Abstract
Anticoagulation is used to treat venous thromboembolism (VTE) in cancer patients, but may be associated with an increased risk of bleeding. VTE recurrence and major bleeding were assessed in cancer patients treated for VTE with the most currently prescribed anticoagulants in clinical practice. Newly diagnosed cancer patients (first VTE 1/1/2013‐05/31/2015) who initiated rivaroxaban, low‐molecular‐weight heparin (LMWH), or warfarin were identified from Humana claims data and observed until end of eligibility or end of data availability. VTE recurrence was a hospitalization with a primary diagnosis of VTE ≥7 days after first VTE. Major bleeding events on treatment were identified using validated criteria. Cohorts were compared using Kaplan–Meier rates at 6 and 12 months and Cox proportional hazards models. Cohorts were adjusted for their differences at baseline. A total of 2428 patients (rivaroxaban: 707; LMWH: 660; warfarin: 1061) met inclusion criteria. Patient characteristics were well balanced after weighting. There was a trend for lower VTE recurrence rates in rivaroxaban users compared to LMWH users at 6 months (13.2% vs. 17.1%; P = .060) and significantly lower at 12 months (16.5% vs. 22.2%; P = .030) [HR: 0.72, 95% CI: (0.52‐0.95); P = .024]. VTE recurrence rates were also lower for rivaroxaban than warfarin users at 6 months (13.2% vs. 17.5%; P = .014) and 12 months (15.7% vs. 19.9%; P = .017) [HR: 0.74, 95% CI: (0.56‐0.96); P = .028]. Major bleeding rates were similar across cohorts. This real‐world analysis suggests cancer patients with VTE treated with rivaroxaban had significantly lower risk of recurrent VTE and similar risk of bleeding compared to those treated with LMWH or warfarin.
1. INTRODUCTION
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is the second leading cause of death for cancer patients.1 It is estimated that the annual incidence of VTE is approximately 1 out of 200 in a population of cancer patients.2 When compared to the general population, patients with cancer have a 4.1‐fold risk of thrombosis and those undergoing chemotherapy have a 6.5‐fold risk.3, 4 Furthermore, the risk of recurrence after a first episode of VTE is higher in cancer patients than in those without underlying malignancy.5
Anticoagulant therapy is the key option for treatment and secondary prophylaxis of VTE. Current treatment guidelines recommend anticoagulation with low‐molecular‐weight heparin (LMWH) for at least 3 to 6 months in patients with cancer.6, 7 Treatment beyond the initial 6 months should also be considered for patients with metastatic disease and for those receiving chemotherapy. These recommendations are based on previous clinical trials of LMWH and warfarin for the treatment of VTE in cancer patients who showed that LMWH was more effective than warfarin in reducing the risk of recurrent thromboembolism without increasing the risk of bleeding.8, 9 In practice, many patients with cancer are treated for less than the recommended 3 to 6 months and are not treated with a LMWH.10, 11, 12, 13
Direct oral anticoagulant (DOAC) agents are approved for VTE treatment but are not yet endorsed by the guidelines for cancer‐associated thrombosis due to lack of clinical evidence in this patient population. A subgroup analysis of patients with cancer from the EINSTEIN randomized trials, who had DVT or PE, reported that rivaroxaban had similar efficacy and superior safety relative to warfarin.14 A recent meta‐analysis of randomized controlled trials also found that DOACs were as effective and safe as heparin in combination with vitamin K antagonists (VKAs) for the treatment of VTE in this population.15 Another meta‐analysis found that DOACs were as effective and safe as VKAs but may have higher rates of bleeding when compared to LMWH.16, 17
Limited information exists on the effectiveness of currently prescribed anticoagulants in prevention of VTE recurrence in patients with cancer. The objective of this observational study was to compare the risk of VTE recurrence and major bleeding in cancer patients treated with anticoagulants for VTE in a real‐world setting.
2. METHODS
2.1. Data source
Medical and pharmacy claims from the Humana database from January 2007 to June 2015 were used to conduct the analysis. The Humana database includes over 18 million covered lives of commercial and Medicare members in all census regions in the United States, but predominantly in the Midwest and South regions. Over 9 million members have both medical and pharmacy coverage. The present study used data elements such as demographics, enrollment history, inpatient and outpatient claims, emergency department visits, and pharmacy claims for commercial and Medicare Advantage Part D members. Data were de‐identified and data collection complied with the requirements of the Health Insurance Portability and Accountability Act (HIPAA).
