Gynecologic carcinosarcomas are uncommon malignancies that have an aggressive biology and lack a standard therapeutic approach. Molecular analyses have shown recurrent alterations in chromatin remodeling genes, but clinical support for therapeutic significance is lacking. This article is the first report of a case of a patient with refractory uterine carcinosarcoma that responded to immune checkpoint inhibitor therapy.
Abstract
Gynecologic carcinosarcomas, previously known as malignant mixed Müllerian tumors, are uncommon malignancies that demonstrate an aggressive biology and lack a standard therapeutic approach. Molecular analyses have revealed recurrent alterations in chromatin remodeling genes, but clinical support for therapeutic significance is lacking. We prospectively identified a patient with refractory uterine carcinosarcoma whose tumor was subject to molecular profiling at diagnosis and again at radiographic progression. Initial molecular testing did not assess tumor mutational burden, DNA polymerase ɛ (POLE), or microsatellite status. After the failure of several lines of chemotherapy, comprehensive genomic profiling of a repeat biopsy identified two missense mutations of the exonuclease domain of POLE (P286R and T323A). Tumor mutational burden was elevated (169 mutations per DNA megabase), consistent with an ultramutator phenotype. As seen in previously reported POLE‐endometrioid cases, our patient harbored alterations in PIK3CA, ARID1A, and PTEN and was microsatellite stable, with appreciable tumor‐infiltrating lymphocytes. She achieved an ongoing durable response with pembrolizumab. This is the first report of programmed cell death protein 1 response in uterine carcinosarcoma.
Key Points.
Uterine carcinosarcoma is an uncommon and aggressive histologic variant of endometrial carcinoma with a poor prognosis.
Inactivating DNA polymerase ɛ (POLE) mutations have been associated with high tumor mutational burden (TMB) and response to immune checkpoint inhibition.
To the authors' knowledge, this is the first report of response to immune checkpoint inhibitor therapy in a patient with uterine carcinosarcoma.
This case further supports expanding genomic profiling to include assessment of tumor mutational burden across tumor types, given the potential for immune checkpoint inhibitor therapy in TMB‐high tumors.
Patient Story
Inactivating DNA polymerase ɛ (POLE) mutations have been associated with high tumor mutational burden (TMB) uterine endometrioid adenocarcinomas. When restricted to uterine carcinosarcoma, an elevated TMB has been observed at variable rates [1], [2]. Our case provides orthogonal support for the responsiveness of tumors with elevated TMB to immune checkpoint inhibitors, regardless of histology. The importance and evolution of comprehensive genomic profiling is highlighted by the clinical impact of microsatellite instability (MSI), POLE, and TMB in subsequent testing for our patient.
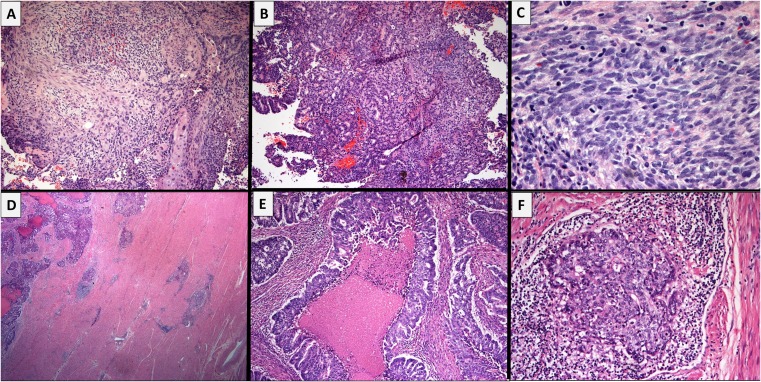
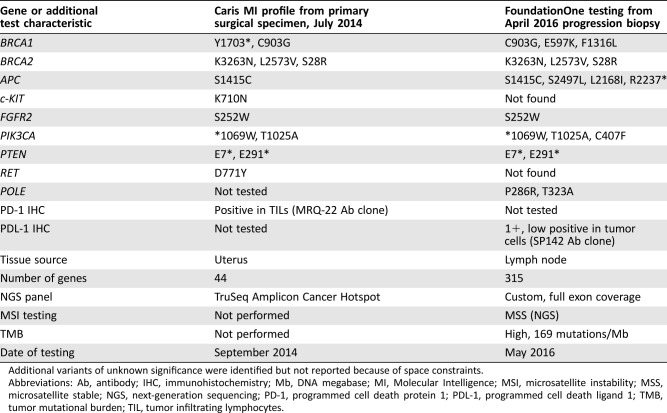
