Summary
IgE‐mediated allergic reactions involve the activation of effector cells, predominantly through the high‐affinity IgE receptor (FcεRI) on mast cells and basophils. Although the mast cell is considered the major effector cell during acute allergic reactions, more recent studies indicate a potentially important and specific role for basophils and their migration which occurs rapidly upon allergen challenge in humans undergoing anaphylaxis. We review the evidence for a role of basophils in contributing to clinical symptoms of anaphylaxis and discuss the possibility that basophil trafficking during anaphylaxis might be a pathogenic (to target organs) or protective (preventing degranulation in circulation) response. Finally, we examine the potential role of basophils in asthma exacerbations. Understanding the factors that regulate basophil trafficking and activation might lead to new diagnostic and therapeutic strategies in anaphylaxis and asthma.
1. INTRODUCTION
1.1. Of mice and not men: the relevance of murine basophils to human basophils
HIGHLIGHTS.
Basophils in mice display substantial differences in morphology, function and immunomodulatory roles in comparison with human basophils. This highlights major pitfalls in extrapolating from animal basophil models to acute allergic reactions in humans.1, 2
Despite an increasing number of studies using mouse models demonstrating an important role for basophils in orchestrating pro‐allergic Th2‐type immune responses and mediating chronic allergic inflammation, extrapolation to humans is highly problematical (Table 1). This is because of substantial differences in basophil morphology and relative expressions of various cell surface receptors, as well as different outcomes of their subsequent stimulation.1, 2 While recent studies suggest that murine basophils produce similar inflammatory mediators to human basophils,3 sensitivities to the biological effects of these mediators differ from one species to another. For example, Berman & Munoz showed that the LD50 of histamine (thought to be an important mediator of anaphylaxis) in mice was >20 mg/mouse 4—a sensitivity several orders of magnitude lower than that in humans. This may have contributed to the relative paucity of studies assessing the role of basophils in anaphylaxis, given that basophils are relatively uncommon in comparison with their tissue‐fixed mast cell counterparts in both mice and humans. However, despite their relative rarity, human basophils are at least one order of magnitude more sensitive to IgE‐mediated provocation than mast cells.5
Table 1.
Differences in the pathophysiology of anaphylaxis in murine models compared to humans (adapted from Turner and Campbell113)
| Murine models | Mediators and mechanisms | Humans |
|---|---|---|
| Polymeric IgA (low serum levels) IgD, IgE, IgMIgG1, IgG2a, IgG2b, IgG3 | Immunoglobulins | Monomeric IgA, 2 serotypes (IgA1, IgA2), IgA1 abundant in serumIgD, IgE, IgMIgG1, IgG2, IgG3, IgG4 |
| Yes | High‐affinity IgE receptor (FcεRI) on mast cells and basophils | Yes |
| No | FcεRI receptor on antigen‐presenting cells | Yes |
| Yes | IgE‐dependent anaphylaxis | Yes |
| Yes | IgG‐dependent anaphylaxis | No evidence for IgG‐mediated activation of human mast cells. If present, likely to require very high levels of antigen exposure |
| Very high: in murine models of peanut allergy, dose/weight equivalent to a human eating ≅1000 peanuts! | Allergen dose required through oral exposure to cause anaphylaxis | Very low doses (mgs), for example, for peanut allergy, 10% of individuals react to 1/70 of a peanut |
| + | Sensitivity to histamine | ++++ |
| Yes | Anaphylaxis inhibited by H1‐antihistamines | Little clinical evidence for this. Significant interspecies differences exist in histamine receptor pharmacology. |
| Yes | Basophils secrete Platelet Activating Factor (PAF) | Data inconsistent |
1.1.1. IgE‐ versus IgG‐mediated anaphylaxis
Multiple pathways of anaphylaxis are described in mice. It has been shown that, upon capture of IgG‐allergen complexes, mouse basophils release platelet activating factor (PAF) that increases vascular permeability, leading to anaphylactic shock. In vivo depletion of basophils protects mice from fatal IgG‐mediated anaphylactic shock, but has no effect on IgE‐mediated anaphylaxis. Thus, Tsujimura and Karasuyama 6, 7 postulated that there are two major distinct pathways of anaphylaxis in mice: one is mediated by basophils, allergen‐IgG‐FcγRII‐III receptor interactions and PAF release, whereas the other is mediated by mast cells, allergen‐IgE‐FcεRI receptor interactions and histamine release. Previous murine studies similarly showed that only mast cells contributed to IgE‐mediated anaphylaxis.8, 9 There are also alternative IgG pathways of murine anaphylaxis, mediated by IgG‐FcγRIII‐macrophages or IgG‐FcγRIV‐neutrophil interactions.10, 11 In addition, the role of neutrophils was also demonstrated in peanut‐induced anaphylaxis in mice.12 More recently, Finkelman et al reviewed the evidence for IgG versus IgE‐mediated anaphylaxis in mice, arguing that dose of allergen is an important factor in determining the precise mechanism of induction.13
In sharp contrast, human basophils cannot be activated through IgG receptors, as their function is inhibited by IgG‐mediated triggering via FcγRIIb receptors which are the predominant IgG receptor subtype on these cells.14, 15 Moreover, allergen‐specific IgG antibodies are of questionable pathogenic relevance 16 and are more associated with blocking the effects of allergen‐specific IgE.17, 18 Furthermore, there is little evidence that human anaphylaxis is in any way mediated by IgG antibodies in relation to either macrophages or neutrophils. Evidence for PAF production by human (as opposed to murine) basophils is also limited and inconsistent.19, 20
1.1.2. Antigen presentation
Murine basophils appear to be able to present antigens through MHC class II‐dependent interactions.21, 22, 23 However, the role of murine basophils as IL‐4‐releasing antigen‐presenting cells (APC) is limited by the observations that basophils and dendritic cells (DCs) could efficiently co‐operate, where basophils produce IL‐4, whereas DCs present antigens.24, 25 Eckl‐Dorna et al26 and Kitzmuller et al27 compared the antigen‐presenting properties of different human cell types including basophils. Human basophils were not able to present allergens to T lymphocytes, whereas a mixture of APCs depleted of basophils did. Furthermore, human basophils lacked the machinery to uptake, process and present allergens, although a small increase of MHC‐II was seen after incubating the basophils with both IFN‐γ and IL‐3. There are some reports that basophils in patients with systemic lupus erythematosus express MHC‐ II,28 but these data are not confirmed in other studies.29 In addition, human basophils lack protease‐activated receptor expression (PAR), and PAR ligands fail to induce activation of these cells.30 In contrast, PAR activators, such as papain, which have been used in many of the mouse models, are able to elicit murine basophil‐mediated Th2 response.21
2. THE ROLE OF BASOPHILS IN LOCAL ACUTE ALLERGIC REACTIONS
Local allergen challenge induces a prompt migration of basophils to the site of allergic inflammation.
