Abstract
Aims
Insulin therapy is indicated for people with Type 1 diabetes mellitus; however, treatment‐related weight gain and hypoglycaemia represent barriers to optimal glycaemic management. This study assessed the health economic value of maintained reductions in HbA1c, BMI and hypoglycaemia incidence among the UK Type 1 diabetes population.
Methods
The Cardiff Type 1 Diabetes Model was used to estimate lifetime costs, life‐years and quality‐adjusted life‐years (QALYs) for individuals with Type 1 diabetes at different baseline HbA1c, BMI and hypoglycaemic event rates. Results were discounted at 3.5%, and the net monetary benefit associated with improving Type 1 diabetes management was derived at £20 000/QALY gained. Per‐person outputs were inflated to national levels using UK Type 1 diabetes prevalence estimates.
Results
Modelled subjects with an HbA1c of 86 mmol/mol (10.0%) were associated with discounted lifetime per‐person costs of £23 795; £12 649 of which may be avoided by maintaining an HbA1c of 42 mmol/mol (6.0%). Combined with estimated QALY gains of 2.80, an HbA1c of 42 mmol/mol (6.0%) vs. 86 mmol/mol (10.0%) was associated with a £68 621 per‐person net monetary benefit. Over 1 year, unit reductions in BMI produced £120 per‐person net monetary benefit, and up to £197 for the avoidance of one non‐severe hypoglyceamic event.
Conclusions
Maintained reductions in HbA1c significantly alleviate the burden associated with Type 1 diabetes in the UK. Given the influence of weight and hypoglycaemia on health economic outcomes, they must also be key considerations when assessing the value of Type 1 diabetes technologies in clinical practice.
What's new?
This study demonstrated the burden of inadequate Type 1 diabetes management, and quantified the value of reducing HbA1c, weight and hypoglycaemia frequency among the UK Type 1 diabetes population.
Significant cost savings, quality‐adjusted life‐year gains and net monetary benefit were predicted in those who achieve HbA1c targets recommended in national guidelines; nevertheless, any incremental improvement in glycaemic management substantially reduced the burden of Type 1 diabetes mellitus on individuals and healthcare systems.
Given the influence of weight and hypoglycaemia on health economic outcomes, these factors should also be key considerations when assessing the value of Type 1 diabetes technologies.
What's new?
This study demonstrated the burden of inadequate Type 1 diabetes management, and quantified the value of reducing HbA1c, weight and hypoglycaemia frequency among the UK Type 1 diabetes population.
Significant cost savings, quality‐adjusted life‐year gains and net monetary benefit were predicted in those who achieve HbA1c targets recommended in national guidelines; nevertheless, any incremental improvement in glycaemic management substantially reduced the burden of Type 1 diabetes mellitus on individuals and healthcare systems.
Given the influence of weight and hypoglycaemia on health economic outcomes, these factors should also be key considerations when assessing the value of Type 1 diabetes technologies.
Introduction
Type 1 diabetes mellitus is a chronic autoimmune disorder associated with significant morbidity and mortality, and an estimated loss in life expectancy of 11–13 years among young adults in the United Kingdom (UK) 1. The management of the condition and its long‐term complications impose significant direct costs on the National Health Service (NHS), and its societal burden is associated with additional indirect costs. While Type 1 diabetes affected 400 000 individuals in the UK during 2010–2011, recent projections suggest that Type 1 diabetes prevalence will rise to 652 000 individuals by 2035–2036, and the economic burden of the condition will grow accordingly 2. Between 2010–2011 and 2035–2036, the direct healthcare costs of Type 1 diabetes are predicted to rise from £1.0 billion to £1.8 billion, with indirect costs of Type 1 diabetes‐related morbidity and mortality predicted to rise from £900 million to £2.4 billion during the same period 2.
Micro‐ and macrovascular complications account for approximately 70% of NHS expenditure on Type 1 diabetes management 2. These include potentially avoidable complications that arise as a consequence of poor glycaemic control; thus, improved disease management may produce significant cost savings for the UK public healthcare system. Treatment regimens that mimic physiological insulin secretion serve to control HbA1c levels in people with Type 1 diabetes, with a view to preventing the long‐term health complications associated with the condition. However, exogenous insulin is often associated with weight gain and the incidence of hypoglycaemic events, which in turn are barriers to optimal glycaemic management 3, 4. Updated guidance from the National Institute for Health and Care Excellence (NICE) recommend insulin therapy in combination with diet and lifestyle changes for the management of Type 1 diabetes, which collectively aim to normalize HbA1c levels, control weight and minimize cardiovascular risk factors 5.
Although NICE guidance published in 2004 recommended that Type 1 diabetes therapy should aim to reduce HbA1c levels to < 59 mmol/mol (7.5%), the 2015–2016 National Diabetes Audit found that 71% of people with Type 1 diabetes in England and Wales failed to achieve and maintain this goal 6. Since 2015, NICE guidelines now recommend that adults with Type 1 diabetes aim for a target HbA1c of 48 mmol/mol (6.5%) or below 5; a level of glycaemic control that only 8% currently reach 6. Despite the difficulty faced by individuals and their clinicians to achieve a stricter treatment target, health economic modelling has recently demonstrated the value of making modest, incremental improvements to glycaemic control in Type 1 diabetes. Based on current UK population data, it was estimated that an achievable HbA1c reduction of 4 mmol/mol (0.4%) could avoid 81 000 microvascular and 7000 macrovascular events over 25 years 7. Fewer complications were subsequently associated with per‐person cost savings between £2057 and £4136 over 25 years, which equated to a potential cost avoidance of £995 million for the total UK population 7. Importantly, the economic impact of differing levels of glycaemic control, in addition to treatment effects, on individual outcomes and quality of life remain poorly characterized.