A 55‐year‐old woman was diagnosed with a uterine carcinosarcoma after presenting with postmenopausal bleeding in July 2014 (Fig. 1A–C). She underwent curative attempt total abdominal hysterectomy, bilateral salpingo‐oophorectomy, and pelvic lymphadenectomy. Surgical pathology revealed an International Federation of Gynecology and Obstetrics stage IIIA (pT3aN0M0) uterine carcinosarcoma with lymphovascular invasion (Fig. 1D, E). Surgical margins were negative. However, an 8‐week postoperative positron emission tomography and computed tomography in September 2014 revealed persistent and recurrent nodal metastases. Re‐resection was attempted but not possible. Broad molecular hot‐spot testing from the patient's original specimen was not considered to reveal actionable therapeutic alterations (Table 1).
Figure 1.
Results of biopsies. Panels (A–C) show the endometrial biopsy performed for postmenopausal bleeding in July 2014. The tumor shows a biphasic pattern of epithelial (carcinomatous) and spindled (sarcomatous) differentiation. (D, E): The hysterectomy specimen from September 2014 shows a large, friable, fungating tumor with >50% myometrial invasion. The tumor consists predominantly of crowded, fused, and cribriform glands, as well as solid nests making up >50% of the tumor and large areas of comedonecrosis, consistent with an endometrioid‐type, International Federation of Gynecology and Obstetrics grade 3, endometrial adenocarcinoma. This matches the carcinomatous component seen on the previous biopsy. (F): The recurrence biopsy from April 2016 shows predominantly carcinomatous differentiation with some areas of spindled pattern consistent with the original diagnosis of uterine carcinosarcoma. Tumor nests are accompanied by a moderate peritumoral lymphocytic response; however, tumor infiltrating lymphocytes are, by and large, non‐brisk.
Table 1. Comparison of molecular testing results conducted on original surgical specimen and repeat testing from progression biopsy in a case of endometrial carcinosarcoma.
Additional variants of unknown significance were identified but not reported because of space constraints.
Abbreviations: Ab, antibody; IHC, immunohistochemistry; Mb, DNA megabase; MI, Molecular Intelligence; MSI, microsatellite instability; MSS, microsatellite stable; NGS, next‐generation sequencing; PD‐1, programmed cell death protein 1; PDL‐1, programmed cell death ligand 1; TMB, tumor mutational burden; TIL, tumor infiltrating lymphocytes.
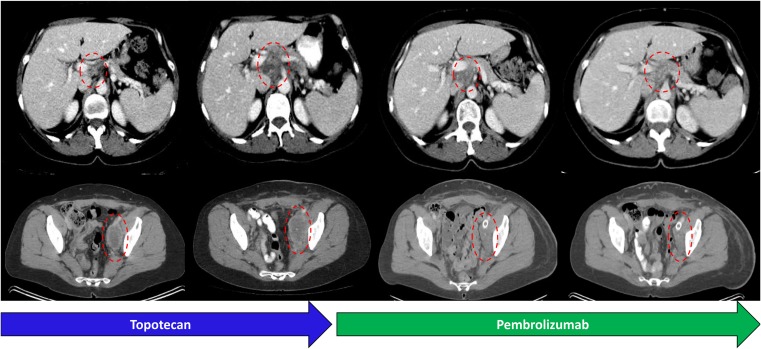
Beginning in late 2014, the patient was treated with several lines of systemic therapy, initially carboplatin and paclitaxel, followed by gemcitabine and oxaliplatin and later single‐agent topotecan. Ultimately, in 2016, she developed increasing abdominal pain and significant left lower extremity lymphedema with radiographic disease progression (Fig. 2). Given the widespread and symptomatic progression despite several lines of conventional cytotoxic chemotherapy, a new biopsy was submitted for comprehensive genomic profiling (CGP; Fig. 1E; FoundationONE, Foundation Medicine, Cambridge, MA, http://www.foundationone.com), revealing an elevated tumor mutational burden as well as inactivating missense mutations in the exonuclease domain of POLE.
