Abstract
Objective
Tofacitinib is an oral JAK inhibitor indicated for the treatment of rheumatoid arthritis (RA). We characterized lymphoma events in the tofacitinib RA clinical development program.
Methods
Lymphoma events (up to March 2015) were identified from 19 tofacitinib studies (2 phase I, 9 phase II, 6 phase III, and 2 long‐term extension) of patients with moderate to severe RA. Patients in these studies received tofacitinib dosed at 1–30 mg twice daily or 20 mg once daily, as monotherapy or with conventional synthetic disease‐modifying antirheumatic drugs. Lymphoma incidence rates (IRs; number of patients with events/100 patient‐years) and standardized incidence ratios (SIRs) were calculated. A descriptive case–matched control analysis (1:4) was performed to identify potential risk factors for lymphoma.
Results
A total of 6,194 patients received tofacitinib (19,406 patient‐years of exposure, 3.4 years median treatment duration). Nineteen lymphomas occurred (IR 0.10 [95% confidence interval (95% CI) 0.06–0.15]), with no increase observed with time of exposure. The age‐ and sex‐adjusted SIR of lymphoma was 2.62 (95% CI 1.58–4.09) (Surveillance, Epidemiology, and End Results [SEER] program database). The clinical characteristics of the 19 lymphomas were typical for the RA population. Three lymphomas were positive for Epstein‐Barr virus, 8 were negative, 2 were equivocal, and 6 were untested. Numerically, more lymphoma cases had a history of Sjögren's syndrome and were positive for anti–cyclic citrullinated protein and rheumatoid factor at baseline versus matched controls. The mean corticosteroid dose was higher for lymphoma cases versus controls.
Conclusion
In the tofacitinib RA clinical development program, lymphoma rates were stable over time and there were minimal differences in the baseline characteristics of patients with and without lymphoma.
Introduction
Malignant lymphomas are seen at increased rates in patients with certain autoimmune diseases, including Sjögren's syndrome and rheumatoid arthritis (RA), with the relative risk for patients with RA reported to be between 1.5 and 4 1. Patients with RA who have longstanding and high levels of inflammatory activity and poorly managed disease have an increased risk of lymphoma 2. Furthermore, infection with viruses, including Epstein‐Barr virus (EBV), occurs more frequently in patients with compromised immune systems, including those with RA, and EBV is associated with various types of lymphoma 3.
Significance & Innovations.
We characterized incidences of lymphoma occurring in the tofacitinib RA clinical development program, which represents 19,406 patient‐years of tofacitinib exposure and a median treatment duration of 3.4 years.
Incidence rates (IRs), standardized incidence ratios (SIRs), and clinical characteristics for the 19 lymphoma events occurring in the development program were typical for this patient population. The IRs and SIRs did not increase with time of exposure and were similar to those observed in RA populations treated with biologic disease‐modifying antirheumatic drugs.
Appropriate Epstein‐Barr virus (EBV) testing was performed in 13 of 19 cases. Three lymphomas were positive for EBV association (1 Hodgkin's lymphoma and 2 B cell lymphomas). In 2 cases, the results of EBV testing were equivocal, and 8 cases were negative.
There were minimal differences between the patient profiles of patients with and without lymphoma in the tofacitinib RA clinical development program.
Although the overall risk of lymphoma is increased in patients with RA, whether there is a causal relationship between immunomodulatory therapies for RA, including conventional synthetic disease‐modifying antirheumatic drugs (DMARDs) and biologic DMARDs, and increased lymphoma rates is unclear, as it is difficult to distinguish between the effects of therapy, other confounders (i.e., concomitant medications), and the underlying disease pathogenesis 4. The difficulty arises due to the fact that patients treated with the most potent immunosuppressants are typically those with the highest disease activity and thus at the greatest risk of lymphoma 5. Accordingly, there is a need to monitor the incidence of lymphoma during the clinical development of immunomodulatory therapies for the treatment of RA and in real‐world treatment.
Tofacitinib is an oral JAK inhibitor indicated for the treatment of RA. JAKs are intracellular protein kinases that mediate cytokine signaling for a broad range of cellular functions, including hematopoiesis and immune cell growth and development 6, and JAK inhibition blocks signaling for a number of proinflammatory cytokines fundamental to the pathogenesis of RA 7. Safety events of special interest related to JAK involvement in immune function, including lymphoma, require close monitoring during the development of immunomodulatory agents.
The efficacy and safety of tofacitinib for the treatment of RA have been reported in phase I 8, 9, phase II 10, 11, 12, 13, 14, phase III 15, 16, 17, 18, 19, 20, and long‐term extension (LTE) studies with up to 105 months of observation (January 2016 data cutoff) 21, 22, 23. Overall malignancy rates (excluding nonmelanoma skin cancer [NMSC]) in the tofacitinib RA clinical program were not elevated compared with rates from the Surveillance, Epidemiology, and End Results (SEER) program database 24. The most commonly occurring malignancies (other than NMSC) were lung cancer and breast cancer, consistent with what is expected in the general population 25. Furthermore, overall malignancy rates with tofacitinib were similar to those observed with biologic DMARDs in an interim analysis of real‐world registry data 26. We conducted an analysis of lymphoma events to characterize lymphomas that occurred during the tofacitinib RA clinical development program.
