Abstract
There remains a large unmet need for new therapies in the treatment of heart failure with reduced ejection fraction (HFrEF). In the early drug development phase, the therapeutic potential of a drug is not yet fully understood and trial endpoints other than mortality are needed to guide drug development decisions. While a true surrogate marker for mortality in heart failure (HF) remains elusive, the successes and failures of previous trials can reveal markers that support clinical Go/NoGo decisions.
Pathophysiologically, HF is defined as the inability of the heart to provide adequate perfusion to the body and its tissues. Clinically, HF is defined as a syndrome with typical signs (pulmonary congestion, peripheral edema, and elevated jugular venous pressure) and symptoms (dyspnea, ankle swelling, and fatigue), which result from abnormal cardiac structure or function.1, 2 HF is a highly prevalent disease, affecting 5.8 million people in the USA and nearly 15 million in Europe.3, 4 The symptoms and the need for frequent hospitalizations in HF cause a significant burden on individual patients. At the same time, HF is a major public health concern due to the need for frequent and intense healthcare resource utilization.1, 2, 4, 5 Further, despite the use of currently available therapies, the prognosis of patients with HF is considerably poor, with 5‐year survival rates of 50%, a prognosis even worse than that of patients with advanced cancer or stroke.6
In HF, echocardiography has traditionally been used to quantify left ventricular ejection fraction (EF; derived as stroke volume/end‐diastolic volume) which is then used to define two types of patient populations: patients with HF and reduced EF (HFrEF) and those with HF and preserved EF (HFpEF). The distinction is not only important because EF constitutes an important prognostic factor, but also because patients with HFpEF seem to respond differently to available therapies than patients with HFrEF.7, 8 Currently, there are no approved drugs for treatment of HFpEF. All major trials on new drugs in patients with HF were conducted in patients with HFrEF (mainly EF ≤35%).1, 2 For the purpose of this review, the authors focused on the chronic HFrEF population.
Our review aims to define a set of criteria and parameters that can be used to support Go/NoGo decisions during early clinical development of new molecular entities (NME) for chronic HFrEF. We aimed to identify factors with a positive predictive value for early development by analyzing clinical parameters and biomarkers during the initial phase of the development for NMEs that achieved a clinical benefit (usually morbidity and mortality) during their confirmatory part of drug development. As the potential HF treatments are directed towards specific therapeutic targets right from the beginning, the traditional and often‐used pathway to expand a proven cardiovascular therapy approved for an existing indication (e.g., hypertension) is not covered in this review. Further, it is well understood that encouraging early clinical, functional and biomarker data supporting a Go decision are followed by investigation of the dose–response of beneficial effects and the compatibility with other state‐of‐the‐art HF therapies and constitute an equally important next milestone.9, 10, 11
CURRENT PHARMACOTHERAPY FOR HEART FAILURE
The goals of treatment in patients with HF are to improve signs and symptoms and quality of life (QoL), prevent hospital readmissions, and reduce mortality rates. For the technical success of new drugs (regulatory approval) as well as the commercial success of a compound, evidence of reduction of mortality is unquestionably the most desirable objective.
Current pharmacotherapy for HFrEF mainly includes drugs that modify (or at least attenuate) the disease process and prolong survival, and those that only ameliorate the clinical signs and symptoms of HF. The first group of drugs that are guideline‐recommended treatments with consistently proven long‐term benefits12 include 1) blockers of the renin‐angiotensin‐aldosterone system (RAAS) such as angiotensin converting enzyme inhibitors (ACEI), angiotensin II receptor type 1 (AT1) antagonists (ARBs), and mineralocorticoid receptor antagonists (MRAs); 2) blockers of the adrenergic system, namely, beta adrenoceptor blockers (BBs); 3) the angiotensin receptor‐neprilysin inhibitor (ARNI); and 4) hydralazine and isosorbide dinitrate (in self‐identified African‐American patients or in those intolerant to ACEI and ARB). Sacubitril/valsartan (previously known as LCZ696), the first‐in‐class ARNI, simultaneously inhibits neprilysin, an enzyme responsible for degradation of several vasoactive peptides including natriuretic peptides, and blocks the AT1 receptor. It is the most recent addition to the armamentarium of HFrEF drugs and was more effective than enalapril in reducing morbidity and mortality in patients with HFrEF in the PARADIGM‐HF trial.13 Other drugs such as diuretics, digoxin, and nitrates are used for symptomatic relief in patients with HFrEF without any demonstrated mortality benefit.1 An overview of established therapies in HFrEF is presented in Table 1.
Table 1.
Modes of action of currently available therapies for HFrEF
| Drugs with proven morbidity or mortality benefit | Drugs with symptomatic benefit but no proven mortality benefit | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Class/ molecular mode of action | Angiotensin‐converting enzyme inhibitors | Angiotensin II receptor type 1 antagonists | Beta‐1‐adrenoceptor antagonists/ blockers | Mineralo‐corticoid receptor antagonists | Angiotensin receptor‐neprilysin (neutral endopeptidase) inhibitor | Combination of isosorbide dinitrate (ISDN) and hydralazinea | Na+/K+ ATPase inhibitors | Inhibition of water/sodium reabsorption in the kidney | Organic nitric oxide donors | If blockers |
| Class short name | ACEI | AT1‐RA/ ARB | BB | MRA | ARNI | Nitrate plus direct arterial vasodilator | Digitalis | Natriuretics/ Diuretics | Nitrates | Selective sinus node inhibitor |
| Postulated mechanism in HF | Inhibition of the detrimental long‐term effects of RAAS activation | Blockade of detrimental long‐term effects of elevated angiotensin II levels | Reduced sympathetic nervous system activity, reduced renin production and release | Synergistic hemodynamic and natriuretic effects with ACEIs, reduced RAAS effects via reduction of tissue ACE activity and the AT1 receptor density | Simultaneous inhibition of angiotensin II via AT1 blockade and increase of beneficial vasoactive substrates including NPs via inhibition of neprilysin | Combination of preferential venous pooling with subsequent pre‐load reduction through ISDN and afterload reduction via direct vasodilation through hydralazine | Positive inotrope through increase of intracellular Ca2+ in cardiomyocytes | Increased excretion of sodium and water to reduce congestion and decrease preload to heart | Increased availability of NO, leads to afterload and preferential preload reduction, venous pooling to reduce congestion and volume overload of the heart | Inhibition of a selective sinoatrial pacemaker current reduces heart rate |
| Examples | Captopril, Enalapril, Lisinopril, Ramipril | Losartan, Olmesartan, Candesartan, Valsartan | Metoprolol, Bisoprolol, Nebivolol, Carvedilol | Spironolactone Eplerenone | Sacubitril/ valsartan | BiDila | Digoxin, Digitoxin | Furosemide Torasemide, Hydrochloro‐thiazide, Indapamide | Glyceryl trinitrate, Isosorbide‐mononitrate | Ivabradine |
ACE, angiotensin converting enzyme; ACEI, angiotensin converting enzyme inhibitor; ARNI, angiotensin receptor‐neprilysin inhibitor; ATI, angiotensin II receptor type 1; BB, beta‐blockers; HFrEF, heart failure and reduced ejection fraction; MRA, mineralocorticoid receptor antagonist; NO, nitric oxide; NP, natriuretic peptide; RA/ARB, renin‐angiotensin/angiotensin receptor blocker; RAAS: renin‐angiotensin‐aldosterone system; NEP, neutral endopeptidase/neprilysin.
