相比有所改善。
此方案需进行进一步考察。
摘要
背景.表皮生长因子受体(EGFR)在头颈部鳞状细胞癌(HNSCC)癌变中起关键作用。我们开展此临床研究的目的是检验以下假设:向复发性和/或转移性HNSCC患者的一线顺铂和多西他赛治疗中添加埃罗替尼得到的缓解率为至少50%, 表示相对于历史对照有所改善。
方法.复发性和/或转移性HNSCC患者(存在至少一处可测量病灶, 未接受过针对复发性和/或转移性疾病的既往化疗, 在入组之前>6个月完成了既往综合治疗且体能状态≤2)接受顺铂、多西他赛和埃罗替尼治疗多达六个周期, 随后接受埃罗替尼维持治疗, 直至出现疾病进展。主要终点是缓解率。
结果.50例患者入组研究(42例男性, 12例从不吸烟, 19例患有口咽癌)。治疗周期中位数为5, 31例患者开始接受埃罗替尼维持治疗;14例患者需要降低埃罗替尼剂量。客观缓解率为62%, 中位无进展生存期和总生存期分别为6.1个月和11.0个月。毒性特征与研究药物的已知副作用一致。
结论.该研究达到其主要终点, 且与历史对照相比缓解率有所改善。这些结果可为针对复发性和/或转移性HNSCC治疗方案的进一步评价提供支持。
Discussion
This single‐arm, single‐institution, phase II study was designed to test the hypothesis that the addition of erlotinib to first‐line cisplatin and docetaxel for patients with recurrent and/or metastatic HNSCC would yield a response rate of at least 50%, representing an improvement over historical controls (the response rate to cisplatin and docetaxel alone observed in a previous trial led by the MD Anderson Cancer Center was 40%). The primary endpoint of the study was met, with an observed objective response rate of 62%. Moreover, the median progression‐free survival (6.1 months) and overall survival (11.0 months) achieved by our patient cohort compared favorably with historical controls with chemotherapy alone.
These results are in accordance with a phase I/II clinical trial evaluating the combination of cisplatin and erlotinib in this setting, which showed a response rate of 21%, considered to be higher than what would be expected with cisplatin alone. Our data add to the growing body of evidence showing that EGFR tyrosine kinase inhibitors have limited activity as monotherapy but may improve outcomes when combined with cytotoxic agents, especially when given in the frontline setting.
In addition to tyrosine kinase inhibitors, EGFR antibodies have also been investigated in phase III trials of recurrent and/or metastatic HNSCC. Currently the combination of platinum, 5‐fluorouracil, and cetuximab is considered a standard first‐line treatment, given improvements in response rates (20% vs. 36%), median progression‐free survival (3.3 vs. 5.6 months), and overall survival (7.4 vs. 10.1 months), when compared with platinum plus 5‐fluorouracil alone. The results of our clinical trial are comparable to those obtained with platinum, 5‐fluorouracil, and cetuximab. One advantage of the regimen studied herein is the use of a drug that can be administered orally or via feeding tube, obviating the need for weekly infusions, especially in the maintenance setting for patients who achieve longer‐term disease control. On the other hand, 40% of our patients experienced diarrhea (10%, grade 3 or 4), which probably resulted from additive toxicities of docetaxel and erlotinib and deserves attention and careful support.
The limited sample size of this trial (with only 19 patients with oropharynx cancer) precludes any conclusions about the efficacy of the regimen in disease that is positive versus negative for human papilloma virus, and this parameter has not been evaluated.
In summary, the results presented herein provide support for further investigations of the regimen. Indeed, on the basis of these findings, we launched and completed a randomized, double‐blind, placebo‐controlled phase II study of platinum and docetaxel with or without erlotinib. Results recently presented in abstract form confirmed the superiority of the erlotinib‐containing arm. Taken together, the data from the single‐arm and randomized phase II trials provide strong rationale for the use of platinum, docetaxel, and erlotinib as frontline therapy for recurrent and/or metastatic HNSCCs.