2.2. Study design
A retrospective longitudinal cohort design was used to compare the risk of VTE recurrence and major bleeding events among cancer patients treated with different anticoagulants. Newly diagnosed patients with cancer (defined as at least one inpatient stay or two outpatient visits with a diagnosis of cancer with a prior washout period of 6 months) and with a first VTE (index VTE) after 2013 were identified. VTE can be an early sign of cancer, so the index VTE had to occur within a 30‐day window before the first cancer diagnosis or at any time after the first cancer diagnosis. Patients with one or more dispensing of an anticoagulant agent within 7 days after their index VTE diagnosis (termed the index anticoagulant therapy) were selected and classified into LMWH, warfarin, or rivaroxaban treatment group (other agents [including unfractionated heparin, fondaparinux, apixaban, dabigatran, and edoxaban] were not included due to low utilization). Patients who received LMWH for a short duration as a bridging agent were classified into the warfarin treatment group. Patients with a prior VTE diagnosis or with an anticoagulant (i.e., warfarin, rivaroxaban, LMWH, unfractionated heparin, fondaparinux, apixaban, dabigatran, and edoxaban) dispensing before their index VTE were excluded from the study.
The observation period for the analysis of VTE recurrence spanned from the initiation of an anticoagulant therapy until the earliest of either the end of data availability (June 2015) or the end of eligibility. Using an intent‐to‐treat approach, treatment switches from the index anticoagulant to another anticoagulant (i.e., initiate a new anticoagulant after discontinuing index anticoagulant therapy [gap of greater than 60 days between the end of the days of supply of a dispensing and the start date of the next dispensing]) were permitted. A sensitivity analysis was performed where the observation period was censored at treatment switch. The observation period for the analysis of major bleeding spanned from the initiation of an anticoagulant therapy until the earliest of either treatment nonpersistence (discontinuation), the end of eligibility, or the end of data availability. Patients needed to be continuously enrolled on both a medical and pharmacy health plan 6 months before their cancer diagnosis until the end of the observation period.
2.3. Study endpoints
The effectiveness endpoint was VTE recurrence, defined as a hospitalization with a primary diagnosis of VTE at least 7 days after the index VTE. The safety endpoint was major bleeding. Major bleeding events were identified based on a primary diagnosis ICD‐9‐CM code for any bleeding at a gastrointestinal, genitourinary, cerebral, or other relevant site, as identified by a validated algorithm developed by Cunningham et al. designed to identify hospitalizations related to bleeding.18 A major bleeding event was also identified by the algorithm if a patient had a primary diagnosis for selected conditions such as chronic or unspecified duodenal ulcer with perforation, esophagitis, and acute posthemorrhagic anemia, and a secondary diagnosis of bleeding at one of the bleeding sites identified above. The use of bleeding diagnoses has shown a positive predictive value of 89% to 99% in Cunningham's validation study.18
2.4. Statistical analysis
Descriptive analyses were conducted to compare patient demographics and baseline clinical characteristics between groups. Mean, standard deviation, and median were reported for continuous variables; frequency and proportion were reported for categorical variables. Statistical differences between groups were assessed using standardized differences.
Three comparisons were performed among the anticoagulant agents: LMWH compared to rivaroxaban, LMWH compared to warfarin, and rivaroxaban compared to warfarin. To reduce the potential for confounding between groups, the inverse probability of treatment weights (IPTW) approach was used based on propensity scores for each comparison. Weighting patients by the inverse probability of the treatment received creates a synthetic sample independent of the baseline covariate.19 Each patient was assigned a weight such that, in the weighted pseudo‐population, the distribution of measured confounders was similar between the compared treatment groups. To calculate IPTWs, the probability (i.e., the propensity score) of receiving LMWH (vs. rivaroxaban; comparison 1) was first estimated using a multivariate logistic regression model conditional on baseline covariates, including age, sex, type of cancer, very high‐risk, and high‐risk cancer types for VTE, region, race, time from cancer to VTE diagnosis, time to anticoagulant initiation, setting of VTE diagnosis (inpatient, outpatient, or emergency room), type of VTE (DVT, PE, or both), treatment with an antineoplastic agent, and Charlson comorbidity index (CCI). As an exploratory analysis to better understand the risk of major bleeding in cancer patients unrelated to anticoagulation, a fourth comparison was made between patients with VTE treated with any of the anticoagulant agents and patients who did not have VTE and were not treated with any anticoagulant agent. For this fourth comparison, patients were matched 1:1 based on their propensity scores.
Kaplan–Meier curves for time from the index date to first recurrent VTE event were compared between groups in the IPTW‐weighted populations. Kaplan–Meier rates for VTE recurrences at 6 and 12 months after the index date were compared between groups using log‐rank tests. For the major bleeding analysis, Kaplan–Meier rates at 3 and 6 months were evaluated and compared between groups. Weighted Cox proportional hazards models (i.e., time to event analysis) were used to compare the time to recurrence or major bleeding between groups. Patients were censored at the end of the observation period if no event occurred. Nonparametric bootstrap procedures with 499 replications were used to estimate 95% confidence intervals (CIs) and P‐values.