2.1. Nose
Basophils have been identified in the nasal washes of patients with allergic rhinitis (AR) and are thought to be an important source of histamine in responses to allergen challenge.31, 32 Braunstahl et al demonstrated that segmental bronchoprovocation in non‐asthmatic allergic rhinitis patients affects mast cell and basophil numbers in nasal and bronchial mucosa.33 The number of basophils increased significantly after challenge, whereas the numbers of mast cells decreased, probably because of the limited immunohistochemical detection (by tryptase and chymase staining) of mast cells after degranulation. At the same time, this study 33 also demonstrated a decrease in the percentage of blood basophils, which might suggest an influx of basophils from the blood into the nasal and bronchial mucosa after the challenge. Interestingly, successful grass pollen immunotherapy is associated with inhibition of seasonal increases in basophils and eosinophils, but not mast cells or neutrophils, within the nasal epithelium of AR patients.34
2.2. Skin
The skin might be an important route of allergen exposure,35 especially in the case of skin barrier disruption,36 and significant increases in the numbers of basophils were previously observed 6 hours after intradermal injection of allergen, 37 or, in patch‐test skin sites, for house dust mite allergen.38 Furthermore, basophil infiltration into skin lesions seems to be more common than previously thought, indicating that they may play important roles in a variety of inflammatory skin diseases.39 Higher number of basophils were detected in inflammatory skin diseases where eosinophils are present,39 and those observations are consistent with a recent study which demonstrated a significant correlation between airway basophils and eosinophils in asthma patients.40, 41
3. ASSESSING THE ROLE OF BASOPHILS IN SYSTEMIC ALLERGIC REACTIONS
3.1. Human experimental models of acute allergic reactions
HIGHLIGHTS.
The combination of controlled allergen challenge and emergency department‐based studies may be the optimal model to investigate anaphylaxis in humans.
3.1.1. Controlled allergen challenge studies
Currently, there are two models for studying anaphylaxis in humans: emergency department (ED) studies and controlled challenge models (mostly to food, but also to Hymenoptera venom).42 Smith et al 43 performed the first prospective human study during sting challenge‐induced systemic allergic reactions, which was followed by a series of similarly designed studies by van der Linden et al in the 1990s.44, 45, 46 However, for safety reasons, in controlled allergen challenge studies, patients with previous anaphylaxis are often excluded from challenge studies due to the potential for life‐threatening reactions.47 Furthermore, in the oral food challenge model, the reaction severity at challenge is also limited by the controlled nature of the challenge (allergen exposure is usually terminated at the onset of objective symptoms) and administration of pharmacologic interventions to treat the symptoms. Consequently, two studies which investigated the role of basophils in human anaphylaxis (after insect sting or food challenge), involved only a very limited number of patients who experienced severe reactions after challenge.48, 49 However, studies in the challenge setting do have the advantage of allowing comparison with prereaction samples, optimal sampling and controlling potentially confounding factors (including acute treatment, where blood samples can often be taken prior to treatment).50
Allergen‐induced reactions often manifest themselves as an early asthmatic response, and bronchial allergen challenge may be another model for study of basophils during the acute allergic reaction.51 In addition, nasal allergen challenges could also be employed as an experimental set‐up to study the role of basophils in local allergic reactions.31, 52
3.1.2. Emergency department‐based studies
The ED‐based anaphylaxis study was first described by Lin et al,53 and then adapted by others.50, 54, 55, 56 Patients with anaphylaxis are studied prospectively at the time of presentation to the ED, with sample collection typically occurring 1 to 2 hours after onset of symptoms, and usually after initial treatment and stabilization.50, 53, 55, 56 Patients with the most severe reactions including hypoxaemia or hypotension can be investigated,50, 56 although this is typically after initial stabilization and treatment (usually with adrenaline). In the case of field‐treatment of anaphylaxis, patients are very often treated with systemic corticosteroids and antihistamines as well.50, 57, 58 Corticosteroids have broad immunological effects, albeit much delayed compared to other anti‐allergic therapies. With respect to basophils, corticosteroids inhibit their pro‐allergic functions,50, 59, 60 and this might be an important confounder.
4. THE ROLE OF BASOPHILS IN ANAPHYLAXIS
4.1. Basophil activation
HIGHLIGHTS.
Studies of anaphylaxis investigating human basophil activation in vivo are required.
4.1.1. Secretion of mediators of allergic inflammation
The current evidence for basophil degranulation resulting in anaphylaxis in humans is very limited. However, there are several important indirect observations. Total tryptase (which is produced by mast cells, but not basophils) is within normal limits in up to 30% of patients with anaphylaxis. The proportion of patients with normal tryptase is even higher in the case of food‐induced anaphylaxis (even when blood samples are optimally timed),61 or in the case of positive oral food challenge in which symptoms of anaphylaxis are observed.62, 63 From these data, some authors speculate that, at least in some patients, the anaphylactic episode may primarily involve basophil and not mast cell degranulation.42 However, there are several other possible reasons for this discrepancy. For example, in the case of localized (eg in the gut) rather than generalized mast cell degranulation, tryptase may enter the circulation less efficiently. A further level of complexity is added by reports that some mast cells express less tryptase (ie those present in the respiratory epithelium, alveolar wall and small intestinal mucosa) than others (eg in the skin, heart and perivascular tissue), and that in some subjects tryptase may be eliminated very rapidly.42
Several studies have assessed the impact of the anti‐IgE monoclonal antibody omalizumab (which prevents IgE from binding to the high‐affinity IgE receptor) on the acute allergic response to nasal allergen or oral food challenge models, which has allowed an evaluation of the relative contribution of basophils and mast cells.52, 64 Using titrated skin prick testing to assess mast cell responses, and histamine release assays after in vitro allergen stimulation to assess basophil responses, these studies demonstrated that a reduction in symptoms occurred when the basophil—rather than mast cell—response was reduced. These results indirectly suggest a potentially important role for basophils in acute allergic reactions. However, in vitro stimulation cannot directly show that basophils are involved in acute allergic response in different target organs.
4.1.2. Expression of proteins on the plasma membrane
Allergen stimulation of basophils induces the appearance of a number of plasma proteins 65 that can be detected by mAbs and flow cytometry. Increased cell surface CD63 expression is most commonly used to assess the degranulation of basophils,66 for which there are several commercial kits (basophil activation tests).67 Additional options to assess basophil activation include measurement of CD203c and CD11b, which are located in a rapidly expressed vesicular compartment that is distinct from the histamine‐containing granules, and CD69, which is not related to secretion but is expressed when basophils are exposed to cytokines, such as IL‐3.65
Under in vitro basophil stimulation experiments with different types of stimuli (allergens, anti‐IgE, anti‐FcεRI mAbs or fMLP), upregulation of CD63 generally parallels degranulation and histamine release.66, 67
However, the situation in vivo is not so clear. Turner et al68 reported increased expressions of CD63, CD107a and CD203c on basophils following double‐blind, placebo‐controlled peanut challenge in 13 peanut‐allergic subjects (P < .01). This is consistent with data from another food challenge study which included 12 subjects with IgE to galactose‐alpha‐1,3‐galactose who experienced a delayed clinical response to mammalian meat.49 Two subjects experienced anaphylaxis, and 8 experienced mild reactions. Nine of those subjects (including 2 asymptomatic) showed increased expression of CD63 (median 30% basophils, range 17%‐67%). However, in the same study, 5 of 13 healthy controls, without IgE to galactose‐alpha‐1,3‐galactose, showed comparable increase in CD63 expression (median 34% basophils, range 17%‐46%) after meat challenge, but without any clinical symptoms.