Efficacy demonstrated for self‐identified African‐Americans in the A‐HeFT study.34
EVOLUTION OF PATHOPHYSIOLOGICAL CONCEPTS IN HEART FAILURE AND NEW THERAPEUTIC STRATEGIES
In the 1980s, HFrEF was considered primarily a hemodynamic disorder, and therapeutic interventions that acutely improved pump function were believed to provide long‐term clinical benefit. Therefore, the therapeutic goal was to increase cardiac output (CO) by either increasing contractility or reducing peripheral resistance using vasodilators.14 This concept was questioned when a number of controlled clinical trials conducted in the 1990s showed that drugs that improved hemodynamics did not necessarily show long‐term clinical benefits with regard to reduction in mortality.15, 16
These observations and the dramatic clinical effects with ACEIs and BBs in large interventional studies caused a paradigm shift in the pathophysiological concepts of HFrEF.17, 18, 19, 20 Until recently, blockers of the neurohormonal systems (RAAS and adrenergic system) were considered the most effective therapeutic options.2 Data from the recently completed PARADIGM‐HF study comparing sacubitril/valsartan with enalapril provided evidence that in addition to blocking the detrimental effects related to sustained RAAS activation, inhibition of neprilysin and subsequent enhancement of beneficial effects of vasoactive peptides further reduced morbidity and mortality in patients with HFrEF.13
An overview of therapeutic principles tested as potentially new medicines for HFrEF are presented in Table 2. Despite encouraging primary pharmacology data and pathophysiological fit, research on these therapeutic principles had to be discontinued, or their use restricted to the symptomatic management of patients with acute HF because of the lack of efficacy or safety concerns. Drugs currently being evaluated in ongoing studies have been discussed elsewhere and are not part of this review.21
Table 2.
Drugs that showed encouraging results in the early phase of clinical heart failure trials
| Class | Molecules | Class/molecular mode of action | Postulated mechanism in HF | Clinical effects, safety/efficacy in HF |
|---|---|---|---|---|
| Endothelin receptor antagonists (ETRAs) | Bosentan, Darusentan, Tezosentan | Bosentan, Tezosentan: mixed endothelin‐1 (ET1) A/B receptor antagonists Darusentan: selective ET1A receptor antagonist | Antagonism of ET1‐A receptors to functionally antagonize the vasoconstrictor effects of ET Agonists of ET1‐B receptors leads to vasodilatation via release of NO and prostacyclin | Despite pharmacological differences between ET1A‐selective (Darusentan) and mixed ETRAs (Bosentan, Tezosentan), none of them could reduce morbidity or mortality rates in mid‐sized dose‐finding trials on HF35, 36, 37 |
| Vasopressin receptor antagonist (VRAs, vaptans) | Tolvaptan, Conivaptan, Lixivaptan | VRAs block the vasoconstriction caused by VP and block renal water reabsorption. They are vasodilatory and aquaretic in nature | Decrease PCWP and RAP, increase water excretion |
Tolvaptan: • EVEREST trial: neither positive nor negative effect on all‐cause mortality or combined endpoint of CV mortality or subsequent hospitalization for worsening HF38 • METEOR study: No effects on LVEF or volumes39 Conivaptan: Lowering of PCWP, RAP, and PAP after a single dose.40 No data on the long‐term use on HF patients available Lixivaptan: No trial on morbidity and mortality conducted |
| Soluble guanylyl‐cyclase modulators (sGC modulators) | Cinaciguat, Vericiguat | Activate sGC, increase levels of soluble cGMP and lead to vasodilatation | Reduce BP, PAP, PCWP, increase CO, preserve GFR41 |
Cinaciguat:
• Reduced BP, PCWP, PVR, and increased CI. Reduction in BP led in part to severe hypotension42 • COMPOSE program (consisting of 3 independent RCTs): BP reductions without improvement in dyspnea or CI. Program was prematurely stopped43 Vericiguat: • SOCRATES‐REDUCED trial: Patients who received vericiguat did not meet the primary endpoint of lowering NT‐proBNP levels44 |
| Prostacyclin analogs (prostanoids) | Epoprostenol | Prostanoids are direct vasodilators of pulmonary and systemic arterial vascular beds, inhibit platelet aggregation | Increase CI, decrease PAP, RAP, PVR | Epoprostenol: Reduced PCWP and SVR and increased CI. The trial was prematurely terminated owing to trend for lower survival rate with epoprostenol15 |
| Calcium sensitizers | Levosimendan, Pimobendan | Inodilators, i.e., combination of calcium sensitization (positive inotrope without affecting calcium transient) and vasodilatation via PDE3 inhibition | Inodilators are positive inotropic agents, reduce preload/afterload, and increase coronary and organ blood flow |
Levosimendan: Despite reductions in BNP levels and mortality benefits suggested by several meta‐analyses,45, 46 RCTs with levosimendan either indicated an increased risk of adverse cardiovascular events47 or were neutral48
Pimobendan: The PICO trial showed increased exercise capacity but increased mortality rate after treatment 49 |
| Natriuretic peptides (BNP analogues) | Recombinant BNP, Nesiritide | BNP is the endogenous ligand for natriuretic peptide‐A receptors, stimulation of GC leads to increased cGMP | Body's physiological reaction to pressure or volume overload. Vasodilatation in venous and arterial beds |
Nesiritide:
• ASCEND‐HF and the FUSION II trial did not demonstrate a reduction of mortality rates. • No indication of increased CV risk, supporting its use in the treatment of patients with acute decompensated HF who have dyspnea at rest or with minimal activity50, 51 |
| Phosphodiesterase 3‐ inhibitor (PDE3I) | Amrinone Milrinone | PDE3I prevents degradation of cAMP to AMP, cAMP stimulates PKA which provides vasodilatation, increases intracellular calcium (positive inotropic), and activates SERCA (positive lusitropic) |
Amrinone: Reduction in cardiac afterload, increase in CO, reduction in left ventricular filling pressure, no changes in BP and HR Milrinone: Increases CI, reduces PCWP52 |
Amrinone: • Beneficial acute hemodynamic effects were not reproducible after 12‐weeks of administration. No change in NYHA class, LVEF, and mortality rates. Adverse events (nausea, vomiting, and diarrhea) with amrinone were frequent and led to need for treatment down‐titration or discontinuation53 Milrinone: • Despite beneficial hemodynamic effects, long‐term use was associated with increased frequency of ventricular arrhythmias and reduced survival duration.16, 54 Milrinone is approved for short‐term IV use in acute decompensated HF |
| Partial PDE3 inhibitor, an ion‐channel modifier | Vesnarinone |
Complex mechanism of action with following components: ‐ Weak PDE3 inhibition ‐ Prolongation of action potential duration ‐ Increases intracellular sodium and calcium concentrations ‐Inhibition of cytokine production |
Increases CI, reduced PCWP, increases exercise capacity | Vesnarinone was associated with a dose‐dependent increase in mortality in chronic HF patients55 |
