Abstract
Lessons Learned.
Results suggest that the combination of bevacizumab plus temozolomide is active in terms of response rate, survival, performance, quality of life, and cognition in elderly patients with glioblastoma multiforme with poor performance status.
Whether this combination is superior to temozolomide alone remains to be demonstrated by a randomized study.
Background.
The optimal treatment of glioblastoma multiforme (GBM) in patients aged ≥70 years with a Karnofsky performance status (KPS) <70 is not established. This clinical trial evaluated the efficacy and safety of upfront temozolomide (TMZ) and bevacizumab (Bev) in patients aged ≥70 years and a KPS <70.
Materials and Methods.
Patients aged ≥70 years with a KPS <70 and biopsy‐proven GBM were eligible for this multicenter, prospective, nonrandomized, phase II trial of older patients with impaired performance status. Treatment consisted of TMZ administered at 130–150 mg/m2 per day for 5 days every 4 weeks plus Bev administered at 10 mg/kg every 2 weeks.
Results.
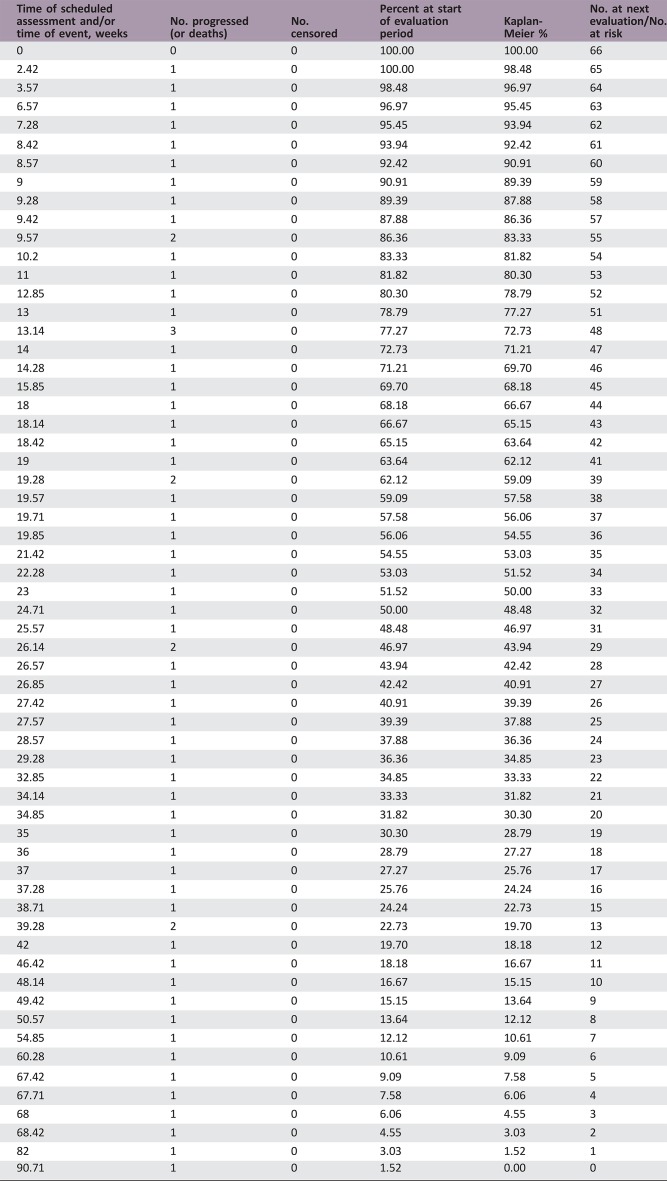
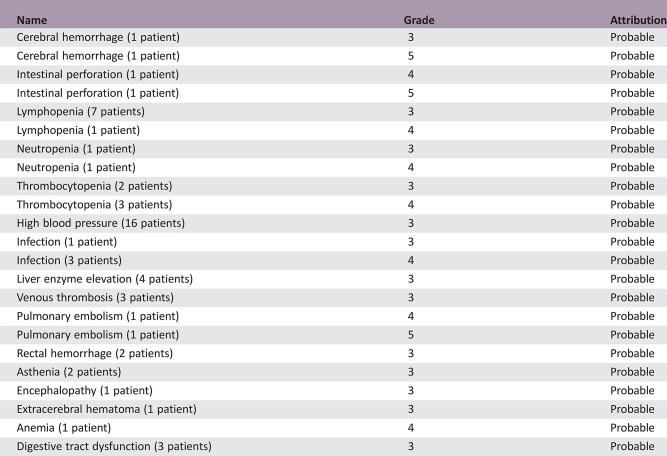
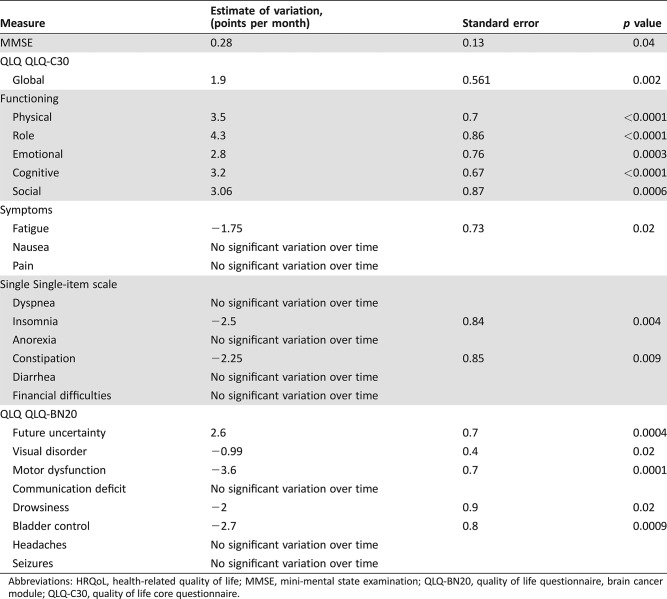
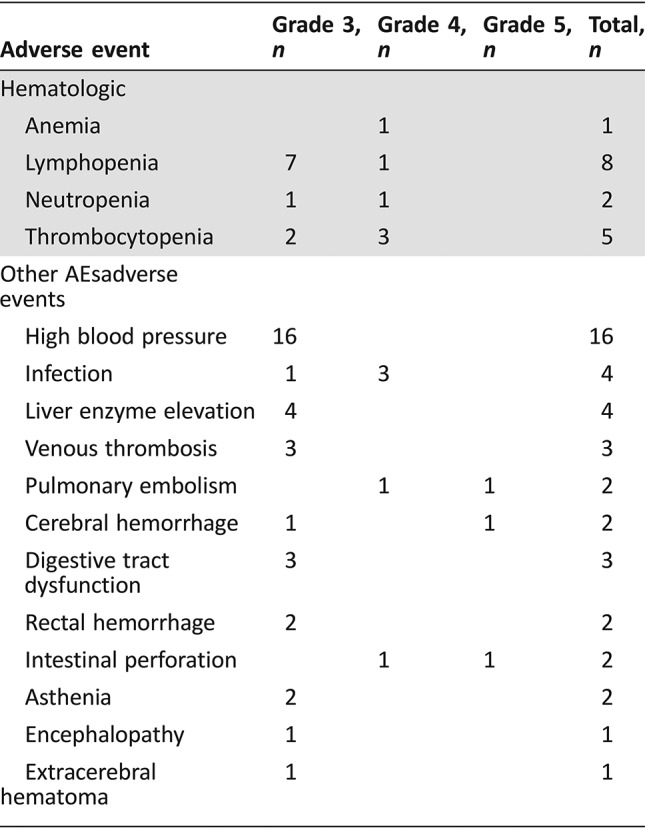
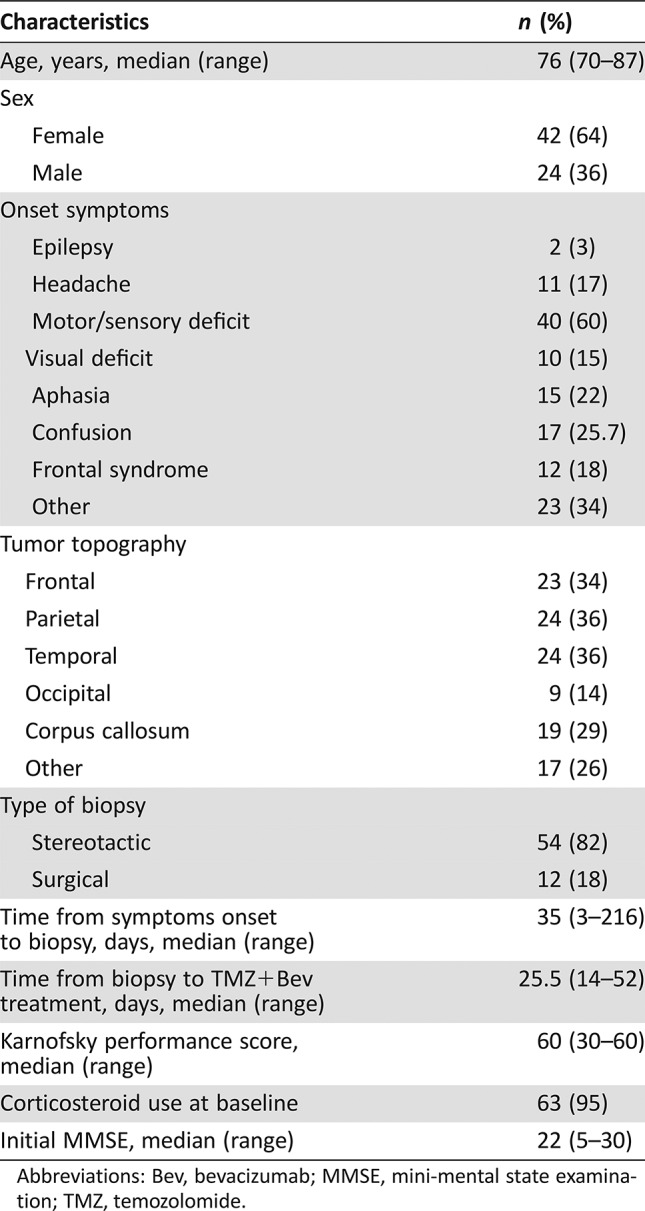
The trial included 66 patients (median age of 76 years; median KPS of 60). The median overall survival (OS) was 23.9 weeks (95% confidence interval [CI], 19–27.6), and the median progression‐free survival (PFS) was 15.3 weeks (95% CI, 12.9–19.3). Twenty‐two (33%) patients became transiently capable of self‐care (i.e., KPS >70). Cognition and quality of life significantly improved over time during treatment. Grade ≥3 hematological adverse events occurred in 13 (20%) patients, high blood pressure in 16 (24%), venous thromboembolism in 3 (4.5%), cerebral hemorrhage in 2 (3%), and intestinal perforation in 2 (3%).
Conclusion.
This study suggests that TMZ + Bev treatment is active in elderly patients with GBM with low KPS and has an acceptable tolerance level.
