 A transition-metal-free unique tandem annulation reaction has been developed for the synthesis of various functionalized 3-hydroxycarbazoles.
A transition-metal-free unique tandem annulation reaction has been developed for the synthesis of various functionalized 3-hydroxycarbazoles.
Abstract
This paper describes a novel synthesis of highly functionalized and diverse carbazoles via transition-metal-free and mild base-promoted condensations of readily available 2-nitrocinnamaldehyde or 2-nitrochalcones with various β-ketoesters or 1,3-diaryl-2-propanones. The method selectively forms four bonds by the intramolecular conjugate addition of an enolate to the enal or chalcone bearing an o-nitro group. This group then undergoes in situ N–O bond cleavage under non-reductive conditions in a one-pot procedure. This protocol allows for the introduction of various functional groups at all positions of the newly formed aromatic ring of the carbazole moiety. The utility of this methodology is further illustrated by the concise synthesis of naturally occurring hyellazole and chlorohyellazole.
Introduction
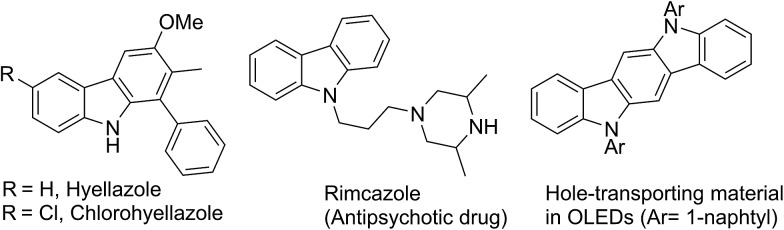
The carbazole framework is found in a wide range of bioactive natural products and pharmaceuticals (Fig. 1).1,2 These carbazole-containing molecules show antiviral,3 antimalarial,4 and antitumor activity.5 Some of them are currently being used as lead compounds for drug development.6 Carbazoles are also used as building blocks for the synthesis of functional materials, such as organic light-emitting diodes (OLED), because of their wide band gap, high luminescence efficiency, and allowing flexible modification of the parent skeleton.7,8
Fig. 1. Selected naturally occurring, pharmaceutical, or electroactive carbazoles.
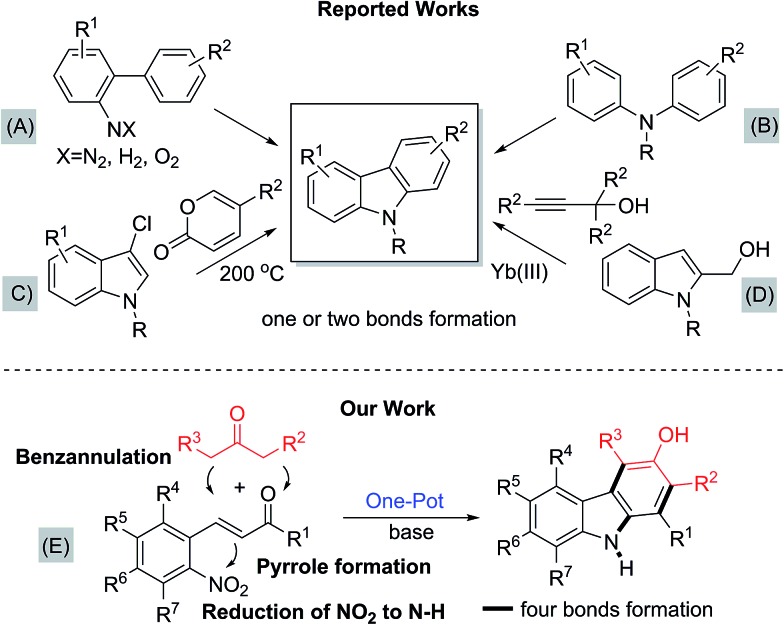
Owing to the importance and usefulness of these carbazole-based compounds, various approaches for their construction have been developed. The general and representative strategies can be classified into two main types depending on how the carbazole ring is constructed. The first strategy relies on the formation of a C–C or a C–N bond to construct the middle pyrrole ring starting from arene building blocks (methods A and B, Fig. 2).9–16 Also, the reaction of arynes with nitrosoarene and the nitrogenation of biphenyl halides have been reported.17 The second strategy involves the installation of a new aromatic ring onto functionalized indole derivatives via benzannulation (methods C and D, Fig. 2).18–23
Fig. 2. Diverse synthetic routes for carbazoles.
Despite their own merits, most, if not all, of these methods suffer from certain drawbacks, including low tolerance of functionality, limited substrate scope, not-easily accessible starting materials, the necessity of complex and expensive transition-metal catalysts, and harsh reaction conditions. In particular, many existing methods require either highly elaborated biaryls or biarylamines to construct the central pyrrole moiety or pre-functionalized indole derivatives for benzannulation. Therefore, more environmentally benign and modular multi-bond forming approaches accommodating structurally simple building blocks as the feedstock are highly sought-after to improve on these shortcomings. In relation to the synthesis of 3-hydroxy carbazoles, iron-mediated reactions have also been reported.24 A recently-reported rhodium-catalyzed tandem annulation uses a new approach, where the [5 + 1] cycloaddition of 3-hydroxy-1,4-enynes with CO generates three bonds and two rings.25 Yet, even for this transformation, various 3-hydroxy-1,4-enyne reagents must be prepared by a multi-step route.
