Abstract
In the present multicentre, open‐label, prospective, phase III study, we evaluated the real‐world effectiveness and ease of use of nasal glucagon (NG) in the treatment of moderate/severe hypoglycaemic events (HEs) in adults with type 1 diabetes (T1D). Patients and caregivers were taught how to use NG (3 mg) to treat moderate/severe HEs, record the time taken to awaken or return to normal status, and measure blood glucose (BG) levels over time. Questionnaires were used to collect information about adverse events and ease of use of NG. In the efficacy analysis population, 69 patients experienced 157 HEs. In 95.7% patients, HEs resolved within 30 minutes of NG administration. In all the 12 severe HEs, patients awakened or returned to normal status within 15 minutes of NG administration without additional external medical help. Most caregivers reported that NG was easy to use. Most adverse events were local and of low to moderate severity. In this study, a single, 3‐mg dose of NG demonstrated real‐life effectiveness in treating moderate and severe HEs in adults with T1D. NG was well tolerated and easy to use.
Keywords: adults, hypoglycaemia, nasal glucagon, type 1 diabetes
1. INTRODUCTION
Severe hypoglycaemia is a serious and potentially life‐threatening complication of insulin therapy.1, 2 Patients with type 1 diabetes (T1D) experience ~2 symptomatic hypoglycaemic events (HEs)/week and 1 severe HE/year.2, 3 Fear of hypoglycaemia is a major barrier to the achievement of glycaemic targets in people with T1D.1, 4 Carbohydrate ingestion may not be possible in severe hypoglycaemia where the person may be unconscious or having a seizure.5 In this scenario, caregivers can either inject glucagon or wait for professional help. The currently available glucagon kits require reconstitution prior to administration.1, 6 Nasal glucagon (NG), a needle‐free, single‐use, compact, portable, drug‐device combination consisting of 3 mg dry glucagon powder that does not require reconstitution, is currently under development as a rescue medication for severe hypoglycaemia.1 NG (3 mg) produced a rapid increase in blood glucose (BG) levels and was non‐inferior to intramuscular glucagon (1 mg) in a previous study in adults with insulin‐induced hypoglycaemia.5 The aim of the present study was to evaluate the real‐world effectiveness, tolerability and ease of use of NG (3 mg nasal glucagon; Eli Lilly and Company) in the treatment of moderate and severe HEs in adults with T1D.
2. MATERIALS AND METHODS
This was a prospective, single‐arm, real‐world study. A severe HE was defined as an event during which the person with diabetes is clinically incapacitated and requires third‐party assistance. A moderate HE was defined as an event during which the person with diabetes shows signs and symptoms of neuroglycopaenia with BG ≤3.3 mmol/L (60 mg/dL) at or near the time of treatment. The study was conducted at 9 centres from May 2014 to July 2015 according to the principles of Good Clinical Practice and was registered in http://clinicaltrials.gov (NCT02171130).
Adults (aged 18‐75 years) with T1D of >1‐year duration and a body mass index ≥18.5 and ≤35.0 kg/m2 were included; each patient was required to have a caregiver to administer NG as needed. Hypoglycaemia awareness at baseline was evaluated using the Clarke Hypoglycaemia Awareness questionnaire (Appendix S1).7 Patients and caregivers were trained to use NG. Caregivers were instructed to administer a single NG dose when the patient experienced a moderate/severe HE and to measure capillary BG levels at the time of dosing and ~15, 30 and 45 minutes thereafter. Treatment response was defined as awakening (recovering consciousness/recovery from convulsions) and/or returning to normal status within 30 minutes of NG administration, as per caregivers' assessment. Caregivers were required to seek emergency medical aid or use injectable glucagon if the patient did not respond within 30 minutes (or even earlier if needed). Caregivers were asked to complete a Hypoglycaemia Episode Questionnaire (Appendix S2) and a User‐Friendliness Assessment Questionnaire (Appendix S3). Patients had to complete a Nasal Score Questionnaire (Appendix S4) after recovery from the HE.
