Abstract
BACKGROUND
Antibodies targeting the programmed death‐ligand 1 (PD‐L1)/programmed cell death protein 1 (PD‐1) checkpoint may cause adverse events (AEs) that are linked to the mechanism of action of this therapeutic class and unique from those observed with conventional chemotherapy.
METHODS
Patients with advanced solid tumors who were enrolled in the phase 1 JAVELIN Solid Tumor (1650 patients) and phase 2 JAVELIN Merkel 200 (88 patients) trials received avelumab, a human anti–PD‐L1 IgG1 antibody at a dose of 10 mg/kg every 2 weeks. Treatment‐related AEs (TRAEs) were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). In post hoc analyses, immune‐related AEs (irAEs) were identified via an expanded AE list and medical review, and infusion‐related reactions (IRRs) occurring ≤2 days after infusion and symptoms occurring ≤1 day after infusion and resolving ≤2 days after onset were identified based on prespecified Medical Dictionary for Regulatory Activities (MedDRA) terms.
RESULTS
Of the 1738 patients analyzed, grade ≥3 TRAEs occurred in 177 (10.2%); the most common were fatigue (17 patients; 1.0%) and IRR (10 patients; 0.6%). TRAEs led to discontinuation in 107 patients (6.2%) and death in 4 patients (0.2%). Grade ≥3 irAEs occurred in 39 patients (2.2%) and led to discontinuation in 34 patients (2.0%). IRRs or related symptoms occurred in 439 patients (25.3%; grade 3 in 0.5% [9 patients] and grade 4 in 0.2% [3 patients]). An IRR occurred at the time of first infusion in 79.5% of 439 patients who had an IRR, within the first 4 doses in 98.6% of 439 patients who had an IRR, and led to discontinuation in 35 patients (2.0%).
CONCLUSIONS
Avelumab generally was found to be well tolerated and to have a manageable safety profile. A minority of patients experienced grade ≥3 TRAEs or irAEs, and discontinuation was uncommon. IRRs occurred mainly at the time of first infusion, and repeated events were infrequent. Cancer 2018;124:2010‐7. © 2018 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society. This is an open access article under the terms of the http://creativecommons.org/licenses/by-nc/4.0/ License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Keywords: avelumab, immunotherapy, JAVELIN, programmed death‐ligand 1 (PD‐L1), safety
Short abstract
In the current pooled analysis of 1738 patients treated with avelumab in the phase 1 JAVELIN Solid Tumor and phase 2 JAVELIN Merkel 200 trials, the incidences of grade ≥3 treatment‐related adverse events or any‐grade immune‐related adverse events is reported to be low. Avelumab generally is well tolerated and has a manageable safety profile consistent with other anti–programmed death‐ligand 1/programmed cell death protein 1 antibodies.
INTRODUCTION
Immune checkpoint inhibitors (ICIs) that block the programmed death 1 axis (programmed death‐ligand 1 [PD‐L1] and programmed cell death protein 1 [PD‐1]) are important treatment options in numerous tumor types.1 Common treatment‐related adverse events (TRAEs) with anti–PD‐L1/PD‐1 agents include low‐grade fatigue, pruritus, and rash.2, 3 In addition, potentially serious immune‐related AEs (irAEs), such as high‐grade pneumonitis or autoimmune‐like side effects, occur in a minority of patients.2, 3 To the best of our knowledge, the mechanisms underlying toxicities related to ICIs are not fully understood, and agents targeting PD‐L1 or PD‐1 may have differing safety profiles.2
Avelumab, a human anti–PD‐L1 immunoglobulin (Ig) G1 antibody that specifically inhibits PD‐L1/PD‐1 interactions,4 is approved in the United States and European Union for the treatment of metastatic Merkel cell carcinoma (mMCC),5, 6 in Japan for the treatment of MCC that cannot be completely cured with surgery, and in the United States for the treatment of locally advanced or metastatic urothelial carcinoma (UC) that has progressed during or after platinum‐containing chemotherapy.5
JAVELIN Solid Tumor (http://ClinicalTrials.gov identifier NCT01772004) is a large, phase 1 dose‐escalation and dose‐expansion trial of avelumab in patients with advanced tumors. Doses ≤20 mg/kg every 2 weeks (Q2W) were safely administered during the dose‐escalation phase.4 Based on pharmacokinetic, target occupancy, and safety analyses, a dose of 10 mg/kg Q2W was selected for further study.4 Objective responses and disease stabilization with avelumab have been reported in several tumor types, including non‐small cell lung cancer (NSCLC), UC, gastric and ovarian cancer, and mesothelioma,7, 8, 9, 10, 11 and pooled safety data have been presented previously in 1300 patients.12 JAVELIN Merkel 200 (http://ClinicalTrials.gov identifier NCT02155647) is a phase 2 trial of avelumab in patients who experienced disease progression after prior chemotherapy for mMCC; durable responses were observed.13, 14 Herein, we report analyses of pooled safety data from the JAVELIN Solid Tumor and Merkel 200 trials to further characterize the safety profile of avelumab.