With this in mind, the aim of this study was to quantify the health economic burden of elevated HbA1c, BMI and frequency of hypoglycaemic events in people with Type 1 diabetes, expressed in terms of costs, life‐years and quality‐adjusted life‐years (QALYs) at a per‐person and national level. The cost savings and QALY gains associated with incremental, maintained reductions in HbA1c, BMI and hypoglycaemia incidence were subsequently estimated, and the net monetary benefit associated with improved Type 1 diabetes management was derived.
Methods
The Cardiff Type 1 Diabetes Model
The Cardiff Type 1 Diabetes Model has been described in detail previously 8. In brief, it is a fixed‐time‐increment stochastic microsimulation model designed to evaluate the lifetime impact of therapeutic changes for individuals with Type 1 diabetes. Consistent with both established and recently published Type 1 diabetes models 9, risk equations implemented within the Cardiff Type 1 Diabetes Model were adapted to incorporate long‐term epidemiological evidence derived from the Diabetes Control and Complications Trial (DCCT) 10 and follow‐up study (Epidemiology of Diabetes Interventions and Complications; EDIC) 11, in addition to cardiovascular risk equations from the Swedish National Diabetes Registry 12. A flow diagram of the Cardiff Type 1 Diabetes Model is shown in Fig. S1; approaches to simulate Type 1 diabetes‐related complications within the model are summarized in Table S1. Further details regarding the development and validation of the Cardiff Type 1 Diabetes Model are provided in its original publication 8.
Costs and utilities
The direct costs of managing Type 1 diabetes and its complications were implemented within the Cardiff Type 1 Diabetes Model. The occurrence of diabetes‐related events was associated with a reduction in quality of life; the utility decrements applied (additively) were consistent with the default values of the CORE Diabetes Model 13 and those applied in recent guidelines 5. Further details regarding the costs and utilities applied in the model are provided in Tables S2–S4. The utility decrement associated with hypoglycaemia was modelled using regression equations that linked event frequency and severity to utility, via the fear of hypoglycaemia score 14.
Baseline characteristics and time‐dependent risk factors
Two characteristic profiles were modelled, representing an average person with Type 1 diabetes in the UK (‘average UK profile’) and a more recently diagnosed individual (‘younger UK profile’), consistent with those reported in NICE guideline NG17 5 (Table 1). The likelihood of clinical events was influenced by several risk factors; while the model has the capability of allowing modifiable risk factors to change with respect to time, HbA1c, weight, blood pressure and lipid parameters remained constant for this study, in the absence of any specific intervention.
Table 1.
Type 1 diabetes characteristic profiles
| Parameter | Average UK profile | Younger UK profile | Source |
|---|---|---|---|
| Baseline demographics and modifiable risk factors | |||
| Mean age (years) | 42.98 | 27.00 | NICE NG17 5, Nathan et al. 15 |
| Mean duration of diabetes (years) | 16.92 | 9.10 | NICE NG17 5, NDA 16 |
| Proportion women | 0.43 | 0.45 | NICE NG17 5, NDA 16 |
| Proportion smokers | 0.22 | 0.26 | NICE NG17 5, NDA 16 |
| Mean total cholesterol (mg/dl) | 176.50 | 176.50 | NICE NG17 5, Nathan et al. 15 |
| Mean HDL cholesterol (mg/dl) | 50.25 | 50.25 | NICE NG17 5, Nathan et al. 15 |
| Mean SBP (mm Hg) | 128.27 | 121.48 | NICE NG17 5, NDA 16 |
| Mean DBP (mm Hg) | 73.55 | 73.55 | Saunders et al. 17 |
| Mean BMI (kg/m2)a | 27.09 | 24.90 | NICE NG17 5 |
| Clinical history and management (proportion) | |||
| History of CVD | 0.003 | Assumed none | NICE NG17 5, HSCIC 18 |
| History of microalbuminuria | 0.181 | NICE NG17 5, NDA 16 | |
| History of neuropathy | 0.049 | NICE NG17 5, Nathan et al. 15 | |
| Hypertension | 0.880 | NICE NG17 5 | |
| ACE inhibitor therapy | 0.710 | NICE NG17 5 | |
| Annual frequency of hypoglycaemia | |||
| Non‐severe events | 28 | 35.5 | UK Hypoglycaemia Study Group 19 |
| Severe events | 0.46 | 0.22 | |
Weight initialized in the model based on an assumed height of 1.72m2.
ACE, angiotensin converting enzyme; CVD, cardiovascular disease; HSCIC, Health and Social Care Information Centre; NDA, National Diabetes Audit; NICE, National Institute for Health and Care Excellence.