The primary outcome was the proportion of patients awakening or returning to normal status within 30 minutes of NG administration. Because some patients experienced >1 HE, the proportion of events in which patients awakened or returned to normal status within 30 minutes was also assessed. Other outcome measures included change in BG levels, tolerability, and ease of use of NG based on caregivers' assessment.
The efficacy analysis population included eligible patients who received ≥1 NG dose with evaluable response information; HEs in which patients required oral carbohydrates/injectable glucagon/professional medical assistance within 30 minutes and before clinical response, were excluded from the efficacy analysis population. The main safety analysis population comprised eligible patients who experienced ≥1 HE and received ≥1 NG dose. A total of 21 patients were considered ineligible (16 because of a clinical trial material issue resulting in potential under‐dose, 5 because of site termination). SAS 9.3 was used to produce descriptive statistics.
3. RESULTS
Patient disposition and baseline characteristics are shown in Figure S1 and Table S1, respectively. Out of 129 patients enrolled, 101 completed the study; 87 received ≥1 NG dose. The main safety analysis population included 74 patients (179 events); the efficacy analysis population included 69 patients (157 events).
3.1. Efficacy results
A total of 66 patients (95.7%) in the efficacy analysis population awakened or achieved normality within 30 minutes after NG administration in at least 1 evaluable event whereas 64 patients (92.8%) awakened or achieved normality in all evaluable events (Table 1).
Table 1.
Return to normal status or awakening within 30 minutes after nasal glucagon administration in the efficacy analysis population
| Patients who experienced ≥1 moderate/severe HE (number of patients, N = 69) | |
|---|---|
| Number of patients who awakened or returned to normal status within 30 min in at least 1 evaluable event (%) | 66 (95.7) |
| Number of patients who awakened or returned to normal status within 30 min in all evaluable events (%) | 64 (92.8) |
| Moderate/severe HEs (number of events, N = 157) | |
| Number of HEs in which patients awakened or returned to normal status within 30 min (%) | 151 (96.2)a |
| Severe HEs (n = 12) | |
| Time to awaken from severe event where patient was unconscious or had convulsions (n = 10)b, n (%) | |
| <5 min | 1 (10.0) |
| 5 to <10 min | 6 (60.0) |
| 10 to <15 min | 3 (30.0) |
| Time to return to normal status from severe event where patient was conscious (n = 2)c, n (%) | |
| 5 to <10 min | 2 (100) |
| Moderate HEs (n = 145) | |
| Time to return to normal statusd, n (%) | |
| <5 min | 27 (18.6) |
| 5 to <10 min | 43 (29.7) |
| 10 to <15 min | 33 (22.8) |
| 15 to <20 min | 23 (15.9) |
| 20 to <25 min | 7 (4.8) |
| 25 to <30 min | 6 (4.1) |
| 30 to <45 min | 5 (3.4) |
| Other | 1e (0.7) |
Abbreviations: HE, hypoglycaemic event; NG, nasal glucagon.
Details regarding the 6 HEs in which patients did not return to normal status within 30 min are presented in Table S2.
Percentages based on the total number of events where “Time to awaken from severe event where patient was unconscious or had convulsions” was reported (n = 10).
Percentages based on the total number of events where “Time to return to normal status from severe event where patient was conscious” was reported (n = 2).
Percentages based on the total number of moderate events (n = 145).
In this HE, the patient reported persistent headache, which prevented the patient from returning to normal status.
Out of 157 evaluable HEs (145 moderate; 12 severe), 151 events (96.2%) resolved within 30 minutes after NG administration. Mean BG levels increased progressively from 2.7 mmol/L (47.9 mg/dL) at the time of NG administration to 4.7 mmol/L (84.4 mg/dL) after 15 minutes and continued to increase further (Figure S2).