Trial Information
- Disease
Head and neck cancers
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
None
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Single arm
- Primary Endpoint
Overall response rate
- Secondary Endpoint
Progression‐free survival
- Secondary Endpoint
Toxicity
- Additional Details of Endpoints or Study Design
- The overall response rate was the primary endpoint of the study and was determined using RECIST version 1.0. The response status was evaluated after two cycles of treatment and confirmed between 4 and 6 weeks thereafter. A Bayesian design based on predictive probabilities was employed to allow for early stopping if the accumulating evidence suggested treatment ineffectiveness. The maximum calculated sample size was 50 patients, and outcomes were evaluated after the first 15 and 30 patients were treated. If at either interim analysis the predictive probability of a positive study (i.e., posterior probability of response rate >30% at least 90%) was low (<.05), the trial would be stopped. The rule corresponded to stopping the trial if one saw three or fewer responders in 15 patients or 9 or fewer responders in 30 patients. This design provided 92% power with an α of 0.08 to detect a true response rate of 50%.
- Eligible patients were required to have histologically or cytologically confirmed metastatic or recurrent head and neck squamous cell carcinoma, with at least one measurable lesion according to RECIST version 1.0; no prior chemotherapy for metastatic or recurrent disease; completed prior combined modality therapy for at least 6 months; Eastern Cooperative Oncology Group (ECOG) performance status <2; and normal organ and marrow function, including leukocytes >3,000/µL, absolute neutrophil count >1,500/µL, platelets >100,000/µL, hemoglobin ≥ 8 g/dL, total bilirubin within normal institutional limits, aspartate aminotransferase/alanine aminotransferase <2.5 × the institutional upper limit of normal (ULN) if alkaline phosphatase is <ULN (alkaline phosphatase may be up to 4 × ULN if transaminases are <ULN), creatinine <2.0 × ULN or creatinine clearance >60 mL/min/1.73 m2 for patients with creatinine level above institutional normal. Exclusion criteria included HNSCC of the nasopharynx; history of nonpalliative radiation for metastatic and/or recurrent disease; prior anti‐EGFR biologic therapy; brain metastases; pre‐existing grade 2 or greater peripheral neuropathy (by the National Cancer Institute Common Terminology Criteria version 2.0); or uncontrolled intercurrent illness.
- Investigator's Analysis
Active and should be pursued further
Drug Information
- Drug 1
- Generic/Working Name
Cisplatin
- Drug Type
Small molecule
- Drug Class
Platinum compound
- Dose
75 milligrams (mg) per square meter (m2)
- Route
IV
- Schedule of Administration
On day 1 every 21 days for a maximum of six cycles
- Drug 2
- Generic/Working Name
Docetaxel
- Drug Type
Small molecule
- Drug Class
Tubulin/microtubules targeting agent
- Dose
60–75 milligrams (mg) per square meter (m2)
- Route
IV
- Schedule of Administration
- On day 1 every 21 days for a maximum of six cycles. The first six patients received docetaxel 60 mg/m2. Escalation of the docetaxel dose to 75 mg/m2 was allowed for patients experiencing minimal toxicity (grade ≤2) after the first cycle. All subsequent patients were treated with docetaxel 75 mg/m2.
- Drug 3
- Generic/Working Name
Erlotinib
- Drug Type
Biological
- Drug Class
EGFR
- Dose
100–150 milligrams (mg) per flat dose
- Route
Oral
- Schedule of Administration
- Once daily, continuous dosing. The first six patients received erlotinib 100 mg/day. Escalation of the erlotinib dose to 150 mg/day was allowed for patients experiencing minimal toxicity (grade 2) after the first cycle. All subsequent patients were treated with erlotinib 150 mg/day.
Patient Characteristics
- Number of Patients, Male
42
- Number of Patients, Female
8
- Stage
Locoregional recurrence: 31 patients Metastatic disease: 19 patients
- Age
Median (range): 57
- Number of Prior Systemic Therapies
0
- Performance Status: ECOG
-
0 — 7
1 — 41
2 — 2
3 —
Unknown —
- Additional details for Patient and Treatment Characteristics can be found in Tables 1 and 2.
