Cancer treatment can lead to the development of cognitive difficulties in cancer survivors. This article reports the results of a study that aimed to evaluate long‐term cognitive abilities in a cohort of patients who survived after treatment (chemotherapy, radiotherapy, or both compared with orchiectomy alone) for germ cell tumors.
Keywords: Long‐term, Cognitive functioning, Testicular germ‐cell tumors, Survivors, Late toxicity, Radiotherapy, Chemotherapy
Abstract
Background.
Treatment for cancer may lead to development of cognitive difficulties in cancer survivors. This study aimed to evaluate long‐term cognitive functioning (CogF) in germ‐cell tumor (GCT) survivors.
Subjects, Materials, and Methods.
GCT survivors (n = 155) from the National Cancer Institute of Slovakia completed the Functional Assessment of Cancer Therapy Cognitive Function at a median of 10 years of follow‐up (range: 5–32). The study group consisted of survivors receiving a cisplatin‐based chemotherapy, radiotherapy to the retroperitoneal lymph nodes, or both, whereas the control group included survivors treated with orchiectomy only.
Results.
Of the total survivors, 138 received treatment beyond orchiectomy and 17 controls had orchiectomy alone. Any treatment resulted in significantly greater cognitive difficulties on the overall cognitive function score. Treatment with radiotherapy was associated with cognitive declines in overall cognitive functioning and in subscales for perceived cognitive impairment and cognitive impairment perceived by others (both p < .05). The burden of chemotherapy plus radiotherapy or radiotherapy versus controls resulted in the impairment in all cognitive functioning domains (all p < .05). Overall long‐term cognitive impairment was independent of age in the multivariable analysis.
Conclusion.
This prospective study shows that GCT survivors suffer from a long‐term CogF impairment. These results may help guide clinicians’ decisions in treatment and follow‐up of GCTs.
Implications for Practice.
In this study, long‐term survivors of germ‐cell tumors have reported cognitive impairment after curative treatment with radiotherapy and chemotherapy compared with controls who had treatment with orchiectomy only. These data provide an argument against the use of adjuvant radiotherapy for stage I seminoma. Unnecessary overtreatment with chemotherapy and additional radiotherapy after chemotherapy should be avoided.
Introduction
Germ cell tumors (GCTs) represent a unique, exceptionally chemosensitive solid malignancy most commonly diagnosed in men between 20 and 40 years of age [1]. Cisplatin‐based chemotherapy is the mainstay in the treatment of GCTs; about 70%–80% of patients with metastatic testicular cancer can be cured with first‐line treatment, and salvage chemotherapy may cure about 20%–60% of patients with relapsed GCTs [2], [3], [4], [5]. GCT survivors cured with surgery, cisplatin combination chemotherapy, and/or radiotherapy live for decades and may experience late toxicities derived from such treatments. These include secondary malignancies, cardiovascular, neuro‐, renal, and pulmonary toxicity, hypogonadism, infertility, and a decline in quality of life (QoL) [6], [7], [8]. Cognitive abilities were reported to decline with cancer treatment in multiple cancer diagnoses; however, available studies mostly evaluated immediate to short‐term cognitive functioning [9], [10], [11], [12]. The decline in cognitive functioning after adjuvant chemotherapy in colon cancer is transient in a substantial proportion of patients, but may gradually worsen in some [10]. Earlier studies evaluating quality of life in cancer survivors did not assess the long‐term cognitive impairment and used European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire‐C30 (QLQ‐C30) questionnaires or nonvalidated tools exploring the cognitive functioning marginally [13], [14], [15]. Cognition has been reported to be altered 12 months after chemotherapy for nonseminoma GCT, and the impairment was chemotherapy dose‐dependent [16]. Two studies reported decreased cognitive measures in testicular cancer survivors treated with chemotherapy after 2–7 and 10 years of follow‐up [17], [18]. These studies, however, evaluated cognitive functioning in relatively small cohorts of patients and did not provide comprehensive assessment of the impact of all treatment modalities used in the treatment of GCTs. Because the GCT survivorship extends to many decades of life, these young men and their families may suffer from late consequences on their long‐term cognition. The Functional Assessment of Cancer Therapy‐Cognitive Function (FACT‐Cog) developed by Wagner and colleagues is a validated patient‐reported outcomes questionnaire‐based tool assessing the cognitive challenges among patients with cancer [19]. By using the FACT‐Cog, we investigated the impact of chemotherapy, radiotherapy, or both on cognition in a GCT survivor population compared with patients cured with orchiectomy alone as a control group. The assessment of late cognitive impairment in this patient population may help guide decisions in controversial situations, such as adjuvant treatment for stage I GCTs. Herein we present the findings from the largest prospectively assessed cohort of GCT survivors evaluating their cognitive abilities.
