Abstract
This randomized, controlled phase 2 study was conducted to evaluate the analgesic efficacy, safety, and tolerability of single intravenous (IV) doses of 15 mg, 30 mg, and 60 mg meloxicam compared with oral ibuprofen 400 mg and placebo after dental impaction surgery. The primary efficacy end point was the sum of time‐weighted pain intensity differences for 0‐24 hours postdose. Among 230 evaluable subjects, meloxicam IV 60 mg produced the greatest reduction in pain, followed by the 30‐mg and 15‐mg doses. Statistically significant differences in summed pain intensity differences over 24 hours were demonstrated for each active‐treatment group vs placebo (favoring active treatment) and for meloxicam IV 30 mg and 60 mg vs ibuprofen 400 mg (favoring meloxicam IV). Moreover, there was a statistically significant dose response for meloxicam IV 15 mg to 60 mg. The onset of action for meloxicam IV was rapid and sustained; significant differences in pain intensity differences were detected as early as 10 minutes postdose and lasted through the 24‐hour postdose period. Subjects in the meloxicam IV groups were more likely than placebo recipients to achieve perceptible and meaningful pain relief and were less likely to use rescue medication. Patient‐reported global evaluation showed that meloxicam IV 60 mg had the highest rating. There were no deaths, serious adverse events, or discontinuations due to adverse events. The incidence of subjects with ≥1 treatment‐emergent adverse event was greatest in the placebo group, followed by the groups that received ibuprofen, meloxicam IV 15 mg, 30 mg, and 60 mg. Nausea was the most commonly reported treatment‐emergent adverse event. Clinical trial registration number: NCT00945763.
Keywords: acute postoperative pain, cyclooxygenase‐2 inhibitor, dental impaction surgery, intravenous, meloxicam, nonsteroidal anti‐inflammatory drugs, sum of pain intensity differences
Surgical removal of impacted third molars is a pain model used extensively to evaluate analgesic efficacy.1, 2 Advantages of this dental pain model include (1) a standardized surgical procedure resulting in substantial postoperative pain that remains consistent for up to 48 hours after surgery, (2) surgery that generally requires only local anesthetics with or without nitrous oxide, and (3) an abundance of potential study candidates.1, 2 The literature is replete with successful studies of this model's utility to assess the efficacy of many analgesic agents and drug combinations.3, 4, 5, 6, 7, 8
Standard doses of orally administered ibuprofen or acetaminophen, alone or in combination with an opioid (hydrocodone or oxycodone), are routinely used to manage postoperative dental pain.9, 10, 11, 12, 13 When the model includes surgical removal of bilateral mandibular bony impactions, the postsurgical pain generally remains consistent for 48 hours, but the need for analgesia can extend up to 5 days after surgery.1, 2 The intensity and duration of postoperative pain are optimal for assessing the efficacy of analgesics that have limited duration of action and thus require repeated dosing to effectively manage pain.
Ibuprofen and acetaminophen are associated with potential adverse events. The known adverse effects of nonselective (cyclooxygenase [COX]‐1/COX‐2 inhibitors) nonsteroidal anti‐inflammatory drugs (NSAIDs) such as ibuprofen and ketorolac include impaired platelet function and gastrointestinal intolerance.14, 15, 16 Acetaminophen is associated with hepatotoxicity if the dosage exceeds 4000 mg in 24 hours or if taken in addition to 3 or more alcoholic drinks per day.14, 17 Undesirable side effects of opioid analgesics include nausea, drowsiness, dizziness, and constipation.18 Furthermore, opioid use is under intense scrutiny due to the epidemic of opioid medication abuse.18
COX‐2 inhibitors have been shown to provide analgesic efficacy in models of acute pain19 by reducing inflammation and pain caused by the expression of COX‐2 and subsequent reduction in prostaglandin biosynthesis following tissue injury.19 Evidence suggests that COX‐2 inhibitors do not increase the risk of perioperative bleeding complications and are associated with a lower risk of gastrointestinal toxicity than are nonselective NSAIDs.19
Meloxicam is a preferential COX‐2 inhibitor that possesses analgesic, anti‐inflammatory, and antipyretic activities.20, 21, 22 Oral meloxicam is approved by the US Food and Drug Administration for use in rheumatoid arthritis and osteoarthritis. A meloxicam dosage of 7.5 to 15 mg/day is as effective as piroxicam, diclofenac, and naproxen as an anti‐inflammatory and analgesic and is associated with better gastrointestinal tolerability.21 The pharmacokinetic half‐life of meloxicam is approximately 20 hours, which allows for once‐daily dosing.23 Oral meloxicam is not currently approved for the treatment of acute pain because of its onset of action, with an observed peak plasma concentration occurring approximately 5 to 6 hours after administration.20, 21 This delayed plasma peak is due largely to the inherent poor solubility of the orally administered drug.
For some drugs with poor solubility, the limitations associated with poor dissolution rates have been overcome by developing colloidal dispersions of nanometer‐sized particles of the solid form of the drug; decreasing particle size can improve solubility and bioavailability.24, 25 A novel nanocrystal colloidal dispersion formulation of meloxicam that can be administered as an intravenous (IV) bolus has been developed for the management of moderate and severe pain. The nanocrystal formulation increases the dissolution rate of the active meloxicam moiety and provides rapid onset of action, thus rendering this formulation potentially suitable for the treatment of acute pain.26
Because the analgesic and anti‐inflammatory dose‐response curves vary for meloxicam, a series of phase 2 clinical trials were designed to look at a broad range of IV meloxicam doses in a variety of postoperative settings. The analgesic effect of meloxicam IV was investigated in a randomized, double‐blind, placebo‐controlled study in women who underwent open abdominal hysterectomy (NCT01084161).26 In this study, meloxicam IV, at doses of 15 mg to 60 mg, produced statistically significant improvement in both the time‐weighted summed pain intensity difference and the time‐weighted sum of total pain relief over 24 hours compared with placebo and with a morphine dose of 0.15 mg/kg (10 to 15 mg in the average adult). In addition, rescue medication use was lower in all meloxicam IV groups (38.8% to 62.5%) compared with placebo (95.0%) and morphine (76.7%) in the first 24 hours.27
The safety and efficacy of meloxicam IV in a model of hard‐tissue pain were investigated in a randomized, double‐blind, placebo‐controlled, phase 2 trial in subjects with moderate to severe pain following a standardized bunionectomy procedure (NCT02675907).28, 29 Meloxicam IV at doses of 30 mg or 60 mg, administered once daily over 15 to 30 seconds, resulted in a low incidence of adverse events and no reported injection‐related events. Moreover, both doses of meloxicam IV produced rapid onset of analgesic activity (within 15 minutes after administration) and maintained analgesia throughout the 24‐hour dosing period, evidenced by significant differences in the time‐weighted summed pain intensity difference with once‐daily administration during the first 48 hours compared with placebo. Subjects randomized to meloxicam IV 30 mg or 60 mg used fewer doses of rescue medication per subject (8.2 and 6.9 doses, respectively) compared with placebo (11.1 doses); however, this difference was not significant.28, 29
The purpose of the present phase 2 study (NCT00945763) was to evaluate the safety, tolerability, and analgesic efficacy of single doses of meloxicam 15 mg, 30 mg, and 60 mg IV compared with placebo and with oral ibuprofen 400 mg after dental impaction surgery. Efficacy measurements were recorded over the course of the 24‐hour evaluation period following dosing. Safety assessments included clinical laboratory tests, electrocardiograms, measuring vital signs, and monitoring adverse events throughout the study. Use of oral rescue analgesia (hydrocodone/acetaminophen, 5/500 mg) was monitored and recorded.