Primary Assessment Method
- Title
Total Patient Population
- Number of Patients Screened
50
- Number of Patients Enrolled
50
- Number of Patients Evaluable for Toxicity
50
- Number of Patients Evaluated for Efficacy
50
- Evaluation Method
RECIST version 1.0
- Response Assessment CR
n = 4 (8%)
- Response Assessment PR
n = 27 (54%)
- Response Assessment SD
n = 13 (26%)
- Response Assessment PD
n = 3 (6%)
- Response Assessment Other
n = 3 (6%)
- (Median) Duration Assessments PFS
6.11 months, CI: 5.32–7.59
- (Median) Duration Assessments OS
11.0 months, CI: 8.28–14.9
- (Median) Duration Assessments Response Duration
4.89 months
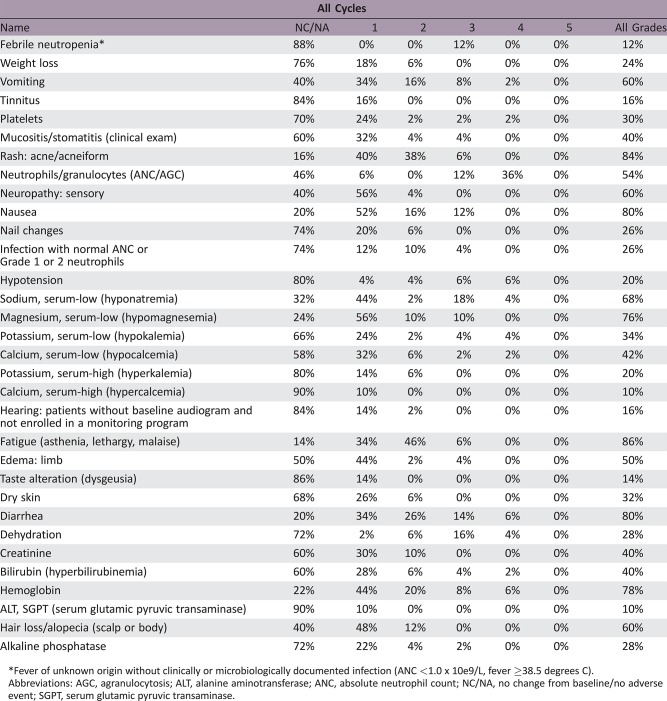
Phase II Experimental Arm Adverse Events
Fever of unknown origin without clinically or microbiologically documented infection (ANC <1.0 x 10e9/L, fever ≥38.5 degrees C).
Abbreviations: AGC, agranulocytosis; ALT, alanine aminotransferase; ANC, absolute neutrophil count; NC/NA, no change from baseline/no adverse event; SGPT, serum glutamic pyruvic transaminase.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Active and should be pursued further
This single‐arm, single‐institution, phase II study was designed to test the hypothesis that the addition of erlotinib to first‐line cisplatin and docetaxel for patients with recurrent and/or metastatic head and neck squamous cell carcinoma (HNSCC) would yield a response rate of at least 50%, representing an improvement over historical controls (the response rate to cisplatin and docetaxel alone observed in a previous trial led by the MD Anderson Cancer Center was 40% [1]). The primary endpoint of the study was met, with an observed objective response rate of 62%. Moreover, the median progression‐free (6.1 months) (Fig. 1) and overall (11.0 months) (Fig. 2) survival achieved by our patient cohort compared favorably with historical controls with chemotherapy alone [1].