Patients and methods
Patients and study design
This analysis included pooled data from 6,194 patients participating in 19 studies of tofacitinib for the treatment of RA: 2 phase I studies 8, 9, 9 phase II studies 10, 11, 12, 13, 27, 28, 29, 30, 31, 6 phase III studies 15, 16, 17, 18, 19, 20, and 2 LTE studies 21, 22. One of the LTE studies (ORAL Sequel) 21 is ongoing; therefore, the study database had not yet been locked; some values may change for the final, locked study database (data cutoff date: March 2015). These studies evaluated various tofacitinib dose regimens. In phase I studies, patients received tofacitinib 10 mg twice daily (BID); in phase II studies, patients received tofacitinib 1, 3, 5, 10, 15, or 30 mg BID, or 20 mg once daily; and in phase III and LTE studies, patients received tofacitinib 5 mg BID or 10 mg BID. Tofacitinib was administered as monotherapy or in combination with background DMARDs (mainly methotrexate) to examine safety and efficacy. In 1 study, patients received atorvastatin as part of the study design (ClinicalTrials.gov identifier: NCT01059864). Concomitant therapy with nonsteroidal antiinflammatory drugs and low‐dose oral glucocorticoids was permitted in the phase II and phase III studies provided they were stably dosed. In the LTE studies, dose adjustment of concomitant therapies was permitted at the investigator's discretion. Concomitant therapy with biologic DMARDs was prohibited in all studies.
Patients participating in the studies were ≥18 years old with active RA at screening and baseline visits. Exclusion criteria were similar across all studies. Patients were excluded if they had a history of, or existing, lymphoproliferative disorder or other malignancy (except for adequately treated or excised nonmetastatic basal cell or squamous cell cancer of the skin or cervical carcinoma in situ). Patients who developed a malignancy were discontinued from the study but were included in standard followup assessments (i.e., until resolution of event or stability). Patients who developed NMSC were permitted to continue treatment provided it was adequately treated or excised nonmetastatic basal cell or squamous cell cancer of the skin or cervical carcinoma in situ.
At baseline, the majority of patients in the index studies were inadequate responders to DMARDs (DMARD‐IR), but some studies also included patients who were methotrexate‐naive (770 patients randomized to tofacitinib in ORAL Start) 16 or inadequate responders to biologic DMARDs (biologic DMARD‐IR; 267 patients randomized to tofacitinib in ORAL Step) 15. Patients who participated in qualifying index studies were eligible to enter 1 of the 2 open‐label LTE studies (the global LTE study ORAL Sequel or the Japanese LTE study NCT00661661) 21, 22.
Lymphoma cases
Lymphoma events occurring in the tofacitinib RA clinical study population up to March 2015 were identified by review of investigator‐reported adverse events (AEs) and output from the central and local histopathology reports. When samples were provided (slides and/or tissue blocks), they were submitted for central histopathology and read by ≥2 board‐certified pathologists as part of the adjudication process. Any discordance between local and central pathology reading was resolved in a malignancy adjudication process in which all available data were reviewed and the event was classified using International Classification of Diseases for Oncology (ICD‐O), version 3, coding. In some cases, samples could not be obtained for reading, and these cases were adjudicated on the basis of information supplied, e.g., local histopathology reports and clinical course, etc. Where documented, the association of lymphoma with EBV was reported and was defined by appropriate testing (i.e., immunohistochemistry or in situ hybridization) performed on an appropriate sample.
The incidence rate (IR; number of unique patients with events per 100 patient‐years of observation) for lymphoma events was based on the number of patients with an event and the total exposure time, censored at the time of event, death, or discontinuation from the study. Some lymphoma events may have occurred following treatment cessation; all of these events were counted in the numerator of IR calculations regardless of when the events occurred, and patients’ total treatment exposure was included in the denominator. The 95% confidence intervals (95% CIs) for IRs were based on exact methods for Poisson distribution adjusted for exposure time. For analysis of IRs by 6‐month intervals, the final 6‐month interval was extended such that there were ≥1,000 patient‐years of exposure to allow sufficient patient‐years for meaningful interpretation. Age‐ and sex‐standardized incidence ratios (SIRs) and 95% CIs were calculated based on the ratio of observed lymphomas in the tofacitinib‐treated population relative to the expected rate in the general population (SEER database, covering the US general population from 1975–2013) 25.
In order to identify whether there were any specific risk factors for lymphoma in this patient population, a descriptive analysis using a nested case–control design was performed. For each lymphoma case, patients without malignancy (controls) were identified from the same analysis population, individually matched for age (at time of lymphoma event) and sex (case to control ratio 1:4). Comparison of patient characteristics between lymphoma cases and controls was based on descriptive statistics; no formal significance testing was conducted.
Results
IRs and SIRs of lymphomas
Overall, this analysis included 6,194 patients treated with tofacitinib, representing a total of 19,406 patient‐years of tofacitinib exposure. Of these patients, 2,239 received an average tofacitinib dose of 5 mg BID (6,870 patient‐years of exposure) and 3,955 received an average dose of 10 mg BID (12,534 patient‐years of exposure). The overall IR (number of patients with events per 100 patient‐years) for lymphoma during this period was 0.10 (95% CI 0.06–0.15). Tofacitinib exposure and IRs of lymphoma during discrete 6‐month periods are shown in Figure 1. The IR remained generally stable over time. The overall SIR for lymphoma was 2.62 (95% CI 1.58–4.09), adjusted for age and sex (Figure 2). Since this analysis was performed, a further data cut including exposure up to 105 months has become available. During this time, no additional lymphoma events have occurred.
Figure 1.