Figure 2.
Response to immune checkpoint inhibition in a case of endometrial carcinosarcoma with an elevated tumor mutational burden and POLE mutation. Axial computed tomography images demonstrate progression in a periportal nodal conglomerate and necrotic pelvic sidewall followed by response after initiation of pembrolizumab, now lasting over 12 months.
Molecular Tumor Board
Uterine carcinosarcomas, formerly known as malignant mixed Müllerian tumors, compose a minority of endometrial cancers and morphologically appear biphasic, containing foci of carcinoma and sarcoma, which gives rise to the carcinosarcoma label [1], [3], [4]. The clinical course for most uterine carcinosarcomas is worse compared with the more common endometrioid adenocarcinomas or even with those with pure serous histology [5], [6], [7]. Surgery remains the mainstay of therapy for localized disease, and advanced disease is managed with systemic chemotherapy, largely carboplatin and paclitaxel [8], [9], [10].
Large collaborative molecular classification efforts such as the Cancer Genome Atlas (TCGA) have improved biologic understanding and identified molecular subtypes across multiple tumor types, including endometrial cancers [11]. Although the endometrial TCGA identified two groups (POLE mutant and hypermutated microsatellite instable [MSI‐H]) with increased numbers of nonsynonymous mutations, the analysis was largely restricted to early‐stage, surgically resected cases with endometrioid or serous histology (360 of 373 cases) [11]. Smaller series have investigated the molecular landscape of uterine carcinosarcomas, and cases with increased TMB have been reported, although clinical response data are lacking [1], [12].
Current National Comprehensive Cancer Network guidelines (version 1.2017) endorse MSI testing for all endometrial cancers, but determination of tumor mutational burden or POLE status is not standard. However, across other tumor types, elevated MSI‐H and elevated TMB have been associated with responsiveness to immune checkpoint inhibitor therapy based on the presumed increase in tumor‐specific neoantigens in these patients [13], [14], [15]. Within this context, tumor tissue from the index patient was submitted for CGP to identify additional therapeutic options.
Genotyping Results and the Interpretation of the Molecular Results
Formalin‐fixed, paraffin‐embedded material from the April 2016 biopsy was sent for CGP using another commercial platform, as previously described [16], [17]. The CGP results revealed a microsatellite‐stable, ultramutated tumor with a TMB of 169 mutations per DNA megabase (Table 1). Two missense mutations of the exonuclease domain of POLE (P286R and T323A) were also identified. Notably, despite the biphasic histologic pattern of the carcinosarcoma, our patient shared clinicopathologic features with POLE‐mutant endometrioid endometrial adenocarcinomas, including mutations in PIK3CA, ARID1A, and PTEN; MSI status; increased TILs; and relative insensitivity to platinum agents, suggesting shared biologic mechanisms despite divergent appearance (Fig. 1, Table 2) [18], [19].