This clinical trial was designed at a time when there was limited evidence of the activity of epidermal growth factor receptor (EGFR)‐targeted agents in HNSCCs, especially in regard to tyrosine kinase inhibitors (TKIs) combined with chemotherapy. During the conduct and after completion of this study, evidence arose that EGFR TKIs as single agents had modest activity in HNSCC progressing after platinum‐based therapy. Soulieres at al. demonstrated a response rate of 4.3% with single‐agent erlotinib [2]. The response rate to single‐agent gefitinib at a dose of 500 mg per day was 10.6% [3]. At a lower dose of 250 mg per day, it was 1.4% [4]. Gefitinib was subsequently compared with methotrexate in a phase III study and showed a response rate of 2.7% at 250 mg per day and 7.6% at 500 mg per day but failed to improve survival compared with the chemotherapy arm [5]. Afatinib elicited a response rate of 10% and improved progression‐free survival (but not overall survival) over methotrexate in a phase III study [6]. Taken together, these data do not support the use of monotherapy with an EGFR TKI in pretreated HNSCC.
Strategies to improve the efficacy of these drugs included investigations of EGFR TKIs earlier in the course of the disease and/or combinations with cytotoxic agents. Our group [7] and others [8] demonstrated, for example, in separate studies in platinum‐naïve, early stage, resectable HNSCC, that erlotinib was associated with response rates of 25%–29%, suggesting that the timing of EGFR TKI exposure may influence activity. Indeed, in the current clinical trial, erlotinib was given as first‐line therapy for recurrent and/or metastatic disease, in patients with no recent exposure to platinum, and showed improved efficacy compared with historical controls. These results are in accordance with a phase I/II clinical trial evaluating the combination of cisplatin and erlotinib in this setting, which showed a response rate of 21%, considered to be higher than what would be expected with cisplatin alone, although the study did not meet the overly optimistic, prespecified target response rate improvement [9]. In contrast, a phase II study in previously treated patients with recurrent and/or metastatic HNSCC showed no benefits of adding gefitinib to docetaxel in regard to survival or response rates [10], again indicating that the use of EGFR TKIs in later lines of therapy may result in suboptimal outcomes.
In addition to TKIs, EGFR antibodies have also been investigated in phase III trials of recurrent and/or metastatic HNSCC [11], [12], [13]. Currently, the combination of platinum, 5‐fluorouracil, and cetuximab is considered a standard first‐line treatment, given improvements in response rates (20% vs. 36%), median progression‐free survival (3.3 vs. 5.6 months) and overall survival (7.4 vs. 10.1 months), when compared with platinum plus 5‐fluorouracil alone [12]. The results of our clinical trial are comparable to those obtained with platinum, 5‐fluorouracil, and cetuximab. One advantage of the regimen studied herein is the use of a drug that can be administered orally or via feeding tube, obviating the need for weekly infusions, especially in the maintenance setting for patients who achieve longer‐term disease control. On the other hand, 40% of our patients experienced diarrhea (10%, grade 3 or 4), which probably resulted from additive toxicities of docetaxel and erlotinib and deserves attention and careful support.
In an attempt to maximize benefit‐risk ratios of EGFR inhibitors in HNSCCs, several groups have investigated candidate biomarkers of efficacy. Human papilloma virus (HPV) status was not found to be a predictive marker of benefit from cetuximab added to platinum and 5‐fluorouracil in one phase III study [14]. In the randomized trial of platinum, 5‐fluorouracil, and panitumumab, the addition of the EGFR antibody was associated with a trend toward improved outcomes primarily in the p16‐negative subgroup [13] (although p16 scoring criteria in that study was different than what has been used in pivotal trials in locally advanced disease). Our study only included 19 patients with oropharynx cancers and we did not evaluate HPV/p16 status. However, given the small sample size and nonrandomized nature of this study, analysis of efficacy according to HPV status would likely be unable to provide meaningful conclusions. EGFR copy number gain failed as a predictive marker of benefit from cetuximab or gefitinib in the phase III studies [5], [15], indicating that the search for biomarkers in this setting is not straightforward and may require a comprehensive evaluation of multiple pathways, an effort that is currently underway using specimens collected during this study.