Methods
The study protocol and written informed consent forms were reviewed and approved by an institutional review board (Aspire IRB, Santee, California) before study initiation. All subjects provided informed consent before completing any study activities. This was a single‐center, randomized, double‐blind, placebo‐ and active‐controlled, single‐dose study conducted in the United States in healthy adult men and women who planned to undergo surgical removal of impacted third molars. All clinical work was conducted in compliance with Good Clinical Practices (as referenced in the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guideline E6), local regulatory requirements, and the principles of the Declaration of Helsinki.
Subjects
Eligible study participants were healthy men and women (aged ≥18 years) who required surgical extraction of more than 2 third molars, at least 1 of which involved partial or complete mandibular bony extraction. To be eligible for randomization to a study group, subjects had to have a baseline pain intensity score of moderate or severe on a 4‐point Likert scale (containing categories of none, mild, moderate, and severe) within 5 hours following completion of the surgery.
Subjects were excluded if they had used aspirin, another NSAID, any other analgesic drug (acting either centrally or peripherally), or a minor tranquilizer, muscle relaxant, or antihistamine within 48 hours before surgery. Additionally, subjects were ineligible to participate if they required any medication to treat chronic pain, had a history of drug or alcohol abuse within 6 months, or had taken antidepressants within 3 weeks of screening. Other exclusion criteria were presence of active peptic ulcer disease, recent history of peptic ulcer disease or gastrointestinal bleeding, or, in women, pregnancy or breastfeeding.
Study Design
Participation entailed a screening visit (1‐21 days before surgery), an inpatient evaluation period of 24 hours following dosing, and a follow‐up phone call 3 to 5 days postdose. On day 1, subjects underwent surgical removal of >2 third molars performed using nitrous oxide at 3 L/min and a local injection of lidocaine with epinephrine. Subjects also received prophylactic antibiotics postsurgically. Following oral surgery, subjects who experienced moderate to severe pain within 5 hours were assigned randomly to a study group. Randomized subjects received the assigned study drug under double‐dummy conditions (ie, each subject had 1 injection and 1 oral dose). Subjects in the placebo group received an IV injection of dextrose 5% in water (D5W [as placebo injection]) plus 2 placebo tablets. Subjects assigned to a meloxicam IV group received an injection of the assigned dose of meloxicam IV plus 2 placebo tablets. Subjects in the ibuprofen group received 2 ibuprofen tablets plus the placebo IV injection of D5W. All subjects were observed on an inpatient basis for 24 hours following dosing with study medication to complete safety and efficacy assessments.
A 2‐part adaptive study design was utilized to determine the lowest effective dose of meloxicam on the linear portion of the analgesic dose‐effect curve. The initial cohort (cohort 1) included 90 subjects: placebo, n = 15; meloxicam IV 15 mg, n = 25; meloxicam IV 60 mg, n = 25; and ibuprofen 400 mg orally, n = 25. After all subjects in cohort 1 completed the study, the blind was broken, and safety and efficacy data for these subjects were analyzed to determine the appropriate dose for the next cohort. Based on the data from cohort 1, subjects in cohort 2 (enrolled sequentially) were randomized to receive placebo (n = 15), meloxicam IV 15 mg (n = 25), meloxicam IV 30 mg (n = 50), meloxicam IV 60 mg (n = 25), or ibuprofen 400 mg orally (n = 25).
Because this was a phase 2 study with a design to facilitate identification of the most effective use of treatment assignments for cohort 2, there was no statistical penalty for obtaining the interim analysis in order to determine the lowest effective acute analgesic dose of meloxicam compared with placebo and ibuprofen.
Assessments
Baseline parameters included demographics, physical examination findings, medical history, medication history, and results of clinical laboratory tests.
Efficacy
The primary efficacy end point was summed pain intensity difference over 24 hours postdose. Pain intensity was measured on a 100‐mm visual analog scale, ranging from “no pain” to “worst possible pain.” Pain intensity was assessed immediately before dosing and at the following postdose time points: 10, 20, 30, and 45 minutes (all ±2 minutes); 1 hour (±2 minutes); 1.5, 2, 3, 4, 6, 8, 10, and 12 hours (all ±5 minutes); and 18 and 24 hours (each ±10 minutes). Summed pain intensity difference was calculated as the sum of time‐weighted pain intensity difference scores in which the weight given to each pain intensity difference score was equal to the elapsed time between assessments.
Secondary end points included pain intensity difference and pain relief at each time point described above. The time‐weighted summed pain intensity difference was assessed for the following intervals: 0‐2, 0‐4, 0‐8, 0‐12, 0‐18, 8‐12, and 12‐18 hours. Pain relief was rated on a 5‐point scale, ranging from 0 (no relief) to 4 (complete relief). The time‐weighted sum of total pain relief scores were assessed for the following intervals: 0‐2, 0‐4, 0‐8, 0‐12, 0‐18, 8‐12, 12‐18, and 0‐24 hours. The times to first perceptible relief and meaningful pain relief were determined using the double‐stopwatch technique. The time to onset of first perceptible relief (time that the first watch was stopped) was defined as the postdose time at which the subject first began to feel pain relief. The time to meaningful pain relief (time that the second watch was stopped) was defined as the postdose time at which the subject began to feel meaningful pain relief in his or her estimation. The time to confirmed first perceptible relief was established only if the subject achieved meaningful pain relief after reporting first perceptible relief.
The time to first use of rescue analgesic was recorded. The patient‐reported global evaluation score for rating the study medication (0 = poor, 1 = fair, 2 = good, 3 = very good, 4 = excellent) was recorded at the time of administration of rescue medication. If no rescue medication was given, the global evaluation score was recorded at the end of the 24‐hour postdose period.
Safety
Safety assessments performed during the 24‐hour postdose period included monitoring adverse events and vital signs (heart rate, systolic and diastolic blood pressure). Vital signs were recorded 10 minutes before dosing (baseline) and at postdose hours 1 (±5 minutes), 8 (±10 minutes), 12 (±10 minutes), 18 (±10 minutes), and 24 (±10 minutes), with subjects sitting or semirecumbent for at least 5 minutes before vital sign assessment. After the 24‐hour evaluation period, subjects were discharged, and a follow‐up phone call was scheduled for 3 to 5 days postdose (study days 4‐6) to record any ongoing adverse events and the use of concomitant medications. Treatment‐emergent adverse events were defined as events that were new or had worsened in severity after the administration of study drug through the end of the study. Treatment‐related treatment‐emergent adverse events were defined as events considered related or possibly related to treatment.