AMP, adenosine monophosphate; BP, blood pressure; cAMP, cyclic adenosine monophosphate; BP, blood pressure; BNP, B‐type natriuretic peptide; cGMP, cyclic guanosine monophosphate; CHF, chronic heart failure; CI, cardiac index; CO, cardiac output; CV, cardiovascular; ET, endothelin; ETRA, endothelin receptor antagonist; EVEREST, Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan; FUSION, Follow‐Up Serial Infusions of Nesiritide; GC, guanylyl cyclase; GFR, glomerular filtration rate; HF, heart failure; HR, heart rate; IV, intravenous; LVEF, left ventricular ejection fraction; METEOR, Multicenter Evaluation of Tolvaptan Effect On Remodeling; NO, nitric oxide; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; NYHA, New York Heart Association; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PDE, phosphodiesterase; PDE3I, PDE3 inhibitor; PKA, protein kinase A; PICO, Pimobendan in Congestive Heart Failure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RCT, randomized controlled trial; REVIVE, Randomized EValuation of Intravenous LeVosimendan Efficacy; sGC, soluble guanylyl cyclase; SOCRATES‐REDUCED, The Soluble Guanylate Cyclase Stimulator in Heart Failure with Reduced Ejection Fraction Study; SURVIVE, The Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support; SVR, systemic vascular resistance; VRA, vasopressin receptor antagonist; VP, vasopressin; SERCA, sarco/endoplasmic reticulum Ca2+‐ATPase.
IMPLICATIONS OF MORTALITY AS THE PRIMARY REGISTRATION ENDPOINT
As HFrEF is a life‐threatening disease, mortality is regarded as the most important single endpoint in phase III studies for evaluation of new drugs for HF. Drugs with other beneficial effects might be approved by regulatory authorities but only under the condition that excess mortality can be excluded. In these cases, mortality (usually all‐cause mortality) helps to assess the drug's safety profile.
Although mortality is an important and easily measurable endpoint, it has several limitations. The main concern of using only mortality as an endpoint in clinical trials is that it refers to the terminal manifestation of HFrEF. Thus, in an outcome study many patients may not contribute to the mortality endpoint, but may have a significantly impaired QoL. Further, the current management of HFrEF with ACEIs/ARBs, BBs, and MRAs has reduced mortality considerably.1, 2 Therefore, if mortality is the primary endpoint, patients with advanced disease have to be enrolled in trials to accrue enough mortality events to provide adequate statistical power within a reasonable period of time. The need to demonstrate a mortality benefit makes it difficult to validate the benefit of a new medication in patients with less pronounced HFrEF or in those with severe HF. Finally, trials in which mortality is the primary endpoint require a large sample size to show a survival benefit of a new drug. Therefore, to evaluate preventative strategies using mortality as an endpoint, large and long‐term phase III clinical trials are needed. Parameters, indices, or biomarkers that are indicative of the potential safety, morbidity, and mortality benefits (or the lack of it) of a new drug have emerged as valuable tools. Such biomarkers are increasingly being recognized as endpoints during the early stages of clinical development (phase I/II) to critically evaluate the developmental path of a new drug.
BIOMARKERS, CLINICAL ENDPOINTS, AND SCORES IN HEART FAILURE
Heerspink et al.22 recently suggested the parameter response efficacy (PRE) score that reflects potential long‐term cardiovascular (CV) or renal benefits and risks and can be used to assess new drugs for a broad range of CV indications. To date, the most frequently used scores for the clinical assessment of the disease stage of patients with HFrEF include the New York Heart Association (NYHA) classification that reflects severity of symptoms and exercise intolerance as assessed by the physician, the Minnesota Living with Heart Failure Questionnaire (MLHFQ), and the Kansas City Cardiomyopathy Questionnaire (KCCQ) that represents how the patient feels.2, 23, 24 Past research has focused on more objective, sensitive, and better quantifiable parameters of disease severity and prognosis. Presently, the spectrum of available measures includes:
Hemodynamic factors: blood pressure (BP), heart rate (HR), cardiac output (CO), systemic vascular resistance (SVR), and pulmonary capillary wedge pressure (PCWP).
Autonomic nervous system markers: heart rate variability, baroreceptor sensitivity, and ventricular repolarization characteristics (QT‐dispersion).
Exercise capacity: 6‐min walk test, treadmill or cycle exercise testing, and spiroergometry.
Soluble biomarkers/neurohormones: norepinephrine, epinephrine, and natriuretic peptides (atrial and brain natriuretic peptides) and high‐sensitivity troponin.
Cardiac imaging indices: left ventricular EF (LVEF) and left ventricular end‐systolic and end‐diastolic pressures (LVESP and LVEDP).
The majority of the parameters and markers mentioned above have been used for clinical purposes spanning from the confirmation of diagnosis of (clinically asymptomatic) HF to the classification of the patients' clinical status and assessment of prognosis. Despite the large number of parameters and indices available, none of them fulfill the hard criteria of a real surrogate, i.e., a biomarker that can replace or predict a real clinical endpoint.25 Nevertheless, experience from previous clinical trials provide useful and relevant information on these markers and their use for early drug development. Three examples may illustrate the value of these markers:
-
1)
LVEF: Changes in LVEF >5% from baseline at 6 months (V‐HeFT I) and 12 months (V‐HEFT II) were found to be strong predictors of mortality.26 No contradictory observations have been published so far.
-
2)
Hemodynamics: All drugs approved for the treatment of HF have long‐term beneficial hemodynamic effects. Nevertheless, a number of controlled clinical trials conducted in the 1990s have shown that drugs that produce striking hemodynamic benefits do not necessarily produce long‐term clinical benefits.15, 16 These findings discouraged the use of hemodynamic variables as surrogate markers for predicting drug efficacy. However, the converse is not true: there are no drugs that worsen hemodynamics and improve long‐term outcomes.
-
3)
Six‐minute walk test: Although the 6‐min walk test was found to predict long‐term mortality and hospitalization rates in patients with left ventricular dysfunction of varying causes and severity,27 other researchers could not confirm these findings.28
Based on the predictive value, these markers can be classified in two categories: 1) those that have positive predictive value (“positive parameter or index”), i.e., a positive/beneficial effect can be inferred from them (indicating potential Go criteria), and 2) those that have a negative predictive value (providing potential NoGo criteria), i.e., an effect on this marker would suggest detrimental effects in humans. It is noteworthy that some parameters or indices (such as hemodynamics, CO, PCWP) that are strong predictors of mortality are not good at predicting therapeutic response.