MATERIALS AND METHODS
Study Design and Patients
JAVELIN Solid Tumor is a global, multicenter, multicohort, open‐label, dose‐escalation, and dose‐expansion phase 1 trial of avelumab in patients with advanced solid tumors. JAVELIN Merkel 200 is a multicenter, international, prospective, open‐label, single‐arm, phase 2 trial of avelumab in patients who experienced disease progression after ≥1 prior line of chemotherapy for mMCC. General and cohort‐specific eligibility criteria are provided in Supporting Table 1.
All patients were enrolled in accordance with approved protocols, international standards of good clinical practice, institutional review board approvals, and institutional safety monitoring. Written informed consent was provided.
Treatments and Assessments
Avelumab was administered at a dose of 10 mg/kg as a 1‐hour intravenous infusion Q2W. Treatment continued until confirmed disease progression, unacceptable toxicity, or the occurrence of any protocol‐specified reason for discontinuation. Treatment was discontinued permanently in the event of any grade ≥3 AE (except for transient [≤6 hours] influenza‐like symptoms or pyrexia controlled with medical management; fatigue, local infusion‐related reaction [IRR], headache, nausea, or emesis that resolved to grade ≤1 within 24 hours; single laboratory values outside the normal range that were unrelated to study treatment and without clinical correlate [except for increased liver enzyme concentrations] that resolved to grade ≤1 within 7 days; and tumor flare, defined as local pain, irritation, or rash localized at sites of known or suspected malignant tissue) or recurring grade 2 TRAEs. Grade 2 AEs were managed by changes in the infusion rate and dose delays, and those that did not resolve to grade ≤1 by the end of the next cycle led to permanent discontinuation of avelumab. A delay of treatment for 0 to 2 days was not considered a dose delay.
AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI‐CTCAE; version 4.0). In comprehensive prospective and post hoc analyses, AEs of special interest, which were defined as irAEs and IRRs and related symptoms, were identified and characterized further. irAEs were identified using a prespecified list of Medical Dictionary for Regulatory Activities (MedDRA) terms and followed by comprehensive medical review. IRRs occurring on the day of or the day after infusion included events reported as IRRs, drug hypersensitivity, or hypersensitivity reactions; IRRs occurring ≤2 days after infusion and symptoms occurring ≤1 day after infusion that had resolved ≤2 days after onset were included in this expanded definition. To prevent a potential occurrence of IRRs, patients received premedication with diphenhydramine and acetaminophen. Treatment algorithms to manage irAEs were provided in the study protocol, and the treatment approach mainly was dependent on severity. For suspected irAEs, adequate evaluation was performed to confirm etiology or exclude other causes. For grade ≥2 irAEs, avelumab could be withheld and corticosteroids administered. If corticosteroids were used to treat an irAE, a taper of ≥1 month was initiated at the time of improvement to grade ≤1. In patients whose irAE could not be controlled with corticosteroid use, the administration of other systemic immunosuppressants was considered. Avelumab was permanently discontinued for serious grade 3/4 or recurrent grade 2 irAEs.5 Additional details for the management of irAEs are provided in Supporting Table 2.
Statistical Analysis
Safety was analyzed in all patients who received ≥1 dose of avelumab. The treatment period was defined from the first dose day (day 1) of trial treatment, ≤30 days after the last dose of trial treatment, or the earliest date of subsequent anticancer therapy minus 1 day, whichever occurred first. In addition, TRAEs were documented through the posttreatment safety follow‐up period and included all treatment‐emergent AEs that occurred <10 weeks after the last dose. Analyses of time to first event of an irAE and time to first occurrence of an irAE of grade ≥3 were provided as simple descriptive analyses (number, median, minimum, maximum, Q1 (25%), and Q3 (75%)). A competing risk analysis was provided to estimate the hazard of irAEs and IRRs over time. To explore potential late‐onset toxicities, the analyses of time to first onset and the competing risk analysis of irAEs included all irAEs observed during the treatment‐emergent period and safety follow‐up period with an onset ≤90 days after last treatment. Death was considered a competing event to the occurrence of an irAE. Results are provided as cumulative incidence rates of time to first event illustrating the cause‐specific hazards for time to first event according to Kalbfleisch and Prentice.15 Summary statistics for time to resolution of AEs were computed using Kaplan‐Meier methods. Time to resolution included all episodes for a patient, and a “+” symbol denoted a censored maximum value. The onset of the first IRR also was analyzed independent of the number of infusions received (ie, the frequencies of IRRs at infusion 1, infusion 2, etc) in relation to the number of patients at risk (patients who received the corresponding infusion without an IRR at an earlier infusion).