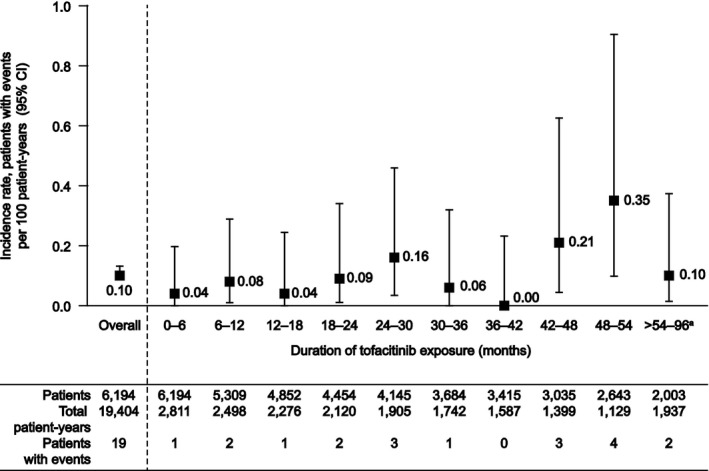
Incidence rates of lymphoma in the tofacitinib clinical program by 6‐month time intervals. Exposure and incidence rates are shown for each discrete 6‐month time period. a = The final reporting period (>54 months) was expanded such that there were at least 1,000 patient‐years of exposure for this period. The final interval included patients with >54 to 96 months of exposure. Since this analysis was performed, a further data cut including exposure up to 105 months has become available. During this time, no additional lymphoma events have occurred. 95% CI = 95% confidence interval.
Figure 2.
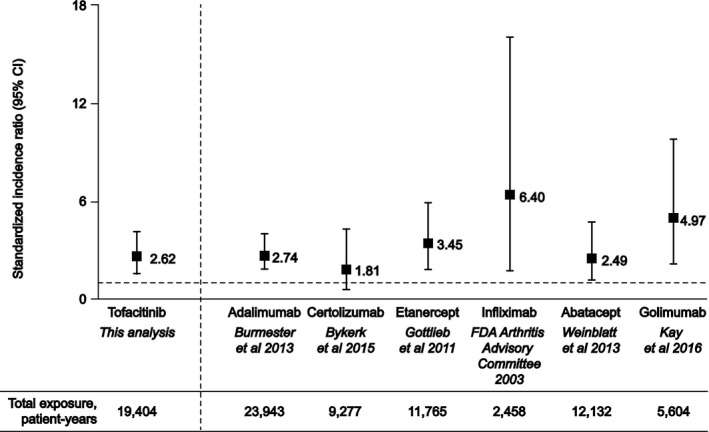
Standardized incidence ratios of lymphoma with tofacitinib clinical trials compared with published clinical trials data for biologic disease‐modifying antirheumatic drugs used to treat rheumatoid arthritis. Clinical trials data for tofacitinib, adalimumab 32, certolizumab pegol 33, etanercept 37, infliximab 34, abatacept 35, and golimumab 36 are standardized against the Surveillance, Epidemiology, and End Results (SEER) program database 25. For golimumab, combined data for 50‐mg and 100‐mg dose groups are shown. Data for tofacitinib are adjusted for age and sex. The broken horizontal line represents standardized incidence ratio = 1.0, i.e., no difference in lymphoma rate versus the US general population. 95% CI = 95% confidence interval; FDA = Food and Drug Administration.
Observed lymphoma events
Overall, 19 lymphoma events occurred during the tofacitinib RA clinical development program. The clinical features and characteristics for each of these events are summarized in Table 1. There were 2 cases of Hodgkin's lymphoma and 17 cases of non‐Hodgkin's lymphoma. Of the 17 non‐Hodgkin's lymphomas, there were 14 B cell lymphomas, 2 T cell lymphomas, and 1 case in which the cell type was unknown. Appropriate EBV testing was performed in 13 of 19 cases. Three lymphomas were positive for EBV association (1 Hodgkin's lymphoma and 2 B cell lymphomas); in 2 cases, results of EBV testing were equivocal, and 8 cases were negative. Three patients reported a history of Sjögren's syndrome.
Table 1.
Lymphomas occurring in the tofacitinib RA clinical development programa
| Age, years/sex/raceb | ICD‐O adjudication, histology, topography | Cell type | EBV presencec | History of SS | MTX use (days)d | GC use (days)d | Outcomee |
|---|---|---|---|---|---|---|---|
| Tofacitinib 5 mg BID | |||||||
| 1. 52/F/Asian | Hodgkin's lymphoma, NOS | NA | Positive | No | 390 | 440 | Recovering/resolving |
| M9650/39 | |||||||
| C77.2, abdominal lymph node | |||||||
| 2. 64/M/white | B‐cell chronic lymphocytic leukemia/small lymphocytic lymphoma | T cellf | NA | No | 0 | 0 | Not recovered/not resolved |
| M9823/35 | |||||||
| C42.1, bone marrow | |||||||
| 3. 71/F/white | Malignant lymphoma, non‐Hodgkin's, NOS | Unknown | NA | No | 1 | 741 | Fatal |
| M9591/39 | |||||||
| C77.9, lymph node, NOS | |||||||
| 4. 79/F/white | Malignant lymphoma, large B‐cell, diffuse, NOS | B cell | Negative | No | 2,685 | 2,929 | Recovering/resolving |
| M9680/36 | |||||||
| C71.0, thalamus | |||||||
| 5. 65/F/white | Malignant lymphoma, non‐Hodgkin's, NOS | B cell | NA | Yes | 2,420 | 486 | Not recovered/not resolved |
| M9591/36 | |||||||
| C34.1, upper lobe, lung | |||||||
| 6. 59/M/white | Malignant lymphoma, large B‐cell, diffuse, NOS | B cell | NA | No | 2,054 | 0 | Fatal |
| M9680/36 | |||||||
| C77.9, lymph node, NOS | |||||||
| Tofacitinib 10 mg BID | |||||||
| 7. 66/M/white | Malignant lymphoma, large B‐cell, diffuse, NOS | B cell | Equivocal | No | 0 | 0 | Not recovered/not resolved |
| M9680/36 | |||||||
| C77.9, lymph node, NOS | |||||||
| 8. 47/F/Asian | Malignant lymphoma, non‐Hodgkin's, NOS | B cell | NA | Yes | 514 | 3 | Not recovered/not resolved |
| M9591/36 | |||||||
| C77.1, mediastinal lymph node; C77.9, lymph node, NOS | |||||||
| 9. 63/F/black | Follicular lymphoma, NOS | B cell | Negative | No | 0 | 7,801 | Recovered/resolved |
| M9690/31 | |||||||
| C09.9, tonsil, NOS | |||||||
| 10. 70/F/white | Malignant lymphoma, large B‐cell, diffuse, NOS | B cell | Positive | No | 2,147 | 0 | Recovering/resolving |
| M9680/36 | |||||||
| C50.9, breast, NOS | |||||||
| 11. 61/F/white | Mantle cell lymphoma | B cell | Negative | No | 3,046 | 3,338 | Recovered/resolved |
| M9673/36 | |||||||
| C09.9, tonsil, NOS | |||||||
| 12. 47/F/white | Malignant lymphoma, non‐Hodgkin's, NOS | B cell | Negative | No | 2,893 | 1,858 | Recovered/resolved |