Six moderate, symptomatic HEs (3.8%) did not resolve within 30 minutes of NG administration. In 4 of these events, patients' BG levels were >3.9 mmol/L (70 mg/dL) within 30 minutes; however, they did not report return to normality because of headache and/or nasal irritation. In 2 events, BG levels increased by ≥1.1 mmol/L (20 mg/dL) and patients returned to normality between 30 and 45 minutes later (Table S2).
Seven patients in the efficacy analysis population reported 12 severe HEs, with BG levels ranging from 1.6 to 3.3 mmol/L (29 to 60 mg/dL) at the time of NG administration. In all severe HEs, including those in patients who were unconscious or had convulsions, patients awakened or returned to normality within 15 minutes of NG administration (Table S3).
There were 7 events (including 2 severe events) in 6 patients in whom oral carbohydrates were used before treatment success criteria were met (Table S4).
3.2. Safety results
Data on adverse events (AEs) were solicited through questionnaires. In the main safety analysis population, 87.8% patients experienced ≥1 AE. The most common AE was nasal irritation, reported by 82.4% of patients (Table S5); in more than half of these patients, it was short‐lasting and resolved within 1 hour. One patient withdrew from the study because of local nasal AEs. Most AEs were of low to moderate severity. No serious drug‐related AE was reported.
3.3. Ease of use and caregiver satisfaction
In most HEs, caregivers reported that it was easy to understand the kit instructions (91% events) and administer NG (80.5% events). Caregivers were able to administer NG within 30 seconds in 70.4% of events and within 1 minute in 92.7% of events. Overall, in 94.4% of HEs, caregivers were satisfied with NG use (Figure 1). Caregivers expressed willingness to carry NG (97.7% events) and agreed that NG was less intimidating than injectable glucagon (100% events).
Figure 1.
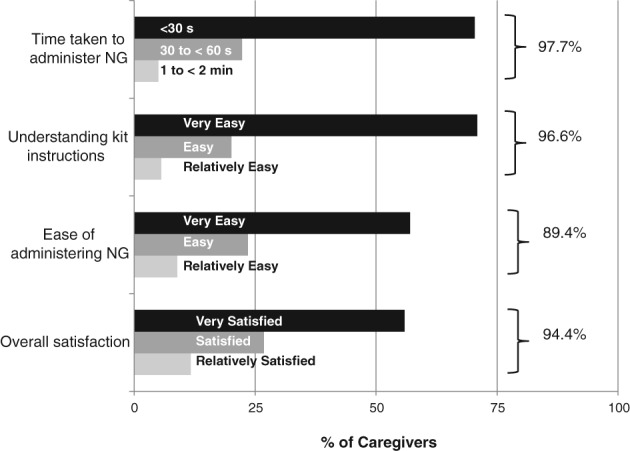
Ease of use of nasal glucagon (NG) and caregiver satisfaction in the main safety analysis population
4. DISCUSSION
In the present real‐world study, we observed that 96.2% of HEs resolved within 30 minutes of NG administration. A previous randomized study in adults with T1D showed that 3‐mg NG was non‐inferior to 1‐mg intramuscular glucagon in treating insulin‐induced hypoglycaemia in a hospital setting.5 Another study in young people with T1D found that NG and injectable glucagon demonstrated similar glycaemic responses.8 The results of the present study, together with previous research, suggest that NG shows promise as an efficacious and effective medication for treatment of severe HEs in the real world.
This is the first clinical trial in which spontaneous, severe HEs were treated using NG. In all 12 severe HEs, patients awakened or returned to normality within 15 minutes of NG administration. No external professional emergency medical assistance was needed in any HE, indicating that caregivers could administer NG and manage the emergency situation themselves. Additionally, caregivers were able to administer NG within 30 seconds in 70.4% of events. These findings suggest that NG has the potential to empower caregivers to effectively manage severe HEs without delay, even in a stressful situation where patients are unconscious or having convulsions.