Abstract
经验总结
结果表明, 对于患多形性恶性胶质瘤且体能状态欠佳的老年患者, 贝伐单抗+替莫唑胺联合治疗在缓解率、生存期、体能、生活质量和认知方面有效。
此联合治疗是否优于替莫唑胺单药治疗有待通过随机研究证明。
摘要
背景.尚未确定Karnofsky体能状态评分(KPS)<70、年龄≥70岁的多形性恶性胶质瘤(GBM)患者的最佳治疗。本临床试验评价了替莫唑胺(TMZ)和贝伐单抗(Bev)初期治疗在年龄≥70岁、KPS<70的患者中的疗效和安全性。
材料和方法.年龄≥70岁、KPS<70且患有经活检证实GBM的患者有资格参加本项针对体能状态受损年长患者的多中心、前瞻性、非随机、II期试验。治疗包括每4周一次、为期5天的TMZ 130–150mg/m2/天给药+每2周一次Bev 10mg/kg给药。
结果.该试验包括66例患者(中位年龄为76岁, 中位KPS为60)。中位总生存期(OS)为23.9周[95%置信区间(CI):19–27.6], 中位无进展生存期(PFS)为15.3周(95% CI:12.9–19.3)。22例(33%)患者暂时能够自我护理(即KPS>70)。治疗期间认知和生活质量得到显著改善。13例(20%)患者发生≥3级血液学不良事件, 16例(24%)患者发生高血压, 3例(4.5%)患者发生静脉血栓栓塞, 2例(3%)患者发生脑出血, 2例(3%)患者发生肠穿孔。
结论.本研究表明TMZ+Bev治疗在KPS较低的GBM老年患者中有效, 且耐受程度可接受。
Discussion
Our study indicates that tolerance to the Bev‐TMZ combination was acceptable in this population. Hematological toxicity greater than grade 3 was reported in 20% of patients. As expected, the most frequent adverse event observed with Bev was high blood pressure, which responded to antihypertensive treatment. Although the rate of thromboembolic events does not appear to be exceedingly high in this bedridden GBM population, the two cases of intestinal perforation can be ascribed to Bev; furthermore, Bev may have played a role in the two cases of cerebral hemorrhage.
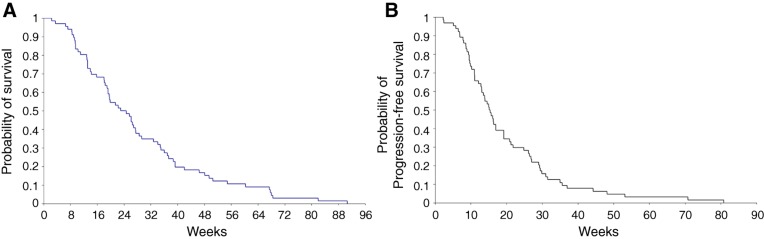
An objective radiological response in one third of patients and the fact that one third of patients also became autonomous and capable of self‐care (i.e., KPS >70) is encouraging and in agreement with the observations of significant improvements in cognition and most quality‐of‐life scales during the treatment period. Additionally, the estimated OS median of 24 weeks (Figure 1) that we found appears higher that the 12 weeks OS that we found in a similar patient population treated with supportive care alone (personal data, unpublished).
Figure 1.
Kaplan‐Meier plot: experimental arm, primary assessment, total patient population. (A): Kaplan‐Meier estimates of overall survival. (B): Kaplan‐Meier estimates of progression‐free survival.
There was a trend for increased PFS and OS in patients with methylated promoter O‐6‐methylguanine‐DNA methyltransferase (MGMT) status; however, in contrast to our previous study that used TMZ alone, this difference did not reach statistical significance. This may reflect a lack of power. On the other hand, it is possible that some patients without methylated MGMT promoter also could benefit from Bev because its action is not believed to be influenced by MGMT methylation status.
Trial Information
- Disease
Brain cancer – primary
- Stage of Disease/Treatment
Primary
- Prior Therapy
None
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Single arm
- Primary Endpoint
Overall survival
- Secondary Endpoint
Progression‐free survival
- Secondary Endpoint
Tolerability
- Secondary Endpoint
Health‐related quality of life
- Secondary Endpoint
Cognitive functioning
- Additional Details of Endpoints or Study Design
- The primary endpoint was median OS, and the secondary endpoints were PFS, tolerance of treatment, health‐related quality of life (European Organisation for Research and Treatment of Cancer [EORTC] quality of life questionnaires: core questionnaire [QLQ‐C30], version 3.0, and brain cancer module [QLQ‐BN20]) and cognitive functioning (mini‐mental state examination [MMSE]). The sample size was based on the accuracy of the median survival estimation. Assuming a median survival of 22 weeks and a minimum follow‐up of 12 months, it was estimated that 70 patients were needed to evaluate survival with a half width of its 95% confidence interval of 14 weeks. All patients who received at least one dose of treatment were included in the analyses. OS was calculated from the date of surgery until death. PFS was defined as the time from surgery to the date of progression or death. The survival distributions were estimated by the Kaplan‐Meier method. The log‐rank test was used to compare OS and PFS according to MGMT status. Prognostic factors for survival were examined by a stepwise Cox regression model. The factors included in the model were age and KPS at inclusion (60 vs. <60). A second Cox regression model was performed on the patients for whom MGMT promoter methylation assessment was available, with MGMT status, age, and KPS as covariates. Changes from baseline of health‐related quality of life scores, MMSE scores, and KPS were analyzed using a mixed‐model analysis, with time as a fixed effect and the patient as a random effect. Logistic regression was used to identify predictive factors of clinical improvement defined by a KPS ≥70 at two consecutive visits. All analyses were performed using SAS software version 8.2 (SAS Institute, Cary, NC). The α level was set at 0.05.