| M9591/36 | |||||||
| C77.0, lymph nodes of head, face, and neck | |||||||
| 13. 64/M/white | Malignant lymphoma, small B lymphocytic, NOS | B cell | NA | No | 1,671 | 1,373 | Not recovered/not resolved |
| M9670/36 | |||||||
| C77.8, lymph nodes of multiple regions | |||||||
| 14. 49/F/white | Marginal zone B‐cell lymphoma, NOS | B cell | Negative | No | 2,293 | 40 | Recovered/resolved |
| M9699/32 | |||||||
| C16.2, body of stomach | |||||||
| 15. 73/F/white | Mature T‐cell lymphoma, NOS | T cell | Negative | No | 1,956 | 631 | Recovered/resolved |
| M9702/35 | |||||||
| C09.9, tonsil, NOS | |||||||
| 16. 55/F/white | Hodgkin's lymphoma, nodular sclerosis, NOS | NA | Negative | No | 121 | 0 | Recovered/resolved |
| M9663/39 | |||||||
| C77.9, lymph node, NOS | |||||||
| 17. 63/F/white | Malignant lymphoma, large B‐cell, diffuse, NOS | B cell | Negative | No | 1,715 | 0 | Not recovered/not resolved |
| M9680/36 | |||||||
| C42.2, spleen | |||||||
| 18. 62/F/white | Marginal zone B‐cell lymphoma, NOS | B cell | Equivocal | No | 2,787 | 28 | Recovered/resolved |
| M9699/39 | |||||||
| C16.9, stomach, NOS; C16.0, gastroesophageal junction | |||||||
| 19. 46/F/white | Follicular lymphoma, NOS | B cell | Positive | Yes | 1,829 | 1,509 | Recovered/resolved |
| M9690/39 | |||||||
| C77.0, submandibular lymph node |
RA = rheumatoid arthritis; ICD‐O = International Classification of Diseases for Oncology; EBV = Epstein‐Barr virus; SS = Sjögren's syndrome; MTX = methotrexate; GC = glucocorticoid; BID = twice daily; F = female; NOS = not otherwise specified; NA = not applicable; M = male.
Age at time of event.
Definitions of EBV attribution are as follows. Positive: clear that appropriate tests (i.e., immunohistochemistry or in situ hybridization) were performed on an appropriate sample and were positive; negative: clear that appropriate tests (i.e., immunohistochemistry or in situ hybridization) were performed on an appropriate sample and were negative; equivocal: immunohistochemistry staining was done for pathology assessment but could not be interpreted with confidence; NA: lymphoma cases where EBV testing was not performed on an appropriate sample (EBV status is therefore unknown).
Total number of days of administration.
Based on information provided by the investigator.
This patient had a rare phenotype of T‐cell chronic lymphocytic leukemia.
In 16 cases, tofacitinib was permanently discontinued due to lymphoma events, as required by protocol. One patient had discontinued tofacitinib due to a prior serious AE (SAE) of pneumonia before the onset of lymphoma. One patient who died had not discontinued tofacitinib at the time of death. In 11 cases, patients recovered or were recovering following discontinuation of tofacitinib and/or medical management of lymphoma, including chemotherapy treatment. This includes 1 patient who recovered having declined chemotherapy and 1 patient who recovered following tonsillectomy without discontinuing tofacitinib, as the event was not initially reported as an SAE. In 6 cases, patients had not recovered from lymphoma events at the time of analysis (4 of whom received treatment for lymphoma). Two patients did not receive chemotherapy, and their lymphoma was stable but without any recovery. Two patients died (neither received treatment for lymphoma). The majority of patients (16 of 19) had received concomitant treatment with methotrexate (1 patient had only received 1 dose), and most patients (13 of 19) had received concomitant therapy with glucocorticoids; 2 patients had received neither methotrexate nor glucocorticoids.
The tofacitinib dosing history for each patient with lymphoma and the time of lymphoma onset, which was based on the date assigned by the reporting investigator rather than the date malignancy was confirmed by biopsy, is shown in Figure 3. Time to onset of events ranged from 149–1,722 days following treatment initiation, and no particular pattern was observed in the onset of lymphoma with respect to time since tofacitinib treatment began. Six lymphoma events occurred in patients who had received tofacitinib dosed predominantly at 5 mg BID. Of these patients, 2 had previously received placebo and 1 had previously received tofacitinib 3 mg BID. Thirteen lymphomas occurred in patients who had received tofacitinib dosed predominantly at 10 mg BID; of these, 4 patients had received tofacitinib 5 mg BID previously, and 2 were receiving tofacitinib 5 mg BID at the time of lymphoma onset.