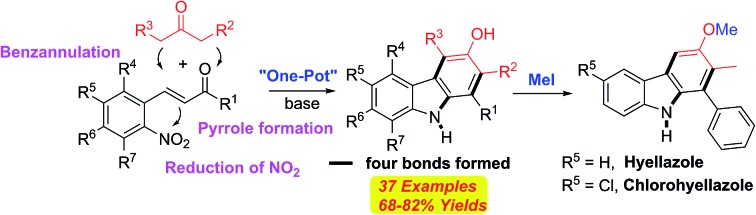
In this regard, the new approach, depicted in E, accommodating a novel double annulation through the consecutive construction of a pyrrole and a benzene moiety reflects further innovation (Fig. 2). A unique feature of the current reaction compared with all other reported pyrrole formations or benzannulations is the formation of the carbazole nitrogen atom by electrophilic attack on a nitro group rather than the use of an amine nucleophile. Herein, we describe a unique tandem annulation followed by N–O bond cleavage without any external reductant for the synthesis of various functionalized 3-hydroxycarbazoles from readily available 2-nitrocinnamaldehyde or 2-nitrochalcone and β-ketoesters or 1,3-diaryl-2-propanone.
Results and discussion
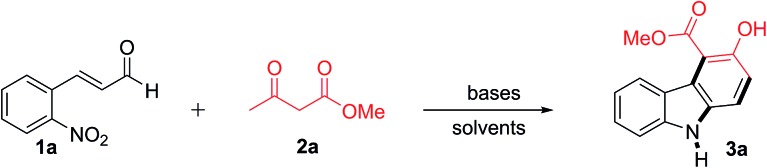
First, the reaction of 2-nitrocinnamaldehyde (1a) and methyl 2-oxobutanoate (2a) was examined with several bases and solvents to optimize the reaction conditions (Table 1). The initial attempt with NaOMe (1 equiv.) in refluxing toluene for 12 h did not provide product 3a (Table 1, entry 1), but produced an intractable mixture. With triethylamine (1 equiv.), product 3a was also not formed (Table 1, entry 2), but with DBU (1 equiv.), 3a was produced in 10% yield (Table 1, entry 3). Encouraged by this result, other bases were screened. With K2CO3 (1 equiv.) for 6 h, the yield of 3a increased to 67% (Table 1, entry 4). The highest yield (81%) was achieved with 1.0 equivalent of Cs2CO3 in refluxing toluene for 4 h (Table 1, entry 5). Increasing the amount of Cs2CO3 to 1.5 equivalents (entry 6) or decreasing it to 0.1 equivalent (Table 1, entry 7) lowered the yield of 3a. Based on these results, this transformation was found to be sensitive towards the base strength used. For example, strong bases like NaOMe (1 equiv.) or DBU (1 equiv.) provided very little or no desired product, while weak bases provided better yields. Among the screened bases, Cs2CO3 was superior in terms of both reaction time and yield for this reaction, probably due to its mild and optimum base strength.26 In two other nonpolar solvents (benzene or dichloroethane), 3a was produced in 35 and 51% yield, respectively, whereas 3a was not obtained in a more polar solvent, such as methanol, DMSO, or water (Table 1, entries 8–12). The structure of 3a was established by spectroscopic analysis. The 1H NMR of 3a showed a characteristic singlet of the OH group at δ 11.12 ppm and another broad singlet for the NH proton at δ 8.17 ppm. The 13C NMR showed the expected characteristic ester carbonyl carbon at δ 171.6 ppm and an aromatic carbon containing OH at δ 157.7 ppm. The structural confirmation of 3a was further evidenced by X-ray crystallographic analysis of the related compound 7a (see ESI†).
Table 1. Optimization of the reaction conditions for the synthesis of carbazole 3a a .
| ||||
| Entry | Base | Solvent | Condition | Yield b (%) |
| 1 | NaOMe (1 equiv.) | Toluene | Reflux, 12 h | 0 |
| 2 | TEA (1 equiv.) | Toluene | Reflux, 12 h | 0 |
| 3 | DBU (1 equiv.) | Toluene | Reflux, 12 h | 10 |
| 4 | K2CO3 (1 equiv.) | Toluene | Reflux, 6 h | 67 |
| 5 | Cs 2 CO 3 (1 equiv.) | Toluene | Reflux, 4 h | 81 |
| 6 | Cs2CO3 (1.5 equiv.) | Toluene | Reflux, 4 h | 78 |
| 7 | Cs2CO3 (0.1 equiv.) | Toluene | Reflux, 12 h | 32 |
| 8 | Cs2CO3 (1 equiv.) | Benzene | Reflux, 12 h | 35 |
| 9 | Cs2CO3 (1 equiv.) | DCE | Reflux, 12 h | 51 |
| 10 | Cs2CO3 (1 equiv.) | MeOH | Reflux, 12 h | 0 |
| 11 | Cs2CO3 (1 equiv.) | DMSO | Reflux, 12 h | 0 |
| 12 | Cs2CO3 (1 equiv.) | Water | Reflux, 12 h | 0 |
aReactions were conducted on a 1.0 mmol scale of 1a.
bIsolated yield.