Statistical Analysis
Summed pain intensity difference over 24 hours postdose, the primary efficacy variable, was the sum of the time‐weighted pain intensity difference scores measured as the intensity change from the baseline pain intensity score. Subjects who received rescue medication before 24 hours had their pain intensity score recorded immediately before the first administration of rescue medication, and the score was carried forward through 24 hours for computation of summed pain intensity difference over 24 hours postdose (ie, last observation carried forward). An analysis of covariance model with treatment as a factor and baseline pain intensity as a covariate was used to analyze the time‐weighted summed pain intensity difference over 24 hours postdose. This model also was applied, as appropriate, to analyses of secondary end points including pain relief, time‐weighted sum of total pain relief, and global evaluation scores.
The time to onset of first perceptible relief and meaningful pain relief within 12 hours after dose initiation was determined using the double‐stopwatch technique. A Cox proportional hazards regression model was used to analyze both variables using treatment as a factor and baseline pain intensity as a covariate. Hazard ratios (HR) and 95%CIs between placebo and each active‐treatment group were estimated. Kaplan‐Meier survival analysis was conducted to estimate time‐to‐event quartiles, and a log‐rank test was performed to identify differences among the groups.
Time‐to‐event data were right censored if a subject withdrew from the study or took rescue medication. Right censoring occurred when a subject's event time (time to the event of interest) was not observed and the observation was imputed to have occurred at a later time than the last known value. For example, if a subject withdrew from the study 8 hours postdose without first perceptible relief, then the event time was censored at 8 hours.
Data for subjects enrolled in cohort 1 and cohort 2 were analyzed separately as well as jointly (pooled). Results of the pooled analysis are presented here.
Safety data were summarized by treatment group without inferential statistics. Adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA; version 12.0).
Results
Demographics
A total of 230 subjects (mean age range, 19.5‐20.4 years) were randomized, treated, and included in the safety and efficacy analyses. Baseline demographic and background characteristics of the study groups are summarized in Table 1. There were more female than male patients (67.4% vs 32.6%, respectively). The majority of subjects were white and not Hispanic or Latino. Thirty‐one patients (13.5%) underwent extraction of 3 third molars, and 199 patients (86.5%) underwent extraction of all 4 third molars. The postoperative baseline pain score was substantial and similar for each study group, ranging from 77.7 to 80.4 on the pain visual analog scale.
Table 1.
Summary of Demographics and Disposition of the Study Population
| Ibuprofen | Meloxicam IV | ||||
|---|---|---|---|---|---|
| Placebo | 400 mg | 15 mg | 30 mg | 60 mg | |
| Characteristics | (n = 30) | (n = 50) | (n = 50) | (n = 50) | (n = 50) |
| Age, y | |||||
| Mean (SD) | 19.9 (3.0) | 19.5 (1.5) | 20.0 (2.8) | 20.4 (2.6) | 19.8 (2.5) |
| Range | (18‐29) | (18‐23) | (18‐32) | (18‐28) | (18‐28) |
| Sex, n (%) | |||||
| Male | 13 (43.3) | 14 (28.0) | 18 (36.0) | 14 (28.0) | 16 (32.0) |
| Female | 17 (56.7) | 36 (72.0) | 32 (64.0) | 36 (72.0) | 34 (68.0) |
| Ethnicity, n (%) | |||||
| Hispanic or Latino | 4 (13.3) | 3 (6.0) | 1 (2.0) | 5 (10.0) | 4 (8.0) |
| Not Hispanic or Latino | 26 (86.7) | 47 (94.0) | 49 (98.0) | 45 (90.0) | 46 (92.0) |
| Race, n (%) | |||||
| White | 26 (86.7) | 45 (90.0) | 48 (96.0) | 48 (96.0) | 45 (90.0) |
| Black | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.0) |
| Asian | 0 (0) | 0 (0) | 1 (2.0) | 0 (0) | 0 (0) |
| Native Hawaiian or other Pacific Islander | 1 (3.3) | 4 (8.0) | 0 (0) | 0 (0) | 2 (4.0) |
| American Indian or Alaskan Native | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 3 (10.0) | 1 (2.0) | 1 (2.0) | 2 (4.0) | 2 (4.0) |
| No. of third molars extracted, n (%) | |||||
| 3 | 6 (20.0) | 7 (14.0) | 2 (4.0) | 9 (18.0) | 7 (14.0) |
| 4 | 24 (80.0) | 43 (86.0) | 48 (96.0) | 41 (82.0) | 43 (86.0) |
| Mean (SD) baseline pain score | 78.0 (13.0) | 79.4 (12.7) | 79.4 (11.9) | 77.7 (13.2) | 80.4 (13.1) |
Efficacy
Statistically significant differences in the time‐weighted summed pain intensity difference over 24 hours postdose (the primary efficacy end point) were seen for each meloxicam IV group and the ibuprofen group compared with the placebo group, in favor of active treatment (P < .001; Figure 1). A positive dose‐response effect on the time‐weighted summed pain intensity difference over 24 hours postdose was observed, corresponding to the dose level of meloxicam IV. Statistically significant differences were observed, favoring meloxicam IV 60 mg over 30 mg (P = .033) and 15 mg (P < .001). Statistically significant differences favoring meloxicam IV 60 mg and 30 mg over ibuprofen (P < .001 and P = .021, respectively) also were noted. The difference between the meloxicam IV 15‐mg group and the ibuprofen group favored meloxicam, but this difference was not statistically significant.
Figure 1.
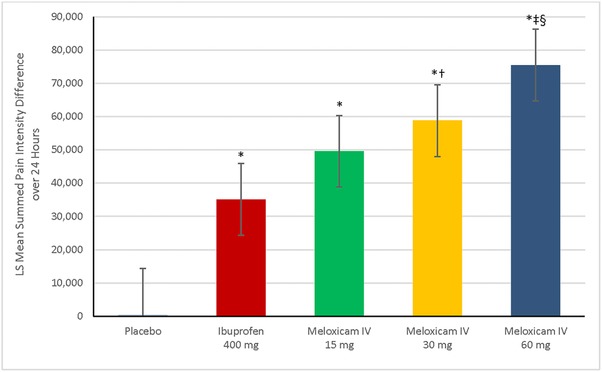
Least squares (LS) mean for summed pain intensity difference over 24 hours postdose, according to study group. Error bars represent the range of the 95%CI. *P < .001 vs placebo. † P = .002 vs ibuprofen 400 mg. ‡ P < .001 vs ibuprofen 400 mg. § P < .001 vs meloxicam IV 15 mg. IV indicates intravenous dosing.