The value and limitations of parameters or indices for dose‐range finding, target engagement, clinical proof of concept, and decision‐making regarding further clinical development have been extensively discussed in the literature.25, 29 Based on general and HFrEF‐specific knowledge on biomarkers, parameters, or indices, it can be concluded that early drug development in HFrEF should focus on ruling out valid NoGo criteria and confirming target engagement and proof of concept. Therefore, we propose an approach that includes defining NoGo criteria that must be ruled out to justify further progression of clinical development, and assessing predictive biomarkers to estimate potential clinical benefits. Figures 1 and 2 illustrate two theoretical outcomes of an early clinical development program. The figures demonstrate that even in the case of evidence of positive pharmacodynamic effects of a drug candidate, the fact that one NoGo criterion has been met may question the whole therapeutic approach and result in discontinuing further clinical development.
Figure 1.

An ideal case suggesting a Go decision.
Figure 2.
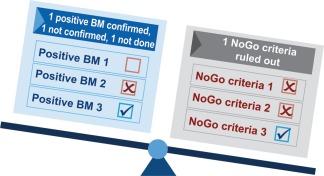
A case suggesting a NoGo decision.
IMPLEMENTATION OF EXPLORATORY CLINICAL DEVELOPMENT PRINCIPLES
Considering the complexity, large size, long treatment duration, and substantial costs of registrational clinical trials assessing morbidity and mortality in patients with HFrEF,13, 30 it is evident that smaller studies are required at earlier development stages to enable well‐informed decisions to either continue development of the new drug or to abandon the program. Due to the smaller size of these early trials, it is not possible to examine the hard endpoints of the registrational trials like morbidity and mortality. Instead, smaller trials will need to look at several markers as indicators of potential efficacy (Go criteria) as well as markers that are indicators of potentially deleterious effects (NoGo criteria).
The MOCHA study is an example of how assessments in a smaller trial of shorter duration can de‐risk the decision to advance a drug candidate into full development.31 In this 6‐month study, carvedilol, the study drug, had no effect on the exercise tolerance test in 350 patients but improved left ventricular function and decreased mortality and hospitalization rates. However, Krum et al.32 identified several beneficial effects with carvedilol therapy in a pilot study with 56 patients with severe HF. In this study all parameters, including symptom scores, functional parameters (6‐min walk test), hemodynamics (stroke volume index, LVEF, pulmonary artery pressure, PCWP, right atrial pressure, and SVR), and preliminary major CV events were all in favor of the intervention.
Based on the above information, it becomes evident that during the early clinical program for development of HFrEF drugs, a number of critical assessments should be performed until a certain level of confidence can be attained that might justify undertaking a trial similar to the MOCHA trial. In general, however, it is important to note that successful drugs will not necessarily always meet all Go criteria but still be viable candidates for phase III development. In contrast, meeting any NoGo criteria clearly raises concerns on the overall benefit, safety, and utility of the therapeutic approach.
Currently, we can identify the following NoGo criteria that must be ruled out by a new drug candidate:
Increase in sympathetic tone, including HR and epinephrine levels.
Activation (reflex) of the RAAS (increase in renin, angiotensin II, aldosterone), provided the mechanism of action of the drug candidate does not involve RAAS inhibition.
Unfavorable effects on hemodynamic parameters (increase in LVEDP, PCWP, pulmonary vascular resistance, or decrease in CO).
Adverse changes in cardiac structure and function and in biomarkers linked to deleterious cardiovascular effects.
Additional parameters or indices may suggest beneficial clinical effects and can help to determine the pharmacodynamically active doses for later clinical studies. These include a mid‐ to long‐term increase in EF.31, 32 Furthermore, circulating biomarkers such as natriuretic peptides, midregional pro‐adrenomedullin (MR‐proADM), ST2, copeptin, galectin 3, and others have been studied for their value in diagnosing HFrEF, determining prognosis, and guiding therapy. However, only BNP, NT‐proBNP, and hs‐TnT have been investigated convincingly in the context of drug development and are currently recommended to be evaluated early in a development program if a new drug candidate has the potential to modify clinically relevant endpoints such as mortality.33 It should be noted, however, that neither of these biomarkers are validated surrogates. Based on the learnings summarized in Table 2 and the above considerations, we propose one set of criteria that might indicate future clinical success and another set that might be a signal for clinical safety concern. Table 3 provides an overview of these criteria and summarizes experience from previous drug development programs. These data allow the derivation of Go/NoGo criteria, which are summarized in Table 4. Importantly, it is recommended to evaluate potential NoGo criteria related to unfavorable hemodynamic effects, sympathetic or neurohormonal activation, LV function, and proarrhythmic potential early on and as appropriate. If the drug candidate does not meet any potential NoGo criteria and is otherwise safe and well tolerated, evidence suggesting a favorable effect on pharmacodynamic endpoints will support a decision to progress a development program. Go criteria should be viewed in the context of the mode of action of the drug candidate, and Table 4 provides examples for potential criteria for which sufficient evidence was available in the literature. These criteria will need to be adapted to a specific compound under development (e.g., BNP as a neprilysin substrate should not be evaluated in the context of predicting response when neprilysin inhibitors are evaluated). The current literature does not support thresholds for Go or NoGo criteria for all biomarkers listed in Table 4, and there are biomarker changes that can neither be attributed to the Go or NoGo category. The magnitude of the effect of the Go criteria will influence decisions to move ahead with a particular project. At the same time, the precise quantum of the change required to drive organizational decision‐making will depend on the particular organization's set‐up, which includes, among other aspects, their R&D model and culture. Continuous evaluation of ongoing development programs may help to close some of these knowledge gaps in the future.
Table 3.