- Baseline assessments included physical and neurological examinations, assessments of KPS, health‐related quality of life questionnaires (EORTC QLQ‐C30, version 3.0, and QLQBN20), cognitive evaluations with MMSE, complete blood counts and blood chemistry tests, and contrast‐enhanced brain computed tomography or magnetic resonance imaging. Patients were assessed every 2 weeks by physical and neurological examinations, complete blood counts, and urine strip tests. The blood chemistry tests, quality of life assessments, KPS evaluations, and MMSEs were repeated every month. Neuroimaging studies were repeated every 2 months, and tumor response was assessed using the response assessment in neuro‐oncology criteria, taking into account the perpendicular diameters of the tumor in contrasted sequences and fluid‐attenuated inversion recovery. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. The EORTC QLQ‐C30 and QLQ‐BN20 were used to assess health‐related quality of life. The QLQ‐C30 questionnaire includes 30 questions comprising five functioning scales (physical, role, emotional, cognitive, and social), three symptom scales (fatigue, vomiting, and pain), and six single‐item scales (dyspnea, insomnia, constipation, anorexia, diarrhea, and financial difficulties). The QLQ‐BN20 questionnaire includes 20 items covering functional deficits, symptoms, toxic effects of treatment, and uncertainty about the future. Questionnaires were filled out by patients when possible or by a companion (always the same) when the patient was unable to complete the form. The MMSE was used as a measure of general cognitive status. Higher scores on this exam, which uses a 30‐point scale, indicated better cognitive function.
- Investigator's Analysis
Active and should be pursued further
Drug Information
- Drug 1
- Generic/Working Name
Temozolomide
- Drug Type
Small molecule
- Drug Class
Alkylating agent
- Dose
130–150 milligrams (mg) per square meter (m2)
- Route
Oral (p.o.)
- Drug 2
- Generic/Working Name
Bevacizumab
- Drug Type
Antibody
- Drug Class
Angiogenesis – VEGF
- Dose
10 mg milligrams (mg) per kilogram (kg)
- Route
IV
- Schedule of Administration
Every 2 weeks
Patient Characteristics
- Number of Patients, Male
24
- Number of Patients, Female
42
- Age
Median (range): 76 years (70–87 years)
- Number of Prior Systemic Therapies
Median: 0
- Other
The median postoperative Karnofsky performance status was 60 (range, 30–60)
Primary Assessment Method
- Title
Total Patient Population
- Number of Patients Screened
71
- Number of Patients Enrolled
66
- Number of Patients Evaluable for Toxicity
66
- Number of Patients Evaluated for Efficacy
66
- Response Assessment CR
n = 1
- Response Assessment PR
n = 20
- Response Assessment SD
n = 24
- Response Assessment PD
n = 15
- (Median) Duration Assessments PFS
15.3 weeks; CI, 12.9–19.3
- (Median) Duration Assessments OS
23.9 weeks; CI, 19–27.6
- Kaplan‐Meier Time Units
Weeks
Kaplan‐Meier Plot Legend
Kaplan‐Meier estimates of overall survival (OS)
Phase II Experimental Adverse Events
- Tolerance to treatment was acceptable, and most adverse events (AEs) were mild to moderate. AEs of grade ≥3 were observed in 37 (56%) patients and are detailed in Table 2. High blood pressure was the most frequent AE and was detected in 16 (24%) patients. It was manageable with antihypertensive medication, and Bev discontinuation was not necessary in any of those cases. Hematological AEs of grade ≥3 were reported in 13 (20%) patients, including neutropenia (n = 2), anemia (n = 1), thrombocytopenia (n = 5), and lymphopenia (n = 8). Fatal myelosuppression was not observed.