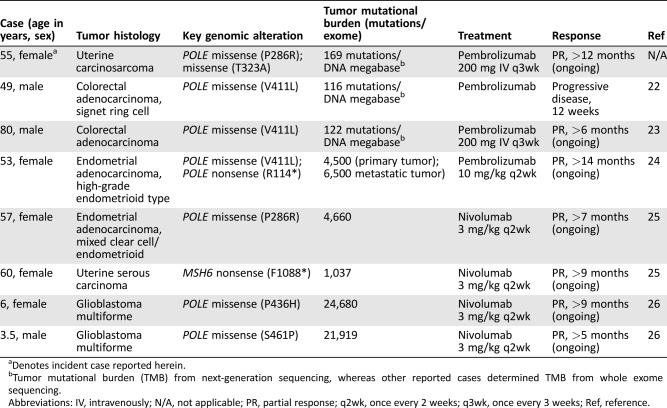
Table 2. Literature review of reported POLE‐mutant, high‐tumor mutational burden, solid tumors with response to immune checkpoint inhibitor therapy.
Denotes incident case reported herein.
Tumor mutational burden (TMB) from next‐generation sequencing, whereas other reported cases determined TMB from whole exome sequencing.
Abbreviations: IV, intravenously; N/A, not applicable; PR, partial response; q2wk, once every 2 weeks; q3wk, once every 3 weeks; Ref, reference.
Potential Strategies to Target the Pathway and Implications for Clinical Practice
POLE‐mutant microsatellite stable (MSS) and MSI‐H endometrial adenocarcinomas have been linked with increased immunogenic mutations [20], [21]. Both POLE‐mutant and MSI‐H endometrial cancers demonstrate enhanced antitumor immune activity with infiltration of T lymphocytes [20], [21], [22], [23], [24]. The histologic appearance of the endometrial tumor is not a reliable predictor of the underlying molecular defect, especially for the POLE‐mutant subgroup, which has been previously reported to show low‐grade or high‐grade endometrioid, serous, or mixed histology. Taken together, these preclinical data provide a scientific rationale for the use of programmed cell death protein 1 (PD‐1) inhibitors among endometrial tumors with a relevant molecular profile, even if they demonstrate uncommon histologic phenotypes. This hypothesis is further supported by several case reports demonstrating significant clinical responses to PD‐1 inhibitors in patients with advanced, recurrent tumors with an ultramutated molecular phenotype (Table 2) [25], [26], [27]. Previously, Castelucci et al. reported two cases of POLE‐mutant colorectal carcinoma with an ultramutated phenotype; one patient experienced partial and durable response to single‐agent pembrolizumab [22]. Our case differs in that the histopathologic classification is uterine carcinosarcoma and expands the description of POLE across tumor types.
As our understanding of the therapeutic relevance of specific genomic features expands, so does the need for more extended molecular assessments. The differing information included in the initial molecular testing (September 2014) and repeat testing (April 2016) likely reflects methodologic differences in testing at the given time points (20‐month interval; Table 1). Although the extent of molecular testing undertaken for this patient in 2014 would be considered beyond standard, it was nonetheless inadequate to identify the elevated tumor mutational burden subsequently identified in 2016. In a 2014 series of 22 gynecologic carcinosarcomas (17 of 22 uterine), two cases harbored oncogenic loss‐of‐function POLE alterations (POLE P286R and V411L); albeit at the time of publication this was not considered clinically actionable, reflecting how our understanding and annotation of genomic information evolves over time [1].
In this case history, the histologic analyses and overlap in genomic alterations identified strongly suggest that both samples shared a clonal origin and that an elevated TMB would have been found in the surgical specimen if this had been assessed on the original test (Fig. 1, Table 1). In fact, genomic profiling may aid in confirming clonality in similar clinical scenarios [28]. Although standard MSI testing captures the nearly 28% of endometrial cancers that are MSI‐H, patients with high TMB and MSS tumors because of POLE‐mediated mechanisms (7%) and others go undetected [11], [21], [25], and thus potentially relevant treatment options remain unexplored. A recent analysis suggests that genomic profiling aids in clinical decision‐making in gynecologic cancers [29].