Gober et al48 evaluated 35 subjects after the in vivo sting challenge, of whom only 1 had a systemic reaction. Despite a significant difference in clinical presentation, the rise in basophil CD63 expression was similar across the group (~2‐ to 3‐fold) and was not related to the severity of the reaction. Interestingly, in the same study, basophils were also examined after in vitro stimulation with insect venom, and the levels of CD63 expression were much greater than after in vivo challenge. A recent ED study showed only a minor increase in CD63 expression on circulating basophils during anaphylaxis, and only one of 31 predominantly venom‐allergic patients had >15% CD63‐activated basophils, despite the fact that the majority experienced a severe anaphylactic reaction with bronchospasm, airway obstruction, hypoxaemia or hypotension, or collapse.50
Vasagar et al69 reported that enhanced in vivo surface CD63 expression on circulating basophils was not associated with increased serum histamine levels, although this observation was in patients with chronic idiopathic urticaria rather than an acute allergic reaction. Human studies using in vivo allergen challenge have shown the ability for basophils to demonstrate increased CD63 expression despite the absence of clinical symptoms of an allergic reaction.48, 49 Moreover, we recently demonstrated a discordance between expression of CD63 on basophils and basophil degranulation (Figure 1).70 Thus, the expression of surface activation markers such as CD63 on basophils may not be synonymous with basophil degranulation: the upregulation of activation markers on basophils may be a “bystander” effect—whereby basophils become activated, either due to direct cross‐linking of IgE on the surface, or perhaps due to other mediators (perhaps mast cell‐derived)—without basophil degranulation occurring (at least in terms of histamine release). The basophils might not therefore release inflammatory mediators which themselves contribute to the symptoms of an allergic reaction.
Figure 1.
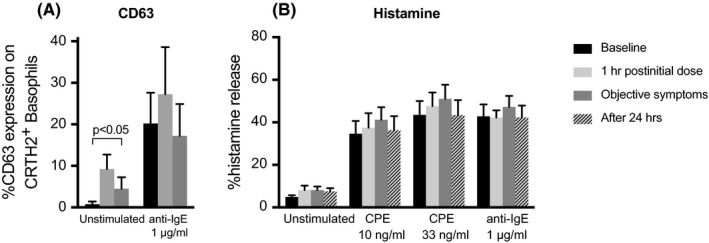
Basophil activation without evidence of degranulation following oral challenge in peanut‐allergic subjects (n = 4). Blood samples were collected prior to, during and 24 h after objective allergic reaction at oral food challenge, as previously described.50 Surface expression of CD63 (A) on basophils was evaluated (without further ex vivo stimulation) by flow cytometry.50 Basophils were isolated by Ficoll‐density centrifugation and purified to over 90% purity by immunomagnetic cell sorting, using a negative selection technique which we have previously described.111 Cells were incubated for 15 min at 37°C before stimulation with crude peanut extract (CPE) or anti‐IgE for 8 min after which histamine release was assessed by spectrofluorometric autoanalysis according to Shore et al 112 (B). Data are shown as mean percentage histamine releases ± SEM. Despite increased surface CD63 expression on ex vivo, unstimulated basophils (A), there was no difference in IgE‐mediated histamine release in the same basophils compared with baseline (B). This implies that circulating basophils have become “activated”—or rather, have increased surface expression of CD63, an activation marker—but with no evidence of degranulation, at least in terms of histamine release. These data presented at the 45th annual meeting of the European Histamine Research Society (EHRS) in Florence, 2016
A number of studies have assessed the basophil CD63 response after in vitro stimulation with allergens,67 including one which suggested that after the completion of venom immunotherapy, changes in the basophil CD63 expression might reflect the induction of tolerance.71 Although these data are interesting, the caveat is that basophil CD63 expression after in vitro stimulation does not readily equate to allergic symptoms and cannot therefore be directly extrapolated to the situation in vivo.
5. BASOPHIL MIGRATION
Basophil migration from the circulation might be a key event during anaphylaxis.
5.1. The mechanism of transendothelial migration of human basophils
Basophil migration comprises of three sequential steps: adhesion to the vascular endothelium, transendothelial migration and locomotion towards target sites in extravascular tissues. For adhesion to the vascular endothelium, basophils express α4β1, α5β1, β2 and α4β7 integrins that interact with different ligands on the endothelium such as VCAM 1, fibronectin and ICAM 1‐3.72, 73 IL‐3, a major basophil priming and growth factor, can also upregulate the expression of β2, and thus augments β2 integrin‐mediated adhesiveness for endothelium. Regarding basophil migration and the recruitment of basophils to the sites of allergic inflammation, basophil‐directed chemokines play the most critical roles, by virtue of inducing transendothelial migration and directional movement. It was postulated that CCL2, CCL5 and CCL11 chemokines play a primary role in basophil migration.72, 73 However, a detailed study by Ikura et al on the migration of freshly isolated human basophils across vascular endothelial cell monolayers showed that the CCR3 ligand CCL11 and the CCR2 ligand CCL2 elicited the most potent migratory response.74 Importantly, there was a significant difference in the cellular specificity of these chemokines, as they bind to different chemokine receptors.73, 75, 76, 77 CCL11 binds to the chemokine receptor CCR3, which is present on basophils, mast cells and eosinophils. CCL5 binds both to chemokine receptors CCR1 and CCR3, but with higher affinity to CCR1 than to CCR3. CCR1 is also present both on basophils and on eosinophils. CCL2 binds to the chemokine receptor CCR2, which is present on basophils but is undetectable on human eosinophils 78 and it fails to induce eosinophil transendothelial migration. Therefore, in contrast to CCL11 and CCL5, which also induce eosinophil migration, CCL2 preferentially induces basophil migration and may represent a unique mechanism for the selective migration of human basophils.
5.2. Migration during anaphylaxis
The results of the experimental allergen challenge in the nose, airways and skin have demonstrated the influx of basophils to inflammatory sites several hours after allergen exposure.31, 37, 51, 79 A recent study has suggested that basophils migrate from the circulation during anaphylaxis, both in ED and controlled allergen challenge models.50 In the ED study, which included predominantly venom‐allergic adult patients, there was a substantial reduction (80%) in circulating basophils during anaphylactic reactions, and these findings were replicated in peanut‐allergic individuals experiencing allergic reactions during double‐blind placebo‐controlled peanut challenge. In contrast to previous studies which monitored basophils at sites of allergen challenge,31, 37, 51, 79 this study50 assessed basophils in the peripheral blood, including absolute basophil count measured by flow cytometry using microbeads, with basophils identified as CD123+HLA‐DR‐ 80 or CRTh2+CD303‐CD123+ cells.81 Basophil migration was confirmed using whole blood gene expression analysis of genes which are specific for basophils, including the α‐subunit of the high‐affinity IgE receptor (FCER1A), carboxypeptidase A3 (CPA3) and histidine decarboxylase (HDC).50 FcεRI is expressed on mast cells and basophils as tetramers (αβγ2) as well as on antigen‐presenting cells, although at substantially lower levels and only as trimmers (αγ2).82 CPA3 is expressed in mast cells and basophils and may be expressed in populations of T cell progenitors and thymic T cells.83 HDC catalyses the formation of histamine from L‐histidine, and in hematopoietic cell lineages, the gene is expressed only in mast cells and basophils.84 Importantly, the expression of all three genes significantly decreased during anaphylaxis, and correlated with the absolute number of circulating basophils, indicating that the decrease in whole blood gene expression of FCER1A, CPA3 and HDC was due to reduced number of basophils in blood.