Analyses
The model was used to evaluate the impact of stepwise reductions in HbA1c from 86 mmol/mol (10.0%) to 42 mmol/mol (6.0%), non‐severe hypoglycaemic events from 100 to zero, severe hypoglycaemic events from five to zero, and BMI from 35 kg/m2 to 25 kg/m2, on costs, life‐years and/or QALYs. Modelled changes in HbA1c from baseline were applied over the first 6 months and maintained over a lifetime (up to 80 years). Changes in hypoglycaemia incidence and BMI were modelled over 1 year; outputs were then scaled to estimate lifetime benefits in an average UK person with Type 1 diabetes. Outputs were discounted at 3.5% annually and reported on a per‐person basis. Using predicted cost savings and QALY gains, net monetary benefit (NMB) was additionally derived, based on a conventional threshold for cost‐effectiveness in the UK. Model analyses were treatment‐independent and did not consider pharmacological management costs; therefore, NMB represents both the burden associated with inadequate Type 1 diabetes management, and the amount that could be spent to achieve a given reduction in HbA1c, BMI and hypoglycaemia frequency, and associated QALY gains, whilst maintaining cost‐effectiveness at a willingness‐to‐pay threshold of £20 000 per additional QALY gained.
To quantify the national level burden of HbA1c in people with Type 1 diabetes, costs, life‐years and QALYs were predicted for a person in each of four HbA1c categories [< 48 mmol/mol (<6.5%); 48–58 mmol/mol (6.5%–7.5%); 58–86 mmol/mol (7.5%–10.0%); ≥ 86 mmol/mol (≥10.0%)], using modelled HbA1c levels of 6.0%, 7.0%, 8.75% and 10.0%, respectively. Health economic outcomes associated with HbA1c were then estimated at a national level, by scaling per‐person outputs to the UK Type 1 diabetes population. The total diabetes population (comprising people with Type 1 and Type 2 diabetes) was estimated using 2015 diabetes prevalence data for England 20, Wales 21, Scotland 22 and Northern Ireland 23. The Type 1 diabetes population (approximately 10% of all people with diabetes 20, 22) was stratified into the four HbA1c categories, according to distributions reported by the National Diabetes Audit 2014–2015 24.
Results
Value of improved glycaemic control
Figure 1 demonstrates the impact of reducing HbA1c from 86 mmol/mol (10.0%) to 42 mmol/mol (6.0%) on per‐person costs, life‐years and QALYs. An average UK person with Type 1 diabetes and an HbA1c of 86 mmol/mol (10.0%) was expected to cost a discounted total of £23 795 over a lifetime. Lowering HbA1c from 86 mmol/mol (10.0%) to 42 mmol/mol (6.0%) in 11 mmol/mol (1.0%) increments was associated with gross cost savings of £3746, £3538, £2983 and £2382 (totalling £12 649). Predicted cost savings were attributed to the avoidance of diabetes‐related complications; a breakdown of discounted per‐person costs are provided in Fig. S2. For a unit reduction in HbA1c from 86 mmol/mol (10.0%) to 75 mmol/mol (9.0%), predicted cost savings were largely driven by reduced costs of neuropathy (−£2492; −66.5%), retinopathy (−£901; −24.1%) and nephropathy (−£503; −13.4%), while some additional costs were incurred for severe hypoglycaemia (+£121; +3.2%), cardiovascular disease (+£17; +0.4%) and ketoacidosis (+£13; +0.3%). Despite overall cost savings, monetized QALY gains were the major driver of NMB associated with improved glycaemic control. Lowering HbA1c from 86 mmol/mol (10.0%) to 42 mmol/mol (6.0%) in 11 mmol/mol (1.0%) units led to incremental QALY gains of 0.84, 0.77, 0.66 and 0.53 (totalling 2.80); and associated NMB of £20 518, £18 935, £16 175 and £12 993 (totalling £68 621), respectively.
Figure 1.
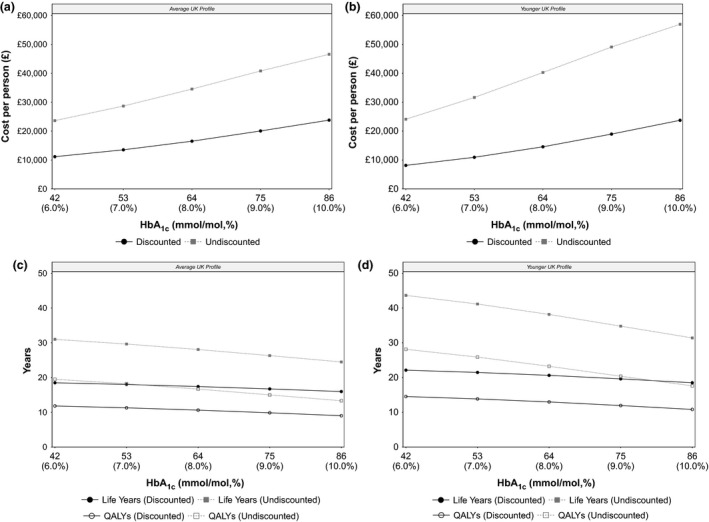
Per‐person costs, life‐years and quality‐adjusted life‐years (QALYs) in people with Type 1 diabetes according to HbA1c level. Incremental improvements in HbA1c were associated with reduced lifetime costs for people with an average UK profile (a) and a younger UK profile (b). Reducing HbA1c from 86 mmol/mol (10.0%) to 42 mmol/mol (6.0%) was also associated with improvements in life‐years and QALYs for people with an average UK profile (c) and a younger UK profile (d). Circles represent discounted costs, life‐years and QALYs; squares represent undiscounted costs, life‐years and QALYs.