The observation that histologic evaluation is often subject to interobserver variability and may not be a reliable predictor of biologic behavior is a driving consideration in adding molecular characteristics to the classification system for endometrial tumors. Clinical examples of broad genomic tumor analyses, which identify unexpected oncogenic alterations within an anatomic or histologic subtype that subsequently respond to molecularly paired therapy, are accumulating despite variations of methodology and concerns about temporal and geographic sample heterogeneity [30], [31], [32], [33].
This raises an important question: should all tumors with highly elevated TMB, regardless of histology, be considered for immune‐mediated therapies? We recognize that prospective clinical studies are needed to answer this question, but mounting evidence warrants pan‐cancer study. In fact, the nationwide American Society of Clinical Oncology TAPUR trial (NCT02693535) was recently amended to match pembrolizumab to any tumor with a pathogenic POLE mutation, and an elevated TMB immunotherapy arm is planned as well.
Patient Update
Based on this high TMB, the index patient was treated with pembrolizumab after informed consent discussion. She achieved rapid symptomatic improvement with decreasing lymphedema and an excellent radiographic partial response, with 65% decrease by immune‐related response criteria and 40% by RECIST version 1.1 criteria (Fig. 2) [34], [35]. She had continued pembrolizumab for over 12 months at the time of manuscript submission.
Glossary of Genomic Terms and Nomenclature
Comprehensive genomic profiling: Assay that examines multiple classes of genomic alterations across a broad panel of cancer‐related genes
Missense mutations: A point mutation in which a single nucleotide alteration results in a codon for a different amino acid
Microsatellite instability: Pattern of genetic hypermutability that results from dysfunctional DNA mismatch repair
Nonsynonymous mutation: A nucleotide mutation that results in altered amino acid sequence
Tumor mutational burden: Total number of genetic mutations per coding area of a tumor genome
Ultramutator phenotype: Genetic profile associated with high tumor mutational burden
Contributed equally
Footnotes
For Further Reading: Andrew Sharabi, Sangwoo Shawn Kim, Shumei Kato et al. Exceptional Response to Nivolumab and Stereotactic Body Radiation Therapy (SBRT) in Neuroendocrine Cervical Carcinoma with High Tumor Mutational Burden: Management Considerations from the Center For Personalized Cancer Therapy at UC San Diego Moores Cancer Center. The Oncologist 2017;22:631–637.
Key Points.
- High‐grade, large‐cell neuroendocrine carcinoma of the cervix is an ultra‐rare malignancy that carries a grim prognosis.
- Next‐generation sequencing may reveal key mutations in MSH2 genes amongst others. MSH2 mutations target the DNA mismatch repair process and can predispose patients to malignancies with high mutational burdens.
- Immunotherapy combined with radiation therapy can elicit a significant response, both within and outside the field of radiation. The latter is termed the “abscopal” effect, perhaps mediated by radiation‐induced cross presentation of tumor antigens resulting in immune activation.
- Sequencing of blood‐derived ctDNA showed a high number of alterations, and tissue sequencing confirmed a high tumor mutational burden as a consequence of a mismatch repair gene defect. This observation led to a therapeutic “match” with an anti‐programmed cell death protein 1 antibody combined with SBRT, resulting in a durable (10+ months), near‐complete remission in a patient with advanced chemotherapy‐refractory disease.