RESULTS
Patients
As of the data cutoff date of June 9, 2016, a total of 1738 patients had received avelumab monotherapy (Supporting Table 3): 1650 patients in the phase 1 JAVELIN Solid Tumor trial and 88 patients in the phase 2 JAVELIN Merkel 200 trial. The dose‐expansion phase of the JAVELIN Solid Tumor trial was still enrolling patients at the data cutoff and included 16 cohorts (Supporting Table 1); the majority of patients (1601 patients; 97.0%) had >3 months of follow‐up. Enrollment of patients into JAVELIN Merkel 200 was complete at the time of data cutoff, and all patients had ≥9 months of follow‐up.
At the time of analysis, treatment was ongoing in 287 patients (16.5%). Patients had received a median of 6 infusions of avelumab (range, 1‐63 infusions) and the median duration of treatment was 12.0 weeks (range, 2.0‐137.9 weeks). Dose delays of 3 to 6 days occurred in 88 patients (5.1%). At the time of analysis, a total of 1451 patients had discontinued treatment predominantly due to disease progression (1016 patients; 58.5%). All 1738 patients were included in the safety analysis.
Overall Safety and Incidence of AEs
The overall safety profile is summarized in Supporting Table 4. Any‐grade all‐causality AEs occurred in 1697 patients (97.6%) and were grade ≥3 in 1008 patients (58.0%). TRAEs occurred in 1164 patients (67.0%) and were grade ≥3 in 177 patients (10.2%). The most common TRAEs of any grade were fatigue and IRR, which were observed in 307 patients (17.7%) and 295 patients (17.0%), respectively (Table 1). The incidences of all AEs and TRAEs are shown in Supporting Tables 5 and 6, respectively. TRAEs led to permanent treatment discontinuation in 107 patients (6.2%); the most common were IRR (32 patients; 1.8%), gamma‐glutamyl transferase increase (7 patients; 0.4%), alanine aminotransferase increase (4 patients; 0.2%), blood creatine phosphokinase increase (4 patients; 0.2%), and fatigue (4 patients; 0.2%). Serious TRAEs occurred in 108 patients (6.2%), most commonly IRR (15 patients; 0.9%), pneumonitis (11 patients; 0.6%), pyrexia (6 patients; 0.3%), and adrenal insufficiency (5 patients; 0.3%). The incidences of all serious AEs and TRAEs are shown in Supporting Tables 7 and 8, respectively.
Table 1.
Most Common Treatment‐Related Adverse Events
|
N = 1738 No. (%) |
||
|---|---|---|
| TRAEa | Any Grade | Grade ≥3 |
| Any TRAE | 1164 (67.0) | 177 (10.2) |
| Fatigue | 307 (17.7) | 17 (1.0) |
| IRRb | 295 (17.0) | 10 (0.6) |
| Nausea | 150 (8.6) | 2 (0.1) |
| Diarrhea | 123 (7.1) | 5 (0.3) |
| Chills | 116 (6.7) | 0 |
| Pyrexia | 106 (6.1) | 0 |
| Decreased appetite | 90 (5.2) | 3 (0.2) |
| Hypothyroidism | 87 (5.0) | 3 (0.2) |
| AST increased | 38 (2.2) | 8 (0.5) |
| Lipase increased | 25 (1.4) | 17 (1.0) |
| GGT increased | 17 (1.0) | 10 (0.6) |
Abbreviations: AST, aspartate aminotransferase; GGT, gamma‐glutamyl transferase; IRR, infusion‐related reaction; TRAE, treatment‐related adverse event.
TRAEs of any grade occurring in ≥5% of patients or TRAEs of grade ≥3 occurring in ≥0.5% of patients are shown (graded according to NCI‐CTCAE [version 4.0]).
Frequency of IRRs reported is based on the MedDRA preferred term and not the composite definition for IRR as an adverse event of special interest, which included events reported as IRRs, drug hypersensitivity, or hypersensitivity reactions occurring on the day of or the day after infusion as well as signs and symptoms of an IRR that occurred on the day of infusion and resolved within 2 days.