Estimated costs increased for a more recently diagnosed individual. A person with a ‘younger UK profile’ and an HbA1c of 86 mmol/mol (10.0%) was estimated to incur a total discounted lifetime cost of £23 739. Gross cost savings when HbA1c was reduced from 86 mmol/mol (10.0%) to 42 mmol/mol (6.0%) in 11 mmol/mol (1.0%) increments were estimated to be £4772, £4394, £3634 and £2800 (totalling £15 601). Predicted cost savings were similarly attributed to the avoidance of adverse events; a breakdown of discounted per‐person costs are provided in Fig. S3. For a unit reduction in HbA1c from 86 mmol/mol (10.0%) to 75 mmol/mol (9.0%), the majority of cost savings were related to the reduced costs of neuropathy (−£2871; −63.9%), nephropathy (−£1260; −28.1%) and retinopathy (−£855; −19.0%), while some additional costs were incurred for ketoacidosis (+£381; +8.5%) and cardiovascular disease (+£113; +2.5%). However, the predicted value of improved glycaemic control was largely driven by monetized utility gains; lowering HbA1c from 86 mmol/mol (10.0%) to 42 mmol/mol (6.0%) in 11 mmol/mol (1.0%) units was associated with incremental QALY gains of 1.12, 1.04, 0.87 and 0.69 (totalling 3.72), and NMB of £26 711, £24 753, £20 774 and £16 385 (totalling £88 624), respectively.
At a national level, 447 338 people were estimated to have Type 1 diabetes in the UK, comprising 38 706 people (8.7%) with HbA1c < 48 mmol/mol (< 6.5%), 95 035 (21.2%) with HbA1c 48–58 mmol/mol (6.5%–7.5%), 242 971 (54.3%) with HbA1c 58–86 mmol/mol (7.5%–10.0%), and 70 625 (15.8%) with HbA1c ≥ 86 mmol/mol (≥ 10.0%). When modelled with an ‘average UK profile’, the Type 1 diabetes subpopulation with HbA1c ≥ 86 mmol/mol (≥ 10.0%; N = 70 625) was estimated to cost £1.7 billion in total; a maintained HbA1c reduction to 42 mmol/mol (6.0%) was associated with a gross cost saving of £0.9 billion. If an HbA1c level of 42 mmol/mol (6.0%) could be achieved and maintained among those with HbA1c ≥ 48 mmol/mol (≥ 6.5%; N = 408 632), the total costs associated with Type 1 diabetes‐related complications could be reduced by more than half (gross cost saving of £3.0 billion). Table 2 provides national level costs, life‐years and QALYs for each HbA1c cohort, modelled with an ‘average UK profile’ and ‘younger UK profile’. Figures S4–S6 additionally illustrate per‐person and national level costs, life‐years and QALYs according to HbA1c category, respectively.
Table 2.
National level costs, life‐years and quality‐adjusted life‐years for UK populations with Type 1 diabetes, stratified by HbA1c category
| HbA1c | |||||||
|---|---|---|---|---|---|---|---|
| < 48 mmol/mol (< 6.5%) | 48–58 mmol/mol (6.5–7.5%) | 58–86 mmol/mol (7.5–10.0%) | ≥ 86 mmol/mol (≥ 10.0%) | Sum of all categories | |||
| Modelled HbA1c value (%) | 6.00 | 7.00 | 8.75 | 10.00 | NA | ||
| Population size | 38 706 | 95 035 | 242 971 | 70 625 | 447 338 | ||
| Costs | |||||||
| Discounted | Average UK profile | Total | £431 439 676 | £1 285 670 103 | £4 645 594 362 | £1 680 558 443 | £8 043 262 583 |
| Incrementala | – | £226 349 970 | £1 937 296 097 | £893 326 834 | £3 056 972 901 | ||
| Younger UK profile | Total | £314 993 860 | £1 039 538 661 | £4 330 124 053 | £1 676 557 223 | £7 361 213 797 | |
| Incrementala | – | £266 129 639 | £2 352 797 043 | £1 101 799 876 | £3 720 726 558 | ||
| Undiscounted | Average UK profile | Total | £914 263 609 | £2 724 199 930 | £9 531 959 140 | £3 288 530 807 | £16 458 953 486 |
| Incrementala | – | £479 395 146 | £3 792 805 591 | £1 620 308 684 | £5 892 509 422 | ||
| Younger UK profile | Total | £932 782 369 | £3 007 575 099 | £11 419 107 379 | £4 021 985 457 | £19 381 450 305 | |
| Incrementala | – | £717 300 932 | £5 563 705 071 | £2 319 972 855 | £8 600 978 858 | ||
| Life‐years | |||||||
| Discounted | Average UK profile | Total | 715 940 | 1 711 181 | 4 108 159 | 1 127 950 | 7 663 230 |