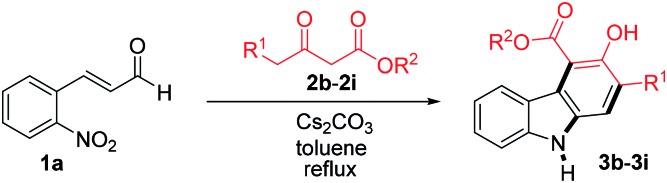
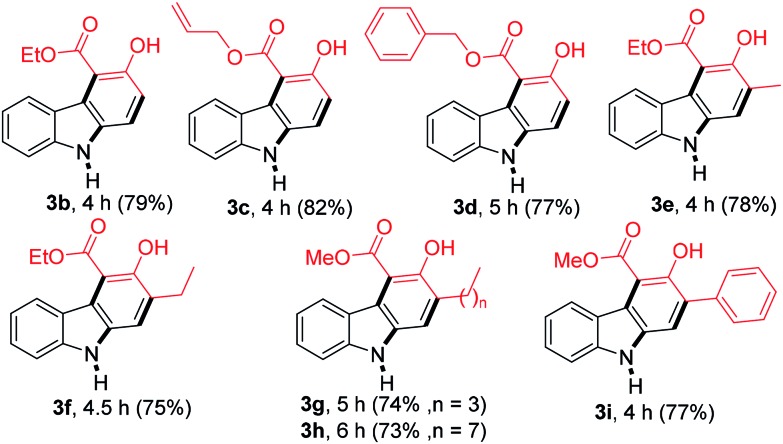
With the optimized conditions in hand, the generality of this reaction was explored by employing different β-ketoesters 2b–2i (Table 2). Reaction of 2-nitrocinnamaldehyde (1a) with several β-ketoesters such as ethyl 2-oxobutanoate (2b), allyl 3-oxobutanoate (2c) and benzyl 3-oxobutanoate (2d), afforded the desired products 3b–3d in 79, 82 and 77% yield, respectively. Moreover, the reactions of other β-ketoesters such as ethyl 3-oxopentanoate (2e), ethyl 3-oxohexanoate (2f), methyl 3-oxooctanoate (2g), methyl 3-oxododecanoate (2h), and methyl 3-oxo-4-phenylbutanoate (2i) provided the desired carbazoles 3e–3i in 73–78% yield.
Table 2. Formation of carbazole 3b–3i by the reaction of 2-nitrocinnamaldehyde 1a with various β-ketoesters 2b–2i a .
|
|
aReactions were performed on a 1.0 mmol scale according to the standard conditions described in Table 1.
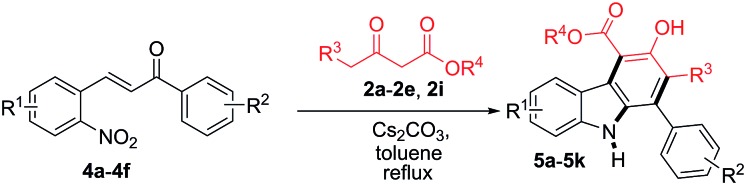
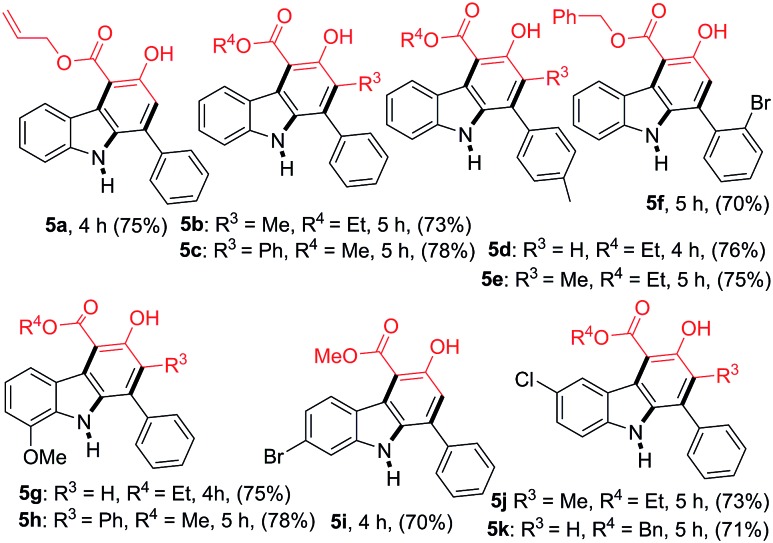
The scope of the reaction was further extended by employing a series of 2-nitrochalcones and β-ketoesters (Table 3). When 2-nitrochalcone 4a was treated with allyl 3-oxobutanoate (2c), ethyl 3-oxopentanoate (2e) or 3-oxo-4-phenylbutanoate (2i) under optimized reaction conditions, the desired products 5a, 5b and 5c were formed in 75, 73 and 78% yield respectively. Furthermore, 2-nitrochalcones 4b–4c, bearing electron-donating or -withdrawing groups such as a methyl or bromo substituent on the 1-phenyl group, and β-ketoesters 2b, 2d and 2e also provided the desired products 5d–5f in 76, 75, and 70% yield, respectively. In addition, 2-nitrochalcones 4d–4f, having electron-donating or -withdrawing groups such as a methoxy, bromo and chloro substituent on the 3-phenyl group, produced the expected carbazoles 5g–5k in good yield (70–78%).