Table 2. Number of patients with grade ≥ 3 adverse events.
Serious Adverse Events
Serious Adverse Events Legend
Serious adverse events included deep venous thrombosis (three cases), pulmonary embolism (two cases, including one fatal), intestinal perforation (two cases, including one fatal), and cerebral hemorrhage (two cases, including one fatal grade 5 toxicity).
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Active and should be pursued further
Elderly patients aged 65 years and older account for approximately 45% of patients with glioblastoma multiforme (GBM) [1], and this figure is expected to rise concurrently with the aging population of most countries [2]. Unfortunately, few trials have been performed in this setting [3], [4], [5], [6], [7]. In elderly patients with good functional status (Karnofsky performance status [KPS] >70 or Eastern Cooperative Oncology Group score 0–2), the standard treatment is now the combination of radiotherapy (RT) and temozolomide (TMZ; concomitant and adjuvant). In elderly patients with poor functional status at the time of diagnosis (KPS <70), there is no standard treatment. One trial suggested that accelerated 1‐week RT could be of help, but this trial mixed various populations, including younger patients and elderly patients in good clinical condition [8]. In elderly, bedridden patients with unresectable GBM, the benefit of radiotherapy is unproven and questionable; indeed, it requires daily trips to the hospital, resulting in increased fatigue and an increased risk of acute complications, including intracranial hypertension and herniation, particularly when high doses per fraction are used. In this very difficult patient population, we previously showed that TMZ alone was associated with improvement in functional status in one third of cases and appeared to increase survival compared with supportive care alone [9].
Bevacizumab (Bev) is an antiangiogenic monoclonal antibody targeting vascular endothelial growth factor that is commonly used in GBM. Although recent phase III studies did not show a significant effect of adding Bev to alkylating agents (TMZ or nitrosoureas) on overall survival (OS), a favorable impact of this combination on progression‐free survival was demonstrated both as a first‐line treatment and in the recurrent setting [10], [11], [12], [13], [14], [15], [16].
Treatment of GBM in elderly patients with impaired functional status is challenging. In a recursive partitioning analysis, the estimated median OS in elderly patients with GBM with a KPS <70 without surgical resection was only 10 weeks (95% CI, 9–13.5) [17]. In a recent phase II trial focusing on this frail elderly population, we found that TMZ alone was helpful with a median OS of 25 weeks (95% CI, 19–28); 25% of patients became able to provide self‐care and had significant improvements in quality of life and cognition before disease progression [9].
In this study, we evaluated the efficacy and safety of the upfront combination of TMZ + Bev as an initial treatment for elderly patients with GBM and impaired functional status (KPS <70). Bev has a well‐known steroid‐sparing effect, presumably because of the blood‐brain and blood‐tumor barrier restoration [10]. Indeed, corticosteroids could be reduced (in 56% of cases) or even discontinued (10%) in our patients with inoperable tumors. This steroid‐sparing effect is clearly favorable given the poor tolerance of elderly patients for steroids [18].
Although the analysis included only 66 patients (5 patients were excluded because they did not take any dose of treatment), the precision obtained at the end of the trial of the estimation of the median survival was higher than expected (standard error of 5 weeks instead of 14 weeks). Additionally, the estimated OS median of 24 weeks that we found appears higher that the 12 weeks of OS that we found in a similar patient population treated with supportive care alone (personal data, unpublished). However, it is comparable to the 25 weeks that we reported in similar patients receiving TMZ alone [9]. Whether this combination is superior to TMZ alone remains to be demonstrated by a randomized study.
Figures and Tables
Table 1. Baseline patient characteristics.
Abbreviations: Bev, bevacizumab; MMSE, mini‐mental state examination; TMZ, temozolomide.
Table 3. Variations in MMSE and HRQoL scores over time before progression.