3. RESULTS
3.1. Patient characteristics
A total of 2428 cancer patients who developed a VTE and were treated with anticoagulant agents within 7 days of VTE diagnosis were identified, including 1061 treated with warfarin, 707 with rivaroxaban, and 660 with LMWH. Median duration of therapy with LMWH (1.0 month) was shorter than for rivaroxaban (3.0 months) and warfarin (3.5 months). Weighted groups were similar in terms of baseline characteristics (Table 1). The mean age was 73 years and about half of patients were women. PE represented between 25% to 30% of the index VTE and DVT represented between 55% and 60%. The remaining 13% to 15% of patients were diagnosed with both PE and DVT. About 10% of patients were diagnosed with a cancer type (stomach, pancreas, or brain) associated with a very high risk of developing VTE and one‐third were diagnosed with a cancer type (lung, lymphatic, gynecologic, bladder, testicular, or renal) associated with a high risk of developing VTE. Prior surgeries,13 including major surgery, abdominopelvic surgery, and neurosurgery or orthopedic surgery, as well as provoked VTE,13 were also generally similar between groups. Some differences were observed in selected comorbidities; patients in the LMWH group had lower rates of hypertension, COPD, and diabetes compared to patients in the rivaroxaban or warfarin groups during the 6‐month baseline period.
Table 1.
Patient characteristics of the weighted cohortsa ,b
| Characteristics | Rivaroxaban cohort(N = 685) | LMWH cohort (N = 682) | Std. diff. (%) | Rivaroxaban cohort (N = 892) | Warfarin cohort (N = 876) | Std. diff. (%) | LMWH cohort (N = 856) | Warfarin cohort (N = 865) | Std. diff. (%) |
|---|---|---|---|---|---|---|---|---|---|
| Demographicsb | |||||||||
| Age, years, mean ± SD | 72.7 ± 8.8 | 72.6 ± 10.8 | 1.2 | 73.4 ± 10.2 | 73.3 ± 9.3 | 0.8 | 72.3 ± 11.7 | 72.4 ± 9.4 | 1.2 |
| Gender, female, % | 51.6 | 51.5 | 0.1 | 48.7 | 48.4 | 0.5 | 50.1 | 50.4 | 0.5 |
| Diagnosis of high‐risk cancer during the baseline period,c % | |||||||||
| Very high risk | 13.7 | 12.5 | 3.4 | 8.7 | 8.7 | 0.1 | 12.9 | 13.4 | 0.8 |
| Stomach | 2.5 | 2.5 | 0.4 | 1.9 | 1.6 | 2.0 | 2.7 | 2.0 | 0.7 |
| Pancreas | 6.3 | 7.0 | 3.2 | 4.2 | 4.2 | 0.2 | 7.3 | 6.3 | 6.2 |
| Brain tumor | 4.9 | 3.4 | 7.5 | 2.7 | 2.9 | 1.7 | 3.3 | 5.0 | 7.4 |
| High risk | 34.7 | 34.9 | 0.3 | 29.1 | 29.1 | 0.1 | 33.7 | 33.8 | 2.1 |
| Lung | 19.7 | 20.1 | 1.4 | 15.7 | 15.5 | 0.5 | 19.1 | 19.2 | 0.3 |
| Lymphoma | 5.5 | 5.9 | 2.3 | 4.5 | 4.7 | 0.9 | 6.1 | 5.6 | 2.6 |
| Gynecologic | 6.8 | 6.9 | 0.9 | 6.1 | 4.9 | 4.9 | 6.5 | 5.1 | 5.7 |
| Bladder | 3.8 | 3.6 | 1.1 | 3.5 | 4.5 | 5.1 | 3.7 | 4.5 | 7.5 |
| Testicular | 0.2 | 0.1 | 3.5 | 0.3 | 0.3 | 1.1 | 0.1 | 0.4 | 10.0 |
| Renal | 0.6 | 0.7 | 2.0 | 0.4 | 0.2 | 5.1 | 0.7 | 0.2 | 5.0 |