Incidence of Death
A total of 911 patients (52.4%) died, and disease progression was the most common reason for death (744 patients; 42.8%). A total of 59 patients (3.4%) died because of an AE not related to treatment. The primary cause of death was unknown/missing in 104 patients (6.0%). The investigator considered TRAEs to be the primary cause of death in 4 patients (0.2%). One patient with gastric cancer experienced autoimmune hepatitis with peritoneal metastases and ascites. Of 2 patients with metastatic breast cancer, 1 experienced liver metastases and acute liver failure and the other, who had liver, lung, and soft tissue metastases and a prior/ongoing history of respiratory disorder (cough, dyspnea, and pneumonia), experienced respiratory distress. One patient with UC experienced treatment‐related pneumonitis, with ongoing Clostridium difficile colitis and diverticulitis not related to study treatment. In addition, 1 patient with NSCLC died after the treatment period due to acute respiratory failure.
irAEs and Management
Any‐grade irAEs occurred in 247 patients (14.2%); these were grade ≥3 in 39 patients (2.2%) and considered serious in 43 patients (2.5%) (Table 2). The most common irAEs were thyroid disorder (98 patients; 5.6%) and rash (90 patients; 5.2%). Other irAEs (eg, colitis, hepatitis, pneumonitis, adrenal insufficiency, and myositis) occurred in <2% of patients. The cumulative incidence of the time to first onset of immune‐related thyroid disorders and rash (any grade), with death as the competing event, are shown in Figure 1, which is representative of time‐to‐onset analyses for other irAEs. The median time to first onset of thyroid disorders (98 patients) was 12.1 weeks (range, 2.0‐55.7 weeks), 9.1 weeks (range, 0.1‐101.1 weeks) in patients with rash (90 patients), 8.9 weeks (range, 0.3‐49.9 weeks) in patients with colitis (26 patients), and 10.7 weeks (range, 0.4‐47.0 weeks) in patients with pneumonitis (21 patients). Among patients with irAEs, the median occurrence of irAEs per patient was 1 (range, 1‐10 irAEs), and 71 patients (4.1%) had >1 irAE.
Table 2.
Immune‐Related Adverse Events by Category
| irAEa |
N = 1738 No. (%) |
|||
|---|---|---|---|---|
| Any Grade | Grade 3 | Grade 4 | Grade 5 | |
| Any irAE | 247 (14.2) | 32 (1.8) | 4 (0.2) | 3 (0.2) |
| Rash | 90 (5.2) | 1 (0.1) | 0 | 0 |
| Colitis | 26 (1.5) | 7 (0.4) | 0 | 0 |
| Pneumonitis | 21 (1.2) | 5 (0.3) | 1 (0.1) | 1 (0.1) |
| Hepatitis | 16 (0.9) | 11 (0.6) | 0 | 2 (0.1) |
| Endocrinopathies | 106 (6.1) | 6 (0.3) | 0 | 0 |
| Thyroid disorders | 98 (5.6) | 3 (0.2) | 0 | 0 |
| Adrenal insufficiency | 8 (0.5) | 1 (0.1) | 0 | 0 |
| Type 1 diabetes mellitus | 2 (0.1) | 2 (0.1) | 0 | 0 |
| All other irAEs | 19 (1.1) | 5 (0.3) | 3 (0.2) | 0 |
| Blood CPK increased | 5 (0.3) | 1 (0.1) | 2 (0.1) | 0 |
| Myositis | 5 (0.3) | 1 (0.1) | 1 (0.1) | 0 |
| Psoriasis | 5 (0.3) | 1 (0.1) | 0 | 0 |
| Guillain‐Barré syndrome | 1 (0.1) | 1 (0.1) | 0 | 0 |
| Systemic inflammatory response syndrome | 1 (0.1) | 1 (0.1) | 0 | 0 |
Abbreviations: CPK, creatine phosphokinase; irAE, immune‐related adverse event.
Categories with an incidence of irAEs of grade ≥3 are shown (graded according to NCI‐CTCAE [version 4.0]).
Figure 1.
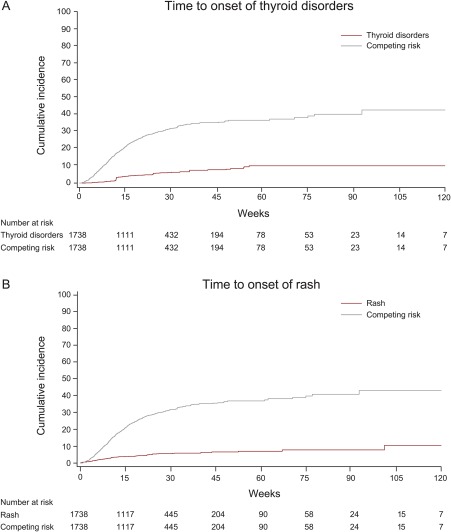
Time to first onset of the most common immune‐related adverse events of any grade. Representative graph of the cumulative incidence of (A) thyroid disorders and (B) rash with death as the competing risk.