Subjects, Materials, and Methods
Patients
As a part of an ongoing translational study (Protocol IZLO‐1), we evaluated the impact of chemotherapy, radiotherapy, or both on cognitive function in male patients cured from GCT. The study included GCT survivors followed up at the National Cancer Institute in Bratislava, Slovakia, who were treated from 1983 to 2012. All survivors who were at least 5 years from the completion of their last treatment for GCT were included. Survivors treated for central nervous system metastases were excluded from this trial.
To assess the impact of distinct types of treatment modalities, patients were distributed into the following categories: orchiectomy only (OBS = control group); radiotherapy only (RAT); chemotherapy only (CHT); orchiectomy only plus radiotherapy (OBS + RAT); orchiectomy only plus chemotherapy only (OBS + CHT); chemotherapy plus radiotherapy (CHT + RAT); any treatment; any chemotherapy ± radiotherapy (CHT + CHTRT); and any radiotherapy ± chemotherapy (RAT + CHTRT). The effect of cumulative burden of cisplatin‐based chemotherapy was assessed in groups of <400 mg/m2 versus ≥400 mg/m2 and <200 mg/m2 versus ≥200 mg/m2 of cisplatin and in a subgroup of patients receiving ≥400 mg/m2 cisplatin versus the control group.
The study was approved by the Institutional Review Board (IRB) of the National Cancer Institute of Slovakia and patients were consented according to the IRB‐approved protocol and enrolled between September 2015 and April 2017.
Measures
Patients filled out the FACT‐Cog v.3 questionnaire during their annual follow‐up visit. The questionnaire was filled out in a separate office in a stable, controlled environment and was the same for all participants. Patients were alone in the office—only a study nurse was available during the procedure.
For all subjects, data regarding age, tumor histology, treatment type, and delivery, clinical examination, and cognitive functioning questionnaires FACT‐Cog v.3 were collected at the clinic location and the clinical trials office during the annual follow‐up. FACT‐Cog consists of four domains evaluating a range of self‐reported cognitive functions: perceived cognitive impairment (CogPCI), perceived cognitive abilities (CogPCA), QoL affected by cognitive impairment (CogQoL), and cognitive impairment perceived by others (CogOth). Overall cognitive function score is the sum of the four mentioned subscales. The scale of cognitive impairment shows the higher impairment with the lower reported number (19).
Statistical Analysis
Patients’ characteristics were tabulated. For comparison of baseline characteristics for patients and controls, t tests were used for continuous variables. Because the data were not normally distributed, we used nonparametric Mann‐Whitney U test or Kruskal‐Wallis test as appropriate for analyses of associations between the subscales for cognitive impairment and different treatments. Negative binominal regression was used for multivariable analysis.
A median follow‐up period was calculated as a median observation time among all survivors at the time of their last follow‐up visit. All reported p values were two‐sided. A p value <.05 was considered as significant. Statistical analyses were performed using NCSS 10, 2015 software (Hintze J, 2015, Kaysville, UT).
Results
Patients’ Characteristics
The study population consisted of 155 GCT survivors with a median age of 41 years (range: 21–77 years). Patients’ characteristics are shown in Table 1. Thirteen patients (8%) who relapsed received more than one line of chemotherapy. Twelve patients (8%) treated with adjuvant radiotherapy only received radiation of 20 Gy to retroperitoneal lymph nodes. One patient (0.6%) had radiotherapy of 30 Gy to the retroperitoneal disease that relapsed after first‐line chemotherapy, and three patients (2%) had curative radiotherapy of 30 Gy for a stage II seminoma, subsequently receiving chemotherapy for contralateral second primary GCT. Four patients (2.5%) had adjuvant radiotherapy and subsequent chemotherapy for relapse. Survivor groups were well balanced on education and marital status. Patients who were treated with chemotherapy and/or radiotherapy were older compared with patients treated with orchiectomy only (median: 41 vs. 33 years, p < .01).
Table 1. Patients’ characteristics.