| Type of index VTE,b % | |||||||||
| PE | 29.9 | 30.0 | 0.3 | 25.2 | 25.8 | 1.4 | 26.9 | 27.1 | 0.5 |
| DVT | 55.2 | 55.5 | 0.5 | 60.8 | 60.3 | 1.0 | 58.9 | 58.0 | 1.7 |
| PE and DVT | 14.9 | 14.5 | 1.1 | 14.0 | 13.9 | 0.4 | 14.3 | 14.9 | 1.7 |
| Region, % | |||||||||
| South | 61.6 | 61.7 | 0.2 | 58.1 | 58.6 | 1.1 | 54.2 | 53.5 | 1.3 |
| Midwest | 25.1 | 25.2 | 0.3 | 27.0 | 27.4 | 1.0 | 30.7 | 30.9 | 0.5 |
| Northeast | 2.9 | 3.0 | 0.3 | 2.2 | 2.1 | 0.8 | 3.0 | 2.8 | 1.2 |
| West | 10.3 | 10.1 | 0.9 | 12.7 | 11.8 | 2.6 | 12.2 | 12.8 | 1.8 |
| Quan‐Charlson comorbidity index, mean ± SD | 4.8 ± 2.9 | 4.8 ± 3.1 | 1.6 | 4.5 ± 3.3 | 4.5 ± 2.6 | 0.1 | 4.9 ± 3.5 | 4.9 ± 2.7 | 0.2 |
| Selected baseline comorbidities, % | |||||||||
| Hypertension | 72.2 | 66.3 | 12.9 | 72.3 | 75.1 | 6.9 | 65.0 | 75.2 | 22.0 |
| COPD | 32.7 | 25.8 | 15.3 | 30.4 | 27.6 | 5.9 | 25.9 | 30.5 | 10.0 |
| Diabetes | 33.1 | 26.2 | 15.3 | 33.3 | 31.2 | 4.4 | 25.8 | 32.4 | 14.4 |
| Congestive heart failure | 15.0 | 11.7 | 9.9 | 15.8 | 13.1 | 7.9 | 11.8 | 13.8 | 6.0 |
| Liver diseases | 17.4 | 20.1 | 6.8 | 14.3 | 13.0 | 3.6 | 21.1 | 14.8 | 16.7 |
| Renal | 16.1 | 13.6 | 7.0 | 16.1 | 19.6 | 9.1 | 13.1 | 19.9 | 16.2 |
| Obesity | 12.2 | 11.3 | 2.9 | 11.7 | 13.0 | 3.9 | 12.1 | 13.6 | 4.3 |
| Provoked VTEd ,f | 8.7 | 8.1 | 2.2 | 9.2 | 12.3 | 9.9 | 8.1 | 12.0 | 13.1 |
| Atrial fibrillation/flutter | 7.1 | 6.7 | 1.7 | 7.7 | 8.5 | 3.1 | 7.2 | 8.6 | 5.1 |
| Stroke/TIA | 4.8 | 3.5 | 6.5 | 3.5 | 5.4 | 9.4 | 3.8 | 5.8 | 9.5 |
| Prior surgery,d % | |||||||||
| Major surgery | 10.8 | 11.0 | 0.5 | 10.4 | 11.5 | 3.4 | 12.4 | 12.3 | 0.7 |
| Abdominopelvic surgery | 14.6 | 17.3 | 7.3 | 13.6 | 15.9 | 6.8 | 18.1 | 17.2 | 2.4 |
| Neurosurgery or orthopedic surgery | 1.9 | 1.0 | 6.4 | 2.5 | 3.1 | 3.4 | 1.3 | 2.7 | 10.4 |
LMWH: Low‐molecular‐weight heparin; Std. diff.: Standardized difference; SD: Standard deviation; VTE: Venous thromboembolism; PE: Pulmonary embolism; DVT: Deep vein thrombosis; COPD: Chronic obstructive pulmonary disease; TIA: Transient ischemic attack.
Notes:
Other patient characteristics included in the propensity score included race, region, time from first cancer to initial VTE, time to anticoagulant initiation, year of first VTE, setting in which the VTE was diagnosed (inpatient, outpatient, or emergency room), type of cancer at index date (solid or hematologic), and treatment with an antineoplastic agent.
The weighted cohorts are reported with different Ns than the unweighted cohorts.
Evaluated at the index date.
Not mutually exclusive.
Evaluated during the 30‐day period prior to the index VTE.
Defined as an index VTE with trauma, acute spinal cord injury, fracture, estrogen therapy, pregnancy/postpartum state, oral contraceptive use, neurosurgery, or orthopedic surgery.