Table 3. Formation of carbazoles 5a–5k from various 2-nitrochalcones (4a–4f) and several β-ketoesters (2a–2e and 2i) a .
|
|
aReactions were performed on a 1.0 mmol scale according to the standard conditions described in Table 1.
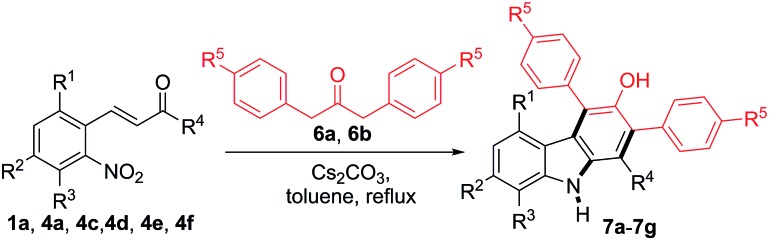
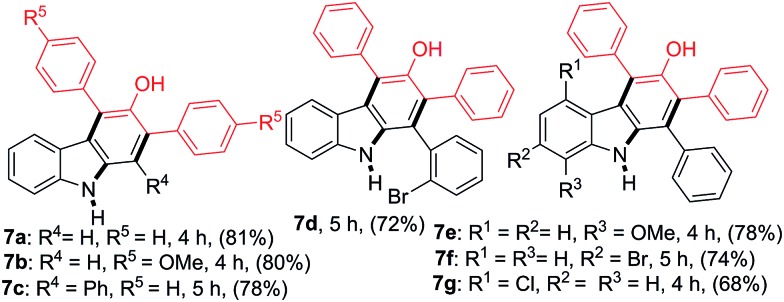
The reactions between 2-nitrocinnamaldehyde (1a) or one of the 2-nitrochalcones (4a, 4c, 4d, 4e and 4f) and 1,3-diarylpropan-2-ones 6a and 6b were examined to further demonstrate the versatility of this carbazole formation (Table 4). The reaction of 1a with 6a or 6b in refluxing toluene for 4 h afforded the corresponding products 7a–7b in 81 and 80% yield, respectively. Similarly, the treatment of the nitrochalcones (4a, 4c, 4d, 4e, and 4f) with 6a or 6b provided the products 7c–7g in the range of 68–78% yield.
Table 4. Formation of carbazoles 7a–7g from 1a or the 2-nitrochalcones and 6a or 6b a .
|
|
aReactions were performed on a 1.0 mmol scale according to the standard conditions described in Table 1.
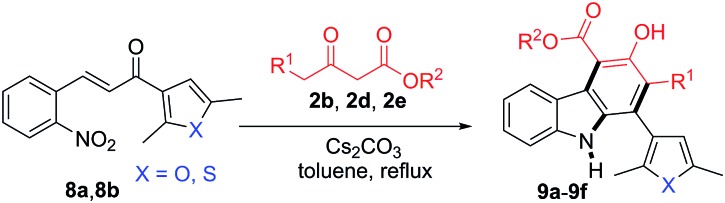
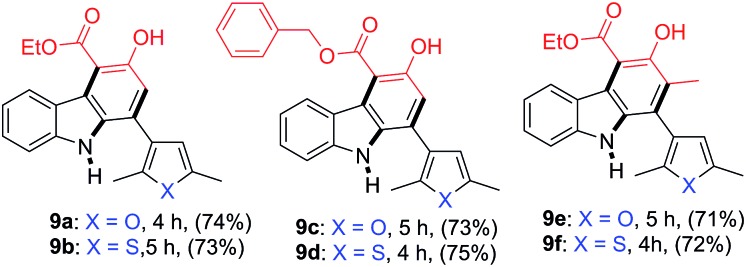
Having confirmed the general applicability of the reaction by using 2-nitrocinnamaldehyde and the 2-nitrochalcones as starting materials, the possibility of using the 2-nitrochalcones bearing a heteroatom was examined, which would lead to the formation of carbazole derivatives with extended structural space. To our delight, the reactions of 8a or 8b with β-ketoesters 2b, 2d, and 2e provided the expected products 9a–9f in the range of 71–75% yield (Table 5).
Table 5. Formation of carbazoles 9a–9f from various 2-nitrochalcones (8a and 8b) and β-ketoesters (2b, 2d and 2e) a .
|
|
aReactions were performed on a 1.0 mmol scale according to the standard conditions described in Table 1.