Overview of indicators of early safety and efficacy from successful drug development programs
| Parameter | Description | Rationale | Trial (Change/ Time) |
|---|---|---|---|
| Ejection Fraction | EF is the percentage of volume ejected during systole (SV) divided by the volume remaining at the end of diastole (end‐diastolic volume) | Reduced EF is a pathognomonic sign of HFrEF and a prognostic marker associated with worse outcome. Improvement of EF is expected to increase the efficiency of the heart, leading to unloading of the heart (with reductions in sympathetic‐adrenergic drive) and lower heart rate due to increase in SV (prolonging diastole and thus improving energetic balance for the myocardium). Drugs that improved morbidity and mortality rates (ACEIs BBs, and MRAs) in clinical trials increased EF |
Enalapril, +3% at 12 weeks56
ACEI in conjunction with nitrates, +7–10% at 2.7±2 years57 Carvedilol, dose‐dependent increase of up to 5% at 6 months (MOCHA trial) 31 Metoprolol, +0.3% at 12 weeks58 MRAs, +1.2–6 % MRAs including spironolactone, eplerenone, canrenone, +3.2% at 7.3±3.5 months (meta‐analysis of 14 RCTs) 59Valsartan, +4% at 27 months (Val‐HeFT trial) 30 |
| Systemic vascular resistance | SVR refers to the resistance to blood flow in the systemic circulation | An increase in SVR contributes to an increased afterload on the ventricle that leads to adverse ventricular remodeling |
Many drugs for HF have vasodilatory properties (e.g., ACEIs, ARBs and nitrates) and have demonstrated reductions in SVR An increase in SVR would be of concern60 |
| Blood pressure | BP is a function of SVR and cardiac output | An increase in BP contributes to an increased afterload on the ventricle that leads to adverse ventricular and vascular remodeling |
Many drugs for HF have anti‐hypertensive properties (e.g., ACEIs, ARBs, BBs, diuretics, and nitrates) An increase in BP would be of concern |
| BNP/NT‐proBNP | The biologically active natriuretic peptide BNP and its inactive precursor NT‐proBNP are released upon increase in myocardial wall stress or stretch. BNP is eliminated by NEP‐mediated degradation and renal clearance; NT‐proBNP is predominantly renally eliminated. Therefore, BNP needs to be interpreted with caution in patients treated with an angiotensin receptor‐ neprilysin inhibitor (ARNI; e.g., sacubitril/valsartan). |
BNP/NT‐proBNP are utilized to diagnose heart failure. BNP values at hospital admission for HF, at hospital discharge and serial changes have been shown to predict HF morbidity and mortality. In addition, guiding HF therapy according to BNP/NT‐proBNP values is associated with lower cardiovascular events, in particular in elderly patients. Drugs that improve morbidity and mortality rates have been shown to reduce NT‐proBNP levels |
Decrease in BNP by 25% and in NT‐proBNP by 40% are considered to be biologically meaningful61, 62
ARNI: Sacubitril/valsartan (NT‐proBNP, change from baseline): PARADIGM‐HF63 −32% at 4 weeks after randomization −35% at 8 months after randomization Short term assessment64 −47% at 1 week −44% at 3 weeks RAAS inhibition: 1. Enalapril (NT‐proBNP, change from baseline): PARADIGM‐HF63 −8% at 4 weeks after randomization −13% at 8 months after randomization CARMEN65 −29% (BNP) at 6 months −34% (NT‐proBNP) at 6 months 2. Valsartan (BNP, change from baseline): Val‐HeFT66 −19% at 4 months −13% at 12 months −9% at 24 months Beta‐blockade: Inconsistent results, but most BBs reduce BNP/NT‐proBNP 1. Carvedilol: COPERNICUS67 No difference in median NT‐proBNP compared to placebo, but nearly −15% at 3 months and nearly −25% at 6 months after uptitration from individual baseline values CARMEN65 +14% at 6 months (BNP) +19% at 6 months (NT‐proBNP) 2. Atenolol (NT‐proBNP change from baseline) 68 −29% at 6 months −31% at 12 months −38% at 24 months 3. Metoprolol and Carvedilol (NT‐proBNP change from baseline) 69 ~ −35% at 12 weeks ~ −45% at 52 weeks No difference between BBs; values estimated for combined group 4. Metoprolol and Carvedilol: COMET trial showed NT‐proBNP reduction to <400pg/mL subsequent to treatment with a BB resulted in lower mortality (RR 0.32) 70 5. Metoprolol (BNP, change from baseline; RESOLVD‐Pilot study): +27% at 24 weeks71 MRA: Spironolactone (change in BNP) −23% change at 3 and 6 months from baseline (RALES) 72 −32% over placebo at 3 months73 approximately 50 pg/mL mean decrease at 3 months from baseline74 Metanalysis of MRAs (n = 3,929): −37 pg/mL in BNP with MRAs vs. control75 |
| hs‐TnT | Increased levels of cardiac troponins are indicative of cardiomyocyte injury. Proposed causes include myocardial ischemia, toxicity of neurohormones, cytokines or oxidation byproducts, or release due to apoptosis or increased cell permeability resulting from increased ventricular wall stress 76 |
In chronic heart failure, hs‐TnT is detectable in more than 90% of patients, of whom 50% present with elevated hs‐TnT indicative of ongoing myocardial injury66, 76
Elevated levels of circulating hs‐TnT are independently associated with clinical events and worse outcome |
In stable HF, persistent serial hsTnT ≥0.01 ng/mL over 1 year was associated with an increased risk of events (OR 3.77) in the following year77
Change in hs‐TnT with drugs: Sacubitril/valsartan (PARADIGM‐HF) 63 −10% at 4 weeks after randomization −9% at 8 months after randomization Enalapril (PARADIGM‐HF) 63 −2% at 4 weeks after randomization +1.5% at 8 months after randomization |
| Heart rate increase | Epidemiologically higher HR is associated with increased all‐cause mortality. HR increase is a predictor of worse outcome in HFrEF |
Increased HR leads to increased oxygen demand, shortened diastolic relaxation, and suboptimal ventricular filling Decrease in HR prolongs the diastolic relaxation phase, improves diastolic filling, and reduces oxygen consumption |
Based on data from the Copenhagen Male Study from SHIFT, a 2–5 bpm difference seems to be relevant78, 79 |
| Pulmonary capillary wedge pressure | PCWP is used clinically as a surrogate of left atrial filling pressures | Increases in PCWP suggest increased filling pressures indicative of abnormal strain on the heart | Any increase in PCWP32, 60 |
| Proarrhythmic potential | Sudden cardiac arrest is a leading cause of mortality in patients with HF | A drug with proarrhythmic potential would be contraindicated in patients with HF who are at increased risk of sudden cardiac arrest | Any evidence of proarrhythmic potential54, 80 |
| Plasma epinephrine | One of two catecholamines (norepinephrine is the other one) that mediate the sympathetic nervous system | Increased plasma epinephrine levels are indicative of increased sympathetic activation that is maladaptive in patients with heart failure | Any evidence of sympathetic nervous system activation |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor‐neprilysin inhibitor; BB, beta‐blocker; BP, blood pressure; bpm, beats per minute; BNP, B‐type natriuretic peptide; EF, ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; hs‐TnT, high sensitivity troponin T; MRA, mineralocorticoid receptor antagonist; NEP, neutral endopeptidase/neprilysin; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; OR, odds ratio; PCWP, pulmonary capillary wedge pressure; RAAS, renin‐angiotensin aldosterone system; RCT, randomized controlled trial; RR, relative risk; SV, stroke volume; SVR, systemic vascular resistance.
Table 4.