The meloxicam IV 60‐mg group had the greatest reduction in pain intensity from baseline as measured by the time‐weighted summed pain intensity difference values for postdose hourly intervals 0‐2, 0‐4, 0‐8, 0‐12, 0‐18, 8‐12, and 12‐18, followed by the 30‐mg group, the 15‐mg group, the ibuprofen group, and the placebo group. Statistically significant differences between each meloxicam IV group and placebo and between ibuprofen and placebo were seen for each time interval, in favor of active treatment (P < .001). For each time interval, statistically significant differences were seen between meloxicam IV 60 mg and ibuprofen and between meloxicam IV 30 mg and ibuprofen, favoring meloxicam IV (P < .01). Analyses performed at intervals throughout the first 8 hours after dosing provided opportunity to evaluate analgesic effect at intervals inclusive of the ibuprofen 400 mg duration of action (Figure 2). In comparison to ibuprofen, meloxicam IV doses of 30 mg and 60 mg produced statistically significant reductions in the time‐weighted summed pain intensity differences during the postdose hourly intervals 0‐2, 0‐4, and 0‐8 (P ≤ .005). The differences between meloxicam IV 15 mg and ibuprofen were statistically significant for 0‐2, 0‐12, 0‐18, and 8‐12 hours postdose (P < .05). The differences between 60 mg and 15 mg meloxicam IV were statistically significant at 0‐12, 0‐18, 0‐24, 8‐12, and 12‐18 hours (P < .05), as were the differences between 60 mg and 30 mg meloxicam IV at 12‐18 and 0‐24 hours (P < .05).
Figure 2.
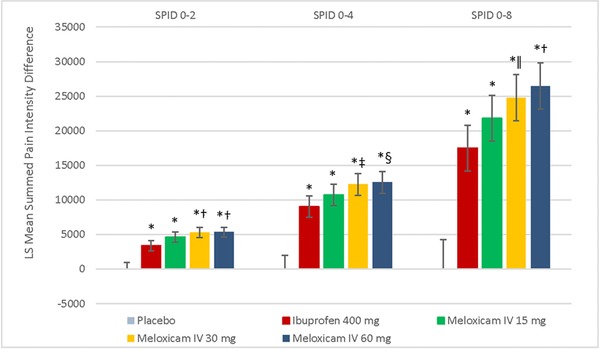
Least squares (LS) mean for summed pain intensity differences (SPID) for time intervals 0‐2 hours, 0‐4 hours, and 0‐8 hours according to study group. Error bars represent the range of the 95%CIs. *P < .001 vs placebo. † P < .001 vs ibuprofen 400 mg. ‡ P = .005 vs ibuprofen 400 mg. § P = .002 vs ibuprofen 400 mg. ǁ P = .003 vs ibuprofen 400 mg. IV indicates intravenous dosing.
Least‐squares (LS) mean values of the time‐weighted sum of total pain relief scores over 24 hours demonstrated the same pattern as the time‐weighted summed pain intensity difference over 24 hours values and were statistically significantly higher for each meloxicam IV group vs the placebo group (P < .001; Figure 3). Statistically significant differences also were noted for meloxicam IV 60 mg vs 15 mg (P < .01) and for each dose of meloxicam IV vs ibuprofen (P < .001, P < .001, and P = .028 for 60 mg, 30 mg, and 15 mg, respectively).
Figure 3.
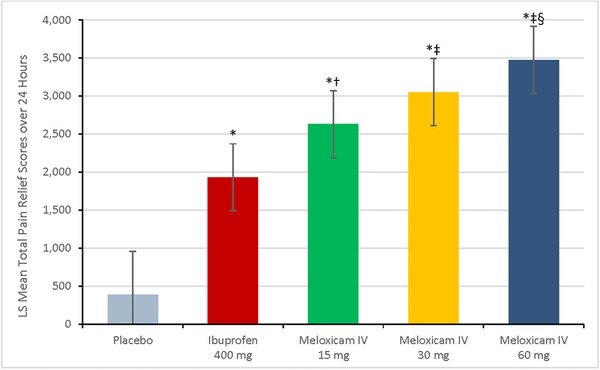
Least squares (LS) mean for the summed time‐weighted pain relief scores over 24 hours postdose, according to study group. Error bars represent the range of the 95%CIs. *P < .001 vs placebo. † P = .028 vs ibuprofen 400 mg. ‡ P < .001 vs ibuprofen 400 mg. § P = .008 vs meloxicam IV 15 mg. IV indicates intravenous dosing.
There were statistically significant differences in individual pain intensity difference values for each active treatment vs placebo at every assessment point in the study (Figure 4A). Differences were apparent as early as 10 minutes postdose (Figure 4B) and continued through the 24‐hour observation period. In general, the decreases in pain intensity were greatest after treatment with meloxicam IV 60 mg, followed by 30 mg, 15 mg, and ibuprofen. Peak pain intensity difference for all active‐treatment groups occurred between 2 and 3 hours postdose (Figure 4A).
Figure 4.
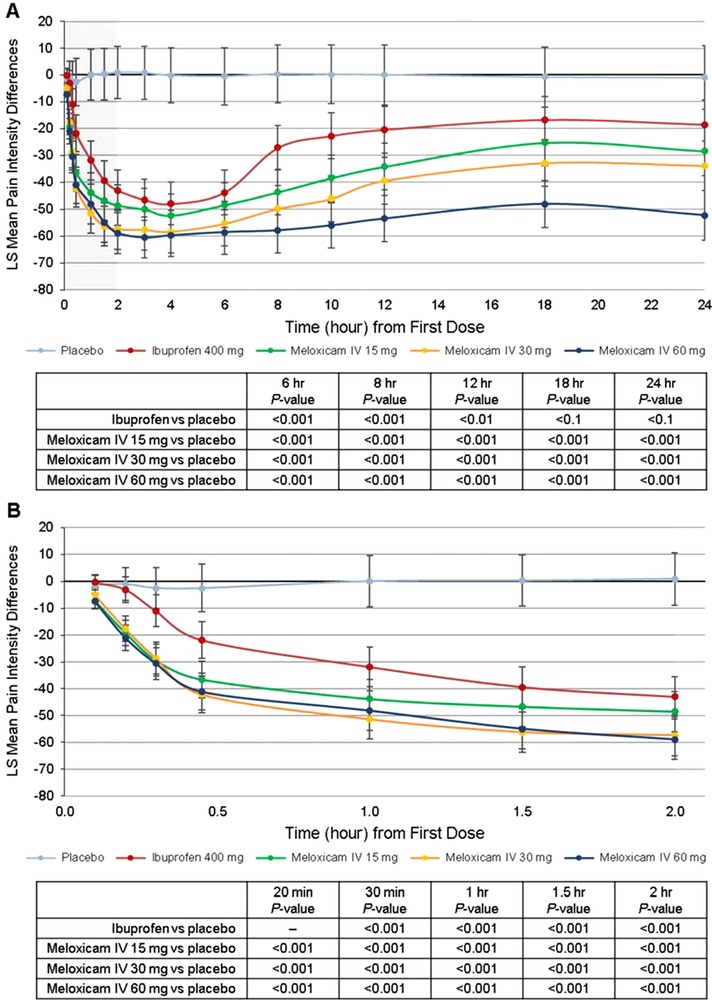
Summary of least squares (LS) mean pain intensity differences over (A) the first 24 hours and (B) the first 2 hours. Error bars represent the range of the 95%CIs. IV indicates intravenous dosing.