3.2. VTE recurrence
During the follow‐up period, 13% of patients treated with rivaroxaban, 18% of patients treated with LMWH, and 18% of patients treated with warfarin experienced a recurrent VTE event (Table 2). Compared to LMWH users, VTE recurrence rates were lower for rivaroxaban users at 6 months (17.1% vs. 13.2%; P = .060), although the difference was not statistically significant, and at 12 months (22.2% vs. 16.5%; P = .030), at which point the difference was statistically significant (Figure 1). The overall rate of VTE recurrence was 28% lower in the rivaroxaban group compared to the LMWH group (hazard ratio [HR]: 0.72, CI: 0.52‐0.95; P = .024). Compared to warfarin, rates of VTE recurrences among rivaroxaban users were significantly lower at 6 months (17.5% vs. 13.2%; P = .014) and 12 months (19.9% vs. 15.7; P = .017; Figure 1). Rivaroxaban users were 26% less likely to have a VTE recurrence compared to warfarin users (HR: 0.74, 95% CI: 0.56‐0.96; P = .028). Risk of VTE recurrence was similar for patients treated with LMWH and warfarin (HR: 1.02, 95% CI: 0.77‐1.30; P = .896). In the sensitivity analysis, in which patient follow‐up was censored at treatment switch, rivaroxaban users were also at significantly lower risk for VTE recurrence relative to LMWH (HR: 0.71, 95% CI: 0.51‐0.96; P = .020) and to warfarin (HR: 0.74, 95% CI: 0.58‐0.95; P = .032) users. There was no risk difference for VTE recurrence between LMWH relative to warfarin users (HR: 1.01, 95% CI: 0.79‐1.33; P = .828).
Table 2.
VTE recurrence and major bleeding events
| Number of events (%) | |||||
|---|---|---|---|---|---|
| Rivaroxaban versus LMWH | LMWH (N = 682) | Rivaroxaban (N = 685) | HR | 95% CIa | P‐valuea |
| VTE recurrence | |||||
| Primary diagnosis in a hospitalization | 120 (17.6) | 90 (13.1) | 0.72 | 0.52‐0.95 | .024 |
| Sensitivity with follow‐up truncated at treatment switch | 105 (15.4) | 88 (12.9) | 0.71 | 0.51‐0.96 | .020 |
| Major bleedingb | 28 (4.1) | 46 (6.7) | 1.03 | 0.64‐1.65 | .917 |
| Number of events (%) | |||||
|---|---|---|---|---|---|
| Rivaroxaban versus warfarin | Warfarin (N = 876) | Rivaroxaban (N = 892) | HR | 95% CIa | P‐valuea |
| VTE recurrence | |||||
| Primary diagnosis in a hospitalization | 157 (17.9) | 119 (13.3) | 0.74 | 0.56‐0.96 | .028 |
| Sensitivity with follow‐up truncated at treatment switch | 154 (17.6) | 117 (13.1) | 0.74 | 0.58‐0.95 | .032 |
| Major bleedingb | 65 (7.5) | 63 (7.0) | 1.01 | 0.71‐1.43 | .961 |
| Number of events (%) | |||||
|---|---|---|---|---|---|
| Warfarin versus LMWH | LMWH (N = 856) | Warfarin (N = 865) | HR | 95% CIa | P‐valuea |
| VTE recurrence | |||||
| Primary diagnosis in a hospitalization | 156 (18.2) | 156 (18.1) | 1.02 | 0.77‐1.30 | .896 |
| Sensitivity with follow‐up truncated at treatment switch | 139 (16.3) | 154 (17.8) | 1.01 | 0.79‐1.33 | .828 |
| Major bleedingb | 39 (4.4) | 63 (7.2) | 1.04 | 0.69‐1.57 | .836 |
VTE: Venous thromboembolism; LMWH: Low‐molecular‐weight heparin; HR: Hazard ratio; CI: Confidence interval.
Notes:
Statistical difference between cohorts (95% CI and P‐value) were obtained using nonparametric bootstrap procedure methods with 499 replications.
From the first anticoagulant dispensing to the earliest between end of eligibility, end of data availability (June 2015), or treatment nonpersistence (i.e., after the end of the days of supply of the first dispensing for which the next dispensing of the index medication, if any, was more than 60 days later). For patients receiving LMWH/warfarin as an index therapy, persistence was evaluated based on warfarin therapy.
Figure 1.
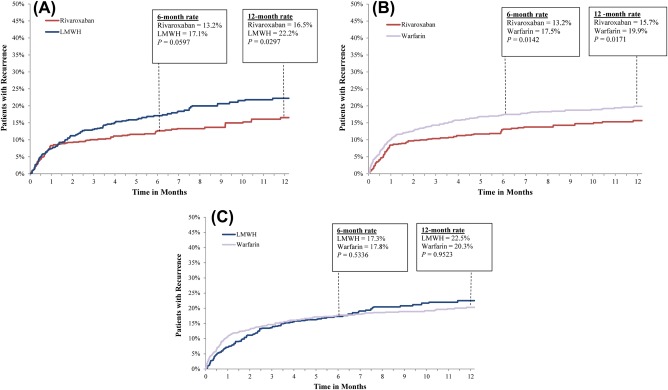
Rates of VTE recurrences. LMWH: low‐molecular‐weight heparin; VTE: venous thromboembolism
3.3. Bleeding events
Rates of major bleeding for LMWH and rivaroxaban users were 8.3% and 8.2%, respectively, at 6 months, with an HR of 1.03 (95% CI: 0.64‐1.65; P = .917; Table 2 and Figure 2). In the comparison between LMWH and warfarin users, major bleeding rates were 8.5% and 8.6%, respectively, at 6 months with an HR of 1.04 (95% CI: 0.69‐1.57; P = .836). The rate of major bleeding was also similar for rivaroxaban and warfarin users—9.0% and 8.7%, respectively, at 6 months with an HR of 1.01 (95% CI: 0.71‐1.43; P = .961). The majority of bleeding events were gastrointestinal in each group (Supporting Information Table S1).