After an irAE, 39 of 247 patients (15.8%) had 1 dose interruption and 9 patients (3.6%) had ≥2 dose interruptions. A total of 109 patients (44.1%) were treated with a systemic corticosteroid for irAEs: 71 (28.7%) with ≥40 mg of prednisone or equivalent and 35 (14.2%) with <40 mg of prednisone or equivalent (the dose level was missing in 3 patients [1.2%]). Five patients (2.0%) were treated with a nonsteroidal immunosuppressant medication: 1 patient experienced events of increased blood creatine phosphokinase and autoimmune disorder and received cyclosporine in addition to corticosteroids; 1 patient experienced events of diarrhea and autoimmune disorder and subsequently received everolimus as further anticancer therapy after the completion of corticosteroid treatment; 1 patient with rheumatoid arthritis received methotrexate and leflunomide in addition to corticosteroids; 1 patient experienced myositis and was treated with methotrexate in addition to corticosteroids; and 1 patient experienced events of psoriasis and maculopapular rash and received only tacrolimus. A total of 379 irAEs were observed in 247 patients, with 134 of 379 irAEs (35.3%) resolved at the time of data analysis; irAEs had resolved in 69 of 247 patients (27.9%). The median time to resolution of all events was not estimable (range, 1‐783+ days). Overall, irAEs led to treatment discontinuation in 34 patients (2.0%).
IRRs, Related Symptoms, and Management
IRRs and related symptoms occurred in 439 patients (25.3%); these were grade 3 in 9 patients (0.5%) and grade 4 in 3 patients (0.2%). No grade 5 IRRs occurred. In these 439 patients, 79.5% had an IRR at the time of first infusion, 98.6% had onset within the first 4 infusions, and 63 patients (14.4%) and 17 patients (3.9%), respectively, had at least 2 or 3 infusions with IRRs. IRRs led to dose interruption in 152 patients (8.7%), an infusion rate reduction in 124 patients (7.1%), and discontinuation in 35 patients (2.0%). Of 439 patients who had an IRR, 432 received medication, including a systemic corticosteroid in 109 patients (24.8%). After a protocol amendment introduced after the initiation of this trial, 1615 patients (92.9%) received premedication with diphenhydramine and acetaminophen (modified based on local guidelines) before the first infusion of avelumab. IRRs were most common during the first infusion, and the incidence was similar in patients with (20.1%) and without (19.5%) premedication. However, premedication appeared to decrease IRR severity because the incidence of grade ≥3 IRRs was observed to be slightly higher in patients without premedication at the time of first infusion (0.3% vs 1.6%) (Fig. 2).
Figure 2.
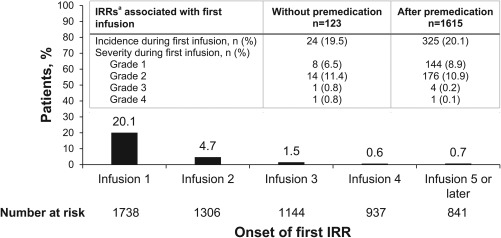
Infusion‐related reactions (IRRs). Onset and incidence/severity with and without premedication. The incidence and severity of IRRs occurring at the time of first infusion in patients treated with avelumab, with and without premedication with diphenhydramine and acetaminophen (Inset: table), and the time to first onset of an IRR. aIRRs occurring on the day of or the day after infusion included events reported as IRRs, drug hypersensitivity, or hypersensitivity. In addition, signs and symptoms of an IRR that occurred on the day of infusion and resolved within 2 days were included. No events were grade 5.
DISCUSSION
Avelumab generally was well tolerated and had a manageable safety profile in a large population of patients with advanced solid tumors. The incidence of grade ≥3 TRAEs or irAEs of any grade was low. irAEs and IRRs (most commonly occurring during the first 2 infusions; reported to occur at the time of first infusion in 79.5% of 439 patients who had an IRR) generally were low grade, manageable, and reversible; treatment discontinuation rarely was required.