5.3. The importance of chemokines and allergen‐IgE stimulation for basophil migration
The results of experimental allergen challenge in various organs 31, 37, 51, 79 and recent anaphylaxis studies reveal that basophils migrate during acute allergic reactions. However, the specific mechanism(s) at play causing basophil migration during allergic reactions is unclear. In the previously mentioned study assessing human anaphylaxis,50 the major basophil chemotactic factors, including the CCR2 ligand CCL2, and the CCR3 ligands CCL11 and CCL5, were evaluated. Interestingly, during anaphylaxis (in an ED experimental set‐up), only an increase in CCL2 was observed, and increases of this chemokine significantly correlated with a decrease in circulating basophils. The CCL2 increase was also replicated in peanut‐allergic individuals undergoing food challenge. In contrast, no changes were evident for CCL5 and CCL11, which could affect other effector cells such as eosinophils, and no evidence of migration of other cell types (including lymphocytes, neutrophils and eosinophils) was observed. The CCL11 results were consistent with another recent study which demonstrated no changes in CCL11 during anaphylaxis.55 These observations suggest that the mechanism of anaphylaxis‐related basophil migration might be CCL2 selective, although the source of CCL2 is currently unknown. It is tempting to speculate that the CCL2 might be mast cell 85 or eosinophils derived.86
Suzukawa et al87 found that human peripheral basophils migrate in response to IgE‐mediated stimulation, and that the concentrations (of either anti‐IgE, anti‐FcεRI or allergen) required to induce migration are less than that required for degranulation, an observation consistent with previous findings from another research group.88 A migration‐enhancing action arising from subthreshold FcεRI cross‐linkage was also demonstrated in murine mast cells.89 These observations suggest that IgE‐mediated basophil migration could be induced without activation and degranulation of basophils in circulation. In case of degranulation and histamine release, binding of histamine to H4 receptors further enhances migration.90 Repeated exposure over long periods to subthreshold allergen concentrations may also result in basophil desensitization (anergy).91, 92 The effects of this phenomenon are not yet clear regarding basophil migration. However, we did not observe any basophil desensitization, in terms of histamine release, from the peanut‐allergic donor basophils shown in Figure 1. This may be due phenotypic differences in basophils, the nature of allergen‐IgE interaction and other parameters which still need to be addressed.
5.4. What is the clinical relevance of basophil migration?
The importance of basophil migration is currently unclear, and we do not know where or when basophil activation and degranulation occurs during anaphylaxis. The observations that IgE‐mediated basophil migration might occur without degranulation,87, 88 and that migration out of the circulation may occur at the onset of symptoms,50 might be consistent with the hypothesis that basophils migrate to the site of allergen exposure where activation and degranulation could occur, thereby contributing to the clinical presentation (Figure 2). This is consistent with clinical observations of different severities and end‐organ patterns of anaphylaxis which suggest that local rather than generalized mast cell and/or basophil degranulation may predominate in some individuals.42
Figure 2.
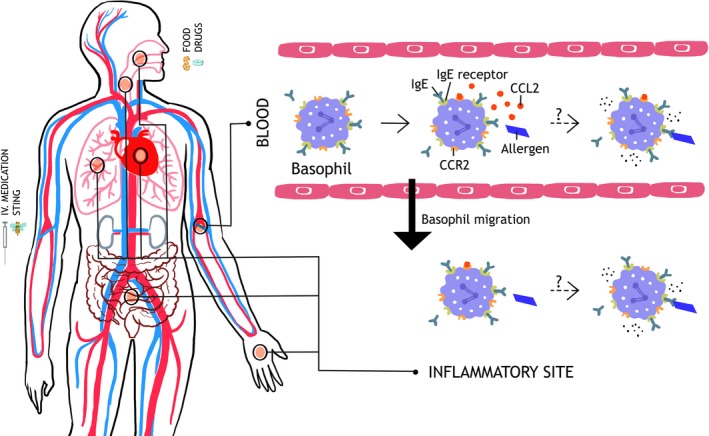
Hypothetical role of basophil migration in anaphylaxis. Upon allergen challenge, basophil‐directed chemokine CCL2 (possibly secreted from mast cells) induces a rapid migration of basophils out of the circulation. This may reactogenic, with migration to target organs resulting in activation and degranulation. Alternatively, the migration may be a protective response, removing basophils from the circulation so that they are unable to degranulate in response to circulating allergen. CCL2, chemokine (C‐C motif) ligand 2; CCR2, C‐C chemokine receptor type 2; IgE, Immunoglobulin E; IgE receptor, high‐affinity IgE receptor (FcεRI)
However, it is also possible that basophil migration occurs as a protective response, preventing their activation and degranulation in the circulation which thus limits systemic degranulation and protects patients against severe anaphylaxis (Figure 2). Unfortunately, we are currently unable to answer these essential questions without the labelling of basophils and tracking their migration in vivo during an acute allergic reaction. Such inflammatory cellular migration, in the case of eosinophils and neutrophils, including the kinetics of cellular influx/efflux into the lungs and other organs, was recently studied and imaged over 4 hours in vivo, either in control subjects 93 or during allergen challenge in atopic asthmatics.94 However, our attempts to undertake similar studies in human volunteers have been limited by poor uptake of radiotracers by human basophils.
6. THE ROLE OF BASOPHILS IN ASTHMA
Basophil infiltration in the airways and subsequent activation or immunomodulatory roles might be an important part of asthma pathogenesis and/or exacerbation.
6.1. Early and late asthmatic response
Inhalation of allergen leads to an early asthmatic response, which is associated with a decrease in lung function that occurs within 2 hours, caused by the release histamine and cysteinyl leukotrienes from mast cells.95 In some patients, the early response is followed by a late asthmatic response, a decline in lung function that occurs during the subsequent 24 hours. The late response is caused by the continued release of mast cell and/or basophil mediators, as well as by the infiltration of inflammatory cells, which produce cytokines and other mediators, resulting in prolonged swelling of the airway mucosa and aggravating of the airway obstruction.95
6.2. Basophil activation during asthma exacerbations
Previous studies examined the changes in expression of plasma proteins on circulating basophils during asthma exacerbation 96 or after inhalation allergen challenge.97 Both scenarios are associated with increased CD203c expression on circulating basophils, but no differences were demonstrated for CD63. Suzuki et al 40 analysed basophils in induced sputum from patients with eosinophilic asthma and showed increased surface expression of both CD203c and CD63. However, this study was performed only on stable patients who had no exacerbations for at least the preceding two months. Salter et al98 also demonstrated increased expression of CD203c in blood, bone marrow and sputum basophils after allergen challenge. However, CD203c is located only in a rapidly expressed vesicular compartment that is distinct from the histamine‐containing granules,65 and thus, it can be not concluded whether basophil degranulation and/or secretion of immediate mediators occurs during asthma exacerbation.
6.3. Migration of basophils during asthma exacerbation
Basophils are increased in induced sputum of asthmatic patients 40, 41, 99 as well as in the sputum or bronchoalveolar lavage (BAL) fluid during exacerbation or after allergen challenge of asthma patients.51, 98, 100, 101, 102 Basophils were also observed in the lungs of patients with fatal asthma.103 This suggests that basophils infiltrate lung tissue in asthma patients. Basophils are increased in the sputum not only from allergic but also of non‐allergic asthmatic patients.40, 41, 99 The highest numbers of basophils were observed in the lungs of patients with eosinophilic asthma, and there is a strong positive correlation between sputum basophil and eosinophil counts.40, 41 Moreover, Suzuki et al40 demonstrated a higher sensitivity, specificity, positive predictive value and negative predictive value of sputum basophil counts for the discrimination of an eosinophilic asthma phenotype than blood eosinophil count and exhaled nitric oxide. As basophil‐derived IL‐4 has been shown to regulate the infiltration of eosinophils,104 one could speculate that early basophil migration into the lungs during exacerbations might be important for subsequent infiltration of eosinophils to airway inflammation. Furthermore, basophil‐derived IL‐4 might also play a role for activation of group 2 innate lymphoid cells (ILC2s).105, 106, 107 Hence, human basophils may be essential players in the pathogenesis of asthma (Figure 3).108
Figure 3.