| Incrementala | – | 1 253 294 | 3 650 271 | 670 063 | 5 573 628 | ||
| Younger UK profile | Total | 856 353 | 2 040 321 | 4 832 873 | 1 306 883 | 9 036 429 | |
| Incrementala | – | 1 582 433 | 4 374 985 | 848 996 | 6 806 414 | ||
| Undiscounted | Average UK profile | Total | 1 199 905 | 2 817 259 | 6 503 596 | 1 729 530 | 12 250 291 |
| Incrementala | – | 2 359 372 | 6 045 709 | 1 271 643 | 9 676 723 | ||
| Younger UK profile | Total | 1 689 392 | 3 911 532 | 8 669 779 | 2 217 770 | 16 488 473 | |
| Incrementala | – | 3 453 645 | 8 211 892 | 1 759 882 | 13 425 419 | ||
| QALYs | |||||||
| Discounted | Average UK profile | Total | 457 345 | 1 072 505 | 2 444 180 | 636 847 | 4 610 878 |
| Incrementala | – | 615 160 | 1 986 836 | 179 502 | 2 781 499 | ||
| Younger UK profile | Total | 562 836 | 1 316 273 | 2 968 217 | 764 552 | 5 611 877 | |
| Incrementala | – | 858 928 | 2 510 872 | 307 207 | 3 677 007 | ||
| Undiscounted | Average UK profile | Total | 755 029 | 1 730 417 | 3 750 648 | 940 775 | 7 176 869 |
| Incrementala | – | 1 273 072 | 3 293 304 | 483 430 | 5 049 806 | ||
| Younger UK profile | Total | 1 089 043 | 2 459 685 | 5 129 012 | 1 243 380 | 9 921 119 | |
| Incrementala | – | 2 002 340 | 4 671 667 | 786 035 | 7 460 042 | ||
Incremental costs, life‐years and quality‐adjusted life‐years (QALYs) calculated with respect to the UK Type 1 diabetes population with HbA1c < 48 mmol/mol (< 6.5%).
Value of reduced frequency of hypoglycaemia
In people experiencing no more than 10 non‐severe hypoglycaemic events, one fewer event was associated with one‐year QALY gains ranging between 0.001 (reduction from 10 to nine events) and 0.01 (reduction from one to zero events). As no costs were applied to the incidence of non‐severe hypoglycaemia in this study, the predicted value of reduced event frequency was driven entirely by monetized QALY gains. Thus, at a willingness‐to‐pay threshold of £20 000 per QALY, one fewer non‐severe event equated to incremental NMB ranging between £27 and £197 (Fig. 2). If modelled reductions in non‐severe hypoglycaemia frequency were maintained in an average UK person with HbA1c of 75 mmol/mol (9.0%), this would translate to lifetime QALY gains of between 0.023 (reduction from 10 to nine events) and 0.164 (reduction from one to zero events), and associated incremental NMB of £452–£3289.
Figure 2.
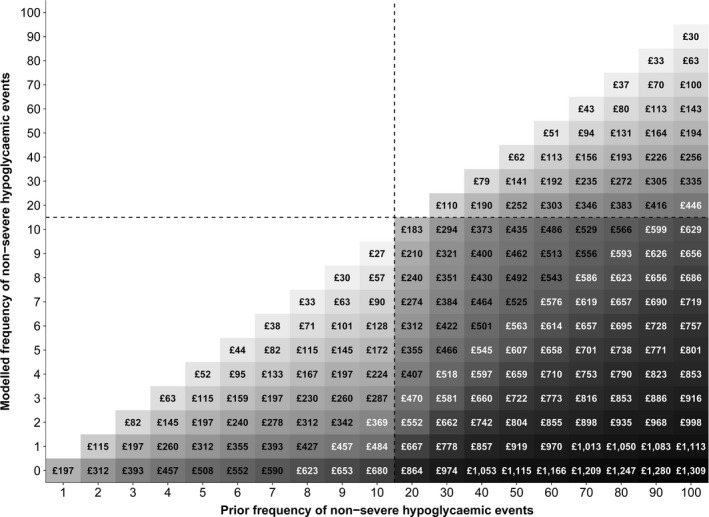
Discounted value of reduced non‐severe hypoglycaemic event incidence in people with Type 1 diabetes (net monetary benefit at £20 000 per quality‐adjusted life‐year gained).
In people experiencing 20–100 non‐severe hypoglycaemia events, 10 fewer events resulted in one‐year QALY gains ranging between 0.001 (reduction from 100 to 90 events) and 0.009 (reduction from 20 to 10 events), and associated incremental NMB of £30–£183 (Fig. 2). If modelled reductions in non‐severe hypoglycaemia frequency were maintained in an average UK person with HbA1c of 75 mmol/mol (9.0%), this would translate to lifetime QALY gains of between 0.025 (reduction from 100 to 90 events) and 0.153 (reduction from 20 to 10 events), and associated incremental NMB of £495–£3068.
In people with no more than five severe hypoglycaemic events, the per‐person cost saving was £344 for every severe hypoglycaemic event avoided. Complete avoidance of severe hypoglycaemia was associated with a 0.01 QALY gain over one year; thus, incremental NMB ranged between £544 (reduction from one to zero events) and £1921 (reduction from five to zero events) (Fig. S7). In an average UK person with HbA1c of 75 mmol/mol (9.0%), maintained avoidance of severe hypoglycaemia over a lifetime would translate to 0.167 QALYs gained, and incremental NMB between £9104 (reduction from one to zero events) and £32 136 (reduction from five to zero events).