The most common TRAEs included fatigue, nausea, diarrhea, and increased serum biomarkers and generally were consistent with TRAEs reported in other trials of anti–PD‐L1/PD‐1 antibodies that enrolled patients with advanced tumors.2 In a phase 1 dose‐escalation trial of nivolumab (anti–PD‐1) monotherapy in 296 patients with advanced solid tumors, approximately 70% of patients experienced TRAEs of any grade; of these, the most common included fatigue (24%), rash (12%), diarrhea (11%), pruritus (10%), decreased appetite (8%), and nausea (8%), and 14% of patients experienced a TRAE that was grade 3 to 4. irAEs occurred in 122 patients (41%), were grade 3 to 4 in 18 patients (6%), and included pneumonitis, colitis, hepatitis, and thyroiditis. Three treatment‐related deaths occurred due to pneumonitis (2 in patients with NSCLC and 1 in a patient with colorectal cancer).16 In a multidose/multischedule phase 1 study of pembrolizumab (anti–PD‐1) in 30 patients with advanced solid tumors, approximately 70% of patients experienced TRAEs of any grade; the most common included fatigue (33%), nausea (23%), pruritus (17%), decreased appetite (13%), and diarrhea and hypothyroidism (7% each). No grade 3 to 4 TRAEs were observed. irAEs occurred in 17% of patients and included grade 1 fatigue, erythema, and hypothyroidism and grade 2 gastritis and pneumonitis.17
Although conclusions drawn from cross‐study comparisons should be made with caution, and to the best of our knowledge the number of pan‐tumor clinical studies of ICI monotherapy is limited, this analysis of a large population of patients across a broad scope of tumor types suggests that avelumab was associated with an incidence of irAEs that is consistent with that of other ICIs.16, 17 Differences may be attributed to the specificity of avelumab for PD‐L1, which leaves PD‐L2/PD‐1 interactions intact and allows for maintenance of immune homeostasis18, 19; however, additional evidence is required to fully understand the contribution of PD‐L2. Furthermore, differences in study design, eligibility criteria, and methodology for defining irAEs may account for variations in incidences across trials of ICI monotherapy. Patient and disease characteristics also affect the spectrum and frequency of AEs. The safety profile of ICIs varies by tumor type and disease histology, with pneumonitis occurring more often in patients with lung cancer and vitiligo and colitis more commonly noted in patients with melanoma.2, 20 The phase 1 multicohort monotherapy data for this class of antibodies support the growing body of evidence attributing a manageable safety outcome for patients treated with anti–PD‐L1/PD‐1 antibodies.
The expanded definition of IRRs used in the current analysis, which aggregates IRR, drug hypersensitivity, hypersensitivity reactions, and signs and symptoms of an IRR occurring on the day of infusion and the day after as well as possible signs and symptoms of IRRs on the day of infusion, appears to differ from the definitions used in other studies and may have contributed to the higher rate of any‐grade IRRs observed with avelumab (25.3%). For example, in the phase 1 dose‐escalation trial of nivolumab monotherapy described previously, IRRs were defined as IRR or hypersensitivity; events of any grade occurred in 3% of patients (grade 3‐4 in <1%).16 In a study of pembrolizumab in patients with advanced NSCLC, IRRs were monitored using a single preferred term; any‐grade IRRs occurred in 3% of patients (grade 3‐5 in <1%).21 Similarly, a study of durvalumab in patients with metastatic UC also reported IRRs using a single preferred term; any‐grade IRRs occurred in 3.3% of patients (grade 3 in 1 patient [1.6%]).22 Despite using an expanded definition of IRRs in the current analysis, the incidence of grade ≥3 IRRs was similar to that in studies that used limited or single‐term definitions of IRR (ie, <1%). It is interesting to note that the majority of IRRs in the current study were mild to moderate in severity, occurred after the first or second infusion, and did not lead to discontinuation. The incidence was similar in patients with or without premedication. However, premedication appeared to decrease IRR severity; the incidence of grade ≥3 IRRs was higher in patients without premedication at the time of first infusion (0.3% vs 1.6%). The rate of repeated events was low, with 14.4% and 3.9% of patients, respectively, experiencing IRRs with 2 or 3 infusions.
To the best of our knowledge, avelumab is the first fully human anti–PD‐L1 antibody and contains a wild‐type IgG1 Fc region, which engages Fc‐γ receptors on natural killer cells to induce tumor‐directed, antibody‐dependent, cell‐mediated cytotoxicity (ADCC).23, 24 Anti–PD‐1 IgG4 antibodies (nivolumab and pembrolizumab) have demonstrated poor affinity for natural killer cells, and anti–PD‐L1 IgG1 antibodies (atezolizumab and durvalumab) were developed to minimize or disable ADCC.25, 26, 27 Additional investigation is required to define the role of humanization of avelumab and the contribution of ADCC to the overall antitumor effects, as well as the safety profile, of avelumab.