Author Contributions
Conception/design: Munveer S. Bhangoo, Peter Boasberg, Samuel J. Klempner
Provision of study material or patients: Pareen Mehta, Julia A. Elvin, Siraj M. Ali, Winnie Wu, Samuel J. Klempner
Collection and/or assembly of data: Munveer S. Bhangoo, Peter Boasberg, Julia A. Elvin, Siraj M. Ali, Samuel J. Klempner
Data analysis and interpretation: Munveer S. Bhangoo, Peter Boasberg, Pareen Mehta, Julia A. Elvin, Siraj M. Ali, Winnie Wu, Samuel J. Klempner
Manuscript writing: Munveer S. Bhangoo, Peter Boasberg, Pareen Mehta, Julia A. Elvin, Siraj M. Ali, Winnie Wu, Samuel J. Klempner
Final approval of manuscript: Munveer S. Bhangoo, Peter Boasberg, Pareen Mehta, Julia A. Elvin, Siraj M. Ali, Winnie Wu, Samuel J. Klempner
Disclosures
Julia A. Elvin: Foundation Medicine (E, OI); Siraj M. Ali: Foundation Medicine (E, OI), Incysus (SAB); Samuel J. Klempner: Foundation Medicine (H), Lilly Oncology (C/A), Boston Biomedical, Leap Therapeutics (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Jones S, Stransky N, McCord CL et al. Genomic analyses of gynaecologic carcinosarcomas reveal frequent mutations in chromatin remodelling genes. Nat Commun 2014;5:5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao S, Bellone S, Lopez S et al. Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial‐mesenchymal transition. Proc Natl Acad Sci USA 2016;113:12238–12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arend R, Doneza JA, Wright JD. Uterine carcinosarcoma. Curr Opin Oncol 2011;23:531–536. [DOI] [PubMed] [Google Scholar]
- 4. Jin Z, Ogata S, Tamura G et al. Carcinosarcomas (malignant mullerian mixed tumors) of the uterus and ovary: A genetic study with special reference to histogenesis. Int J Gynecol Pathol 2003;22:368–373. [DOI] [PubMed] [Google Scholar]
- 5. George EM, Herzog TJ, Neugut AI et al. Carcinosarcoma of the ovary: Natural history, patterns of treatment, and outcome. Gynecol Oncol 2013;131:42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kernochan LE, Garcia RL. Carcinosarcomas (malignant mixed Müllerian tumor) of the uterus: Advances in elucidation of biologic and clinical characteristics. J Natl Compr Canc Netw 2009;7:550–556; quiz 557. [DOI] [PubMed] [Google Scholar]
- 7. Callister M, Ramondetta LM, Jhingran A et al. Malignant mixed Müllerian tumors of the uterus: Analysis of patterns of failure, prognostic factors, and treatment outcome. Int J Radiat Oncol Biol Phys 2004;58:786–796. [DOI] [PubMed] [Google Scholar]
- 8. Galaal K, van der Heijden E, Godfrey K et al. Adjuvant radiotherapy and/or chemotherapy after surgery for uterine carcinosarcoma. Cochrane Database Syst Rev 2013;2:CD006812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Powell MA, Filiaci VL, Rose PG et al. Phase II evaluation of paclitaxel and carboplatin in the treatment of carcinosarcoma of the uterus: A Gynecologic Oncology Group study. J Clin Oncol 2010;28:2727–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lacour RA, Euscher E, Atkinson EN et al. A phase II trial of paclitaxel and carboplatin in women with advanced or recurrent uterine carcinosarcoma. Int J Gynecol Cancer 2011;21:517–522. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research Network ; Kandoth C, Schultz N, Cherniack AD et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Growdon WB, Roussel BN, Scialabba VL et al. Tissue‐specific signatures of activating PIK3CA and RAS mutations in carcinosarcomas of gynecologic origin. Gynecol Oncol 2011;121:212–217. [DOI] [PubMed] [Google Scholar]
- 13. Rosenberg JE, Hoffman‐Censits J, Powles T et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: A single‐arm, multicentre, phase 2 trial. Lancet 2016;387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Le DT, Uram JN, Wang H et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rizvi NA, Hellmann MD, Snyder A et al. Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015;348:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frampton GM, Fichtenholtz A, Otto GA et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013;31:1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hall MJ, Gowen K, Sanford EM et al. Evaluation of microsatellite instability (MSI) status in gastrointestinal (GI) tumor samples tested with comprehensive genomic profiling (CGP). J Clin Oncol 2016;34(suppl 4):528A. [Google Scholar]