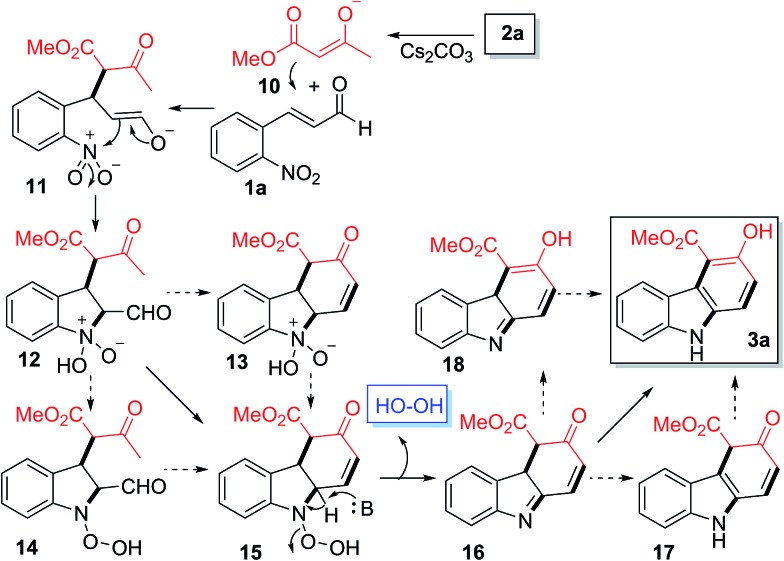
We propose that the formation of the observed carbazole products may involve a mechanism shown in Scheme 1. In a basic medium, enolate 10 derived from 2a undergoes Michael addition onto 1a to give the new enolate intermediate 11, which subsequently reacts with the nitro group to form the bicyclic intermediate 12.27 The reorganization of the O–N–OH moiety in 12 to N–O–OH would generate 15via13 or 14. The base-induced elimination of the hydrogen peroxide from 15 would generate 16, which would then undergo sequential double tautomerization via17 or 18 to generate the observed product 3a.
Scheme 1. Proposed mechanism for the formation of 3a.
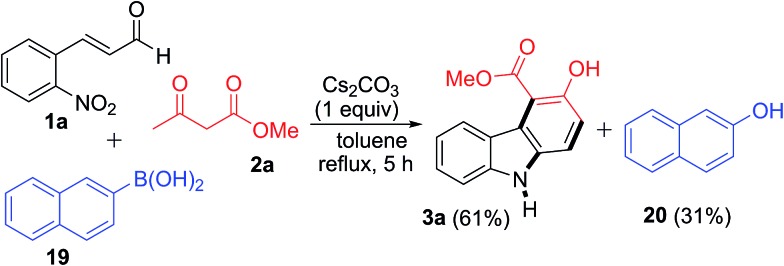
To obtain evidence for the formation of H2O2 during the reaction sequence, a control experiment was carried out with added aryl boronic acid (Scheme 2). To our delight, this reaction involving 1a, 2a and 2-naphthyl boronic acid 19 under the standard reaction conditions provided product 3a (61%) together with 2-naphthol 20 in 31% yield. The formation of 2-naphthol 20 implies the existence of in situ generated H2O2 in the reaction, although other mechanistic possibilities cannot be excluded.28
Scheme 2. Control experiment to detect H2O2 in the reaction pathway.
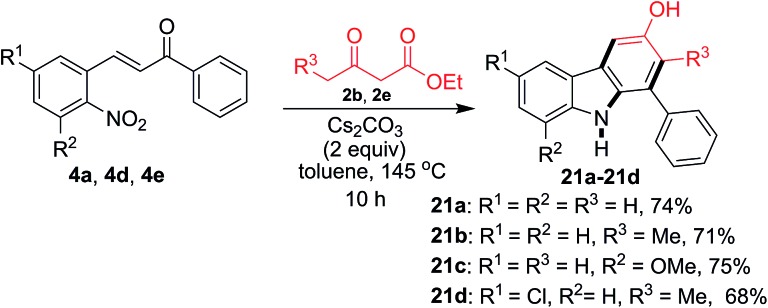
Next, we broaden the carbazole structures to those that do not carry a carboethoxy group at the 4-position (Scheme 3). By carrying out the reaction at a higher temperature (145 °C) for a prolonged time using 2 equivalents of Cs2CO3 for decarboethoxylation, carbazoles 21a–21d were obtained in 68–75% yield.
Scheme 3. Formation of the decarboethoxylated carbazoles 21a–21d from various 2-nitrochalcones and β-ketoesters.
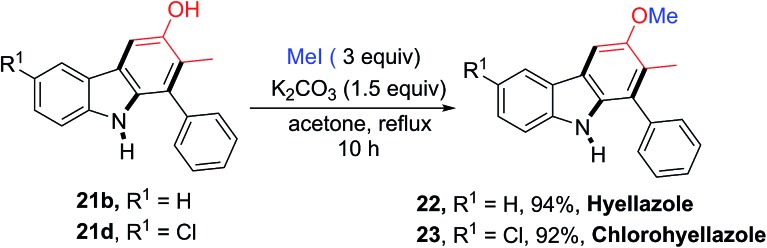
The utility of this new protocol was demonstrated by the conversion of 21b and 21d to biologically active natural products (Scheme 4). Upon treating 21b and 21d with iodomethane in refluxing acetone in the presence of K2CO3, hyellazole (22) and chlorohyellazole (23) were obtained in 94% and 92% yields, respectively. Our concise synthesis of hyellazole and chlorohyellazole was achieved in two steps from commercially available starting materials in 67% and 63% overall yields, respectively. This protocol has several advantages such as higher yields, lower cost, fewer steps, transition metal-free, and environmentally benignity.29,30 The identity of these two natural products was confirmed by the comparison of their spectroscopic data with those previously reported.29,30
Scheme 4. Synthesis of naturally occurring hyellazole (22) and chlorohyellazole (23).