Hypoglycaemia has been associated with cardiac arrhythmias and increased risk of death in critically ill, hospitalized patients.9, 10 Recently, there has been increased awareness about the importance of clinically significant hypoglycaemia (identified by the International Hypoglycaemia Study Group as BG <3.0 mmol/L [54 mg/dL]) with its associated risk of potential harm.11 Recent literature has also reported that hypoglycaemia with BG <2.8 mmol/L (50 mg/dL) is associated with increased mortality; therefore, this cutoff could be an objective, measurable criterion by which to detect dangerous hypoglycaemia in a broader group of patients at risk of severe hypoglycaemia.12 In the present study, all patients who experienced moderate HEs had neuroglycopaenic symptoms and were at a risk of negative clinical outcomes; therefore, these moderate symptomatic events can be considered serious enough to represent surrogates for severe events.
Nausea and vomiting, well‐known side effects of glucagon, were reported in 23% and 9.5% of patients, respectively. Local AEs such as transient nasal irritation, were commonly reported with NG; many of these were of mild to moderate severity. Although nasal irritation was perceived as severe by 16.2% patients, this should be seen in the context of the potential benefit of NG as a life‐saving, rescue medication.
Caregivers reported that NG was easy to administer (80.5% events). They were able to administer the medication successfully within 30 seconds in the majority of cases (70.4% events) and were satisfied with NG use (94.4% events). Our findings are similar to those of a recent severe hypoglycaemia simulation study,13 which compared the user‐friendliness and success rate for administration of NG with injectable glucagon when administered to mannequins in a simulated severe hypoglycaemia situation. That study found that a higher percentage of people (caregivers and acquaintances) were able to administer NG as compared to injectable glucagon. Furthermore, the average time taken to administer NG was less than the time taken to administer injectable glucagon.13
Injectable glucagon has been the treatment of choice for hypoglycaemia, and its efficacy is well established1; however, injectable glucagon requires reconstitution and injection. In contrast, NG is needle‐free and ready‐to‐use, and a single 3‐mg dose can be used in both adult and paediatric patients independent of body weight.14 Our data showed that the 3‐mg NG drug‐device combination was not only efficacious but also showed effectiveness in a real‐world scenario. All patients with severe hypoglycaemia recovered within 15 minutes of NG administration, and all caregivers were able to use it successfully, confirming its effectiveness in the hands of the intended user.
A potential limitation of this pragmatic study is that it had a single arm; however, the protocol allowed the use of intramuscular glucagon, if needed. Additionally, there was a provision to call for emergency medical aid if the patient did not respond. This allowance to access emergency medical services in case of non‐response served as an alternative rescue option, which was not required in any HE. Because it has already been shown that NG is non‐inferior to injectable glucagon,5 the design of the present study was appropriate to investigate its effectiveness in the real world.
This phase III, real‐world study suggests that a single 3‐mg dose of NG may be a viable and effective option in treating severe hypoglycaemia in adults with T1D. Approximately 70% of caregivers were able to administer NG within 30 seconds; >90% were able to administer it within 1 minute and no alternative external medical assistance was needed, confirming the effectiveness of NG in the hands of the final user. NG was found to be effective and reasonably well tolerated in adults with T1D.
Supporting information
Figure S1. Patient disposition.
Figure S2. Blood glucose levels after NG administration, mean (±SD).
Table S1. Baseline demographics and diabetes history of patients included in the Main Safety Analysis Population.
Table S2. Summary of moderate hypoglycemic events in which patients did not return to normal status in 30 minutes after NG administration in the efficacy analysis population.
Table S3. Summary of severe hypoglycemic events in the efficacy analysis population.
Table S4. Summary of hypoglycemic events in which patients ingested oral carbohydrates.
Table S5. Adverse events solicited through questionnaires in the main safety analysis population.