Meta‐analyses of the safety and tolerability of anti–PD‐L1/PD‐1 agents corroborate findings of improved safety profiles of ICIs compared with chemotherapy regimens used to treat patients with advanced cancers.28, 29, 30, 31 In a meta‐analysis of 3450 patients from 7 randomized controlled trials, treatment with ICIs was associated with a lower incidence of any‐grade AEs compared with chemotherapy (67.6% vs 82.9%) and grade 3 to 4 AEs (11.4% vs 35.7%), and treatment discontinuation occurred less frequently with anti–PD‐L1/PD‐1 agents compared with chemotherapy (4.5% vs 11.1%).28 The incidence of TRAEs associated with ICIs ranged from 9% to 31%, with a pooled rate of 16% (95% confidence interval, 12%‐21%).30 Treatment with ICIs was associated with a higher incidence of any‐grade and grade 3 to 4 irAEs (ie, rash, aspartate aminotransferase increase, hypothyroidism, colitis, and pneumonitis) compared with non‐ICI regimens.31 The incidence of IRRs and hypersensitivity reactions occurring with avelumab and other ICIs was similar to or less than the incidence reported with other systemic and targeted treatments, including commonly used taxane‐based chemotherapy.32 Overall, treatment with anti–PD‐L1/PD‐1 antibodies was better tolerated than chemotherapy; was associated with a lower incidence of TRAEs; and, although associated with an increased incidence of irAEs, offered a favorable risk‐benefit profile.
Conclusions
This pooled analysis of 1738 patients with advanced solid tumors confirms that avelumab was well tolerated and had a manageable safety profile similar to that of other anti–PD‐L1/PD‐1 antibodies. The incidences and rates of discontinuation due to grade ≥3 TRAEs or any‐grade irAEs were low. IRRs for the most part occurred at the time of first infusion and were manageable, and the percentage of patients with repeated events was low.
FUNDING SUPPORT
Sponsored by Merck KGaA (Darmstadt, Germany) and part of an alliance between Merck KGaA and Pfizer Inc (New York, New York). Medical writing support was provided by ClinicalThinking Inc (Hamilton, New Jersey) and funded by Merck KGaA and Pfizer Inc.
CONFLICT OF INTEREST DISCLOSURES
Jeffrey R. Infante reports personal fees as an employee of Janssen Oncology for work performed outside of the current study. Matthew H. Taylor has acted as a paid consultant and received honoraria from Trillium Pharma; has received honoraria from Eisai Inc and Bristol‐Myers Squibb; and has acted as a paid member of the advisory boards for and received honoraria from Blue Print Medicines, Loxo Oncology, and Novartis for work performed outside of the current study. Deborah J. Wong reports grants from Merck Serono during the conduct of the current study. Janice M. Mehnert reports consultancy for Merck, Sharp & Dohme and Amgen; reimbursement for travel and accommodations from EMD Serono and Merck, Sharp & Dohme; other relationships with Amgen, EMD Serono, and Merck KGaA; honoraria from Genentech and EMD Serono; Janice M. Mehnert's institution received research funding from Merck KGaA, Sanofi, Novartis, Polynoma, Immunocore, Amgen, and AstraZeneca. Anja H. Loos and Isabell Speit report personal fees as employees of Merck KGaA for work performed outside of the current study. Helga Koch reports personal fees as an employee of Merck KGaA and EMD Serono for work performed outside of the current study. All other authors have no relevant disclosures.
AUTHOR CONTRIBUTIONS
Karen Kelly: Investigation and writing–review and editing. Jeffrey R. Infante: Conceptualization, methodology, investigation, data curation, writing–review and editing, visualization, and supervision. Matthew H. Taylor: Investigation and writing–review and editing. Manish R. Patel: Investigation and writing–review and editing. Deborah J. Wong: Investigation, data curation, writing–review and editing, and supervision. Nicholas Iannotti: Investigation, resources, data curation, writing–review and editing, supervision, and project administration. Janice M. Mehnert: Investigation and writing–review and editing. Anja H. Loos: Conceptualization, methodology, software, validation, formal analysis, data curation, writing–review and editing, visualization, and project administration. Helga Koch: Conceptualization, methodology, validation, and writing–review and editing. Isabell Speit: Conceptualization, methodology, and formal analysis. James L. Gulley: Conceptualization, methodology, investigation, resources, data curation, writing–reviewing and editing, supervision, and funding acquisition.
Supporting information
Additional supporting information may be found in the online version of this article.
Supporting Information 1
Presented in part at the American Society of Clinical Oncology Annual Meeting; June 2‐6, 2017; Chicago, IL.
We thank the patients and their families, investigators, coinvestigators, and the study teams at each of the participating centers and at Merck KGaA (Darmstadt, Germany) and EMD Serono (Billerica, Massachusetts; a business of Merck KGaA, Darmstadt, Germany).