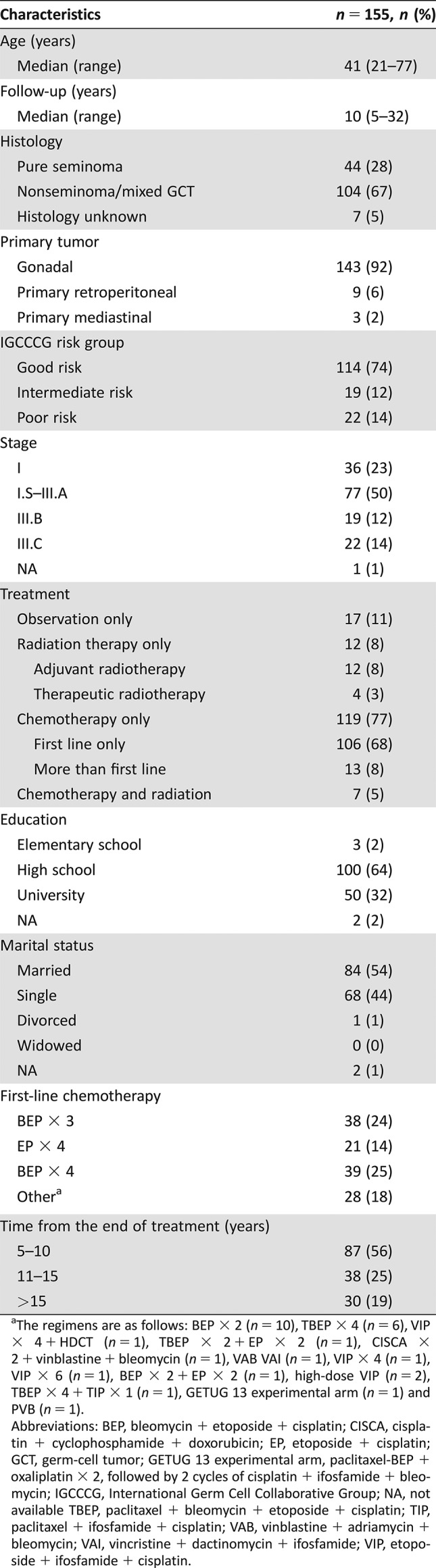
The regimens are as follows: BEP × 2 (n = 10), TBEP × 4 (n = 6), VIP × 4 + HDCT (n = 1), TBEP × 2 + EP × 2 (n = 1), CISCA × 2 + vinblastine + bleomycin (n = 1), VAB VAI (n = 1), VIP × 4 (n = 1), VIP × 6 (n = 1), BEP × 2 + EP × 2 (n = 1), high‐dose VIP (n = 2), TBEP × 4 + TIP × 1 (n = 1), GETUG 13 experimental arm (n = 1) and PVB (n = 1).
Abbreviations: BEP, bleomycin + etoposide + cisplatin; CISCA, cisplatin + cyclophosphamide + doxorubicin; EP, etoposide + cisplatin; GCT, germ‐cell tumor; GETUG 13 experimental arm, paclitaxel‐BEP + oxaliplatin × 2, followed by 2 cycles of cisplatin + ifosfamide + bleomycin; IGCCCG, International Germ Cell Collaborative Group; NA, not available TBEP, paclitaxel + bleomycin + etoposide + cisplatin; TIP, paclitaxel + ifosfamide + cisplatin; VAB, vinblastine + adriamycin + bleomycin; VAI, vincristine + dactinomycin + ifosfamide; VIP, etoposide + ifosfamide + cisplatin.
The Impact of Radiotherapy on CogF
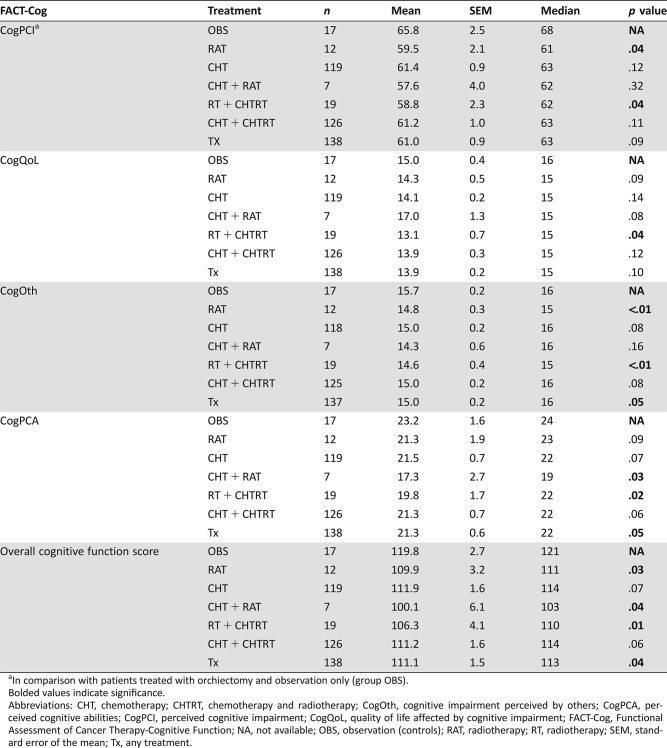
Survivors treated with RAT versus OBS had a decrease in CogPCI (p = .04) and CogOth (p < .01), whereas survivors that received CHTRT had decreased CogPCA (p = .03; Table 2). Survivors treated with RAT ± CHTRT reported the statistical significant decline in all subscales of FACT‐Cog compared with patients treated with orchiectomy only (all p < .05). When we analyzed CogF in survivors receiving RAT or CHTRT versus survivors receiving CHT or OBS, we observed a decline in CogOth (p = .04).