Figure 3.
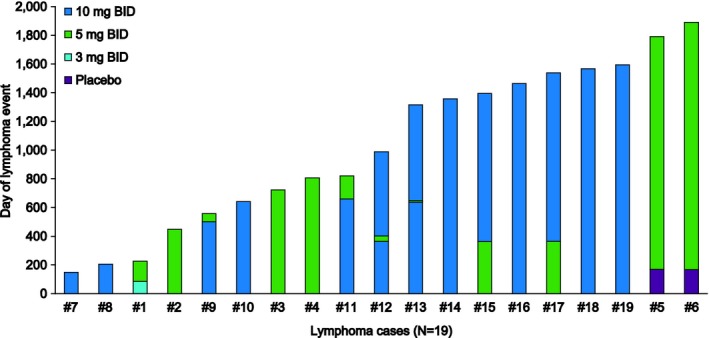
Tofacitinib dose history for patients with lymphoma events in the tofacitinib rheumatoid arthritis clinical development program. Case numbers correspond to those given in the first column of Table 1. Periods during which patients stopped tofacitinib treatment are included within the prior dose. The longest no‐dose period was 16 days. BID = twice daily.
Lymphoma case–control analysis
The demographics and baseline disease characteristics of the patients with lymphoma and matched control patients are shown in Table 2. The baseline disease characteristics were generally similar between the lymphoma case and control groups, including disease duration, daily tofacitinib dose, duration of tofacitinib exposure, and disease severity, as measured by Health Assessment Questionnaire disability index and the 4‐variable Disease Activity Score in 28 joints using the erythrocyte sedimentation rate scores. Compared with the control group, the lymphoma case group had numerically greater proportions of patients with anti–cyclic citrullinated protein (anti‐CCP; 10/19 [52.6%] cases versus 34/76 [44.7%] controls) and rheumatoid factor (RF; 14/16 [87.5%] cases versus 49/67 [73.1%] controls) positivity (baseline RF status was available for 16 patients in the lymphoma case group and 67 patients in the control group), and a greater proportion of lymphoma cases had a history of Sjögren's syndrome (3/19 [15.8%] cases versus 5/76 [6.6%] controls). The mean weekly dose of methotrexate was similar between the 2 groups, and the mean daily corticosteroid dose was numerically higher in the lymphoma case group than in the control group. Compared with the control group, a numerically greater proportion of patients in the lymphoma case group had used 2 DMARDs; numerically greater proportions of controls had used 0 DMARDs, 1 DMARD, and >2 DMARDs than in the lymphoma case group. The proportion of patients with prior biologic DMARD use was the same in the 2 groups.
Table 2.
Baseline demographics and disease characteristics of patients with lymphoma events and age‐ and sex‐matched controls from the tofacitinib RA clinical development programa
| Lymphoma (n = 19) | Controls (n = 76) | |
|---|---|---|
| Median age, years (range) | 60 (42–76) | 60 (42–78) |
| Sex, % female | 78.9 | 78.9 |
| Race, % white | 78.9 | 72.4 |
| Average tofacitinib dose, mg/day | 16.2 ± 4.6 | 16.9 ± 3.9 |
| Tofacitinib treatment duration, years | 2.2 ± 1.7 | 2.6 ± 1.5 |
| Body mass index, kg/m2 | 28.2 ± 6.8 | 28.0 ± 5.5 |
| RA disease duration, years | 8.1 ± 6.5 | 8.3 ± 7.6 |
| HAQ DI score | ||
| At baseline | 1.2 ± 0.6 | 1.5 ± 0.7 |
| At time lymphoma was reported | 0.9 ± 0.6 | 0.8 ± 0.7 |
| Four‐variable DAS28‐ESR score | ||
| At baseline | 6.1 ± 1.3 | 6.3 ± 1.1 |
| At time lymphoma was reported | 3.6 ± 1.0 | 3.5 ± 1.1 |
| Concomitant methotrexate, % | 84.2 | 65.8 |
| Weekly dose, mg | 16.0 | 15.0 |
| Corticosteroid daily dose, mg | 7.3 | 5.1 |
| Anti‐CCP positivity, % | 52.6 | 44.7 |
| RF positivity, % | 87.5 | 73.1 |
| History of Sjögren's syndrome, %b | 15.8 | 6.6 |
| No. DMARDs used previously, % | ||
| 0 | 5.3 | 11.8 |
| 1 | 36.8 | 39.5 |
| 2 | 31.6 | 15.8 |
| >2 | 26.3 | 32.9 |
| Prior biologic DMARD use, % | 21.1 | 21.1 |
Values are the mean ± SD unless otherwise indicated. RA = rheumatoid arthritis; HAQ DI = Health Assessment Questionnaire disability index; DAS28‐ESR = Disease Activity Score in 28 joints using the erythrocyte sedimentation rate; CCP = cyclic citrullinated peptide; RF = rheumatoid factor; DMARDs = disease‐modifying antirheumatic drugs.
For patients with lymphoma, history of Sjögren's syndrome was specifically queried at the time of lymphoma event. Collection of Sjögren's syndrome history was not systematically performed for patients in the matched control group.