Value of reduced BMI
Costs associated with weight change were not applied in model analyses; thus, the predicted value of reduced BMI was driven entirely by monetized utility gains. Reducing BMI by 1 kg/m2 was associated with a 0.006 QALY gain over one year, which corresponded to an incremental NMB of £120 for each unit reduction in BMI. Therefore, reducing BMI from 35 kg/m2 to 25 kg/m2 equated to an incremental NMB of £1200 per year, at a willingness‐to‐pay threshold of £20 000 per QALY. If modelled weight change was maintained in an average UK person with HbA1c of 75 mmol/mol (9.0%), each unit reduction in BMI would translate to a lifetime QALY gain of 0.100, and associated incremental NMB of £2007.
Discussion
Recent studies have reported the health economic value of improved disease management in Type 2 diabetes 25, and estimated the potential costs avoided that a modest HbA1c reduction would achieve within the UK healthcare system 7. In the present analysis, we quantified the health economic burden of inadequate Type 1 diabetes management in the UK, and estimated the value associated with incremental HbA1c improvements, fewer hypoglycaemic events and reduced BMI in people with Type 1 diabetes. Analyses using the Cardiff Type 1 Diabetes Model found that unit reductions in HbA1c, BMI and frequency of hypoglycaemia were independently associated with per‐person cost savings, QALY gains and NMB. Monetized QALY gains were the major driver of NMB associated with improved Type 1 diabetes management, while predicted cost savings were attributed to the avoidance of long‐term health complications that arise as a consequence of poor glycaemic control.
National guidelines recommend that adults with Type 1 diabetes aim to lower HbA1c to ≤ 48 mmol/mol (≤ 6.5%) 5, and the present study highlighted the considerable health economic benefits that could be achieved in those who successfully reach and maintain this ‘ideal’ HbA1c target. However, NICE additionally recognize that several factors, including comorbidities, risk of complications and history of hypoglycaemia, represent barriers to achieving optimal glycaemic control; and subsequently advocate the use of individualized HbA1c targets in these cases 5. With this in mind, we demonstrated that unit reductions in HbA1c were associated with substantial per‐person cost savings, and gains in life‐years and QALYs, regardless of whether NICE treatment targets were met. The feasibility of realizing such benefits is highly individualized; nevertheless, the results of this study support the notion that incremental improvements in glycaemic control, in addition to BMI and hypoglycaemia incidence, can potentially alleviate the burden of Type 1 diabetes on individuals and healthcare systems. Analyses were conducted independent of pharmacological management and service delivery costs; and it is likely that strategies to improve glycaemic control, hypoglycaemia event rate and/or body weight will incur additional costs to the NHS. However, if this expenditure falls below the predictions of NMB generated in this study, such interventions would be deemed cost‐effective at a willingness‐to‐pay threshold of £20 000 per additional QALY gained.
People with Type 1 diabetes who experience poor glucose control, high BMI and a high frequency of hypoglycaemia have the greatest risk of adverse clinical outcomes, health disutility and potential resource use. Indeed, previous estimates have indicated that a modest reduction in HbA1c (4 mmol/mol; 0.4%) among people with Type 1 diabetes and HbA1c exceeding 75 mmol/mol (9.0%) may save the NHS £447 million over 25 years 7. Our results additionally illustrate that people with higher baseline HbA1c have the potential to achieve relatively greater QALY gains with improved glycaemic control, while reducing weight and avoiding hypoglycaemia in those with high BMI and hypoglycaemia event rates, respectively, was associated with increasing NMB. Improved population‐based intervention strategies that target such ‘high risk’ individuals may therefore provide the greatest clinical and financial benefits to the healthcare system. However, intervention‐specific research is required to assess the feasibility of improving HbA1c, BMI and/or hypoglycaemia frequency in those with poorly managed Type 1 diabetes, and the resource and health system implications of realizing these targets in such a challenging population.
Injectable basal insulin therapies for Type 1 diabetes currently include intermediate‐acting [neutral protamine Hagedorn (NPH) insulin], long‐acting (glargine and detemir analogues) and ultra‐long‐acting (degludec analogue) agents. Based on evidence that multiple daily insulin injections using long‐acting analogues are more efficacious than NPH insulin in Type 1 diabetes, current NICE guidelines recommend twice‐daily insulin detemir as the optimal basal treatment regimen 5. In those who fail to maintain HbA1c below 69 mmol/mol (8.5%) or experience disabling hypoglycaemia following multiple daily insulin injections, rapid‐acting agents (aspart, glulisine and lispro analogues), delivered using continuous subcutaneous insulin infusion devices, are subsequently advocated 26. Guideline recommendations are partly founded on cost–utility analyses; however, insulin therapy is highly individualized, and drawing direct comparisons between Type 1 diabetes technologies poses several methodological challenges. For example, insulin titration algorithms and levels of care are heterogeneous across studies and not reflective of routine clinical practice 27; and the reporting of hypoglycaemic events in clinical trials is often confounded by ill‐defined HbA1c thresholds and nocturnal periods 28. Our study illustrates the value of reducing HbA1c, hypoglycaemia and body weight irrespective of treatment strategy; therefore, data describing these factors are useful to inform cost‐effectiveness analyses for any therapeutic modality in Type 1 diabetes.