Table 2. Analysis of FACT‐Cog patient‐reported cognitive functioning in testicular germ‐cell tumor survivors.
In comparison with patients treated with orchiectomy and observation only (group OBS).
Bolded values indicate significance.
Abbreviations: CHT, chemotherapy; CHTRT, chemotherapy and radiotherapy; CogOth, cognitive impairment perceived by others; CogPCA, perceived cognitive abilities; CogPCI, perceived cognitive impairment; CogQoL, quality of life affected by cognitive impairment; FACT‐Cog, Functional Assessment of Cancer Therapy‐Cognitive Function; NA, not available; OBS, observation (controls); RAT, radiotherapy; RT, radiotherapy; SEM, standard error of the mean; Tx, any treatment.
The Impact of Chemotherapy on CogF
Chemotherapy alone did not significantly alter CogF compared with the controls (all p > .05); however, there was a nonsignificant trend for impairment in subscales for CogOth (p = .08) and CogPCA (p = .07). The assessment of chemotherapy and chemotherapy plus radiotherapy versus orchiectomy only and radiotherapy only did not show differences in the CogF (Table 2).
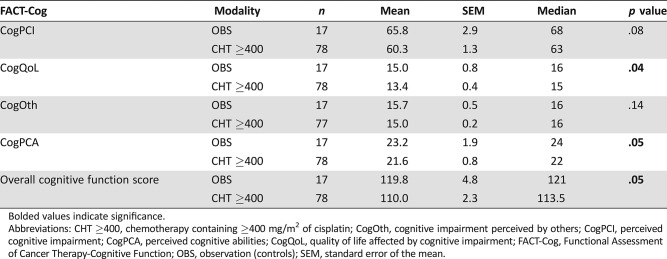
Subsequently, we performed a subgroup analysis of survivors receiving >200 mg/m2 versus ≤200 mg/m2 and ≥400 mg/m2 versus <400 mg/m2 of cisplatin. We did not discover significant differences among these (all p > .05). However, survivors who received ≥400 mg/m2 of cisplatin (n = 78) had a decline in CogQoL and CogPCA domains as well as overall cognitive function score compared with the controls (both p < .05), showing a cognitive impairment in survivors who received higher dosages of chemotherapy (Table 3).
Table 3. Subgroup analysis of cognitive functioning in survivors treated with chemotherapy containing ≥400 mg/m2 of cisplatin versus controls.
Bolded values indicate significance.
Abbreviations: CHT ≥400, chemotherapy containing ≥400 mg/m2 of cisplatin; CogOth, cognitive impairment perceived by others; CogPCI, perceived cognitive impairment; CogPCA, perceived cognitive abilities; CogQoL, quality of life affected by cognitive impairment; FACT‐Cog, Functional Assessment of Cancer Therapy‐Cognitive Function; OBS, observation (controls); SEM, standard error of the mean.
The Impact of Age, Length of Follow‐Up, Education, and Marital Status on CogF
Neither age nor time from treatment was associated with cognitive impairment in the univariate analysis. Education and marital status did not have a statistically significant impact on cognitive functioning in GCT survivors (all p > .05; Table 4).
Table 4. Univariate analysis of overall cognitive function score in association with age, follow‐up, education, and marital status.