Hypothetical role of basophil migration in asthma exacerbation. Following exposure to allergen or respiratory viral infection, basophil chemotactic factors are released in lungs leading to recruitment of basophils from the circulation to the airways where they may contribute to the early asthmatic response. In some patients, a Th2‐type immune response orchestrated by basophils, mast cells and infiltration of eosinophils can cause late asthmatic response, resulting in prolonged swelling of the airway mucosa and aggravating the airway obstruction. CCL2, chemokine (C‐C motif) ligand 2; CCR2, C‐C chemokine receptor type 2; IgE, Immunoglobulin E; IgE receptor, high‐affinity IgE receptor (FcεRI); IL‐4, interleukin 4
The source of basophils in the airways of asthmatic patients should be circulating basophils, but there is no current direct experimental evidence which can confirm basophil migration from the circulation to the airways during asthma exacerbations. Assessing the basophil absolute count and/or whole blood expression of genes specific for basophils during asthma exacerbation or after allergen challenge, and comparing them with baseline values, would be an obvious approach. In anaphylaxis models, the induction of migration seems to be related to IgE‐ and FceRI‐cross‐linking upon allergen contact.87 However, this might not be the case in asthma, as basophils are also increased in the airways of non‐allergic asthmatics,40, 41, 99 and the most common cause of asthma exacerbations is not allergens but respiratory viral infections. Interestingly, recent reports suggest 109 that for basophil development or homoeostasis, TSLP may play an important role (in addition to IL‐3‐dependent mechanisms), operating in a non‐IgE‐dependent manner. Epithelial cell‐derived TSLP stimulates various aspects of basophil functions including, at least in part, basophil activation in asthma patients, in addition to other important epithelial cytokines (alternatively spliced variants of IL‐33 and IL‐25).98, 99 Furthermore, it has recently been shown that in vitro TSLP‐primed basophil migrate to CCL11 chemokine by upregulation of CCR3 expression.98
Finally, basophil chemotactic factors such as CCL2 may also be important for basophil migration in asthma patients, similar to anaphylaxis.50 This is supported by recent observations that CCL2 is released by airway smooth muscles in asthma patients, and that levels of CCL2 are increased in the serum of asthma patients.110 However, substantially broader studies are required to confirm or refute these speculations.
7. CONCLUSIONS
Recent publications have highlighted the importance of human basophils by providing compelling evidence that these cells contribute substantially to anaphylaxis and asthma exacerbations. Understanding the factors that regulate basophil trafficking and activation might lead to new diagnostic and therapeutic strategies in anaphylaxis and asthma.
ACKNOWLEDGEMENTS
PK and MR are supported by Slovenian Research Agency (reference P3‐0360 and J3‐6787). PJT is in receipt of a Clinician Scientist award funded by the UK Medical Research Council (reference MR/K010468/1). BFG is in receipt of an award funded by the UK Medical Research Council (reference WM/3306381). We are grateful to Dr Mohamed Shamji at Imperial College London for contributing to the data in Figure 1, which was presented at the 45th annual meeting of the European Histamine Research Society (EHRS) in Florence, 2016. PJT and AC are supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS, NIHR or the Department of Health.
Korošec P, Gibbs BF, Rijavec M, Custovic A, Turner PJ. Important and specific role for basophils in acute allergic reactions. Clin Exp Allergy. 2018;48:502–512. https://doi.org/10.1111/cea.13117
REFERENCES
- 1. Lee JJ, McGarry MP. When is a mouse basophil not a basophil? Blood. 2007;109:859‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Knol EF, Gibbs BF. Basophils and antigen presentation: of mice and not men? Allergy. 2012;67:579‐580. [DOI] [PubMed] [Google Scholar]
- 3. Gurzeler U, Rabachini T, Dahinden CA, et al. In vitro differentiation of near‐unlimited numbers of functional mouse basophils using conditional Hoxb8. Allergy. 2013;68:604‐613. [DOI] [PubMed] [Google Scholar]
- 4. Bergman RK, Munoz JJ. Increased histamine sensitivity in mice after administration of endotoxins. Infect Immun. 1977;15:72‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schulman ES, MacGlashan DW, Schleimer RP, et al. Purified human basophils and mast cells: current concepts of mediator release. Eur J Respir Dis Suppl. 1983;128(Pt 1):53‐61. [PubMed] [Google Scholar]
- 6. Tsujimura Y, Obata K, Mukai K, et al. Basophils play a pivotal role in Immunoglobulin‐G‐mediated but not Immunoglobulin‐E‐mediated systemic anaphylaxis. Immunity. 2008;28:581‐589. [DOI] [PubMed] [Google Scholar]
- 7. Karasuyama H, Mukai K, Tsujimura Y, Obata K. Newly discovered roles for basophils: a neglected minority gains new respect. Nat Rev Immunol. 2009;9:9‐13. [DOI] [PubMed] [Google Scholar]
- 8. Dombrowicz D, Flamand V, Miyajima I, Ravetch JV, Galli SJ, Kinet JP. Absence of Fc epsilonRI alpha chain results in upregulation of Fc gammaRIII‐dependent mast cell degranulation and anaphylaxis. Evidence of competition between Fc epsilonRI and Fc gammaRIII for limiting amounts of FcR beta and gamma chains. J Clin Invest. 1997;99:915‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE‐ or IgG1‐dependent passive anaphylaxis. J Clin Invest. 1997;99:901‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506‐515. [DOI] [PubMed] [Google Scholar]
- 11. Jönsson F, Mancardi DA, Kita Y, et al. Mouse and human neutrophils induce anaphylaxis. J Clin Invest. 2011;121:1484‐1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reber LL, Marichal T, Mukai K, et al. Selective ablation of mast cells or basophils reduces peanut‐induced anaphylaxis in mice. J Allergy Clin Immunol. 2013;132:881‐888.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finkelman FD, Khodoun MV, Strait R. Human IgE‐independent systemic anaphylaxis. Journal of Allergy and Clinical Immunology. 2016;137:1674‐1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bruhns P, Frémont S, Daëron M. Regulation of allergy by Fc receptors. Curr Opin Immunol. 2005;17:662‐669. [DOI] [PubMed] [Google Scholar]
- 15. Malbec O, Cassard L, Albanesi M, et al. Trans‐inhibition of activation and proliferation signals by Fc receptors in mast cells and basophils. Sci Signal. 2016;9:ra126‐ra126. [DOI] [PubMed] [Google Scholar]
- 16. Hochwallner H, Schulmeister U, Swoboda I, et al. Patients suffering from non‐IgE‐mediated cow's milk protein intolerance cannot be diagnosed based on IgG subclass or IgA responses to milk allergens. Allergy. 2011;66:1201‐1207. [DOI] [PubMed] [Google Scholar]