Conclusions
A highly efficient, transition-metal-free, modular and operationally simple tandem annulation process was developed for the synthesis of diverse carbazole derivatives starting from readily available 2-nitrocinnamaldehydes or 2-nitrochalcones and β-ketoesters or 1,3-diaryl-2-propanones. This synthetic approach for the rapid construction of various functionalized carbazoles involves the intramolecular addition of an enolate to a nitro group and a unique in situ N–O bond cleavage under non-reductive conditions. As an application of this new synthetic methodology, a concise synthesis of naturally occurring bioactive hyellazole and chlorohyellazole has been realized in two steps.
Supplementary Material
Acknowledgments
This research was supported by the Nano Material Technology Development Program through the Korean National Research Foundation (NRF) funded by the Korean Ministry of Education, Science, and Technology (2012M3A7B4049675) and by the Korea government (MSIP) (NRF-2014R1A2A1A11052391).
Footnotes
†Electronic supplementary information (ESI) available: Experimental procedures, characterization data, 1H NMR and 13C NMR spectra for synthesized compounds. X-ray structure and data for 7a. CCDC 1046362. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc02407b
References
- (a) Knölker H.-J., Reddy K. R. Chem. Rev. 2002;102:4303. doi: 10.1021/cr020059j. [DOI] [PubMed] [Google Scholar]; (b) Knölker H.-J. Curr. Org. Synth. 2004;1:309. [Google Scholar]; (c) Knölker H.-J. Chem. Lett. 2009;38:8. [Google Scholar]
- (a) Ito C., Itoigawa M., Sato A., Hasan C. M., Rashid M. A., Tokuda H., Mukainaka T., Nishino H., Furukawa H. J. Nat. Prod. 2004;67:1488. doi: 10.1021/np0400611. [DOI] [PubMed] [Google Scholar]; (b) Maneerat W., Ritthiwigrom T., Cheenpracha S., Promgool T., Yossathera K., Deachathai S., Phakhodee W., Laphookhieo S. J. Nat. Prod. 2012;75:741. doi: 10.1021/np3000365. [DOI] [PubMed] [Google Scholar]
- Schmidt A. W., Reddy K. R., Knölker H.-J. Chem. Rev. 2012;112:3193. doi: 10.1021/cr200447s. [DOI] [PubMed] [Google Scholar]
- Li W.-S., McChesney J. D., El-Feraly F. S. Phytochemistry. 1991;30:343. [Google Scholar]
- (a) Janosik T., Wahlstrom N., Bergman J. Tetrahedron. 2008;64:9159. [Google Scholar]; (b) Chao W. R., Yean D., Amin K., Green C., Jong L. J. Med. Chem. 2007;50:3412. doi: 10.1021/jm070040e. [DOI] [PubMed] [Google Scholar]
- (a) Pieper A. A., McKnight S. L., Ready J. M. Chem. Soc. Rev. 2014;43:6716. doi: 10.1039/c3cs60448a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Takeuchi T., Oishi S., Watanabe T., Ohno H., Sawada J.-I., Matsuno K. J. Med. Chem. 2011;54:4839. doi: 10.1021/jm200448n. [DOI] [PubMed] [Google Scholar]
- (a) Reddy R. A., Baumeister U., Keith C., Tschierske C. J. Mater. Chem. 2007;17:62. [Google Scholar]; (b) Li J., Grimsdale A. C. Chem. Soc. Rev. 2010;39:2399. doi: 10.1039/b915995a. [DOI] [PubMed] [Google Scholar]
- (a) Liu X., Xu Y., Jiang D. J. Am. Chem. Soc. 2012;134:8738. doi: 10.1021/ja303448r. [DOI] [PubMed] [Google Scholar]; (b) Huang H., Fu Q., Pan B., Zhaang S., Wang L., Chen J., Ma D., Yang C. Org. Lett. 2012;14:4786. doi: 10.1021/ol3020286. [DOI] [PubMed] [Google Scholar]; (c) Tsvelikhovsky D., Buchwald S. L. J. Am. Chem. Soc. 2011;133:14228. doi: 10.1021/ja206229y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Ackermann L., Althammer A. Angew. Chem., Int. Ed. 2007;46:1627. doi: 10.1002/anie.200603833. [DOI] [PubMed] [Google Scholar]; (b) Bedford R. B., Betham M. J. Org. Chem. 2006;71:9403. doi: 10.1021/jo061749g. [DOI] [PubMed] [Google Scholar]