Statistically significant differences in pain intensity difference, favoring the 15‐mg and 60‐mg doses of meloxicam IV vs placebo, were seen at each time point analyzed (P < .05 and P < .01 at 10 minutes, respectively; P < .001 at all other time points), and favoring meloxicam IV 30 mg vs placebo at all time points (P < .001) except 10 minutes postdose. The comparison of pain intensity difference data for meloxicam IV and oral ibuprofen showed statistically significant benefits for meloxicam IV at the 60‐mg dose vs ibuprofen (P < .05) at each postdose time point, the 30‐mg dose vs ibuprofen (P < .05) at each time point except 4 and 6 hours postdose, and the 15‐mg dose vs ibuprofen (P < .05) at 10, 20, 30, 45, and 60 minutes postdose.
Statistically significant differences in the time‐weighted sum of pain relief scores were noted between meloxicam IV 60 mg and 15 mg at 8, 10, 12, 18, and 24 hours postdose (P < .05) and between meloxicam IV 60 mg and 30 mg at 12, 18, and 24 hours postdose (P < .05). There were no statistically significant differences in the time‐weighted sum of pain relief scores over 24 hours postdose between the 30‐mg and 15‐mg doses.
The LS mean individual scores for pain relief in the 3 meloxicam IV groups followed the same pattern as pain intensity difference values throughout the study. The difference between each meloxicam IV dose and placebo was statistically significant at every time point (P < .01); the difference between meloxicam IV 60 mg and ibuprofen was statistically significant at all time points (P < .05) except 4 hours postdose. Statistically significant differences in pain relief also were seen between meloxicam IV 30 mg and ibuprofen at time points (P < .05) other than 3, 4, and 6 hours postdose and between meloxicam IV 15 mg and ibuprofen at time points (P < .05) other than 1.5, 2, 3, 4, and 6 hours postdose.
The time to confirmed first perceptible relief is shown in Figure 5. Log‐rank analysis demonstrated a statistically significant difference between each meloxicam IV dose group and placebo (P < .0001). Meaningful pain relief was experienced by 10% of the placebo group, 78% of the ibuprofen group, 92% of the meloxicam IV 60‐mg group, 86% of the meloxicam IV 30‐mg group, and 74% of the meloxicam IV 15‐mg group (Figure 6). Log‐rank analysis of active treatment vs placebo showed that the difference in meaningful pain relief between each active treatment and placebo was statistically significant (P ≤ .0017). HR analysis indicated that subjects who received meloxicam IV 60 mg were approximately 20 times more likely than placebo subjects to experience meaningful pain relief (HR 19.8; 95% CI 4.8‐81.9); those who received meloxicam IV 30 mg and 15 mg were approximately 12 and 9 times more likely (respectively) than were placebo subjects to have meaningful pain relief (30 mg, HR 11.8; 95% CI 3.7‐38.3; 15 mg, HR 9.0; 95% CI 2.7‐29.8). In a Cox model with treatment as a factor and baseline pain intensity as a covariate, statistically significant differences in time to meaningful pain relief were detected for each meloxicam IV group vs placebo (P < .001), for ibuprofen vs placebo (P < .01), and for meloxicam IV 60 mg vs ibuprofen (P <.01).
Figure 5.
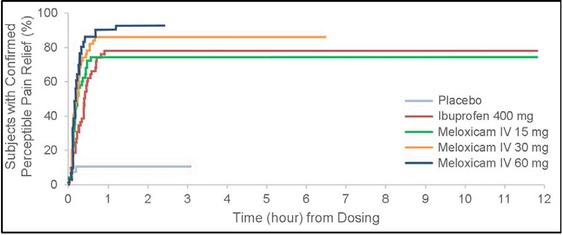
Survival analysis of time to confirmed perceptible pain relief. Data were censored if a subject withdrew or took rescue medication. IV indicates intravenous dosing.
Figure 6.
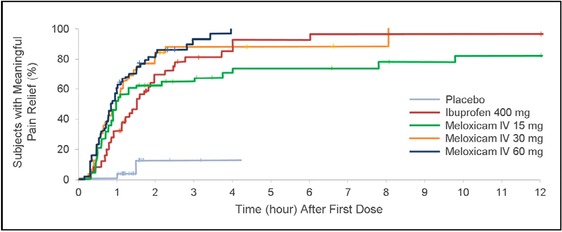
Survival analysis of time to meaningful pain relief. Data were censored if a subject withdrew or took rescue medication. IV indicates intravenous dosing.
Rescue medication (oral hydrocodone/acetaminophen 5/500 mg) was taken by 93.3% of subjects in the placebo group during the 24‐hour postdose evaluation period, compared with 28% of the meloxicam IV 60‐mg group, 58% of the meloxicam IV 30‐mg and 15‐mg groups, and 72% of the ibuprofen group (Figure 7). The difference in use of rescue medication between each active treatment and placebo was statistically significant (P < .001). Relative to placebo, rescue medication use during the 24‐hour observation period was reduced 93% by meloxicam IV 60 mg (HR 0.07; 95% CI 0.03‐0.15; P < .001), 86% by meloxicam IV 30 mg (HR 0.14; 95% CI 0.08‐0.24; P < 0.001), 87% by meloxicam IV 15 mg (HR 0.13; 95% CI 0.07‐0.23; P < .001), and 79% by ibuprofen (HR 0.21; 95% CI 0.12‐0.36; P < .001). Rescue medication use was 75% lower with meloxicam IV 60 mg vs ibuprofen (HR 0.25; 95% CI 0.13‐0.47; P < .001).
Figure 7.
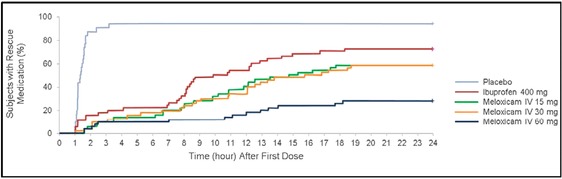
Survival analysis of time to first use of rescue medication. Data were censored if a subject withdrew or took rescue medication. IV indicates intravenous dosing.
Patient‐reported LS mean global evaluation scores for rating the study medication (0 = poor, 1 = fair, 2 = good, 3 = very good, 4 = excellent) were highest for meloxicam IV 60 mg (3.1; 95% CI 2.8‐3.4), followed by meloxicam IV 30 mg (2.8; 95% CI 2.5‐3.2), meloxicam IV 15 mg (2.4; 95% CI 2.1‐2.8), ibuprofen (2.1; 95% CI 1.8‐2.5), and placebo (0.3; 95% CI, –0.1 to 0.7). Statistically significant differences in global evaluation scores for rating study medication were detected between each meloxicam IV dose and placebo and between ibuprofen and placebo, in favor of active treatment (P < .001). Although meaningful differences on this scale have not been established, the scores do demonstrate statistically significant differences between meloxicam IV 60 mg and ibuprofen (P < .001) and between meloxicam IV 30 mg and ibuprofen (P = .003), both favoring meloxicam IV. Moreover, the difference was significant between meloxicam IV 60 mg and 15 mg (P = .007), favoring 60 mg.
Given the disparity in sex distribution across study groups, an analysis by sex was conducted. No significant differences in any efficacy parameter were found between male and female subjects.