Figure 2.
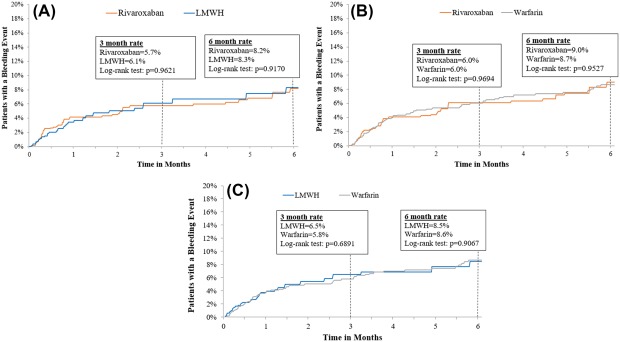
Rates of major bleeding events. LMWH: low‐molecular‐weight heparin
To understand the rates of major bleeding that are not related to anticoagulation in patients with cancer, a pooled cohort of patients treated with all three anticoagulants (2428) were matched to cancer patients who did not have VTE nor received anticoagulation. The rates of major bleeding at 3 months were 5.9% and 2.6% in the anticoagulated and control cohorts, respectively. At 6 months, the rates were 8.7% and 4.2% in the anticoagulated and control cohorts, respectively.
4. DISCUSSION
The intent of this claims analysis study was to assess the benefits and risks associated with VTE treatment among currently prescribed anticoagulants in patients with cancer across US clinical practices. Patients treated with LMWH had a similar risk of recurrent VTE as patients treated with warfarin. However, patients treated with rivaroxaban were 28% and 26% less likely to suffer recurrent VTE compared to LMWH and warfarin, respectively. There was no increased risk of major bleeding for any of the observed anticoagulants in comparison to each other, with cumulative incidence rates of 5.9% and 8.7% at 3 and 6 months, respectively. The exploratory analysis suggests that patients with cancer are at risk of bleeding irrespective of anticoagulant use.
Two large prospective randomized controlled trials have previously demonstrated that LMWH (dalteparin and tinzaparin) is associated with a lower risk of recurrent VTE than warfarin in cancer patients, but there were differences in the magnitude of risk reduction between the two studies. The CLOT study reported a 52% lower risk of recurrent VTE in patients receiving dalteparin when compared to warfarin over a 6‐month study period (P = .002).8 The CATCH study reported a 35% decreased risk of VTE in patients receiving tinzaparin when compared to warfarin over a 6‐month study period, but the result did not reach statistical significance (P = .07).9 With respect to bleeding risk, the rates of major bleeding in the LMWH and warfarin arms of CLOT and CATCH were similar to the findings reported in the current study. One potential explanation for the inferior efficacy of LMWHs observed in the current study is that patients are suboptimally treated in real‐world settings. The median duration of therapy with LMWHs (1 month) was significantly shorter than with oral anticoagulants such as rivaroxaban (3 months) and warfarin (3.5 months). This trend is in line with other studies reporting that the duration of therapy for LMWH tends to be shorter than for oral anticoagulants10, 11, 12, 20 and, furthermore, suggests that most cancer patients on LMWH are not getting the generally recommended 3 to 6 months of treatment in a real‐world clinical practice setting. In addition, a separate large claims analysis also has reported a higher persistence rate at 6 months for oral anticoagulants among cancer patients when compared to LMWHs (61% vs. 37%).13 A reason patients may be stopping treatment with LMWH early could be the higher cost of the injectable as compared to the oral agents. Injectable medications are usually set at a higher copay than oral medications, so patients may consider discontinuing LMWH if the copay proves cost prohibitive.21
The cumulative rates for major bleeding at 6 months observed in the current study (8.5%) appear similar to those reported in the CLOT study (4‐6%) and higher than those reported in the CATCH study (3%).8 This discrepancy between the two randomized controlled trials and the current study may be explained, in part, by differences in patient demographics. Patients in the current study cohorts were generally older than in the randomized trials, with a mean population age of 73 years compared to 63 years in CLOT and 59 years in CATCH. In addition, many patients in this study had comorbidities that are known risk factors for bleeding, such as hypertension (65‐75%), liver disease (13‐21%), renal disease (13‐20%), congestive heart failure (12‐15%), and diabetes (26‐33%).22
There are no clinical trial data that directly compare LMWHs and rivaroxaban in patients with cancer, although such trials are currently being conducted. However, several small studies corroborate the perceived benefits of rivaroxaban over LMWHs in patients with cancer. A retrospective analysis of electronic medical records for cancer patients with VTE reported that rivaroxaban had similar safety (absence of bleeding) and efficacy (rethrombosis) when compared to a LMWH plus warfarin (P = .54 and P = .25, respectively) or to LMWH alone (P = .46 and P = .29, respectively).23 A separate prospective cohort study assessed rivaroxaban in 200 patients with cancer‐associated thrombosis and reported 6‐month cumulative incidence rates of 4.4% for new and recurrent VTEs and 2.2% for major bleeding.24 Finally, another prospective cohort study in 296 evaluable patients treated with rivaroxaban for DVT or PE reported similar VTE recurrence rates between those with and without an active malignancy (3.3% vs. 2.8%, P = .533).25 The result suggests the relative efficacy of rivaroxaban may be higher among cancer patients who have been reported to have a 3‐fold greater risk of VTE compared to patients without cancer.5 Furthermore, the risk of recurrent VTE noted in prospective cohort studies of rivaroxaban in the treatment of cancer‐associated VTE also appear numerically lower than those reported in the LMWH arms of the CLOT (8%) and CATCH (7.2%) studies.24, 25 These studies together provide further support for the use of rivaroxaban over LMWHs and warfarin to protect against VTE without further increasing bleeding risk in cancer patients. These results suggest rivaroxaban could be an effective treatment option, with potentially better patient adherence as indicated by time on therapy, for lowering the risk of VTE recurrence in cancer patients while having a similar bleeding risk to that of LMWHs. Head‐to‐head comparisons of LMWHs and DOACs in randomized clinical trials are currently ongoing and will provide further insights into their efficacy and safety in patients with cancer.
Several limitations of the current study should be noted. Cancer stage data was not available; therefore, the analysis did not control for this variable, although the assumption is that case severity is evenly distributed across treatment cohorts. In addition, the Cunningham criteria have not been validated in patients with cancer‐associated thrombosis and thus could potentially under‐ or over‐estimate the incidence of major bleeding events. The HRs remain valid, however, as the Cunningham criteria were applied to all three cohorts equally. A limitation of the methodology was that deaths from cancers were not considered as a competing risk but the assumption was that the rates were the same across treatment cohorts. Additional limitations to note include the possibility of billing inaccuracies and missing data in the claims records. It is possible that the number of recurrent thrombotic events was underestimated due to under‐coding of VTE events. However, in this study, recurrent VTE was determined based only on the primary diagnosis during hospitalization. The use of this strict coding definition for VTE gives us greater confidence that only “true‐positive” VTE events were captured, but likely underestimates the number of recurrent thrombotic events because asymptomatic or less symptomatic VTE may be diagnosed and treated in outpatient settings. However, we believe it is reasonable to assume that the prevalence of such issues is similar between the study cohorts. Finally, an inherent limitation of observational studies is that adjustments in the multivariate analyses could only account for observable factors.
5. CONCLUSIONS
This real‐world claims analysis is the first to compare the effectiveness and safety of the most‐commonly prescribed anticoagulants across US clinical practices for cancer‐associated thrombosis. Compared to clinical trials, LMWH did not show superiority over warfarin at preventing VTE recurrence in the real‐world setting, a result that may be driven by the suboptimal duration of treatment observed in the current analysis. Patients who initiated rivaroxaban had significantly lower risk of recurrent VTE compared to those treated with LMWH or warfarin and experienced similar rates of major bleeding. This observation suggests rivaroxaban could be a valuable alternative to LMWH for the treatment and secondary prophylaxis against VTE in cancer patients. Further investigation is warranted to determine the benefits and risks of rivaroxaban and other DOACs for treatment of cancer‐associated thrombosis.
CONFLICT OF INTERESTS
M. B. Streiff reports having received nonfinancial support from Janssen (study design, statistical analysis, manuscript preparation) during the conduct of the study; outside of the submitted work, he reports having received grants and consulting fees from Janssen, consulting fees from Daiichi‐Sankyo and Portola, and grants from Boehringer‐Ingelheim, Portola, and Roche. C. Crivera, J. Schein, and D. Milentijevic are employees of Janssen Scientific Affairs, LLC, and are shareholders of Johnson & Johnson. D. Yannicelli was an employee of Janssen Pharmaceuticals, Inc. at the time of the study. W. W. Nelson was an employee of Janssen Scientific Affairs, LLC, at the time of the study and is a shareholder of Johnson & Johnson. J. Fortier, F. Laliberté, and P. Lefebvre are employees of Analysis Group, a company that received research grants from Janssen Scientific Affairs, LLC, during the conduct of the study. A. A. Khorana has received honoraria for consulting from Janssen, Bayer, Pfizer, Halozyme, AngioDynamics, and Leo Pharma and research funding (to institution) from Amgen; A. A. Khorana would also like to acknowledge additional research support from Sondra and Stephen Hardis Endowed Chair in Oncology Research. K. McCrae has nothing to disclose.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
Technical editorial assistance was provided by Shannon O'Sullivan, ELS, of MedErgy, and was supported by Janssen Scientific Affairs, LLC.