Discussion
In this analysis of lymphoma events occurring during the tofacitinib RA clinical development program, IRs for lymphoma were stable over time, which was consistent with the findings of an integrated analysis of all malignancies occurring in the tofacitinib RA program 24. The IR for lymphoma in the tofacitinib RA program was similar to rates observed in long‐term clinical studies of patients with RA treated with the biologic DMARDs adalimumab 32, certolizumab pegol 33, infliximab 34, abatacept 35, and golimumab 36, where IRs ranged from 0.05–0.16 patients with events per 100 patient‐years. The SIR of lymphoma calculated for tofacitinib based on comparison against the US SEER database was within range of SIRs observed in these clinical studies of biologic therapies (SIRs ranged from 1.81–6.40; see Figure 2) 32, 33, 34, 35, 36, 37, although comparison should be made with caution, owing to variability in the study designs and patient populations analyzed in these studies. SIRs for lymphoma were also consistent with rates observed in meta‐analyses and observational studies of biologic DMARDs for the treatment of RA, where SIRs for lymphoma ranged from 1.7–5.99 5, 38.
The histologic subtype of the observed lymphoma events in the present analysis was generally consistent with that expected in patients with RA; lymphomas were predominantly B cell in origin and observed in typically affected sites 1. Two of the B cell lymphomas in this analysis were EBV positive. For context, the rate of EBV association reported in the literature for B cell lymphomas is 4–16% in patients with RA treated with methotrexate 3, 39. This analysis identified only a relatively small number of lymphoma cases, and EBV attribution relied on different techniques conducted in a variety of laboratories; appropriate samples for centralized testing were frequently not available, and local testing was inconsistently performed and not centrally reviewed. Additional influences on EBV association with lymphoma include geographic location 40, affected cell type 41, and affected anatomic location 42, and these factors should also be considered when interpreting the observed rate of EBV‐associated lymphoma. There were 5 lymphoma events that occurred in the tofacitinib renal transplant program 43, 44; 4 occurred in patients positive for EBV; however, these patients received an initial higher dose of tofacitinib and multiple concomitant immunosuppressive therapies in addition to tofacitinib. In addition, it is recognized that approximately 70% of post‐transplant lymphoproliferative disorders are positive for EBV 45.
The case–control analysis performed in this study found that more lymphoma cases than controls were positive for RF and anti‐CCP biomarkers at baseline. Furthermore, there were more lymphoma cases with a history of Sjögren's syndrome than in the control group; however, there may be bias in the collection of Sjögren's syndrome history from patients reporting lymphoma. Although there were also minimal differences in the profile of prior RA treatments between cases and controls, it is not known if high levels and duration of inflammatory activity rather than RA therapy was a major determinant of lymphoma risk, given that prior to entry into the tofacitinib clinical trials, the level of disease management for each patient was not known; this could be a critical factor in determining lymphoma risk 2. Thus, it remains difficult to differentiate the contributory effects of therapy and of the underlying disease etiology to lymphoma risk using RA clinical trials data, as patients in clinical trials are normally receiving, or have previously received, some kind of immunomodulatory therapy for RA.
A limitation of this analysis was that previous history of malignancy, including lymphoma, or related lymphoproliferative disorders, was an exclusion criterion of the clinical trials from which the patient population in this analysis was pooled. Patients who developed lymphoma during the course of the trials were to discontinue, limiting our ability to assess the risk of secondary or recurrent lymphomas with continued tofacitinib treatment. Second, with a relatively small number of lymphoma events identified in the tofacitinib RA population, no further risk factor analyses could be conducted, given the limited sample size. Accordingly, conclusions are based on comparisons of descriptive statistics only, and it was not possible to conduct formal significance testing in the case–control analysis, nor to conduct analysis of different lymphoma subtypes or other clinical characteristics of lymphoma events. An additional consequence of the relatively small number of lymphoma events was that for the analysis of IRs during discrete 6‐month periods, exposure during each period was also relatively low and could significantly fluctuate with even 1 event within a time interval. While this analysis included studies with placebo treatment groups, exposure in the placebo groups was not sufficient to evaluate rates of long latency events including lymphoma. Accordingly, analyses of open‐label extension study data are important to perform. Although disease activity and inflammatory load were assessed at baseline in all of the studies pooled in this analysis, detailed information regarding level of inflammation and level of disease management control over a prolonged period of time preceding patients’ entry into the studies was not collected. This information may be important given the association of lymphoma risk with high inflammatory activity 2, but it remains challenging to understand these risks within clinical trials populations, as there are very few surrogate baseline characteristics to understand poor and/or prolonged history of uncontrolled inflammatory disease. In addition, radiographic assessment of RA‐related erosions was only performed in 2 of the studies included in this analysis; thus, full lymphoma risk with radiographic disease status could not be properly assessed.
A limitation of the SIR analysis performed was that SIRs of lymphoma in this global patient population were calculated based on comparison with the SEER database, which is based on data from the US general population. Due to the recognized limitations of making comparisons with the global population (GLOBOCAN) database (i.e., potential bias introduced by underreporting and failure of diagnosis, particularly in less developed countries) 46, a SIR calculation comparison with the GLOBOCAN database was not performed. It is also acknowledged that the cross‐study comparison of SIRs of lymphoma with tofacitinib and with biologic DMARDs used to treat RA should be interpreted cautiously due to the limitations in comparing rates across different patient populations, study designs, and extents of exposure. A limitation of the case–control analysis was that the controls were matched based on age and sex, and it is known that matching may introduce bias by causing the baseline characteristics of the 2 groups to be more similar than they otherwise would be 47. However, these 2 matching criteria are mandatory when studying an outcome such as lymphoma, where the incidence is heavily dependent on age and sex.