- 17. Custovic A, Soderstrom L, Ahlstedt S, Sly PD, Simpson A, Holt PG. Allergen‐specific IgG antibody levels modify the relationship between allergen‐specific IgE and wheezing in childhood. J Allergy Clin Immunol. 2011;127:1480‐1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Curin M, Weber M, Thalhamer T, et al. Hypoallergenic derivatives of Fel d 1 obtained by rational reassembly for allergy vaccination and tolerance induction. Clin Exp Allergy. 2014;44:882‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lie WJ, Homburg CHE, Kuijpers TW, et al. Regulation and kinetics of platelet‐activating factor and leukotriene C4 synthesis by activated human basophils. Clin Exp Allergy. 2003;33:1125‐1134. [DOI] [PubMed] [Google Scholar]
- 20. Betz SJ, Lotner GZ, Henson PM. Generation and release of platelet‐activating factor (PAF) from enriched preparations of rabbit basophils; failure of human basophils to release PAF. J Immunol. 1980;125:2749‐2755. [PubMed] [Google Scholar]
- 21. Sokol CL, Chu N‐Q, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen‐presenting cells for an allergen‐induced T helper type 2 response. Nat Immunol. 2009;10:713‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perrigoue JG, Saenz SA, Siracusa MC, et al. MHC class II‐dependent basophil‐CD4 + T cell interactions promote T(H)2 cytokine‐dependent immunity. Nat Immunol. 2009;10:697‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoshimoto T, Yasuda K, Tanaka H, et al. Basophils contribute to T(H)2‐IgE responses in vivo via IL‐4 production and presentation of peptide‐MHC class II complexes to CD4 + T cells. Nat Immunol. 2009;10:706‐712. [DOI] [PubMed] [Google Scholar]
- 24. Hammad H, Plantinga M, Deswarte K, et al. Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097‐2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang H, Cao W, Kasturi SP, et al. The T helper type 2 response to cysteine proteases requires dendritic cell‐basophil cooperation via ROS‐mediated signaling. Nat Immunol. 2010;11:608‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eckl‐Dorna J, Ellinger A, Blatt K, et al. Basophils are not the key antigen‐presenting cells in allergic patients. Allergy. 2012;67:601‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kitzmüller C, Nagl B, Deifl S, et al. Human blood basophils do not act as antigen‐presenting cells for the major birch pollen allergen Bet v 1. Allergy. 2012;67:593‐600. [DOI] [PubMed] [Google Scholar]
- 28. Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 2010;16:701‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dijkstra D, Hennig C, Witte T, Hansen G. Basophils from humans with systemic lupus erythematosus do not express MHC‐II. Nat Med. 2012;18:488‐489. [DOI] [PubMed] [Google Scholar]
- 30. Falcone FH, Morroll S, Gibbs BF. Lack of protease activated receptor (PAR) expression in purified human basophils. Inflamm Res. 2005;54(Suppl 1):S13‐S14. [DOI] [PubMed] [Google Scholar]
- 31. Iliopoulos O, Baroody FM, Naclerio RM, Bochner BS, Kagey‐Sobotka A, Lichtenstein LM. Histamine‐containing cells obtained from the nose hours after antigen challenge have functional and phenotypic characteristics of basophils. J Immunol. 1992;148:2223‐2228. [PubMed] [Google Scholar]
- 32. Busse WW, Swenson CA, Sharpe G, Koschat M. Enhanced basophil histamine release to concanavalin A in allergic rhinitis. J Allergy Clin Immunol. 1986;78(1 Pt 1):90‐97. [DOI] [PubMed] [Google Scholar]
- 33. Braunstahl GJ, Overbeek SE, Fokkens WJ, et al. Segmental bronchoprovocation in allergic rhinitis patients affects mast cell and basophil numbers in nasal and bronchial mucosa. Am J Respir Crit Care Med. 2001;164:858‐865. [DOI] [PubMed] [Google Scholar]
- 34. Wilson DR, Irani AM, Walker SM, et al. Grass pollen immunotherapy inhibits seasonal increases in basophils and eosinophils in the nasal epithelium. Clin Exp Allergy. 2001;31:1705‐1713. [DOI] [PubMed] [Google Scholar]
- 35. Smith AR, Knaysi G, Wilson JM, Wisniewski JA. The skin as a route of allergen exposure: part I. Immune components and mechanisms. Curr Allergy Asthma Rep. 2017;17:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tordesillas L, Goswami R, Benedé S, et al. Skin exposure promotes a Th2‐dependent sensitization to peanut allergens. J Clin Invest. 2014;124:4965‐4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Irani AM, Huang C, Xia HZ, et al. Immunohistochemical detection of human basophils in late‐phase skin reactions. J Allergy Clin Immunol. 1998;101:354‐362. [DOI] [PubMed] [Google Scholar]
- 38. Mitchell EB, Chapman M, Pope FM, Crow J, Jouhal S, Platts‐Mills TE. Basophils in allergen‐induced patch test sites in atopic dermatitis. Lancet. 1982;319:127‐130. [DOI] [PubMed] [Google Scholar]
- 39. Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, Yokozeki H. Basophil recruitment and activation in inflammatory skin diseases. Allergy. 2011;66:1107‐1113. [DOI] [PubMed] [Google Scholar]
- 40. Suzuki Y, Wakahara K, Nishio T, Ito S, Hasegawa Y. Airway basophils are increased and activated in eosinophilic asthma. Allergy. 2017;72:1532‐1539. [DOI] [PubMed] [Google Scholar]
- 41. Brooks CR, van Dalen CJ, Hermans IF, Gibson PG, Simpson JL, Douwes J. Sputum basophils are increased in eosinophilic asthma compared with non‐eosinophilic asthma phenotypes. Allergy. 2017;72:1583‐1586. [DOI] [PubMed] [Google Scholar]
- 42. Simons FER, Frew AJ, Ansotegui IJ, et al. Risk assessment in anaphylaxis: current and future approaches. J Allergy Clin Immunol. 2007;1(Suppl):S2‐S24. [DOI] [PubMed] [Google Scholar]
- 43. Smith PL, Kagey‐Sobotka A, Bleecker ER, et al. Physiologic manifestations of human anaphylaxis. J Clin Invest. 1980;66:1072‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van der Linden PW, Hack CE, Kerckhaert JA, Struyvenberg A, van der Zwan JC. Preliminary report: complement activation in wasp‐sting anaphylaxis. Lancet. 1990;336:904‐906. [DOI] [PubMed] [Google Scholar]
- 45. van der Linden PW, Hack CE, Poortman J, Vivié‐Kipp YC, Struyvenberg A, van der Zwan JK. Insect‐sting challenge in 138 patients: relation between clinical severity of anaphylaxis and mast cell activation. J Allergy Clin Immunol. 1992;90:110‐118. [DOI] [PubMed] [Google Scholar]
- 46. van der Linden PW, Hack CE, Struyvenberg A, et al. Controlled insect‐sting challenge in 55 patients: correlation between activation of plasminogen and the development of anaphylactic shock. Blood. 1993;82:1740‐1748. [PubMed] [Google Scholar]