Streiff MB, Milentijevic D, McCrae K, et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol. 2018;93:664–671. https://doi.org/10.1002/ajh.25059
Funding information This study was supported by Janssen Scientific Affairs, LLC
REFERENCES
- 1. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632–634. [DOI] [PubMed] [Google Scholar]
- 2. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23 Suppl 1):I17–I21. [DOI] [PubMed] [Google Scholar]
- 3. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ. 3rd,. Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160(6):809–815. [DOI] [PubMed] [Google Scholar]
- 4. Gerotziafas GT, Mahe I, Elalamy I. New orally active anticoagulant agents for the prevention and treatment of venous thromboembolism in cancer patients. Ther Clin Risk Manag. 2014;10:423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484–3488. [DOI] [PubMed] [Google Scholar]
- 6. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(17):2189–2204. [DOI] [PubMed] [Google Scholar]
- 7. Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition). Chest. 2008;133(6 Suppl):454s–545s. [DOI] [PubMed] [Google Scholar]
- 8. Lee AYY, Levine MN, Baker RI, et al. Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–153. [DOI] [PubMed] [Google Scholar]
- 9. Lee AY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677–686. [DOI] [PubMed] [Google Scholar]
- 10. Delate T, Witt DM, Ritzwoller D, et al. Outpatient use of low molecular weight heparin monotherapy for first‐line treatment of venous thromboembolism in advanced cancer. Oncologist. 2012;17(3):419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farge D, Trujillo‐Santos J, Debourdeau P, et al. Fatal events in cancer patients receiving anticoagulant therapy for venous thromboembolism. Medicine. 2015;94(32):e1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. den Exter PL, Hooijer J, Dekkers OM, Huisman MV. Risk of recurrent venous thromboembolism and mortality in patients with cancer incidentally diagnosed with pulmonary embolism: a comparison with symptomatic patients. J Clin Oncol. 2011;29(17):2405–2409. [DOI] [PubMed] [Google Scholar]
- 13. Khorana AA, McCrae K, Milentijevic D, et al. Current practice patterns and patient persistence on anticoagulant treatments for cancer‐associated thrombosis. Res Pract Thromb Haemost. 2017;1(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN‐DVT and EINSTEIN‐PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37–e46. [DOI] [PubMed] [Google Scholar]
- 15. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta‐analysis. Chest. 2015;147(2):475–483. [DOI] [PubMed] [Google Scholar]
- 16. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct oral anti‐coagulants compared with vitamin‐K inhibitors and low‐molecular‐weight‐heparin for the prevention of venous thromboembolism in patients with cancer: a meta‐analysis study. Int J Cardiol. 2017;230:214–221. [DOI] [PubMed] [Google Scholar]
- 17. Carrier M, Cameron C, Delluc A, Castellucci L, Khorana AA, Lee AY. Efficacy and safety of anticoagulant therapy for the treatment of acute cancer‐associated thrombosis: a systematic review and meta‐analysis. Thromb Res. 2014;134(6):1214–1219. [DOI] [PubMed] [Google Scholar]
- 18. Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20(6):560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khorana AA, Yannicelli D, McCrae KR, et al. Evaluation of US prescription patterns: are treatment guidelines for cancer‐associated venous thromboembolism being followed? Thromb Res. 2016;145:51–53. [DOI] [PubMed] [Google Scholar]
- 21. Kiser K. Oral Anticoagulation Therapy: Cases and Clinical Correlation. 1st ed New York: Springer; 2017. [Google Scholar]
- 22. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2):e419S–e494S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xavier FD, Hoff PMG, Braghiroli MI, et al. Rivaroxaban: an affordable and effective alternative in cancer‐related thrombosis? J Glob Oncol. 2016;3:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mantha S, Laube E, Miao Y, et al. Safe and effective use of rivaroxaban for treatment of cancer‐associated venous thromboembolic disease: a prospective cohort study. J Thromb Thrombolysis. 2017;43(2):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bott‐Kitslaar DM, Saadiq RA, McBane RD, et al. Efficacy and safety of rivaroxaban in patients with venous thromboembolism and active malignancy: a single‐center registry. Am J Med. 2016;129(6):615–619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