Last, in some cases, information specific to each lymphoma event was obtained retrospectively, through supplementary inquiries to the investigator; therefore, some data may be missing and/or tests on samples may not have been consistently conducted. In conclusion, IRs and types of lymphoma observed during the tofacitinib RA clinical development program (with upward of 8 years of exposure) were consistent with expectations in the RA patient population as a whole and did not increase with exposure time. Although efforts were made to ascertain the EBV status of the lymphomas reported, the data do not allow a clear assessment to be made. Continued evaluation of studies of patients with autoimmune diseases, including randomized clinical trials and population‐based observational research, will be important to evaluate the relative contributions of immunosuppressive therapies and underlying disease pathogenesis to lymphoma risk.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Chen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Mariette, Chen, Biswas, Kwok, Boy.
Acquisition of data
Chen, Biswas, Kwok, Boy.
Analysis and interpretation of data
Mariette, Chen, Biswas, Kwok, Boy.
Role of the study sponsor
The studies included in this analysis were funded by Pfizer Inc. This analysis was conceived by the Pfizer Inc. authors in collaboration with the academic authors. Medical writing support under the guidance of the authors was provided by Daniel Binks, PhD, of Complete Medical Communications, and was funded by Pfizer Inc. All authors were involved in interpreting the data and in drafting, reviewing, and developing the manuscript. Publication of this article was not contingent upon approval by Pfizer Inc.
Acknowledgment
The authors acknowledge the contribution of Jamie Geier at Pfizer Inc. in providing input on the epidemiologic aspects of this analysis.
ClinicalTrials.gov identifiers: NCT00147498, NCT00413699, NCT00413660, NCT00960440, NCT00550446, and NCT00603512.
Supported by Pfizer Inc.
Dr. Mariette has received honoraria from Bristol‐Myers Squibb, GlaxoSmithKline, MedImmune, Pfizer Inc., Sanofi, and UCB (less than $10,000 each).
References
- 1. Smedby KE, Baecklund E, Askling J. Malignant lymphomas in autoimmunity and inflammation: a review of risks, risk factors, and lymphoma characteristics. Cancer Epidemiol Biomarkers Prev 2006;15:2069–77. [DOI] [PubMed] [Google Scholar]
- 2. Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum 2006;54:692–701. [DOI] [PubMed] [Google Scholar]
- 3. Starkebaum G. Rheumatoid arthritis and lymphoma: risky business for B cells. J Rheumatol 2007;34:243–6. [PubMed] [Google Scholar]
- 4. Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta‐analysis. Arthritis Res Ther 2015;17:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mariette X, Matucci‐Cerinic M, Pavelka K, Taylor P, van Vollenhoven R, Heatley R, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta‐analysis. Ann Rheum Dis 2011;70:1895–904. [DOI] [PubMed] [Google Scholar]
- 6. Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev 2009;228:273–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP‐690,550). J Immunol 2011;186:4234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Charles‐Schoeman C, Fleischmann R, Davignon J, Schwartz H, Turner SM, Beysen C, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol 2015;67:616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kremer JM, Kivitz AJ, Simon‐Campos JA, Nasonov EL, Tony H, Lee SK, et al. Evaluation of the effect of tofacitinib on measured glomerular filtration rate in patients with active rheumatoid arthritis: results from a randomised controlled trial. Arthritis Res Ther 2015;17:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double‐blind, placebo‐controlled phase IIa trial of three dosage levels of CP‐690,550 versus placebo. Arthritis Rheum 2009;60:1895–905. [DOI] [PubMed] [Google Scholar]
- 11. Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, et al. Phase IIb dose‐ranging study of the oral JAK inhibitor tofacitinib (CP‐690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease‐modifying antirheumatic drugs. Arthritis Rheum 2012;64:617–29. [DOI] [PubMed] [Google Scholar]
- 12. Tanaka Y, Takeuchi T, Yamanaka H, Nakamura H, Toyoizumi S, Zwillich S. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12‐week, randomized, phase 2 study. Mod Rheumatol 2015;25:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez‐Reino J, et al. A phase IIb dose‐ranging study of the oral JAK inhibitor tofacitinib (CP‐690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum 2012;64:970–81. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka Y, Takeuchi T, Yamanaka H, Suzuki M, Nakamura H, Toyoizumi S, et al. Tofacitinib (CP‐690,550), an oral janus kinase inhibitor, as monotherapy in Japanese patients with active rheumatoid arthritis: a 12‐week phase 2b study [abstract]. Arthritis Rheum 2011;63 Suppl 10:S844–5. [Google Scholar]
- 15. Burmester GR, Blanco R, Charles‐Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP‐690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. [DOI] [PubMed] [Google Scholar]
- 16. Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley J, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 17. Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin‐Mola E, et al. Tofacitinib in combination with nonbiologic disease‐modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013;159:253–61. [DOI] [PubMed] [Google Scholar]
- 18. Fleischmann R, Kremer J, Cush J, Schulze‐Koops H, Connell CA, Bradley JD, et al. Placebo‐controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 19. Van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 20. Van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP‐690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve‐month data from a twenty‐four–month phase III randomized radiographic study. Arthritis Rheum 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 21. Wollenhaupt J, Silverfield J, Lee EB, Curtis JR, Wood SP, Soma K, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open‐label, longterm extension studies. J Rheumatol 2014;41:837–52. [DOI] [PubMed] [Google Scholar]
- 22. Yamanaka H, Tanaka Y, Takeuchi T, Sugiyama N, Yuasa H, Toyoizumi S, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open‐label, long‐term extension study. Arthritis Res Ther 2016;18:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wollenhaupt J, Silverfield J, Lee EB, Terry K, Kwok K, Abramsky S, et al. Tofacitinib, an oral Janus kinase inhibitor, in the treatment of rheumatoid arthritis: safety and efficacy in open‐label, long‐term extension studies over 8 years [abstract]. Arthritis Rheumatol 2016;68 Suppl 10:S1110. [Google Scholar]
- 24. Curtis JR, Lee EB, Kaplan IV, Kwok K, Geier J, Benda B, et al. Tofacitinib, an oral Janus kinase inhibitor: analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann Rheum Dis 2016;75:831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al (eds). SEER Cancer Statistics Review, 1975–2013, National Cancer Institute. Bethesda (MD). URL: https://seer.cancer.gov/csr/1975_2013/.