- 47. Kowalski ML, Ansotegui I, Aberer W, et al. Risk and safety requirements for diagnostic and therapeutic procedures in allergology: World Allergy Organization Statement. World Allergy Organ J. 2016;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gober LM, Eckman JA, Sterba PM, et al. Expression of activation markers on basophils in a controlled model of anaphylaxis. J Allergy Clin Immunol. 2007;119:1181‐1188. [DOI] [PubMed] [Google Scholar]
- 49. Commins SP, James HR, Stevens W, et al. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose‐alpha‐1,3‐galactose. J Allergy Clin Immunol. 2014;134:108‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Korosec P, Turner PJ, Silar M, et al. Basophils, high‐affinity IgE receptors and CCL2 in human anaphylaxis. J Allergy Clin Immunol. 2017;140:750‐758.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gauvreau GM, Lee JM, Watson RM, Irani AM, Schwartz LB, O'Byrne PM. Increased numbers of both airway basophils and mast cells in sputum after allergen inhalation challenge of atopic asthmatics. Am J Respir Crit Care Med. 2000;161:1473‐1478. [DOI] [PubMed] [Google Scholar]
- 52. Eckman JA, Sterba PM, Kelly D, et al. Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge. J Allergy Clin Immunol. 2010;125:889‐895.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lin RY, Schwartz LB, Curry A, et al. Histamine and tryptase levels in patients with acute allergic reactions: an emergency department‐based study. J Allergy Clin Immunol. 2000;106(1 Pt 1):65‐71. [DOI] [PubMed] [Google Scholar]
- 54. Vadas P, Gold M, Perelman B, et al. Platelet‐activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28‐35. [DOI] [PubMed] [Google Scholar]
- 55. Stone SF, Cotterell C, Isbister GK, Holdgate A, Brown SGA. Elevated serum cytokines during human anaphylaxis: identification of potential mediators of acute allergic reactions. J Allergy Clin Immunol. 2009;124:786‐92.e4. [DOI] [PubMed] [Google Scholar]
- 56. Vadas P, Perelman B, Liss G. Platelet‐activating factor, histamine, and tryptase levels in human anaphylaxis. J Allergy Clin Immunol. 2013;131:144‐149. [DOI] [PubMed] [Google Scholar]
- 57. Grabenhenrich L, Hompes S, Gough H, et al. Implementation of anaphylaxis management guidelines: a register‐based study. PLoS ONE. 2012;7:e35778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Worm M, Eckermann O, Dölle S, et al. Triggers and treatment of anaphylaxis: an analysis of 4,000 cases from Germany, Austria and Switzerland. Dtsch Arztebl Int. 2014;111:367‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Saavedra‐Delgado AM, Mathews KP, Pan PM, Kay DR, Muilenberg ML. Dose‐response studies of the suppression of whole blood histamine and basophil counts by prednisone. J Allergy Clin Immunol. 1980;66:464‐471. [DOI] [PubMed] [Google Scholar]
- 60. Dunsky EH, Zweiman B, Fischler E, Levy DA. Early effects of corticosteroids on basophils, leukocyte histamine, and tissue histamine. J Allergy Clin Immunol. 1979;63:426‐432. [DOI] [PubMed] [Google Scholar]
- 61. Brown SGA, Stone SF, Fatovich DM, et al. Anaphylaxis: clinical patterns, mediator release, and severity. J Allergy Clin Immunol. 2013;132:1141‐1149.e5. [DOI] [PubMed] [Google Scholar]
- 62. Sampson HA, Mendelson L, Rosen JP. Fatal and near‐fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327:380‐384. [DOI] [PubMed] [Google Scholar]
- 63. Sampson HA, Jolie PL. Increased plasma histamine concentrations after food challenges in children with atopic dermatitis. N Engl J Med. 1984;311:372‐376. [DOI] [PubMed] [Google Scholar]
- 64. Savage JH, Courneya J‐P, Sterba PM, MacGlashan DW, Saini SS, Wood RA. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab‐treated adults with peanut allergy. J Allergy Clin Immunol. 2012;130:1123‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. MacGlashan DW. Basophil activation testing. J Allergy Clin Immunol. 2013;132:777‐787. [DOI] [PubMed] [Google Scholar]
- 66. Knol EF, Mul FP, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol. 1991;88(3 Pt 1):328‐338. [DOI] [PubMed] [Google Scholar]
- 67. Hoffmann HJ, Santos AF, Mayorga C, et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy. 2015;70:1393‐1405. [DOI] [PubMed] [Google Scholar]
- 68. Turner PJ, McMahon O, Switzer A, et al. Marked increase in basophil activation during non‐anaphylactic allergic reactions to peanut in man. J Allergy Clin Immunol. 2015;135(Supp 2):AB33. [Google Scholar]
- 69. Vasagar K, Vonakis BM, Gober LM, Viksman A, Gibbons SP Jr, Saini SS. Evidence of in vivo basophil activation in chronic idiopathic urticaria. Clin Exp Allergy. 2006;36:770‐776. [DOI] [PubMed] [Google Scholar]
- 70. BF G. New Insights into the Intracellular Control of IgE‐Dependent Histamine Release from Human Basophils. 14th annual meeting of the European Histamine Research Society (EHRS), Florence, 2016.
- 71. Eržen R, Košnik M, Silar M, Korošec P. Basophil response and the induction of a tolerance in venom immunotherapy: a long‐term sting challenge study. Allergy. 2012;67:822‐830. [DOI] [PubMed] [Google Scholar]
- 72. Bochner BS, Sterbinsky SA, Briskin M, Saini SS, MacGlashan DW. Counter‐receptors on human basophils for endothelial cell adhesion molecules. J Immunol. 1996;157:844‐850. [PubMed] [Google Scholar]
- 73. Bochner BS, Schleimer RP. Mast cells, basophils, and eosinophils: distinct but overlapping pathways for recruitment. Immunol Rev. 2001;179:5‐15. [DOI] [PubMed] [Google Scholar]
- 74. Iikura M, Ebisawa M, Yamaguchi M, et al. Transendothelial migration of human basophils. J Immunol. 2004;173:5189‐5195. [DOI] [PubMed] [Google Scholar]
- 75. Uguccioni M, Mackay CR, Ochensberger B, et al. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP‐4, and other chemokines. J Clin Invest. 1997;100:1137‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ochensberger B, Tassera L, Bifrare D, Rihs S, Dahinden CA. Regulation of cytokine expression and leukotriene formation in human basophils by growth factors, chemokines and chemotactic agonists. Eur J Immunol. 1999;29:11‐22. [DOI] [PubMed] [Google Scholar]
- 77. Iikura M, Miyamasu M, Yamaguchi M, et al. Chemokine receptors in human basophils: inducible expression of functional CXCR4. J Leukoc Biol. 2001;70:113. [PubMed] [Google Scholar]
- 78. Nagase H, Miyamasu M, Yamaguchi M, et al. Expression of CXCR4 in eosinophils: functional analyses and cytokine‐mediated regulation. J Immunol. 2000;164:5935‐5943. [DOI] [PubMed] [Google Scholar]
- 79. Guo CB, Liu MC, Galli SJ, Bochner BS, Kagey‐Sobotka A, Lichtenstein LM. Identification of IgE‐bearing cells in the late‐phase response to antigen in the lung as basophils. Am J Respir Cell Mol Biol. 1994;10:384‐390. [DOI] [PubMed] [Google Scholar]
- 80. Kosnik M, Silar M, Bajrovic N, Music E, Korosec P. High sensitivity of basophils predicts side‐effects in venom immunotherapy. Allergy. 2005;60:1401‐1406. [DOI] [PubMed] [Google Scholar]