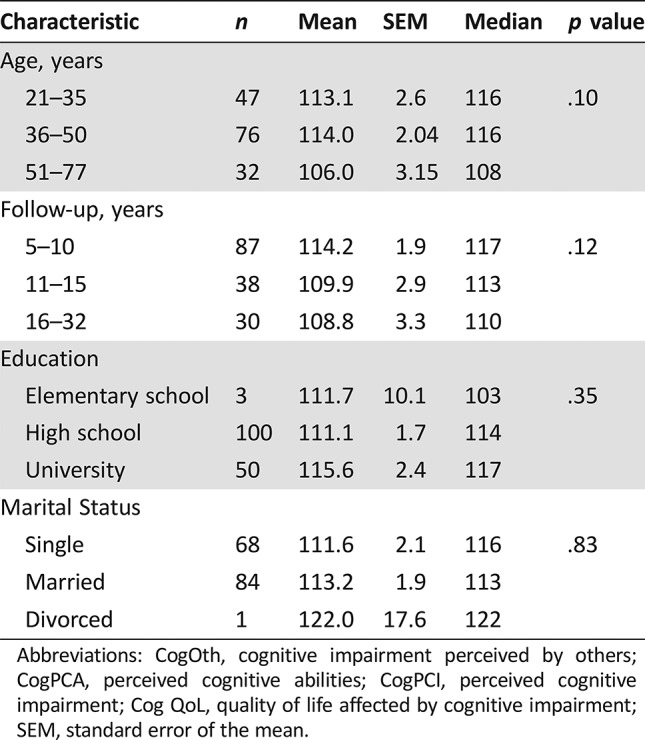
Abbreviations: CogOth, cognitive impairment perceived by others; CogPCA, perceived cognitive abilities; CogPCI, perceived cognitive impairment; Cog QoL, quality of life affected by cognitive impairment; SEM, standard error of the mean.
Overall Cognitive Function Score
Compared with the controls, the overall cognitive function score was altered in survivors treated with radiotherapy, chemotherapy and radiotherapy, and any treatment (all p < .05; Table 2). The long‐term cognitive decline in survivors treated with any treatment was 7.3%, whereas radiotherapy, and chemotherapy plus radiotherapy, caused 8.7% and 16.5% declines, respectively. Additionally, a nonsignificant decline was seen in survivors treated with chemotherapy only (p = .07).
A multivariable analysis showed that decrease of overall cognitive function score was associated with radiation therapy ± chemotherapy, independently of patients’ age (Table 5).
Table 5. Multivariable analysis of overall cognitive function score in association with age and treatment modalities.
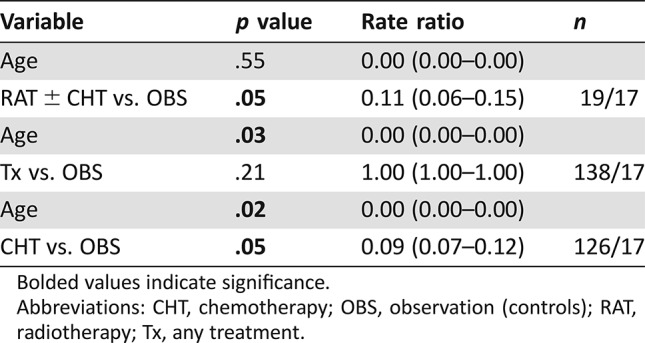
Bolded values indicate significance.
Abbreviations: CHT, chemotherapy; OBS, observation (controls); RAT, radiotherapy; Tx, any treatment.
Discussion
In this largest, prospectively performed observational study using a validated tool in GCT survivors, we observed that self‐reported CogF is a substantial and persistent problem many years after treatment. To our knowledge, no larger studies have comprehensively assessed a long‐term cognition in this survivor population cured at young age using a widely validated questionnaire tool. Despite the fact that GCTs are the most common malignant tumors in young males, the compliance among GCT survivors with the long‐term follow‐up may be insufficient, thus creating a challenge to systematically assess their late treatment sequelae.