Appendix S1. Clarke hypoglycemia awareness survey.
Appendix S2. Hypoglycemia episode questionnaire.
Appendix S3. Assessment of the user‐friendliness of the dry‐mist nasal glucagon questionnaire.
Appendix S4. Nasal score questionnaire.
ACKNOWLEDGMENTS
The authors would like to thank the study participants, caregivers, and healthcare professionals for making the study possible.
Conflict of interest
E.R.S. is the Principal Investigator at University of Minnesota, Minneapolis. She also consults for Eli Lilly, Locemia, Zucara, Sanofi and Novo Nordisk and receives grants from Locemia and Eli Lilly. R.R‐L. is the Principal Investigator at Montreal Clinical Research Institute, Montreal, QC, Canada, and receives research grants from AMG, Astra‐Zeneca, Eli Lilly, Lexicon, Merck, NIH, Novo‐Nordisk and Sanofi‐Aventis. He is on the consulting/advisory panel of Abbott, Amgen, Astra‐Zeneca, Boehringer, Carlina Technologies, Eli Lilly, Janssen, Medtronic, Merck, Novo‐Nordisk, Roche, Sanofi‐Aventis and Takeda. G.M.T. is the Principal Investigator at McGill University Health Center, QC, Canada. J.R.C. is the Principal Investigator at Canadian Centre for Research on Diabetes, Smith Falls, ON, Canada. S.J.W. is the Principal Investigator at CHU de Québec‐Université Laval, Quebec City, QC, Canada. He receives research grants from Astra‐Zeneca, Eli Lilly, Novo‐Nordisk, Sanofi‐Aventis, Bayer, Novartis, Boehringer, Medtronic, Locemia and Diabetes Canada. G.F.G. is the Principal Investigator at Division of Community Endocrinology, Albany Medical College, Albany, New York. He receives research grants from Boehringer, Eli Lilly, Lexicon and Novo Nordisk and speaker honoraria from Boehringer, Dexcom, Janssen, Lilly, Merck & Novo Nordisk. V.C.W. is the Principal Investigator at University of Manitoba, Winnipeg, MB, Canada. He has been involved in clinical trials for Eli Lilly, Locemia, BMS, Astra Zeneca, Janssen, Merck, Novo Nordisk, Sanofi and he is on the advisory boards for Eli Lilly, Merck, Astra Zeneca, Novo Nordisk, Janssen and Sanofi. H.D., C.A.P. and D.C. are employees and stockholders of Locemia Solutions, which funded the study. X.M.Z. is an employee of and stockholder in Eli Lilly, Canada. S.P. is an employee of Eli Lilly, Bangalore, India. S.Z. is an employee of and stockholder in Eli Lilly and Company. C.B.G. is a former employee of and stockholder in Eli Lilly and Company. The authors report no other conflicts of interest.
Author contributions
C.A.P., E.R.S. and H.D. designed the study. E.R.S., R.R.‐L., G.M.T., J.R.C., S.J.W., G.F.G., V.C.W. and H.D. conducted the study. All the authors were involved in interpreting the data as well as drafting and reviewing the manuscript. All the authors have approved the final draft of the manuscript for submission.
Prior presentation
Parts of this study have been presented in the form of an oral presentation at the 77th Scientific Sessions of the American Diabetes Association (ADA 2017), San Diego, California, June 9 to 13, 2017 and as a poster at the 53rd Annual Meeting of the European Association for the Study of Diabetes (EASD 2017), Lisbon, Portugal, September 11 to 15, 2017.