- 18. Hussein YR, Weigelt B, Levine DA et al. Clinicopathological analysis of endometrial carcinomas harboring somatic POLE exonuclease domain mutations. Mod Pathol 2015;28:505–514. [DOI] [PubMed] [Google Scholar]
- 19. Bellone S, Bignotti E, Lonardi S et al. Polymerase epsilon (POLE) ultra‐mutation in uterine tumors correlates with T lymphocyte infiltration and increased resistance to platinum‐based chemotherapy in vitro. Gynecol Oncol 2017;144:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Gool IC, Eggink FA, Freeman‐Mills L et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res 2015;21:3347–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Howitt BE, Shukla SA, Sholl LM et al. Association of polymerase e‐mutated and microsatellite‐instable endometrial cancers with neoantigen load, number of tumor‐infiltrating lymphocytes, and expression of PD‐1 and PD‐L1. JAMA Oncol 2015;1:1319–1323. [DOI] [PubMed] [Google Scholar]
- 22. Castellucci E, He T, Goldstein DY et al. DNA polymerase ɛ deficiency leading to an ultramutator phenotype: A novel clinically relevant entity. The Oncologist 2017;22:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gong J, Wang C, Lee PP et al. Response to PD‐1 blockade in microsatellite stable metastatic colorectal cancer harboring a POLE mutation. J Natl Compr Canc Netw 2017;15:142–147. [DOI] [PubMed] [Google Scholar]
- 24. Eggink FA, Van Gool IC, Leary A et al. Immunological profiling of molecularly classified high‐risk endometrial cancers identifies POLE‐mutant and microsatellite unstable carcinomas as candidates for checkpoint inhibition. Oncoimmunology 2017;6:e1264565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehnert JM, Panda A, Zhong H et al. Immune activation and response to pembrolizumab in POLE‐mutant endometrial cancer. J Clin Invest 2016;126:2334–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Santin AD, Bellone S, Buza N et al. Regression of chemotherapy‐resistant polymerase ɛ (POLE) ultra‐mutated and MSH6 hyper‐mutated endometrial tumors with nivolumab. Clin Cancer Res 2016;22:5682–5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bouffet E, Larouche V, Campbell BB et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol 2016;34:2206–2211. [DOI] [PubMed] [Google Scholar]
- 28. Klempner SJ, Ou SH, Costa DB et al. The clinical use of genomic profiling to distinguish intrapulmonary metastases from synchronous primaries in non‐small‐cell lung cancer: A mini‐review. Clin Lung Cancer 2015;16:334–339.e1. [DOI] [PubMed] [Google Scholar]
- 29. Rodriguez‐Rodriguez L, Hirshfield KM, Rojas V et al. Use of comprehensive genomic profiling to direct point‐of‐care management of patients with gynecologic cancers. Gynecol Oncol 2016;141:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klempner SJ, Gershenhorn B, Tran P et al. BRAFV600E mutations in high‐grade colorectal neuroendocrine tumors may predict responsiveness to BRAF‐MEK combination therapy. Cancer Discov 2016;6:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim ST, Kim SY, Klempner SJ et al. Rapamycin‐insensitive companion of mTOR (RICTOR) amplification defines a subset of advanced gastric cancer and is sensitive to AZD2014‐mediated mTORC1/2 inhibition. Ann Oncol 2017;28:547–554. [DOI] [PubMed] [Google Scholar]
- 32. Planchard D, Besse B, Groen HJM et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)‐mutant metastatic non‐small cell lung cancer: An open‐label, multicentre phase 2 trial. Lancet Oncol 2016;17:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwaederle M, Zhao M, Lee JJ et al. Association of biomarker‐based treatment strategies with response rates and progression‐free survival in refractory malignant neoplasms: A meta‐analysis. JAMA Oncol 2016;2:1452–1459. [DOI] [PubMed] [Google Scholar]
- 34. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 35. Wolchok JD, Hoos A, O'Day S et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune‐related response criteria. Clin Cancer Res 2009;15:7412–7420. [DOI] [PubMed] [Google Scholar]