Potential Go/NoGo criteria to consider during early drug development
| Biomarker | Go criteria | NoGo criteria |
|---|---|---|
| Ejection fraction | Increase | Any significant decrease |
| Systemic vascular resistance | Not identified | Any significant increase |
| Blood pressure | Not identified | Any significant increase |
| BNP/NT‐proBNP | Decrease in dependence of baseline value and mode of action | Any significant increase if not related to mode of action (e.g., neprilysin inhibition) |
| hsTnT | Decrease | Any significant increase |
| Heart rate | Decrease | Sustained increase |
| PCWP | Not identified | Any significant increase |
| Proarrhythmic potential | Not identified | Any evidence of proarrhythmic potential |
| Epinephrine | Not identified | Any significant increase |
BNP, B‐type natriuretic peptide; bpm, beats per minute; hsTnT, high sensitivity troponin T; NT‐proBNP, N‐terminal proBNP; PCWP, pulmonary capillary wedge pressure.
CONCLUSION
Based on current knowledge, we conclude that the exploratory clinical development of new drugs for HFrEF should be done with a balanced approach: a number of predefined NoGo criteria that should be ruled out, and a (smaller) number of parameters, indices, or biomarkers indicative of a clinical benefit (Go criteria) should be determined. With this approach, the transition of a clinical HFrEF project from one stage of development to the next is still associated with considerable development risk. However, individual decisions to expand from smaller and less predictive studies to larger, more costly, but more predictive studies will be based on clinical data and reflect the currently available development knowledge in HFrEF. Because of the complexity of the matter and the relatively large number of different effects that have to be proven and excluded, the implementation of these principles will have to be different for each individual drug candidate.
FUNDING
All authors are employees of Novartis. The editorial support for this article was provided by Roohi Chopra, Novartis.
CONFLICT OF INTEREST
M.H., B.A.Y., and T.H.L are employees of Novartis.
AUTHOR CONTRIBUTIONS
M.H., B.A.Y., and T.H.L. wrote the article. M.H. designed the research; M.H., B.A.Y., and T.H.L. performed the research; M.H., B.A.Y., and T.H.L. analyzed the data. All the authors have contributed to the development and revisions of article. M.E.H. designed and conceptualized the first analysis of data and provided the first draft of the article. B.A.Y. and T.H.L. made substantial contributions to conception and design, and acquisition and analysis and interpretation of data.
ACKNOWLEDGMENTS
The authors thank Dr. Eric C. Svensson, Novartis Institutes for Biomedical Research, for challenging the authors with his excellent questions, and providing valuable feedback on the article. The authors acknowledge Roohi Chopra (Novartis Healthcare Pvt. Ltd., Hyderabad, India) for providing editorial support.
References
- 1. Ponikowski, P . et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 18, 891–975 (2016). [DOI] [PubMed] [Google Scholar]
- 2. Yancy, C.W. et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 62, e147–239 (2013). [DOI] [PubMed] [Google Scholar]
- 3. Braunschweig, F. , Cowie, M.R. & Auricchio, A. What are the costs of heart failure? Europace 13(suppl. 2), ii13–17 (2011). [DOI] [PubMed] [Google Scholar]
- 4. Roger, V.L. Epidemiology of heart failure. Circ. Res. 113, 646–659 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blecker, S. , Paul, M. , Taksler, G. , Ogedegbe, G. & Katz, S. Heart failure‐associated hospitalizations in the United States. J. Am. Coll. Cardiol. 61, 1259–1267 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Askoxylakis, V. et al. Long‐term survival of cancer patients compared to heart failure and stroke: a systematic review. BMC Cancer 10, 105 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cleland, J.G.F. & Clark, A.L. Heart failure—does it matter whether LVEF is reduced? Lancet 380, 1363–1365 (2012). [DOI] [PubMed] [Google Scholar]
- 8. Jumean, M.F. & Konstam, M.A. Heart failure with preserved ejection fraction: what is in a name? Cardiol. Rev. 23, 161–167 (2015). [DOI] [PubMed] [Google Scholar]
- 9. Packer, M. The impossible task of developing a new treatment for heart failure. J. Card. Fail. 8, 193–196 (2002). [DOI] [PubMed] [Google Scholar]
- 10. McMurray, J.J. et al. Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N. Engl. J. Med. 374, 1521–1532 (2016). [DOI] [PubMed] [Google Scholar]
- 11. Investigators, O. et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 358, 1547–1559 (2008). [DOI] [PubMed] [Google Scholar]
- 12. Dobrek, L. & Thor, P. Neuroendocrine activation as a target of modern chronic heart failure pharmacotherapy. Acta Pol. Pharm. 68, 307–316 (2011). [PubMed] [Google Scholar]
- 13. McMurray, J.J.V. et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 371, 993–1004 (2014). [DOI] [PubMed] [Google Scholar]
- 14. Barton, M. & Yanagisawa, M. Endothelin: 20 years from discovery to therapy. Can. J. Physiol. Pharmacol. 86, 485–498 (2008). [DOI] [PubMed] [Google Scholar]
- 15. Califf, R.M. et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: the Flolan International Randomized Survival Trial (FIRST). Am. Heart J. 134, 44–54 (1997). [DOI] [PubMed] [Google Scholar]
- 16. Packer, M. et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N. Engl. J. Med. 325, 1468–1475 (1991). [DOI] [PubMed] [Google Scholar]
- 17. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N. Engl. J. Med. 316, 1429–1435 (1987). [DOI] [PubMed] [Google Scholar]
- 18. Cohn, J.N. et al. A comparison of enalapril with hydralazine‐isosorbide dinitrate in the treatment of chronic congestive heart failure. N. Engl. J. Med. 325, 303–310 (1991). [DOI] [PubMed] [Google Scholar]
- 19. Garg, R. & Yusuf, S. Overview of randomized trials of angiotensin‐converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA 273, 1450–1456 (1995). [PubMed] [Google Scholar]
- 20. Pfeffer, M.A. et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N. Engl. J. Med. 327, 669–677 (1992). [DOI] [PubMed] [Google Scholar]
- 21. George, M. , Rajaram, M. , Shanmugam, E. & VijayaKumar, T.M. Novel drug targets in clinical development for heart failure. Eur. J. Clin. Pharmacol. 70, 765–774 (2014). [DOI] [PubMed] [Google Scholar]