Seaquist ER, Dulude H, Zhang XM, et al. Prospective study evaluating the use of nasal glucagon for the treatment of moderate to severe hypoglycaemia in adults with type 1 diabetes in a real‐world setting. Diabetes Obes Metab. 2018;20:1316–1320. https://doi.org/10.1111/dom.13278
Funding information Locemia Solutions, Montreal, QC, Canada; Eli Lilly and Company, Indianapolis, IN, USA
REFERENCES
- 1. Pontiroli AE. Intranasal glucagon: a promising approach for treatment of severe hypoglycemia. J Diabetes Sci Technol. 2015;9(1):38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cryer PE. Hypoglycemia in type 1 diabetes mellitus. Endocrinol Metab Clin North Am. 2010;39(3):641‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCrimmon RJ, Sherwin RS. Hypoglycemia in type 1 diabetes. Diabetes. 2010;59(10):2333‐2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haugstvedt A, Wentzel‐Larsen T, Graue M, Sovik O, Rokne B. Fear of hypoglycaemia in mothers and fathers of children with type 1 diabetes is associated with poor glycaemic control and parental emotional distress: a population‐based study. Diabet Med. 2010;27(1):72‐78. [DOI] [PubMed] [Google Scholar]
- 5. Rickels MR, Ruedy KJ, Foster NC, et al; T1D Exchange Intranasal Glucagon Investigators. Intranasal glucagon for treatment of insulin‐induced hypoglycemia in adults with type 1 diabetes: a randomized crossover noninferiority study. Diabetes Care. 2016;39(2):264‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kedia N. Treatment of severe diabetic hypoglycemia with glucagon: an underutilized therapeutic approach. Diabetes Metab Syndr Obes. 2011;4:337‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clarke WL, Cox DJ, Gonder‐Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18(4):517‐522. [DOI] [PubMed] [Google Scholar]
- 8. Sherr JL, Ruedy KJ, Foster NC, et al; T1D Exchange Intranasal Glucagon Investigators. Glucagon nasal powder: a promising alternative to intramuscular glucagon in youth with type 1 diabetes. Diabetes Care. 2016;39(4):555‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chow E, Bernjak A, Williams S, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 2014;63(5):1738‐1747. [DOI] [PubMed] [Google Scholar]
- 10. Finfer S, Liu B, Chittock DR, et al; NICE‐SUGAR Study Investigators.Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367(12):1108‐1118. [DOI] [PubMed] [Google Scholar]
- 11. International Hypoglycemia Study Group . Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40(1):155‐157. [DOI] [PubMed] [Google Scholar]
- 12. Cryer PE. Individualized glycemic goals and an expanded classification of severe hypoglycemia in diabetes. Diabetes Care. 2017;40(12):1641‐1643. [DOI] [PubMed] [Google Scholar]
- 13. Yale JF, Dulude H, Egeth M, et al. Faster use and fewer failures with needle‐free nasal glucagon vs. injectable glucagon in severe hypoglycemia rescue: a simulation study. Diabetes Technol Ther. 2017;19(7):423‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deeb LC, Dulude H, Guzman CB, et al. A phase 3 multicenter, open‐label, prospective study designed to evaluate the effectiveness and ease of use of nasal glucagon in the treatment of moderate and severe hypoglycemia in children and adolescents with type 1 diabetes in the home or school setting. Pediatr Diabetes. 2018. https://doi.org/10.1111/pedi.12668 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patient disposition.
Figure S2. Blood glucose levels after NG administration, mean (±SD).
Table S1. Baseline demographics and diabetes history of patients included in the Main Safety Analysis Population.
Table S2. Summary of moderate hypoglycemic events in which patients did not return to normal status in 30 minutes after NG administration in the efficacy analysis population.
Table S3. Summary of severe hypoglycemic events in the efficacy analysis population.
Table S4. Summary of hypoglycemic events in which patients ingested oral carbohydrates.
Table S5. Adverse events solicited through questionnaires in the main safety analysis population.
Appendix S1. Clarke hypoglycemia awareness survey.
Appendix S2. Hypoglycemia episode questionnaire.
Appendix S3. Assessment of the user‐friendliness of the dry‐mist nasal glucagon questionnaire.
Appendix S4. Nasal score questionnaire.