Abbreviations: HRQoL, health‐related quality of life; MMSE, mini‐mental state examination; QLQ‐BN20, quality of life questionnaire, brain cancer module; QLQ‐C30, quality of life core questionnaire.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We thank the patients and their families for their participation in this clinical trial, as well as the data managers, nurses, and physicians involved in the study for their support and care.
Footnotes
ClinicalTrials.gov Identifier: NCT02898012
Sponsor(s): Institut National du Cancer (PHRC program); Roche; Assistance Publique – Hôpitaux de Paris
Principal Investigator: Jean‐Yves Delattre
IRB Approved: Yes
Disclosures
Olivier Chinot: Roche, IPSEN Celldex (C/A), Servier, Astra‐Zeneca (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Dolecek TA, Propp JM, Stroup NE et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 2012;14(suppl 5):v1–v49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fleury A, Menegoz F, Grosclaude P et al. Descriptive epidemiology of cerebral gliomas in France. Cancer 1997;79:1195–1202. [DOI] [PubMed] [Google Scholar]
- 3. Keime‐Guibert F, Chinot O, Taillandier L et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med 2007;356:1527–1535. [DOI] [PubMed] [Google Scholar]
- 4. Malmström A, Grønberg BH, Marosi C et al. Temozolomide versus standard 6‐week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol 2012;13:916–926. [DOI] [PubMed] [Google Scholar]
- 5. Wick W, Platten M, Meisner C et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: The NOA‐08 randomised, phase 3 trial. Lancet Oncol 2012;13:707–715. [DOI] [PubMed] [Google Scholar]
- 6. Perry JR, Laperriere N, O'Callaghan CJ et al. A phase III randomized controlled trial of short‐course radiotherapy with or without concomitant and adjuvant temozolomide in elderly patients with glioblastoma (CCTG CE.6, EORTC 26062‐22061, TROG 08.02, NCT00482677). J Clin Oncol 2016;34(suppl 18):LBA2A. [Google Scholar]
- 7. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 8. Roa W, Kepka L, Kumar N et al. International Atomic Energy Agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 2015;33:4145–4150. [DOI] [PubMed] [Google Scholar]
- 9. Gállego Pérez‐Larraya J, Ducray F, Chinot O et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: An ANOCEF phase II trial. J Clin Oncol 2011;29:3050–3055. [DOI] [PubMed] [Google Scholar]
- 10. Chinot OL, Wick W, Mason W et al. Bevacizumab plus radiotherapy‐temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014;370:709–722. [DOI] [PubMed] [Google Scholar]
- 11. Gilbert MR, Dignam JJ, Armstrong TS et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014;370:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taal W, Oosterkamp HM, Walenkamp AM et al. Single‐agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol 2014;15:943–953. [DOI] [PubMed] [Google Scholar]
- 13. Wick W, Brandes AA, Gorlia T et al. EORTC 26101 phase III trial exploring the combination of bevacizumab and lomustine in patients with first progression of a glioblastoma. J Clin Oncol 2016;34(suppl 15):2001A. [Google Scholar]
- 14. Lou E, Peters KB, Sumrall AL et al. Phase II trial of upfront bevacizumab and temozolomide for unresectable or multifocal glioblastoma. Cancer Med 2013;2:185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Friedman HS, Prados MD, Wen PY. et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733–4740. [DOI] [PubMed] [Google Scholar]
- 16. Chauffert B, Feuvret L, Bonnetain F et al. Randomized phase II trial of irinotecan and bevacizumab as neo‐adjuvant and adjuvant to temozolomide‐based chemoradiation compared with temozolomide‐chemoradiation for unresectable glioblastoma: Final results of the TEMAVIR study from ANOCEF. Ann Oncol 2014;25:1442–1447. [DOI] [PubMed] [Google Scholar]
- 17. Scott JG, Bauchet L, Fraum TJ et al. Recursive partitioning analysis of prognostic factors for glioblastoma patients aged 70 years or older. Cancer 2012;118:5595–5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fardet L, Fève B. Systemic glucocorticoid therapy: A review of its metabolic and cardiovascular adverse events. Drugs 2014;74:1731–1745. [DOI] [PubMed] [Google Scholar]