- 26. Kavanaugh AF, Geier J, Bingham CO III, Chen C, Reed GW, Saunders KC, et al. Real world results from a post‐approval safety surveillance of tofacitinib (Xeljanz): over 3 year results from an ongoing US‐based rheumatoid arthritis registry [abstract]. Arthritis Rheumatol 2016;68 Suppl 10:S2595. [Google Scholar]
- 27. Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH, and the Tofacitinib Study Investigators . Phase II study of tofacitinib (CP‐690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken) 2011;63:1150–8. [DOI] [PubMed] [Google Scholar]
- 28. McInnes IB, Kim HY, Lee SH, Mandel D, Song YW, Connell CA, et al. Open‐label tofacitinib and double‐blind atorvastatin in rheumatoid arthritis patients: a randomised study. Ann Rheum Dis 2014;73:124–31. [DOI] [PubMed] [Google Scholar]
- 29. Winthrop KL, Silverfield J, Racewicz A, Neal J, Lee EB, Hrycaj P, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis 2016;75:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boyle DL, Soma K, Hodge J, Kavanaugh A, Mandel D, Mease P, et al. The JAK inhibitor tofacitinib suppresses synovial JAK1‐STAT signaling in rheumatoid arthritis. Ann Rheum Dis 2015;74:1311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Conaghan PG, Østergaard M, Bowes MA, Wu C, Fuerst T, van der Heijde D, et al. Comparing the effects of tofacitinib, methotrexate and the combination, on bone marrow oedema, synovitis and bone erosion in methotrexate‐naive, early active rheumatoid arthritis: results of an exploratory randomised MRI study incorporating semiquantitative and quantitative techniques. Ann Rheum Dis 2016;75:1024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long‐term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis 2013;72:517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bykerk VP, Cush J, Winthrop K, Calabrese L, Lortholary O, de Longueville M, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis 2015;74:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Centocor . Remicade (infliximab). Presentation to the Food and Drug Administration Arthritis Advisory Committee. URL: http://www.fda.gov/ohrms/dockets/ac/03/slides/3930s1.htm.
- 35. Weinblatt ME, Moreland LW, Westhovens R, Cohen RB, Kelly SM, Khan N, et al. Safety of abatacept administered intravenously in treatment of rheumatoid arthritis: integrated analyses of up to 8 years of treatment from the abatacept clinical trial program. J Rheumatol 2013;40:787–97. [DOI] [PubMed] [Google Scholar]
- 36. Kay J, Fleischmann R, Keystone E, Hsia EC, Hsu B, Zhou Y, et al. Five‐year safety data from 5 clinical trials of subcutaneous golimumab in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 2016;43:2120–30. [DOI] [PubMed] [Google Scholar]
- 37. Gottlieb AB, Gordon K, Giannini EH, Mease P, Li J, Chon Y, et al. Clinical trial safety and mortality analyses in patients receiving etanercept across approved indications. J Drugs Dermatol 2011;10:289–300. [PubMed] [Google Scholar]
- 38. Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum 2007;56:2886–95. [DOI] [PubMed] [Google Scholar]
- 39. Baecklund E, Backlin C, Iliadou A, Granath F, Ekbom A, Amini RM, et al. Characteristics of diffuse large B cell lymphomas in rheumatoid arthritis. Arthritis Rheum 2006;54:3774–81. [DOI] [PubMed] [Google Scholar]
- 40. Hjalgrim H, Friborg J, Melbye M. The epidemiology of EBV and its association with malignant disease In: Arvin A, Campadelli‐Fiumi G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K, editors. Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge: Cambridge University Press; 2007. p. 1–109. [PubMed] [Google Scholar]
- 41. Carbone A, Gloghini A, Dotti G. EBV‐associated lymphoproliferative disorders: classification and treatment. Oncologist 2008;13:577–85. [DOI] [PubMed] [Google Scholar]
- 42. Grywalska E, Rolinski J. Epstein‐Barr virus‐associated lymphomas. Semin Oncol 2015;42:291–303. [DOI] [PubMed] [Google Scholar]
- 43. Lawendy N, Krishnaswami S, Wang R, Gruben D, Cannon C, Swan S, et al. Effect of CP‐690,550, an orally active janus kinase inhibitor, on renal function in healthy adult volunteers. J Clin Pharmacol 2009;49:423–9. [DOI] [PubMed] [Google Scholar]
- 44. Vincenti F, Tedesco Silva H, Busque S, O'Connell P, Friedewald J, Cibrik D, et al. Randomized phase 2b trial of tofacitinib (CP‐690,550) in de novo kidney transplant patients: efficacy, renal function and safety at 1 year. Am J Transplant 2012;12:2446–56. [DOI] [PubMed] [Google Scholar]
- 45. Morscio J, Tousseyn T. Recent insights in the pathogenesis of post‐transplantation lymphoproliferative disorders. World J Transplant 2016;6:505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 47. Pearce N. Analysis of matched case‐control studies. BMJ 2016;352:I969. [DOI] [PMC free article] [PubMed] [Google Scholar]