- 22. Heerspink, H.J.L. , Grobbee, D.E. & de Zeeuw, D. A novel approach for establishing cardiovascular drug efficacy. Nat. Rev. Drug Discov. 13, 942 (2014). [DOI] [PubMed] [Google Scholar]
- 23. Rector, T.S. , Kubo, S.H. & Cohn, J.N. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am. J. Cardiol. 71, 1106–1107 (1993). [DOI] [PubMed] [Google Scholar]
- 24. Green, C.P. , Porter, C.B. , Bresnahan, D.R. & Spertus, J.A. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J. Am. Coll. Cardiol. 35, 1245–1255 (2000). [DOI] [PubMed] [Google Scholar]
- 25. Frank, R. & Hargreaves, R. Clinical biomarkers in drug discovery and development. Nat. Rev. Drug Discov. 2, 566–580 (2003). [DOI] [PubMed] [Google Scholar]
- 26. Cintron, G. , Johnson, G. , Francis, G. , Cobb, F. & Cohn, J.N. Prognostic significance of serial changes in left ventricular ejection fraction in patients with congestive heart failure. The V‐HeFT VA Cooperative Studies Group. Circulation 87, VI17–23 (1993). [PubMed] [Google Scholar]
- 27. Bittner, V. et al. Prediction of mortality and morbidity with a 6‐minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA 270, 1702–1707 (1993). [PubMed] [Google Scholar]
- 28. Rouleau, J.L. et al. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet 356, 615–620 (2000). [DOI] [PubMed] [Google Scholar]
- 29. Danhof, M. , Alvan, G. , Dahl, S.G. , Kuhlmann, J. & Paintaud, G. Mechanism‐based pharmacokinetic‐pharmacodynamic modeling‐a new classification of biomarkers. Pharm. Res. 22, 1432–1437 (2005). [DOI] [PubMed] [Google Scholar]
- 30. Cohn, J.N. , Tognoni, G. & Valsartan Heart Failure Trial, I. A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N. Engl. J. Med. 345, 1667–1675 (2001). [DOI] [PubMed] [Google Scholar]
- 31. Bristow, M.R. et al. Carvedilol produces dose‐related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation 94, 2807–2816 (1996). [DOI] [PubMed] [Google Scholar]
- 32. Krum, H. et al. Double‐blind, placebo‐controlled study of the long‐term efficacy of carvedilol in patients with severe chronic heart failure. Circulation 92, 1499–1506 (1995). [DOI] [PubMed] [Google Scholar]
- 33. Kim, H.‐N. & Januzzi, J.L., Jr. Natriuretic peptide testing in heart failure. Circulation 123, 2015–2019 (2011). [DOI] [PubMed] [Google Scholar]
- 34. Taylor, A.L. et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N. Engl. J. Med. 351, 2049–2057 (2004). [DOI] [PubMed] [Google Scholar]
- 35. Anand, I. et al. Long‐term effects of darusentan on left‐ventricular remodelling and clinical outcomes in the EndothelinA Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double‐blind, placebo‐controlled trial. Lancet 364, 347–354 (2004). [DOI] [PubMed] [Google Scholar]
- 36. McMurray, J.J.V. et al. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA 298, 2009–2019 (2007). [DOI] [PubMed] [Google Scholar]
- 37. Teerlink, J.R. Recent heart failure trials of neurohormonal modulation (OVERTURE and ENABLE): approaching the asymptote of efficacy? J. Cardiac Fail. 8, 124–127 (2002). [DOI] [PubMed] [Google Scholar]
- 38. Konstam, M.A. et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 297, 1319–1331 (2007). [DOI] [PubMed] [Google Scholar]
- 39. Udelson, J.E. et al. Multicenter, randomized, double‐blind, placebo‐controlled study on the effect of oral tolvaptan on left ventricular dilation and function in patients with heart failure and systolic dysfunction. J. Am. Coll. Cardiol. 49, 2151–2159 (2007). [DOI] [PubMed] [Google Scholar]
- 40. Udelson, J.E. et al. Acute hemodynamic effects of conivaptan, a dual V(1A) and V(2) vasopressin receptor antagonist, in patients with advanced heart failure. Circulation 104, 2417–2423 (2001). [DOI] [PubMed] [Google Scholar]
- 41. Stasch, J.‐P. , Pacher, P. & Evgenov, O.V. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123, 2263–2273 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Erdmann, E. et al. Cinaciguat, a soluble guanylate cyclase activator, unloads the heart but also causes hypotension in acute decompensated heart failure. Eur. Heart J. 34, 57–67 (2013). [DOI] [PubMed] [Google Scholar]
- 43. Gheorghiade, M. et al. Cinaciguat, a soluble guanylate cyclase activator: results from the randomized, controlled, phase IIb COMPOSE programme in acute heart failure syndromes. Eur. J. Heart Fail. 14, 1056–1066 (2012). [DOI] [PubMed] [Google Scholar]
- 44. Gheorghiade, M. et al. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES‐REDUCED Randomized Trial. Jama 314, 2251–2262 (2015). [DOI] [PubMed] [Google Scholar]
- 45. Belletti, A. et al. The effect of inotropes and vasopressors on mortality: a meta‐analysis of randomized clinical trials. Br. J. Anaesth. 115, 656–675 (2015). [DOI] [PubMed] [Google Scholar]
- 46. Landoni, G. et al. Effects of levosimendan on mortality and hospitalization. A meta‐analysis of randomized controlled studies. Crit. Care Med. 40, 634–646 (2012). [DOI] [PubMed] [Google Scholar]
- 47. Packer, M. et al. Effect of levosimendan on the short‐termclinical course of patients with acutelydecompensated heart failure. JACC Heart Fail. 1, 103–111 (2013). [DOI] [PubMed] [Google Scholar]
- 48. Mebazaa, A. et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA 297, 1883–1891 (2007). [DOI] [PubMed] [Google Scholar]
- 49. Lubsen, J. et al. Effect of pimobendan on exercise capacity in patients with heart failure: main results from the Pimobendan in Congestive Heart Failure (PICO) trial. Heart 76, 223–231 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yancy, C.W. et al. Safety and efficacy of outpatient nesiritide in patients with advanced heart failure: results of the Second Follow‐Up Serial Infusions of Nesiritide (FUSION II) trial. Circ. Heart Fail. 1, 9–16 (2008). [DOI] [PubMed] [Google Scholar]
- 51. O'Connor, C.M. et al. Effect of nesiritide in patients with acute decompensated heart failure. N. Engl. J. Med. 365, 32–43 (2011). [DOI] [PubMed] [Google Scholar]
- 52. Simonton, C.A. et al. Milrinone in congestive heart failure: acute and chronic hemodynamic and clinical evaluation. J. Am. Coll. Cardiol. 6, 453–459 (1985). [DOI] [PubMed] [Google Scholar]
- 53. Massie, B. et al. Long‐term oral administration of amrinone for congestive heart failure: lack of efficacy in a multicenter controlled trial. Circulation 71, 963–971 (1985). [DOI] [PubMed] [Google Scholar]