In light of this, the present study used an outcome‐focused approach to quantify the health economic value of improving HbA1c, reducing the frequency of hypoglycaemic events and lowering BMI in people with Type 1 diabetes, regardless of the intervention used to achieve these responses. Our results do not support the adoption of any particular therapeutic strategy, but rather support the notion that modest improvements to Type 1 diabetes management can reduce the clinical and financial burdens associated with developing and managing its long‐term health complications. This may be achieved through the use of insulin‐based therapies; however, weight gain and hypoglycaemia are common adverse events and barriers to optimal glycaemic control [3 4]. In line with current NICE guidance, promoting lifestyle changes and educational tools, including the dose‐adjustment for normal eating programme, will additionally assist in managing Type 1 diabetes and its complications 5. Emerging adjunctive therapies, that serve to improve glycaemic control whilst reducing insulin‐related side effects, are currently under investigation and may provide significant health economic value in the future 29.
Several computer simulation models have been developed to characterize Type 1 diabetes, including the CORE Diabetes Model 30, Sheffield Type 1 Diabetes Policy Model 31 and the Economic Assessment of Glycaemic Control and Long‐Term Effects of Diabetes Model 32. By simulating disease progression over a time horizon longer than that feasible in clinical trials, computer modelling represents an important tool to quantify the long‐term health economic outcomes of Type 1 diabetes interventions, and support clinical and reimbursement decision‐making 9. In this study, the value of modest improvements in disease management was quantified using the Cardiff Type 1 Diabetes Model, implemented with risk equations partially derived from DCCT/EDIC study data 10. Model validity is contingent on the data used to inform its development; therefore, caution is required when extrapolating model predictions beyond the scope of DCCT/EDIC observations. However, several baseline characteristics act as predictive risk factors within the Cardiff Type 1 Diabetes Model (including age, gender, age at diabetes onset, HbA1c and weight); and the accuracy and generalizability of its outputs have previously been validated against other new and established Type 1 diabetes models 8. Subsequently, this model represents a plausible approach to assess the value of improving glycaemic control, hypoglycaemia incidence and body weight in populations not reflective of the DCCT/EDIC study cohort, such as those over 60 years of age, or with a long duration of Type 1 diabetes. Furthermore, the impact of weight, hypoglycaemia and HbA1c on predicted QALYs in this study are consistent with published analyses using the Cardiff Type 2 Diabetes Model 25; and the structure and performance of both Cardiff models have undergone rigorous scrutiny at several Mount Hood Diabetes Challenge Meetings 33, 34, 35.
Our study demonstrated the individual impact of HbA1c, weight and hypoglycaemia on health economic outcomes in people with Type 1 diabetes, with all other parameters held constant. Clinically, there is a well‐recognized relationship between glycaemic control, weight and hypoglycaemia in Type 1 diabetes, and the assessment of each factor in isolation subsequently represents one limitation of this analysis. However, the objective of this study was to demonstrate the independent benefits of reducing HbA1c, BMI and hypoglycaemia incidence, and as such, aims to provide clinicians and payers with evidence to quantify the health economic value of improving each factor, both individually and in combination. Although the value of lowering HbA1c was evaluated over a lifetime, BMI and frequency of hypoglycaemia were assessed in the short‐term due to the duration over which such changes may be observed, and the relative delays in the observed impact of each factor on individual outcomes. Nevertheless, QALY gains, cost savings and/or NMB predicted over one year were scaled to illustrate the benefits of managing BMI and hypoglycaemia over a lifetime, in an average UK person with Type 1 diabetes. Furthermore, the impact of BMI and hypoglycaemia on the risk of adverse events including falls and fractures, cardiovascular disease and all‐cause mortality, and their correlation with other risk factors related to Type 1 diabetes, were not considered 36, 37, 38. Although these modelling assumptions may collectively underestimate the true, long‐term value of lowering BMI and hypoglycaemic event incidence in people with Type 1 diabetes, our study nevertheless demonstrates the impact of weight and hypoglycaemia on health‐related quality of life, to which a monetary value has been derived.
Due to the UK payer perspective adopted in the study, societal costs were not quantified, and the strength of the national level estimates reported is contingent on the quality of prevalence data available in the UK. This study did not account for changes in population size since 2015, people with Type 1 diabetes below 17 years of age, nor adjust for Quality and Outcomes Framework disease register estimates in Wales. Cumulatively, these factors may underestimate the true size of the UK Type 1 diabetes population, and therefore the true value associated with improved disease management. The UK perspective of this study may limit the relevance of its economic results to other national settings; however, predicted health utility changes are generalizable, and may be used in conjunction with country‐specific cost data to inform local decision‐making.
In conclusion, this study highlighted the health economic benefits of incremental improvements to HbA1c, BMI and hypoglycaemic event frequency in the UK Type 1 diabetes population. Model analyses found that monetized QALY gains were the major driver of NMB associated with improved Type 1 diabetes management, while predicted cost savings were attributed to the avoidance of its long‐term health complications. Given the influence of weight and hypoglycaemia on health economic outcomes in Type 1 diabetes, these factors must be key considerations when assessing the cost‐effectiveness of health technologies and their efficacy in clinical practice.