Safety
All doses of meloxicam IV appeared safe and generally well tolerated. In general, the adverse events were reported as mild, and there was no clinically meaningful difference in adverse events among the meloxicam IV groups. There were no deaths, serious adverse events, or discontinuations due to adverse events. The incidence of subjects with at least 1 treatment‐emergent adverse event was greatest in the placebo group, followed by the groups that received ibuprofen, meloxicam IV 15 mg, 30 mg, and 60 mg. A summary of treatment‐emergent adverse events appears in Table 2. The incidence of subjects with at least 1 study drug‐related treatment‐emergent adverse event was greatest in the placebo group (16.7%), followed by the ibuprofen group (10.0%) and the meloxicam IV 60‐mg (6.0%), 30‐mg (4.0%), and 15‐mg (2.0%) groups. Nausea was the most commonly reported drug‐related treatment‐emergent adverse event, followed by vomiting. The greatest incidence of nausea was in the placebo group (13.3%), followed by the ibuprofen group (6.0%), and the meloxicam IV 60‐mg group (4.0%), 30‐mg group (2.0%), and 15‐mg group (2.0%). Vomiting was reported most frequently in the placebo group (6.7%), followed by the ibuprofen group (4.0%), and all 3 meloxicam IV groups (2.0% each). All other remaining adverse events were reported by no more than 1 person in each study group.
Table 2.
Summary of All Treatment‐Emergent Adverse Events
| No. (%) of Subjects | |||||
|---|---|---|---|---|---|
| Ibuprofen | Meloxicam IV | ||||
| Placebo | 400 mg | 15 mg | 30 mg | 60 mg | |
| Adverse Event | (n = 30) | (n = 50) | (n = 50) | (n = 50) | (n = 50) |
| Subjects with ≥1 TEAEa | 8 (26.7) | 13 (26.0) | 12 (24.0) | 8 (16.0) | 6 (12.0) |
| Cardiac disorders | |||||
| Bradycardia | 0 (0) | 0 (0) | 0 (0) | 1 (2.0) | 0 (0) |
| Tachycardia | 0 (0) | 0 (0) | 0 (0) | 1 (2.0) | 0 (0) |
| Ear and labyrinth disorders | |||||
| Ear pain | 0 (0) | 1 (2.0) | 0 (0) | 0 (0) | 0 (0) |
| Gastrointestinal disorders | |||||
| Diarrhea | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.0) |
| Nausea | 5 (16.7) | 9 (18.0) | 9 (18.0) | 3 (6.0) | 5 (10.0) |
| Vomiting | 4 (13.3) | 5 (10.0) | 6 (12.0) | 3 (6.0) | 3 (6.0) |
| General disorders and administration‐site conditions | |||||
| Chills | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.0) |
| Fatigue | 0 (0) | 0 (0) | 1 (2.0) | 0 (0) | 0 (0) |
| Hyperthermia | 0 (0) | 0 (0) | 0 (0) | 1 (2.0) | 0 (0) |
| Influenza‐like illness | 0 (0) | 0 (0) | 0 (0) | 1 (2.0) | 0 (0) |
| Infusion site extravasation | 0 (0) | 1 (2.0) | 0 (0) | 0 (0) | 0 (0) |
| Injection site pain | 1 (3.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pyrexia | 0 (0) | 1 (2.0) | 0 (0) | 0 (0) | 0 (0) |
| Infections and infestations | |||||
| Infection | 0 (0) | 0 (0) | 0 (0) | 1 (2.0) | 1 (2.0) |
| Injury, poisoning, and procedural complications | |||||
| Postprocedural complication | 0 (0) | 0 (0) | 0 (0) | 1 (2.0) | 0 (0) |
| Postprocedural hematoma | 0 (0) | 0 (0) | 1 (2.0) | 0 (0) | 0 (0) |
| Nervous system disorders | |||||
| Dizziness | 0 (0) | 1 (2.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Headache | 1 (3.3) | 2 (4.0) | 0 (0) | 0 (0) | 0 (0) |
| Syncope | 0 (0) | 1 (2.0) | 0 (0) | 0 (0) | 0 (0) |
| Tremor | 1 (3.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Respiratory, thoracic, and mediastinal disorders | |||||
| Epistaxis | 0 (0) | 0 (0) | 0 (0) | 1 (2.0) | 1 (2.0) |
| Skin and subcutaneous tissue disorders | |||||
| Hyperhidrosis | 0 (0) | 0 (0) | 1 (2.0) | 0 (0) | 0 (0) |
| Vascular disorders | |||||
| Pallor | 0 (0) | 0 (0) | 0 (0) | 1 (2.0) | 0 (0) |
| Presyncope | 0 (0) | 1 (2.0) | 0 (0) | 1 (2.0) | 0 (0) |
TEAEs were defined as adverse events that were new or had worsened in severity after administration of the study drug.
There were no adverse events at the injection site in any meloxicam IV group (eg, infusion‐site extravasations, pain, or venous thrombosis). Infusion‐site extravasation was reported for 1 patient in the ibuprofen group, and injection‐site pain was reported for 1 patient in the placebo group.
Few abnormalities in vital signs were clinically meaningful. At least 1 systolic blood pressure value was clinically abnormal (≤90 mm Hg, having decreased ≥20 mm Hg from baseline) for 1 subject each in the placebo, ibuprofen, and meloxicam IV 15‐mg groups and for 3 subjects in the meloxicam IV 30‐mg group. At least 1 diastolic blood pressure value was clinically abnormal (≤50 mm Hg, having decreased ≥15 mm Hg from baseline) for 1 subject in the meloxicam IV 15 mg group. No clinically meaningful abnormalities in heart rate were reported. No significant findings were identified from hematologic, multiphasic chemistry, or coagulation tests.
Discussion
The primary objective of this study was to evaluate the analgesic efficacy, safety, and tolerability of single doses of meloxicam IV after surgical removal of impacted third molars. Postoperative pain following surgical removal of impacted third molars is a well‐established clinical model for evaluating the efficacy of analgesics. The pain model demonstrated assay sensitivity that enabled differential efficacy among active treatments. Overall, meloxicam IV 60 mg produced the greatest reduction in pain, followed by meloxicam IV 30 mg and 15 mg. Highly significant differences in pain intensity were seen for active treatment groups vs placebo in every efficacy analysis. Moreover, statistically significant differences in the primary efficacy variable (time‐weighted summed pain intensity difference over 24 hours postdose) were noted for meloxicam IV vs placebo and for ibuprofen vs placebo. Time‐weighted summed pain intensity difference for hours 0‐24 and for other intervals (including hours 12‐18) indicated a prolonged duration of action for meloxicam IV, supportive of once‐daily dosing. This is corroborated by the statistically significant difference in pain intensity difference, in favor of meloxicam IV, observed at time points throughout the present study. There have been only a few studies in which NSAID treatment demonstrated superiority to ibuprofen 400 mg.30, 31, 32 In the present study the analgesic efficacy scores for each dose of meloxicam IV were higher than those for ibuprofen 400 mg, including throughout the first 8 hours after dosing, which were within the ibuprofen 400‐mg duration of analgesic effect.