Haugnes et al. summarized in their review that a permanent decline in neuropsychological performance has not been systematically documented [20]. Our results provide a novel statement and are supported by two studies carried out on a smaller scale. Stouten‐Kemperman et al. assessed a CogF in GCT survivors using a battery of 12 neurocognitive tests and interviews, and their follow‐up averaged at 14 years. They reported a lower CogF in patients treated with chemotherapy versus surgery only using a Mahalanobis distance score (p = .03). However, the study group consisted of only 28 patients, and the impact of other variables such as radiotherapy or chemotherapy dosages was not assessed. Further, the aim of this study was also appointed toward brain assessment, as authors also investigated white matter changes using MRI [17]. Amidi et al. reported cognitive impairment related to verbal learning and memory, visual learning and memory, processing speed, executive functioning, attention, and working memory, but did not observe a difference with different treatments in 72 GCT survivors compared with controls [18]. A large study evaluated cognition in GCT survivors treated 4–21 years after diagnosis, using a nonvalidated in‐depth tool developed by the authors. This study did not provide a comprehensive assessment of different treatment modalities; however, it reported impairment in cognitive function in survivors who received more than five cycles of chemotherapy versus no chemotherapy, similar to our own findings [21]. A study by Schagen et al. evaluated cognitive complaints by GCT survivors at median follow‐up of 3 years. Authors observed a pronounced impairment in patients treated with orchiectomy and chemotherapy, versus orchiectomy alone (p = .038), but did not observe significant changes between groups treated with surgery and chemotherapy versus surgery and radiotherapy (p = .7), or surgery and radiotherapy versus surgery (p = .07) [22]. Because radiotherapy seemed to show a trend toward significance in this study and proved to be the most potent variable to cause cognitive impairment in our study, we can presume the overall negative impact of radiotherapy may worsen with longer follow‐up time. Moreover, the employment of radiotherapy after chemotherapy seems to create the most significant risk in cognitive impairment in this survivor population, as also analogically evidenced by studies reporting the eminent increase in risk for secondary malignancies and cardiovascular disease in patients treated with both modalities [23], [24]. A Norwegian group published findings on the decrease in cognitive complaints after 3 months from the baseline assessment prior to chemotherapy. Subsequent assessment at 12 months reported a return to baseline, suggesting that cognitive dysfunction is transient after provided treatment [25]. However, cognition was assessed by a subset of questions from the EORTC QLQ‐C30 questionnaire, which does not provide such comprehensive assessment as FACT‐Cog. A similar observation was made in patients treated for breast cancer [26]. A prospective study by Olver et al. assessed short‐term cognition in GCT patients at 12 months of follow‐up. Cognition was assessed in 145 patients by CogHealth, a 10 minute online playing card tool, comparing orchiectomy only‐ versus orchiectomy plus chemotherapy‐treated patients. There was no significant difference for objective or self‐reported function between groups 12 months after treatment [27]. Despite using different measures, these reports and our study suggest that the improvement in CogF after an initial decline following treatment is transient, and may worsen in the long‐term perspective. This pattern also suggests a different underlying mechanism of induction for the long‐term impairment in cognition, which needs to be further investigated. Pro‐inflammatory markers and apolipoprotein E (APOE)‐specific genotype were recently proposed as mechanisms for cognitive impairment in GCT patients undergoing chemotherapy, but their role in the long‐term development of cognitive impairment remains unanswered [28]. Researchers addressed the role of genetic variations in genes regulating neuronal function, neurotransmission, and plasticity in Asian women treated with chemotherapy for early breast cancer and discovered two single nucleotide polymorphisms (SNPs), rs6443264 and rs4676371, that were associated with cognitive impairment. These SNPs lie in iatrogenic regions encoding OGG1 and ARPC4 genes, involved in the downstream regulation of neuronal genes and memory formation [29].
Our study has several strengths and limitations. Strengths include the largest prospective cohort of GCT survivors with long‐term follow‐up using a validated tool. A well‐validated FACT‐Cog provides a substantial power to the interpretation of this study. Also, the control group consisting of survivors treated with orchiectomy only provides valuable information about the specific impact of chemotherapy and radiotherapy, opposite to the age‐matched controls. A limitation is a smaller number of patients in some subgroups, which probably prevented the achievement of statistical significance in several attributes, but still provided power to observe treatment‐specific cognitive declines in this cohort. Another limitation is an imbalance in age, which could have contributed to changes in the cognition of survivors; however, cognitive impairment was not associated with age in the multivariable analysis.
Conclusion
We observed significant decline in GCT survivor‐reported long‐term CogF. The most significant changes to the cognition could be attributed to radiotherapy, and chemotherapy with radiotherapy. The causal relationship of CogF and radiotherapy to retroperitoneal lymph nodes is not clear and remains to be explored. We hypothesize that pathophysiological effects of radiation may lead to changes in cytokine signaling, endothelial dysfunction, collagen deposition, and fibrosis, leading to changes in cognition, much like those observed with cardiovascular complications associated with radiotherapy [30]. An argument can be made against the use of adjuvant radiotherapy for stage I seminoma based on this observation. Additional radiotherapy after chemotherapy should not be considered (e.g., radiation to the residual tumor after chemotherapy), as it poses the highest risk for cognitive dysfunction and its role in curative treatment in GCTs is not established. We speculate that cognitive impairment may be similar or worse in patients with therapeutic radiotherapy to retroperitoneal lymph nodes with higher delivery dose compared with the adjuvant radiotherapy; however, our study was underpowered to make a definitive conclusion for this group. Chemotherapy also induced changes in several cognitive domains; however, these trends did not reach statistical significance in our cohort and need to be further validated. Our results indicate that perceived cognitive impairment is a substantial problem for males who were cured from GCTs.