- 54. DiBianco, R. , Shabetai, R. , Kostuk, W. , Moran, J. , Schlant, R.C. & Wright, R. A comparison of oral milrinone, digoxin, and their combination in the treatment of patients with chronic heart failure. N. Engl. J. Med. 320, 677–683 (1989). [DOI] [PubMed] [Google Scholar]
- 55. Cohn, J.N. et al. A dose‐dependent increase in mortality with vesnarinone among patients with severe heart failure. Vesnarinone Trial Investigators. N. Engl. J. Med. 339, 1810–1816 (1998). [DOI] [PubMed] [Google Scholar]
- 56. Chrysant, S.G. , Brown, R.D. , Kem, D.C. & Brown, J.L. Antihypertensive and metabolic effects of a new converting enzyme inhibitor, enalapril. Clin. Pharmacol. Ther. 33, 741–746 (1983). [DOI] [PubMed] [Google Scholar]
- 57. Levine, T.B. , Levine, A.B. , Keteyian, S.J. , Narins, B. & Lesch, M. Reverse remodeling in heart failure with intensification of vasodilator therapy. Clin. Cardiol. 20, 697–702 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eichhorn, E.J. et al. Effect of metoprolol on myocardial function and energetics in patients with nonischemic dilated cardiomyopathy: a randomized, double‐blind, placebo‐controlled study. J. Am. Coll. Cardiol. 24, 1310–1320 (1994). [DOI] [PubMed] [Google Scholar]
- 59. Phelan, D. , Thavendiranathan, P. , Collier, P. & Marwick, T.H. Aldosterone antagonists improve ejection fraction and functional capacity independently of functional class: a meta‐analysis of randomised controlled trials. Heart 98, 1693–1700 (2012). [DOI] [PubMed] [Google Scholar]
- 60. Zelis, R. , Flaim, S.F. , Liedtke, A.J. & Nellis, S.H. Cardiocirculatory dynamics in the normal and failing heart. Annu. Rev. Physiol. 43, 455–476 (1981). [DOI] [PubMed] [Google Scholar]
- 61. Chowdhury, P. , Kehl, D. , Choudhary, R. & Maisel, A. The use of biomarkers in the patient with heart failure. Curr. Cardiol. Rep. 15, 372 (2013). [DOI] [PubMed] [Google Scholar]
- 62. Januzzi, J.L., Jr. The role of natriuretic peptide testing in guiding chronic heart failure management: review of available data and recommendations for use. Arch. Cardiovasc. Dis. 105, 40–50 (2012). [DOI] [PubMed] [Google Scholar]
- 63. Packer, M. et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 131, 54–61 (2015). [DOI] [PubMed] [Google Scholar]
- 64. Kobalava, Z. et al. Pharmacodynamic and pharmacokinetic profiles of sacubitril/valsartan (LCZ696) in patients with heart failure and reduced ejection fraction. Cardiovasc. Ther. 34, 191–198 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rosenberg, J. , Gustafsson, F. , Remme, W.J. , Riegger, G.A.J. & Hildebrandt, P.R. Effect of beta‐blockade and ACE inhibition on B‐type natriuretic peptides in stable patients with systolic heart failure. Cardiovasc. Drugs Ther. 22, 305–311 (2008). [DOI] [PubMed] [Google Scholar]
- 66. Latini, R. et al. Effects of valsartan on circulating brain natriuretic peptide and norepinephrine in symptomatic chronic heart failure: the Valsartan Heart Failure Trial (Val‐HeFT). Circulation 106, 2454–2458 (2002). [DOI] [PubMed] [Google Scholar]
- 67. Hartmann, F. et al. NT‐proBNP in severe chronic heart failure: rationale, design and preliminary results of the COPERNICUS NT‐proBNP substudy. Eur. J. Heart Fail. 6, 343–350 (2004). [DOI] [PubMed] [Google Scholar]
- 68. Stanek, B. et al. Prognostic evaluation of neurohumoral plasma levels before and during beta‐blocker therapy in advanced left ventricular dysfunction. J. Am. Coll. Cardiol. 38, 436–442 (2001). [DOI] [PubMed] [Google Scholar]
- 69. Fung, J.W.H. et al. Effect of beta blockade (carvedilol or metoprolol) on activation of the renin‐angiotensin‐aldosterone system and natriuretic peptides in chronic heart failure. Am. J. Cardiol. 92, 406–410 (2003). [DOI] [PubMed] [Google Scholar]
- 70. Olsson, L.G. et al. Prognostic importance of plasma NT‐pro BNP in chronic heart failure in patients treated with a beta‐blocker: results from the Carvedilol Or Metoprolol European Trial (COMET) trial. Eur. J. Heart Fail. 9, 795–801 (2007). [DOI] [PubMed] [Google Scholar]
- 71. Effects of metoprolol CR in patients with ischemic and dilated cardiomyopathy: the randomized evaluation of strategies for left ventricular dysfunction pilot study. Circulation 101, 378–384 (2000). [DOI] [PubMed] [Google Scholar]
- 72. Rousseau, M.F. et al. Beneficial neurohormonal profile of spironolactone in severe congestive heart failure: results from the RALES neurohormonal substudy. J. Am. Coll. Cardiol. 40, 1596–1601 (2002). [DOI] [PubMed] [Google Scholar]
- 73. Macdonald, J.E. , Kennedy, N. & Struthers, A.D. Effects of spironolactone on endothelial function, vascular angiotensin converting enzyme activity, and other prognostic markers in patients with mild heart failure already taking optimal treatment. Heart 90, 765–770 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Berry, C. et al. Effects of aldosterone receptor blockade in patients with mild‐moderate heart failure taking a beta‐blocker. Eur. J. Heart Fail. 9, 429–434 (2007). [DOI] [PubMed] [Google Scholar]
- 75. Hu, L.‐j. , Chen, Y.‐q. , Deng, S.‐b. , Du, J.‐l. & She, Q. Additional use of an aldosterone antagonist in patients with mild to moderate chronic heart failure: a systematic review and meta‐analysis. Br. J. Clin. Pharmacol. 75, 1202–1212 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. de Boer, R.A. , Daniels, L.B. , Maisel, A.S. & Januzzi, J.L., Jr. State of the art: newer biomarkers in heart failure. Eur. J. Heart Fail. 17, 559–569 (2015). [DOI] [PubMed] [Google Scholar]
- 77. Miller, W.L. , Hartman, K.A. , Burritt, M.F. , Grill, D.E. & Jaffe, A.S. Profiles of serial changes in cardiac troponin T concentrations and outcome in ambulatory patients with chronic heart failure. J. Am. Coll. Cardiol. 54, 1715–1721 (2009). [DOI] [PubMed] [Google Scholar]
- 78. Jensen, M.T. , Suadicani, P. , Hein, H.O. & Gyntelberg, F. Elevated resting heart rate, physical fitness and all‐cause mortality: a 16‐year follow‐up in the Copenhagen Male Study. Heart 99, 882–887 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Swedberg, K. et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo‐controlled study. Lancet 376, 875–885 (2010). [DOI] [PubMed] [Google Scholar]
- 80. Tisdale, J.E. , Patel, R. , Webb, C.R. , Borzak, S. & Zarowitz, B.J. Electrophysiologic and proarrhythmic effects of intravenous inotropic agents. Prog. Cardiovasc. Dis. 38, 167–180 (1995). [DOI] [PubMed] [Google Scholar]