Two important characteristics of a suitable medication for treatment of acute pain are rapid onset of analgesic effect and appropriate duration of analgesia.33 In the present study the onset of action for meloxicam IV was rapid; statistically significant differences in pain intensity difference and pain relief were observed as early as 10 minutes postdose. The median times to confirmed first perceptible relief and to meaningful pain relief were significantly shorter for meloxicam IV than for placebo, and meloxicam IV 60 mg was significantly superior to ibuprofen 400 mg. This rapid onset of action supports further evaluation of meloxicam IV for the treatment of acute postsurgical pain. Furthermore, meloxicam IV resulted in prolonged duration of analgesic action, evidenced by statistically significant differences in pain intensity difference and pain relief throughout the 24‐hour postdose observation period. Another measure of sustained analgesic effect, the time to first use of rescue medication, also demonstrated the sustained analgesic effect of meloxicam IV. The time to first use of rescue medication was longer with higher doses of meloxicam IV, and the 60‐mg dose was significantly better than ibuprofen in this regard.
Subjects treated with meloxicam IV used less opioid rescue medication than did subjects who received ibuprofen or placebo. Although opioids are effective for treating acute pain after surgery, they are associated with various adverse events, including respiratory depression, nausea, vomiting, and elevated risk of accidental overdose and addiction.18 Fast‐ and long‐acting nonopioid analgesic alternatives are needed to effectively reduce pain while minimizing opioid requirements and opioid‐related adverse events. Significantly less rescue medication was used after each tested dose of meloxicam than after placebo. According to patient‐reported global evaluation scores rating study medication, meloxicam IV 60 mg was ranked highest, followed by meloxicam IV 30 mg, meloxicam IV 15 mg, ibuprofen, and placebo.
Although the study was not powered to distinguish among the dose levels of meloxicam IV, the summed pain intensity difference over 24 hours postdose results numerically favored the meloxicam IV 60‐mg dose over the 15‐mg and 30‐mg doses; similarly, the 60‐mg and 30‐mg doses of meloxicam IV were numerically favored vs ibuprofen. To date, this is the only study demonstrating a significant difference between the 30‐mg and 60‐mg doses of meloxicam IV. In other phase 2 clinical trials, no significant difference in efficacy was observed between the 30‐mg and 60‐mg doses.27, 28, 29 Therefore, the 30‐mg dose was selected for future phase 3 clinical studies because it was statistically superior to ibuprofen 400 mg in this study and considered to have the optimal efficacy/safety profile of the doses tested in phase 2 trials.
Investigators determined that each dose of meloxicam IV used in the present trial appeared to be generally well tolerated. No deaths, serious adverse events, or discontinuations due to adverse events were reported during the study. The incidence of treatment‐emergent adverse events was greatest for the ibuprofen and placebo groups. Nausea and vomiting were the most commonly reported treatment‐emergent adverse events among the study population. These adverse events, commonly associated with opioid medications, may be associated with opioid rescue. There was no relation between the dose of meloxicam IV and the frequency of treatment‐emergent adverse events. No infusion‐related adverse events occurred with meloxicam IV, and there were no notable clinically relevant effects on vital signs, laboratory measurements, or ECG assessments.
A potential limitation of the study was that the overall opioid consumption through 48 hours after discharge was not monitored. Additionally, this study was designed to provide support for the regulatory submission, which requires a 24‐hour evaluation period following single doses of each active treatment. Oral ibuprofen 400 mg was selected as the active treatment comparator, which is considered the standard of care for postoperative dental pain, although it has a shorter serum half‐life of just 1.5 to 2 hours, and additional doses of ibuprofen were not permitted during the 24‐hour evaluation period.34 Furthermore, although the superiority of higher doses of ibuprofen compared to 400 mg to treat postoperative dental pain has not been demonstrated in a clinical trial setting, higher doses or multiple doses of ibuprofen may be thought to provide better pain relief and could be investigated in future clinical studies. Summed pain intensity difference scores were right censored if a subject took rescue medication, which may have affected the summed pain intensity difference efficacy results. Further studies with meloxicam IV are needed to demonstrate the robustness of efficacy and to determine whether safety is sustained after multiple‐dose regimens. Such studies should employ models that have pain duration lasting several days, such as the postbunionectomy model.1
Conclusions
Single‐dose treatment with meloxicam IV (15 mg, 30 mg, or 60 mg) achieved the primary end point following surgical removal of impacted third molars. It provided rapid onset of analgesic effect (within 10 minutes), and the duration of effect lasted 24 hours. Each dose of meloxicam IV was statistically significantly better than ibuprofen or placebo for managing pain at early time points and throughout the entire 24‐hour observation period. Meloxicam IV was much more likely than was placebo to result in pain relief that was perceptible and meaningful. Stepwise improvement was noted for each meloxicam IV dose group, and the 60‐mg group had the greatest reduction in pain. The need for opioid rescue medication was statistically significantly lower with meloxicam IV 60 mg than with ibuprofen. Meloxicam IV, even at the highest dose tested (60 mg), appeared generally safe and well tolerated; the incidence of adverse events was low, and there were no deaths, serious adverse events, or adverse‐event–related discontinuations. In conclusion, meloxicam IV appears to provide safe, rapid, long‐acting, and well‐tolerated nonopioid analgesia that may manage acute postoperative pain.
Funding
Funding for this research was provided by Recro Pharma, Inc., Malvern, PA.