In summary, we demonstrated herein that the addition of erlotinib to first‐line cisplatin and docetaxel led to improved response rates compared with historical controls. This clinical trial met its primary endpoint, providing support for further investigations of this regimen. Indeed, on the basis of these findings, we launched and completed a randomized, double‐blind, placebo‐controlled phase II study of platinum and docetaxel with or without erlotinib. Results recently presented in abstract form confirmed the superiority of the erlotinib‐containing arm [16]. Taken together, the data from the described single‐arm phase II trial and the randomized phase II trials provide a strong rationale for the use of platinum, docetaxel, and erlotinib as frontline therapy for recurrent and/or metastatic HNSCCs.
Figures and Tables
Figure 1.
Progression‐free survival (bold line) and 95% confidence interval (dotted lines).
Figure 2.
Overall survival (bold line) and 95% confidence interval (dotted lines).
Table 1. Patient characteristics.
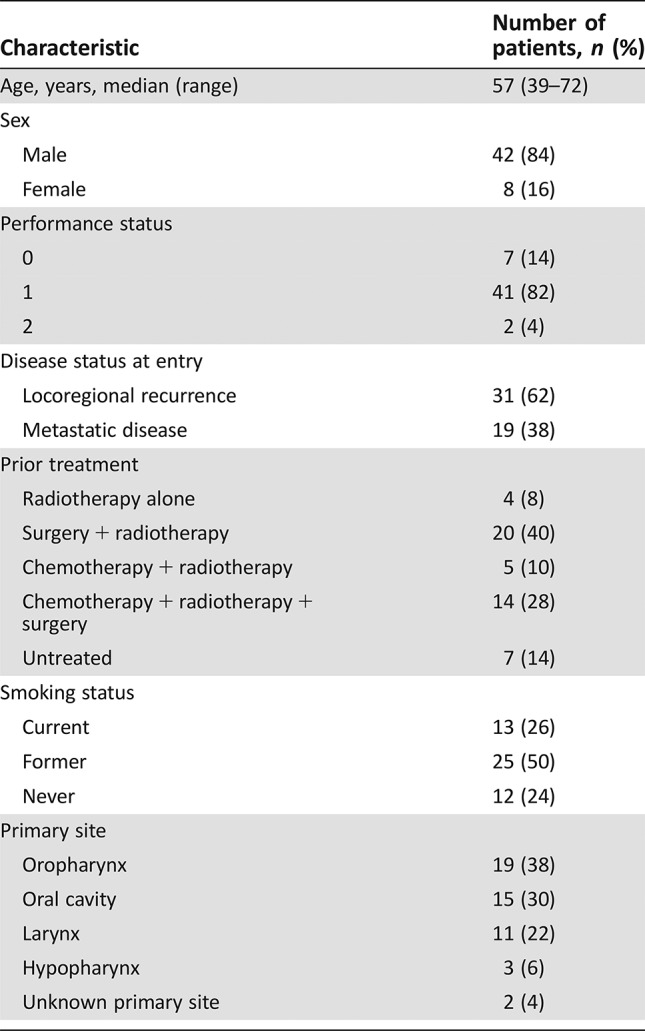
Table 2. Treatment characteristics.
Acknowledgments
This work was supported by NIH grant P30 CA016672, Cancer Prevention Research Institute of Texas grant RP140464, and a grant from the Conquer Cancer Foundation.
Footnotes
ClinicalTrials.gov Identifier: NCT00076310
Sponsor(s): Genentech, OSI Pharmaceuticals, and Sanofi Aventis
Principal Investigator: William Nassib William Jr.