- (a) Pumphrey A. L., Dong H., Driver T. G. Angew. Chem., Int. Ed. 2012;51:5920. doi: 10.1002/anie.201201788. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Stokes B. J., Jovanovic B., Dong H., Richert K. J., Riell R. D., Driver T. G. J. Org. Chem. 2009;74:3225. doi: 10.1021/jo9002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Hernandez-Perez A. C., Collins S. K. Angew. Chem., Int. Ed. 2013;52:12696. doi: 10.1002/anie.201306920. [DOI] [PubMed] [Google Scholar]; (b) Campeau L.-C., Parisien M., Jean A., Fagnou K. J. Am. Chem. Soc. 2006;128:581. doi: 10.1021/ja055819x. [DOI] [PubMed] [Google Scholar]; (c) Trosien S., Bottger P., Siegfried R. W. Org. Lett. 2014;16:402. doi: 10.1021/ol403304t. [DOI] [PubMed] [Google Scholar]; (d) Wang C., Piel I., Glorius F. J. Am. Chem. Soc. 2009;131:4194. doi: 10.1021/ja8100598. [DOI] [PubMed] [Google Scholar]; (e) Kumar V. P., Gruner K. K., Kataeva O., Knölker H.-J. Angew.Chem., Int. Ed. 2013;52:11073. doi: 10.1002/anie.201305993. [DOI] [PubMed] [Google Scholar]
- (a) Takamatsu K., Hirano K., Satoh T., Miura M. Org. Lett. 2014;16:2892. doi: 10.1021/ol501037j. [DOI] [PubMed] [Google Scholar]; (b) Antonchick A. P., Samanta R., Kulikov K., Lategahn J. Angew. Chem., Int. Ed. 2011;50:8605. doi: 10.1002/anie.201102984. [DOI] [PubMed] [Google Scholar]; (c) Jordan-Hore J. A., Johansson C. C. C., Gulias M., Beck E. M., Gaunt M. J. J. Am. Chem. Soc. 2008;130:16184. doi: 10.1021/ja806543s. [DOI] [PubMed] [Google Scholar]; (d) Cho S. H., Yoon S. H., Chang S. J. Am. Chem. Soc. 2011;133:5996. doi: 10.1021/ja111652v. [DOI] [PubMed] [Google Scholar]; (e) Tsang W. C. P., Zheng N., Buchwald S. L. J. Am. Chem. Soc. 2005;127:14560. doi: 10.1021/ja055353i. [DOI] [PubMed] [Google Scholar]
- (a) Nozaki K., Takahashi K., Nakano K., Hiyama T., Tang H.-Z., Fujiki M., Yamaguchi S., Tamao K. Angew. Chem., Int. Ed. 2003;42:2051. doi: 10.1002/anie.200250648. [DOI] [PubMed] [Google Scholar]; (b) Kuwahara A., Nakano K., Nozaki K. J. Org. Chem. 2005;70:413. doi: 10.1021/jo048472+. [DOI] [PubMed] [Google Scholar]
- (a) Cadogan J. I. G., Cameron-Wood M. Proc. Chem. Soc. London. 1962:361. [Google Scholar]; (b) Ragaini F., Cenini S., Gallo E., Caselli A., Fantauzzi S. Curr. Org. Chem. 2006;10:1479. [Google Scholar]; (c) Kuethe J. T., Childers K. G. Adv. Synth. Catal. 2008;350:1577. [Google Scholar]
- Gao H., Xu Q.-L., Yousufuddin M., Ess D. H., Kurti L. Angew. Chem., Int. Ed. 2014;53:2701. doi: 10.1002/anie.201309973. [DOI] [PubMed] [Google Scholar]
- (a) Knölker H.-J., O'Sullivan N. Tetrahedron. 1994;50:10893. [Google Scholar]; (b) Krahl M. P., Jäger A., Krause T., Knölker H.-J. Org. Biomol. Chem. 2006;4:3215. doi: 10.1039/b607792g. [DOI] [PubMed] [Google Scholar]; (c) Forke R., Jäger A., Knölker H.-J. Org. Biomol. Chem. 2008;6:2481. doi: 10.1039/b805451g. [DOI] [PubMed] [Google Scholar]; (d) Forke R., Krahl M. P., Däbritz F., Jäger A., Knölker H.-J. Synlett. 2008:1870. [Google Scholar]; (e) Schmidt M., Knölker H.-J. Synlett. 2009:2421. [Google Scholar]; (f) Hesse R., Gruner K. K., Kataeva O., Schmidt A. W., Knölker H.-J. Chem.–Eur. J. 2013;19:14098. doi: 10.1002/chem.201301792. [DOI] [PubMed] [Google Scholar]
- Chakrabarty S., Chatterjee I., Tebben L., Studer A., Oua Y., Jiao N. Angew. Chem., Int. Ed. Chem. Commun. 2013;2013;5249:2968. 3473. doi: 10.1002/anie.201209447. [DOI] [PubMed] [Google Scholar]
- (a) Ozaki K., Zhang H., Ito H., Lei A., Itami K. Chem. Sci. 2013;4:3416. [Google Scholar]; (b) Abid M., Spaeth A., Torok B. Adv. Synth. Catal. 2006;348:2191. [Google Scholar]
- Guney T., Lee J. J., Kraus G. A. Org. Lett. 2014;16:1124. doi: 10.1021/ol403733n. [DOI] [PubMed] [Google Scholar]