References
- 1. Cooper SA, Desjardins PJ, Turk DC, et al. Research design considerations for single‐dose analgesic clinical trials in acute pain: IMMPACT recommendations. Pain. 2016;157(2):288–301. [DOI] [PubMed] [Google Scholar]
- 2. Cooper SA, Desjardins PJ. The value of the dental impaction pain model in drug development. Methods Mol Biol. 2010;617:175–190. [DOI] [PubMed] [Google Scholar]
- 3. Au AH, Choi SW, Cheung CW, Leung YY. The efficacy and clinical safety of various analgesic combinations for post‐operative pain after third molar surgery: a systematic review and meta‐analysis. PLoS One. 2015;10(6):e0127611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Litkowski LJ, Christensen SE, Adamson DN, et al. Analgesic efficacy and tolerability of oxycodone 5 mg/ibuprofen 400 mg compared with those of oxycodone 5 mg/acetaminophen 325 mg and hydrocodone 7.5 mg/acetaminophen 500 mg in patients with moderate to severe postoperative pain: a randomized, double‐blind, placebo‐controlled, single‐dose, parallel‐group study in a dental pain model. Clin Ther. 2005;27(4):418–429. [DOI] [PubMed] [Google Scholar]
- 5. Malmstrom K, Fricke JR, Kotey P, Kress B, Morrison B. A comparison of rofecoxib versus celecoxib in treating pain after dental surgery: a single‐center, randomized, double‐blind, placebo‐ and active‐comparator‐controlled, parallel‐group, single‐dose study using the dental impaction pain model. Clin Ther. 2002;24(10):1549–1560. [DOI] [PubMed] [Google Scholar]
- 6. Qi DS, May LG, Zimmerman B, et al. A randomized, double‐blind, placebo‐controlled study of acetaminophen 1000 mg versus acetaminophen 650 mg for the treatment of postsurgical dental pain. Clin Ther. 2012;34(12):2247–2258.e2243. [DOI] [PubMed] [Google Scholar]
- 7. Schou S, Nielsen H, Nattestad A, et al. Analgesic dose‐response relationship of ibuprofen 50, 100, 200, and 400 mg after surgical removal of third molars: a single‐dose, randomized, placebo‐controlled, and double‐blind study of 304 patients. J Clin Pharmacol. 1998;38(5):447–454. [DOI] [PubMed] [Google Scholar]
- 8. Shah D, Shah S, Mahajan A, et al. A comparative clinical evaluation of analgesic efficacy of tapentadol and ketorolac in mandibular third molar surgery. Natl J Maxillofac Surg. 2017;8(1):12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taneja P, Pattni A, Pearson D. What's new in… the management of post‐operative pain in dentistry. SAAD Dig. 2015;31:3–7. [PubMed] [Google Scholar]
- 10. Moore PA, Hersh EV. Combining ibuprofen and acetaminophen for acute pain management after third‐molar extractions: translating clinical research to dental practice. J Am Dent Assoc. 2013;144(8):898–908. [DOI] [PubMed] [Google Scholar]
- 11. Derry S, Wiffen PJ, Moore RA. Relative efficacy of oral analgesics after third molar extraction—a 2011 update. Br Dent J. 2011;211(9):419–420. [DOI] [PubMed] [Google Scholar]
- 12. Cooper SA, Engel J, Ladov M, et al. Analgesic efficacy of an ibuprofen‐codeine combination. Pharmacotherapy. 1982;2(3):162–167. [DOI] [PubMed] [Google Scholar]
- 13. Cooper SA, Precheur H, Rauch D, et al. Evaluation of oxycodone and acetaminophen in treatment of postoperative dental pain. Oral Surg Oral Med Oral Pathol. 1980;50(6):496–501. [DOI] [PubMed] [Google Scholar]
- 14. Hersh EV, Moore PA. Adverse drug interactions in dentistry. Periodontol 2000. 2008;46(1):109–142. [DOI] [PubMed] [Google Scholar]
- 15. Pozzi A, Gallelli L. Pain management for dentists: the role of ibuprofen. Ann Stomatol (Roma). 2011;2(3‐4 Suppl):3–24. [PMC free article] [PubMed] [Google Scholar]
- 16. CADTH rapid response reports Ketorolac for Pain Management: A Review of the Clinical Evidence. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2014. [PubMed] [Google Scholar]
- 17. Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N. Acetaminophen‐induced hepatotoxicity: a comprehensive update. J Clin Transl Hepatol. 2016;4(2):131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 suppl):S105–S120. [PubMed] [Google Scholar]
- 19. Wickerts L, Warren Stomberg M, Brattwall M, Jakobsson J. Coxibs: is there a benefit when compared to traditional non‐selective NSAIDs in postoperative pain management? Minerva Anestesiol. 2011;77(11):1084–1098. [PubMed] [Google Scholar]
- 20. Turck D, Busch U, Heinzel G, Narjes H. Clinical pharmacokinetics of meloxicam. Arzneimittelforschung. 1997;47(3):253–258. [PubMed] [Google Scholar]
- 21. Del Tacca M, Colucci R, Fornai M, Blandizzi C. Efficacy and tolerability of meloxicam, a COX‐2 preferential nonsteroidal anti‐inflammatory drug. Clin Drug Invest. 2002;22(12):799–818. [Google Scholar]
- 22. Degner F, Türck D, Pairet M. Pharmacological, pharmacokinetic and clinical profile of meloxicam. Drugs Today. 1997;33(10):739–758. [Google Scholar]
- 23. Turck D, Roth W, Busch U. A review of the clinical pharmacokinetics of meloxicam. Br J Rheumatol. 1996;35(suppl 1):13–16. [DOI] [PubMed] [Google Scholar]
- 24. Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3(9):785–796. [DOI] [PubMed] [Google Scholar]
- 25. Chen H, Khemtong C, Yang X, Chang X, Gao J. Nanonization strategies for poorly water‐soluble drugs. Drug Discov Today. 2011;16(7‐8):354–360. [DOI] [PubMed] [Google Scholar]
- 26. Ochi M, Kawachi T, Toita E, et al. Development of nanocrystal formulation of meloxicam with improved dissolution and pharmacokinetic behaviors. Int J Pharm. 2014;474(1‐2):151–156. [DOI] [PubMed] [Google Scholar]
- 27. Mack R, Freyer A, Du W. An evaluation of the efficacy and safety of N1539, a novel intravenous formulation of nanocrystal meloxicam, in subjects with moderate to severe pain following hysterectomy (abstract 409). J Pain. 2016;17(4s):S77. [Google Scholar]
- 28. Gottlieb IJ, Tunick DR, Mack RJ, et al. An evaluation of the safety and efficacy of N1539, a novel intravenous formulation of NanoCrystal meloxicam, administered by IV push in subjects with moderate to severe pain following bunionectomy. PAINWeek Abstract Book 2016. Postgrad Med. 2016;128(suppl 2):S57–S58. [Google Scholar]
- 29. Gottlieb IJ, Tunick DR, Mack RJ, et al. An evaluation of the safety and efficacy of N1539, a novel intravenous formulation of NanoCrystal meloxicam, administered by IV push in subjects with moderate to severe pain following bunionectomy. PAINWeek National Conference; September 06‐10 2016; Las Vegas, NV. Full poster http://c.eqcdn.com/_a231e0af3576d655e04f95ce92517b26/recropharma/db/204/476/pdf/Phase+II+Bunionectomy_2016+Painweek.pdf [Google Scholar]
- 30. Malmstrom K, Daniels S, Kotey P, Seidenberg BC, Desjardins PJ. Comparison of rofecoxib and celecoxib, two cyclooxygenase‐2 inhibitors, in postoperative dental pain: a randomized, placebo‐ and active‐comparator‐controlled clinical trial. Clin Ther. 1999;21(10):1653–1663. [DOI] [PubMed] [Google Scholar]
- 31. Morrison BW, Christensen S, Yuan W, et al. Analgesic efficacy of the cyclooxygenase‐2‐specific inhibitor rofecoxib in post‐dental surgery pain: a randomized, controlled trial. Clin Ther. 1999;21(6):943–953. [DOI] [PubMed] [Google Scholar]
- 32. Forbes JA, Kehm CJ, Grodin CD, Beaver WT. Evaluation of ketorolac, ibuprofen, acetaminophen, and an acetaminophen‐codeine combination in postoperative oral surgery pain. Pharmacotherapy. 1990;10(6 Pt 2):94s–105s. [PubMed] [Google Scholar]
- 33. Moore ND. In search of an ideal analgesic for common acute pain. Acute Pain. 2009;11(3):129–137. [Google Scholar]
- 34. Becker DE. Pain management: part 1: managing acute and postoperative dental pain. Anesth Prog. 2010;57(2):67–78; quiz 79‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]