IRB Approved: Yes
Disclosures
William N. William Jr.: Roche/Genentech (C/A), Astellas Pharmaceuticals, Eli Lilly, Bristol‐Meyers Squibb, Merck, Astellas Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals (RF), AstraZeneca, Roche, Genentech (H); Anne S. Tsao: Boehringer‐Ingelheim Pharmaceuticals, Bristol‐Myers Squibb, EMD Serono, Genentech BioOncology, Eli Lilly, Merck, Novartis, Roche Laboratories, Takeda Oncology (C/A), Boehringer‐Ingelheim Pharmaceuticals, Bristol‐Meyers Squibb, Genentech, Eli Lilly, Merck (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Glisson BS, Murphy BA, Frenette G et al. Phase II trial of docetaxel and cisplatin combination chemotherapy in patients with squamous cell carcinoma of the head and neck. J Clin Oncol 2002;20:1593–1599. [DOI] [PubMed] [Google Scholar]
- 2. Soulieres D, Senzer NN, Vokes EE et al. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 2004;22:77–85. [DOI] [PubMed] [Google Scholar]
- 3. Cohen EE, Rosen F, Stadler WM et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol 2003;21:1980–1987. [DOI] [PubMed] [Google Scholar]
- 4. Cohen EE, Kane MA, List MA et al. Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res 2005;11:8418–8424. [DOI] [PubMed] [Google Scholar]
- 5. Stewart JS, Cohen EE, Licitra L et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J Clin Oncol 2009;27:1864–1871. [DOI] [PubMed] [Google Scholar]
- 6. Machiels JP, Haddad RI, Fayette J et al. Afatinib versus methotrexate as second‐line treatment in patients with recurrent or metastatic squamous‐cell carcinoma of the head and neck progressing on or after platinum‐based therapy (LUX‐Head & Neck 1): An open‐label, randomised phase 3 trial. Lancet Oncol 2015;16:583–594. [DOI] [PubMed] [Google Scholar]
- 7. William WN Jr, Weber RS, Lee JJ et al. Randomized trial of a short course of erlotinib 150 to 300 mg daily prior to surgery for squamous cell carcinomas of the head and neck (SCCHN) in current, former, and never smokers: Objective responses and clinical outcomes J Clin Oncol 2011;29(suppl 15):5520A. [Google Scholar]
- 8. Thomas F, Rochaix P, Benlyazid A et al. Pilot study of neoadjuvant treatment with erlotinib in nonmetastatic head and neck squamous cell carcinoma. Clin Cancer Res 2007;13:7086–7092. [DOI] [PubMed] [Google Scholar]
- 9. Siu LL, Soulieres D, Chen EX et al. Phase I/II trial of erlotinib and cisplatin in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: A Princess Margaret Hospital phase II consortium and National Cancer Institute of Canada clinical trials group study. J Clin Oncol 2007;25:2178–2183. [DOI] [PubMed] [Google Scholar]
- 10. Argiris A, Ghebremichael M, Gilbert J et al. Phase III randomized, placebo‐controlled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: An Eastern Cooperative Oncology Group trial. J Clin Oncol 2013;31:1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Machiels JP, Subramanian S, Ruzsa A et al. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous‐cell carcinoma of the head and neck after failure of platinum‐based chemotherapy: An open‐label, randomised phase 3 trial. Lancet Oncol 2011;12:333–343. [DOI] [PubMed] [Google Scholar]
- 12. Vermorken JB, Mesia R, Rivera F et al. Platinum‐based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116–1127. [DOI] [PubMed] [Google Scholar]
- 13. Vermorken JB, Stöhlmacher‐Williams J, Davidenko I et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous‐cell carcinoma of the head and neck (SPECTRUM): An open‐label phase 3 randomised trial. Lancet Oncol 2013;14:697–710. [DOI] [PubMed] [Google Scholar]
- 14. Vermorken JB, Psyrri A, Mesía R et al. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: Retrospective analysis of the phase III EXTREME trial. Ann Oncol 2014;25:801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Licitra L, Mesia R, Rivera F, et al. Evaluation of EGFR gene copy number as a predictive biomarker for the efficacy of cetuximab in combination with chemotherapy in the first‐line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: EXTREME study. Ann Oncol 2011;22:1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. William WN, Feng L, Kies MS et al. Randomized, double‐blind, placebo‐controlled, phase II trial of first‐line platinum/docetaxel with or without erlotinib (E) in patients (pts) with recurrent and/or metastatic (R/M) head and neck squamous cell carcinomas (HNSCCs). J Clin Oncol 2017;35(suppl 15):6017A. [Google Scholar]