REFERENCES
- 1. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974‐1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51‐60. [DOI] [PubMed] [Google Scholar]
- 3. Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33:2092‐2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heery CR, O'Sullivan‐Coyne G, Madan RA, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose‐escalation trial. Lancet Oncol. 2017;18:587‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merck KGaA . Bavencio (avelumab) injection [package insert]. Darmstadt, Germany: Merck KGaA; 2017. [Google Scholar]
- 6. Merck KGaA . Bavencio (avelumab) injection [summary of product characteristics]. Darmstadt, Germany: Merck KGaA; 2017. [Google Scholar]
- 7. Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non‐small‐cell lung cancer (JAVELIN Solid Tumor): dose‐expansion cohort of a multicentre, open‐label, phase 1b trial. Lancet Oncol. 2017;18:599‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti‐programmed death‐ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase 1b study. J Clin Oncol. 2017;35:2117‐2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chung HC, Arkenau HT, Wyrwicz L, et al. 2364 avelumab (MSB0010718C), an anti‐PD‐L1 antibody, in patients with advanced gastric or gastroesophageal junction cancer: a phase 1b trial. Eur J Cancer. 2015;51(suppl 3):S457. [Google Scholar]
- 10. Disis ML, Patel MR, Pant S, et al. Avelumab (MSB0010718C; anti‐PD‐L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor phase 1b trial: safety and clinical activity. J Clin Oncol. 2016;34:5533. [Google Scholar]
- 11. Hassan R, Thomas A, Patel MR, et al. Avelumab (MSB0010718C; anti‐PD‐L1) in patients with advanced unresectable mesothelioma from the JAVELIN Solid Tumor phase 1b trial: safety, clinical activity, and PD‐L1 expression. J Clin Oncol. 2016;34:8503. [Google Scholar]
- 12. Kelly K, Heery CR, Patel MR, et al. Avelumab (MSB0010718C; anti‐PD‐L1) in patients with advanced cancer: safety data from 1300 patients enrolled in the phase 1b JAVELIN Solid Tumor trial. J Clin Oncol. 2016;34:3055. [Google Scholar]
- 13. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy‐refractory metastatic Merkel cell carcinoma: a multicentre, single‐group, open‐label, phase 2 trial. Lancet Oncol. 2016;17:1374‐1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaufman HL, Russell JS, Hamid O, et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow‐up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer. 2018;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley & Sons: 1980. [Google Scholar]
- 16. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD‐1 antibody in cancer. N Engl J Med. 2012;366:2443‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK‐3475; anti‐PD‐1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21:4286‐4293. [DOI] [PubMed] [Google Scholar]
- 18. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD‐1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rozali EN, Hato SV, Robinson BW, Lake RA, Lesterhuis WJ. Programmed death ligand 2 in cancer‐induced immune suppression. Clin Dev Immunol. 2012;2012:656340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti‐PD‐1 and anti‐PD‐L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375‐2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garon EB, Rizvi NA, Hui R, et al; KEYNOTE‐001 Investigators . Pembrolizumab for the treatment of non–small‐cell lung cancer. N Engl J Med. 2015;372:2018‐2028. [DOI] [PubMed] [Google Scholar]
- 22. Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti‐programmed cell death ligand‐1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34:3119‐3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boyerinas B, Jochems C, Fantini M, et al. Antibody‐dependent cellular cytotoxicity activity of a novel anti‐PD‐L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res. 2015;3:1148‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fujii R, Friedman ER, Richards J, et al. Enhanced killing of chordoma cells by antibody‐dependent cell‐mediated cytotoxicity employing the novel anti‐PD‐L1 antibody avelumab. Oncotarget. 2016;7:33498‐33511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti‐PD‐1 antibody nivolumab, BMS‐936558, and in vivo toxicology in non‐human primates. Cancer Immunol Res. 2014;2:846‐856. [DOI] [PubMed] [Google Scholar]
- 26. Sharon E, Streicher H, Goncalves P, Chen HX. Immune checkpoint inhibitors in clinical trials. Chin J Cancer. 2014;33:434‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stewart R, Morrow M, Hammond SA, et al. Identification and characterization of MEDI4736, an antagonistic anti‐PD‐L1 monoclonal antibody. Cancer Immunol Res. 2015;3:1052‐1062. [DOI] [PubMed] [Google Scholar]
- 28. Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and tolerability of PD‐1/PD‐L1 inhibitors compared with chemotherapy in patients with advanced cancer: a meta‐analysis. Oncologist. 2017;22:470‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen R, Peng PC, Wen B, et al. Anti‐programmed cell death (PD)‐1 immunotherapy for malignant tumor: a systematic review and meta‐analysis. Transl Oncol. 2015;9:32‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang T, Xie J, Arai S, et al. The efficacy and safety of anti‐PD‐1/PD‐L1 antibodies for treatment of advanced or refractory cancers: a meta‐analysis. Oncotarget. 2016;7:73068‐73079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Velasco G, Je Y, Bosse D, et al. Comprehensive meta‐analysis of key immune‐related adverse events from CTLA‐4 and PD‐1/PD‐L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5:312‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vogel WH. Infusion reactions: diagnosis, assessment, and management. Clin J Oncol Nurs. 2010;14:E10‐E21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supporting Information 1