Acknowledgments
We would like to acknowledge our patients and their families, Zlatica Pekova for administration support, Alzbeta Jancikova, Andrea Krieschova, and Simona Turnova for informed consent collection and oversight of study questionnaires completion. M.CH. and L.V. share the first authorship; J.M. and M.M. are co‐senior authors. This study was presented in part on June 3–7, 2017, at the American Society of Clinical Oncology annual meeting, Chicago, as publication only abstract E21607. This work was supported by the Slovak Research and Development Agency under contract APVV‐15‐0086.
Author Contributions
Conception/design: Michal Chovanec, Beata Mladosievicova, Jozef Mardiak, Michal Mego
Provision of study material or patients: Michal Chovanec, Lucia Vasilkova, Jana Obertova, Patrik Palacka, Katarina Rejlekova, Zuzana Sycova‐Mila, Katarina Kalavska, Daniela Svetlovska, Jozef Mardiak, Michal Mego
Collection and/or assembly of data: Michal Chovanec, Lucia Vasilkova, Lucia Setteyova, Jana Obertova, Patrik Palacka, Katarina Rejlekova, Zuzana Sycova‐Mila, Katarina Kalavska, Daniela Svetlovska, Michal Mego
Data analysis and interpretation: Michal Chovanec, Silvia Cingelova, Michal Mego
Manuscript writing: Michal Chovanec, Michal Mego
Final approval of manuscript: Michal Chovanec, Lucia Vasilkova, Lucia Setteyova, Jana Obertova, Patrik Palacka, Katarina Rejlekova, Zuzana Sycova‐Mila, Katarina Kalavska, Daniela Svetlovska, Silvia Cingelova, Beata Mladosievicova, Jozef Mardiak, Michal Mego
Disclosures
The authors indicated no financial relationships.
References
- 1. Einhorn LH. Treatment of testicular cancer: A new and improved model. J Clin Oncol 1990;8:1777–1781. [DOI] [PubMed] [Google Scholar]
- 2. Motzer RJ, Geller NL, Tan CC et al. Salvage chemotherapy for patients with germ cell tumors. The Memorial Sloan‐Kettering Cancer Center experience (1979–1989). Cancer 1991;67:1305–1310. [DOI] [PubMed] [Google Scholar]
- 3. Motzer RJ, Sheinfeld J, Mazumdar M et al. Paclitaxel, ifosfamide, and cisplatin second‐line therapy for patients with relapsed testicular germ cell cancer. J Clin Oncol 2000;18:2413–2418. [DOI] [PubMed] [Google Scholar]
- 4. Mardiak J, Salek T, Sycova‐ Mila Z et al. Paclitaxel plus ifosfamide and cisplatin in second‐line treatment of germ cell tumors: A phase II study. Neoplasma 2005;52:497–501. [PubMed] [Google Scholar]
- 5. Einhorn LH, Williams SD, Chamness A et al. High‐dose chemotherapy and stem‐cell rescue for metastatic germ‐cell tumors. N Engl J Med 2007;357:340–348. [DOI] [PubMed] [Google Scholar]
- 6. Hanna N, Einhorn LH. Testicular cancer: A reflection on 50 years of discovery. J Clin Oncol 2014;32:3085–3092. [DOI] [PubMed] [Google Scholar]
- 7. Fung C, Fossa SD, Williams A et al. Long‐term morbidity of testicular cancer treatment. Urol Clin North Am 2015;42:393–408. [DOI] [PubMed] [Google Scholar]
- 8. van Leeuwen M, Kieffer JM, Efficace F et al. International evaluation of the psychometrics of health‐related quality of life questionnaires for use among long‐term survivors of testicular and prostate cancer. Health Qual Life Outcomes 2017;15:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janelsins MC, Heckler CE, Peppone LJ et al. Longitudinal assessment of cancer‐related cognitive impairment (CRCI) up to six‐months post‐chemotherapy with multiple cognitive testing methods in 943 breast cancer (BC) patients and controls. J Clin Oncol 2017;35(suppl 15):10014a. [Google Scholar]
- 10. Cruzado JA, Lopez‐Santiago S, Martinez‐ Marin V et al. Longitudinal study of cognitive dysfunctions induced by adjuvant chemotherapy in colon cancer patients. Support Care Cancer 2014;22:1815–1823. [DOI] [PubMed] [Google Scholar]
- 11. Wouters H, Baars JW, Schagen SB. Neurocognitive function of lymphoma patients after treatment with chemotherapy. Acta Oncol 2016;55:1121–1125. [DOI] [PubMed] [Google Scholar]
- 12. Hartl K, Engel J, Herschbach P et al. Personality traits and psychosocial stress: Quality of life over 2 years following breast cancer diagnosis and psychological impact factors. Psychooncology 2010;19:160–169. [DOI] [PubMed] [Google Scholar]