- Yamashita M., Hirano K., Satoh T., Miura M. Org. Lett. 2009;11:2337. doi: 10.1021/ol900736s. [DOI] [PubMed] [Google Scholar]
- Samala S., Mandadapu A. K., Saifuddin M., Kundu B. J. Org. Chem. 2013;78:6769. doi: 10.1021/jo400799b. [DOI] [PubMed] [Google Scholar]
- Wang S., Chai Z., Wei Y., Zhu X., Zhou S., Wang S. Org. Lett. 2014;16:3592. doi: 10.1021/ol501605h. [DOI] [PubMed] [Google Scholar]
- Tsuchimoto T., Matsubayashi H., Kaneko M., Nagase Y., Miyamura T., Shirakawa E. J. Am. Chem. Soc. 2008;130:15823. doi: 10.1021/ja803954e. [DOI] [PubMed] [Google Scholar]
- (a) Knölker H.-J., Bauermeister M., Pannek J.-B. Chem. Ber. 1992;125:2783. [Google Scholar]; (b) Knolker H.-J., Bauermeister M. J. Chem. Soc., Chem. Commun. 1989:1468. [Google Scholar]; (c) Knölker H.-J., Fröhner W. Tetrahedron Lett. 1997;38:1535. [Google Scholar]; (d) Fröhner W., Krahl M. P., Reddy K. R., Knölker H.-J. Heterocycles. 2004;63:2393. [Google Scholar]; (e) Kataeva O., Krahl M. P., Knölker H.-J. Org. Biomol. Chem. 2005;3:3099. doi: 10.1039/b507660a. [DOI] [PubMed] [Google Scholar]; (f) Knott K. E., Auschill S., Jäger A., Knölker H.-J. Chem. Commun. 2009:1467. doi: 10.1039/b821039j. [DOI] [PubMed] [Google Scholar]; (g) Gruner K. K., Hopfmann T., Matsumoto K., Jäger A., Katsukib T., Knölker H.-J. Org. Biomol. Chem. 2011;9:2057. doi: 10.1039/c0ob01088j. [DOI] [PubMed] [Google Scholar]
- Li X., Song W., Tang W. J. Am. Chem. Soc. 2013;135:16797. doi: 10.1021/ja408829y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Poudel T. N., Lee Y. R. Org. Lett. 2015;17:2050. doi: 10.1021/acs.orglett.5b00996. [DOI] [PubMed] [Google Scholar]; (b) Poudel T. N., Lee Y. R. Chem.–Eur. J. 2014;12:919. doi: 10.1039/c3ob41800f. [DOI] [PubMed] [Google Scholar]; (c) Qian J., Yi W., Huang X., Miao Y., Zhang J., Cai C., Zhang W. Org. Lett. 2015;17:1090. doi: 10.1021/ol503615n. [DOI] [PubMed] [Google Scholar]
- (a) Moskalev N., Makosza M. Chem. Commun. 2001:1248. [Google Scholar]; (b) Makosza M. Chem.–Eur. J. 2014;20:5536. doi: 10.1002/chem.201400097. [DOI] [PubMed] [Google Scholar]
- (a) Prakash G. K. S., Chacko S., Panja C., Thomas T. E., Gurung L., Rasul G., Mathew T., Olah G. A. Adv. Synth. Catal. 2009;351:1567. [Google Scholar]; (b) Simon J., Salzbrunn S., Prakash G. K. S., Petasis N. A., Olah G. A. J. Org. Chem. 2001;66:633. doi: 10.1021/jo0015873. [DOI] [PubMed] [Google Scholar]
- (a) Markad S. B., Argade N. P. Org. Lett. 2014;16:5470. doi: 10.1021/ol502721r. [DOI] [PubMed] [Google Scholar]; (b) Kano S., Sugino E., Shibuya S., Hibino S. J. Org. Chem. 1981;46:3856. [Google Scholar]
- (a) Takano S., Suzuki Y., Ogasawara K. Heterocycles. 1981;16:1479. [Google Scholar]; (b) Moody C. J., Shah P. J. Chem. Soc., Perkin Trans. 1. 1989:2463. [Google Scholar]; (c) Danheiser R. L., Brisbois R. G., Kowalczyk J. J., Miller R. F. J. Am. Chem. Soc. 1990;112:3039. [Google Scholar]; (d) Kawasaki T., Nonaka Y., Akahane M., Maeda N., Sakamoto M. J. Chem. Soc., Perkin Trans. 1. 1993:1777. [Google Scholar]; (e) Beccalli E. M., Marchesin A., Pilati T. J. Chem. Soc., Perkin Trans. 1. 1994:579. [Google Scholar]; (f) Knölker H.-J., Baum E., Hopfmann T. Tetrahedron Lett. 1995;36:5339. [Google Scholar]; (g) Choshi T., Sada T., Fujimoto H., Nagayama C., Sugino E., Hibino S. J. Org. Chem. 1997;62:2535. doi: 10.1021/jo962038t. [DOI] [PubMed] [Google Scholar]; (h) Knölker H.-J., Baum E., Hopfmann T. Tetrahedron. 1999;55:10391. [Google Scholar]; (i) Duval E., Cuny G. D. Tetrahedron Lett. 2004;45:5411. [Google Scholar]; (j) Knölker H.-J., Fröhner W., Heinrich R. Synlett. 2004:2705. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.