- 81. Shamji MH, Bellido V, Scadding GW, et al. Effector cell signature in peripheral blood following nasal allergen challenge in grass pollen allergic individuals. Allergy Eur J Allergy Clin Immunol. 2015;70:171‐179. [DOI] [PubMed] [Google Scholar]
- 82. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl. 2):S73‐S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lilla JN, Chen CG, Mukai K, et al. Reduced mast cell and basophil numbers and function in Cpa3‐Cre; Mcl‐1 fl/fl mice. Blood. 2011;118:6930‐6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kuramasu A, Saito H, Suzuki S, Watanabe T, Ohtsu H. Mast cell‐/basophil‐specific transcriptional regulation of human L‐histidine decarboxylase gene by CpG methylation in the promoter region. J Biol Chem. 1998;273:31607‐31614. [DOI] [PubMed] [Google Scholar]
- 85. Feuser K, Thon K‐P, Bischoff SC, Lorentz A. Human intestinal mast cells are a potent source of multiple chemokines. Cytokine. 2012;58:178‐185. [DOI] [PubMed] [Google Scholar]
- 86. Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709‐750. [DOI] [PubMed] [Google Scholar]
- 87. Suzukawa M, Hirai K, Iikura M, et al. IgE‐ and Fc RI‐mediated migration of human basophils. Int Immunol. 2005;17:1249‐1255. [DOI] [PubMed] [Google Scholar]
- 88. Bochner BS, MacGlashan DW, Marcotte GV, Schleimer RP. IgE‐dependent regulation of human basophil adherence to vascular endothelium. J Immunol. 1989;142:3180‐3186. [PubMed] [Google Scholar]
- 89. Taub D, Dastych J, Inamura N, et al. Bone marrow‐derived murine mast cells migrate, but do not degranulate, in response to chemokines. J Immunol. 1995;154:2393‐2402. [PubMed] [Google Scholar]
- 90. Mommert S, Kleiner S, Gehring M, et al. Human basophil chemotaxis and activation are regulated via the histamine H4 receptor. Allergy. 2016;71:1264‐1273. [DOI] [PubMed] [Google Scholar]
- 91. Komiya A, Hirai K, Iikura M, et al. Induction of basophil desensitization in physiological medium: enhancement after IgE‐dependent upregulation of surface IgE binding on basophils. Int Arch Allergy Immunol. 2003;130:40‐50. [DOI] [PubMed] [Google Scholar]
- 92. MacGlashan D, Miura K. Loss of syk kinase during IgE‐mediated stimulation of human basophils. J Allergy Clin Immunol. 2004;114:1317‐1324. [DOI] [PubMed] [Google Scholar]
- 93. Lukawska JJ, Livieratos L, Sawyer BM, et al. Real‐time differential tracking of human neutrophil and eosinophil migration in vivo. J Allergy Clin Immunol. 2014;133(233–239):e1. [DOI] [PubMed] [Google Scholar]
- 94. Lukawska JJ, Livieratos L, Sawyer BM, et al. Imaging inflammation in asthma: real time, differential tracking of human neutrophil and eosinophil migration in allergen challenged, atopic asthmatics in Vivo. EBioMedicine. 2014;1:173‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Roquet A, Dahlén B, Kumlin M, et al. Combined antagonism of leukotrienes and histamine produces predominant inhibition of allergen‐induced early and late phase airway obstruction in asthmatics. Am J Respir Crit Care Med. 1997;155:1856‐1863. [DOI] [PubMed] [Google Scholar]
- 96. Ono E, Taniguchi M, Higashi N, et al. CD203c expression on human basophils is associated with asthma exacerbation. J Allergy Clin Immunol. 2010;125(483–489):e3. [DOI] [PubMed] [Google Scholar]
- 97. Salter BM, Nusca G, Tworek D, et al. Expression of activation markers in circulating basophils and the relationship to allergen‐induced bronchoconstriction in subjects with mild allergic asthma. J Allergy Clin Immunol. 2016;137(936–938):e7. [DOI] [PubMed] [Google Scholar]
- 98. Salter BM, Oliveria JP, Nusca G, et al. Thymic stromal lymphopoietin activation of basophils in patients with allergic asthma is IL‐3 dependent. J Allergy Clin Immunol. 2015;136:1636‐1644. [DOI] [PubMed] [Google Scholar]
- 99. Gordon ED, Simpson LJ, Rios CL, et al. Alternative splicing of interleukin‐33 and type 2 inflammation in asthma. Proc Natl Acad Sci. 2016;113:8765‐8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kimura I, Tanizaki Y, Saito K, Takahashi K, Ueda N, Sato S. Appearance of basophils in the sputum of patients with bronchial asthma. Clin Allergy. 1975;5:95‐98. [DOI] [PubMed] [Google Scholar]
- 101. Maruyama N, Tamura G, Aizawa T, et al. Accumulation of basophils and their chemotactic activity in the airways during natural airway narrowing in asthmatic individuals. Am J Respir Crit Care Med. 1994;150:1086‐1093. [DOI] [PubMed] [Google Scholar]
- 102. Dijkstra D, Hennig C, Hansen G, Biller H, Krug N, Hohlfeld JM. Identification and quantification of basophils in the airways of asthmatics following segmental allergen challenge. Cytom Part A. 2014;85:580‐587. [DOI] [PubMed] [Google Scholar]
- 103. Kepley CL, McFeeley PJ, Oliver JM, Lipscomb MF. Immunohistochemical detection of human basophils in postmortem cases of fatal asthma. Am J Respir Crit Care Med. 2001;164:1053‐1058. [DOI] [PubMed] [Google Scholar]
- 104. Cheng LE, Sullivan BM, Retana LE, Allen CDC, Liang H‐E, Locksley RM. IgE‐activated basophils regulate eosinophil tissue entry by modulating endothelial function. J Exp Med. 2015;212:513‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Motomura Y, Morita H, Moro K, et al. Basophil‐derived interleukin‐4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40:758‐771. [DOI] [PubMed] [Google Scholar]
- 106. Kim BS, Wang K, Siracusa MC, et al. Basophils Promote Innate Lymphoid Cell Responses in Inflamed Skin. J Immunol. 2014;193:3717‐3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hussain M, Borcard L, Walsh KP, et al. Basophil‐derived IL‐4 promotes epicutaneous antigen sensitization concomitant with the development of food allergy. J All Clin Immunol. 2018;141:223‐234. [DOI] [PubMed] [Google Scholar]
- 108. Fux M, von Garnier C. Sputum basophils and asthma diagnosis: dawn of a new era? Allergy. 2017;72:1437‐1439. [DOI] [PubMed] [Google Scholar]
- 109. Siracusa MC, Saenz SA, Hill DA, et al. TSLP promotes interleukin‐3‐independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Singh SR, Sutcliffe A, Kaur D, et al. CCL2 release by airway smooth muscle is increased in asthma and promotes fibrocyte migration. Allergy. 2014;69:1189‐1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gibbs BF, Papenfuss K, Falcone FH. A rapid two‐step procedure for the purification of human peripheral blood basophils to near homogeneity. Clin Exp Allergy. 2008;38:480‐485. [DOI] [PubMed] [Google Scholar]
- 112. Shore PA, Burkhalter A, Cohn VH. A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959;127:182‐186. [PubMed] [Google Scholar]
- 113. Turner PJ, Campbell DE. Epidemiology of severe anaphylaxis: can we use population‐based data to understand anaphylaxis? Curr Opin Allergy Clin Immunol. 2016;16:441‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]