- 13. Aaronson NK, Ahmedzai S, Bergman B et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: A quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 14. Fossa SD, Moynihan C, Serbouti S. Patients' and doctors' perception of long‐term morbidity in patients with testicular cancer clinical stage I. A descriptive pilot study. Support Care Cancer 1996;4:118–128. [DOI] [PubMed] [Google Scholar]
- 15. Hjermstad MJ, Fayers PM, Bjordal K et al. Health‐related quality of life in the general Norwegian population assessed by the European Organization for Research and Treatment of Cancer Core Quality‐of‐Life Questionnaire: The QLQ=C30 (+ 3). J Clin Oncol 1998;16:1188–1196. [DOI] [PubMed] [Google Scholar]
- 16. Wefel JS, Vidrine DJ, Marani SK et al. A prospective study of cognitive function in men with non‐seminomatous germ cell tumors. Psychooncology 2014;23:626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stouten‐Kemperman MM, de Ruiter MB, Caan MW et al. Lower cognitive performance and white matter changes in testicular cancer survivors 10 years after chemotherapy. Hum Brain Mapp 2015;36:4638–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amidi A, Wu LM, Pedersen AD et al. Cognitive impairment in testicular cancer survivors 2 to 7 years after treatment. Support Care Cancer 2015;23:2973–2979. [DOI] [PubMed] [Google Scholar]
- 19. Wagner L, Sweet J, Butt Z et al. Measuring patient self‐reported cognitive function: Development of the Functional Assessment of Cancer Therapy–Cognitive Function Instrument. J Support Oncol 2009;7:W32–W39. [Google Scholar]
- 20. Haugnes HS, Bosl GJ, Boer H et al. Long‐term and late effects of germ cell testicular cancer treatment and implications for follow‐up. J Clin Oncol 2012;30:3752–3763. [DOI] [PubMed] [Google Scholar]
- 21. Skoogh J Jr, Steineck G, Stierner UK et al. Long‐term cognitive function among testicular cancer survivors treated with chemotherapy. J Clin Oncol 2008;26(suppl 15):5035a. [Google Scholar]
- 22. Schagen SB, Boogerd W, Muller MJ et al. Cognitive complaints and cognitive impairment following BEP chemotherapy in patients with testicular cancer. Acta Oncol 2008;47:63–70. [DOI] [PubMed] [Google Scholar]
- 23. van den Belt‐Dusebout AW, de Wit R, Gietema JA et al. Treatment‐specific risks of second malignancies and cardiovascular disease in 5‐year survivors of testicular cancer. J Clin Oncol 2007;25:4370–4378. [DOI] [PubMed] [Google Scholar]
- 24. Haugnes HS, Wethal T, Aass N et al. Cardiovascular risk factors and morbidity in long‐term survivors of testicular cancer: A 20‐year follow‐up study. J Clin Oncol 2010;28:4649–4657. [DOI] [PubMed] [Google Scholar]
- 25. Skaali T, Fossa SD, Dahl AA. A prospective study of cognitive complaints in patients with testicular cancer. Clin Genitourin Cancer 2011;9:6–13. [DOI] [PubMed] [Google Scholar]
- 26. Hermelink K, Kuchenhoff H, Untch M et al. Two different sides of 'chemobrain': Determinants and nondeterminants of self‐perceived cognitive dysfunction in a prospective, randomized, multicenter study. Psychooncology 2010;19:1321–1328. [DOI] [PubMed] [Google Scholar]
- 27. Olver IN, Whitford HS, Kalinowski P et al. Chemotherapy and cognitive function in testicular cancer: A prospective, longitudinal cohort study. J Clin Oncol 2016;34(suppl 15):4547a. [Google Scholar]
- 28. Amidi A, Agerbaek M, Wu LM et al. Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging Behav 2017;11:769–783. [DOI] [PubMed] [Google Scholar]
- 29. NG T, Yeo HL, Shwe M et al. A genome‐wide association study (GWAS) meta‐analysis of chemotherapy‐associated cognitive impairment (CACI) in Asian early‐stage breast cancer patients (ESBC). J Clin Oncol 2017;35(suppl 15):10096a. [Google Scholar]
- 30. Basavaraju SR, Easterly CE. Pathophysiological effects of radiation on atherosclerosis development and progression, and the incidence of cardiovascular complications. Med Phys 2002;29:2391–2403. [DOI] [PubMed] [Google Scholar]