Funding sources
Sponsorship for this study and manuscript preparation were funded by AstraZeneca Pharmaceuticals.
Competing interests
P. McEwan, H. Bennett, K. Bolin and M. Evans have served as consultants to and received research funding from AstraZeneca Pharmaceuticals in relation to this study. K. Bergenheim is an employee of AstraZeneca Pharmaceuticals.
Author contributions
P. McEwan and H. Bennett were involved in the study design, analysis, interpretation of results and writing of the manuscript. K. Bolin and K. Bergenheim were involved in the design, interpretation of results and writing of the manuscript. M. Evans assisted with interpretation of results and editing the manuscript.
Supporting information
Figure S1. Flow diagram of the Cardiff Type 1 Diabetes Model.
Figure S2. Breakdown of discounted per‐person costs related to Type 1 diabetes (average UK profile) at different HbA1c levels.
Figure S3. Breakdown of discounted per‐person costs related to Type 1 diabetes (younger UK profile) at different HbA1c levels.
Figure S4. Discounted per‐person and national level costs related to Type 1 diabetes at different HbA1c levels.
Figure S5. Discounted per‐person and national level life‐years related to Type 1 diabetes at different HbA1c levels.
Figure S6. Discounted per‐person and national level QALYs related to Type 1 diabetes at different HbA1c levels.
Figure S7. Discounted value associated with reduced severe hypoglycaemia event (SHE) incidence in people with Type 1 diabetes (net monetary benefit at £20 000 per QALY gained).
Table S1. Summary of approaches to model Type 1 diabetes related complications in the Cardiff Type 1 Diabetes Model.
Table S2. Costs applied within the Cardiff Type 1 Diabetes Model.
Table S3. Derivation of minor and major amputation costs.
Table S4. Utilities applied within the Cardiff Type 1 Diabetes Model.
Acknowledgements
Editorial assistance in the preparation of this manuscript was provided by Dr Karina Hamilton and Samantha Webster of HEOR Ltd.
Diabet. Med. 35, 557–566 (2018)
References
- 1. Livingstone SJ, Levin D, Looker HC, Lindsay RS, Wild SH, Joss N et al Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008–2010. JAMA 2015; 313: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med 2012; 29: 855–862. [DOI] [PubMed] [Google Scholar]
- 3. DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA 2003; 289: 2254–2264. [DOI] [PubMed] [Google Scholar]
- 4. Snoek F. Barriers to good glycaemic control: the patient's perspective. Int J Obes 2000; 24(Suppl. 3): S12–S20. [DOI] [PubMed] [Google Scholar]
- 5. National Institute for Health and Care Excellence (NICE) . Type 1 Diabetes in Adults: Diagnosis and Managment. NICE guidelines [NG17]. 2015. Available at https://www.nice.org.uk/guidance/ng17 Last accessed 27 February 2017.
- 6. National Health Service . National Diabetes Audit – 2015‐2016: Report 1, Care Processes and Treatment Targets, 2017. Available at http://www.content.digital.nhs.uk/catalogue/PUB23241 Last accessed 27 February 2017.
- 7. Baxter M, Hudson R, Mahon J, Bartlett C, Samyshkin Y, Alexiou D et al Estimating the impact of better management of glycaemic control in adults with Type 1 and Type 2 diabetes on the number of clinical complications, and the associated financial benefit. Diabet Med 2016; 33: 1575–1581. [DOI] [PubMed] [Google Scholar]
- 8. McEwan P, Bennett H, Fellows J, Priaulx J, Bergenheim K. The health economic value of changes in glycaemic control, weight and rates of hypoglycaemia in Type 1 diabetes mellitus. PLoS One 2016; 11: e0162441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henriksson M, Jindal R, Sternhufvud C, Bergenheim K, Sörstadius E, Willis M. A systematic review of cost‐effectiveness models in type 1 diabetes mellitus. Pharmacoeconomics 2016; 34: 569–585. [DOI] [PubMed] [Google Scholar]
- 10. Diabetes Control Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 11. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ et al Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cederholm J, Eeg‐Olofsson K, Eliasson B, Zethelius B, Gudbjörnsdottir S. A new model for 5‐year risk of cardiovascular disease in Type 1 diabetes; from the Swedish National Diabetes Register (NDR). Diabet Med 2011; 28: 1213–1220. [DOI] [PubMed] [Google Scholar]
- 13. Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health 2014; 17: 462–470. [DOI] [PubMed] [Google Scholar]
- 14. Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P. Multivariate models of health‐related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 2006; 22: 1523–1534. [DOI] [PubMed] [Google Scholar]
- 15. Nathan DM, Zinman B, Cleary PA, Backlund JYC, Genuth S, Miller R et al Modern‐day clinical course of type 1 diabetes mellitus after 30 years’ duration: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications and Pittsburgh Epidemiology of Diabetes Complications Experience (1983–2005). Arch Intern Med 2009; 169: 1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Health Service . National Diabetes Audit 2011‐2012, Report 1: Care Processes and Treatment Targets, 2013. Available at http://content.digital.nhs.uk/catalogue/PUB12421 Last accessed 17 March 2017.
- 17. Saunders S, Wallymhamed M, Macfarlane I. Improvements in glycaemic control and cardiovascular risk factors in a cohort of patients with type 1 diabetes over a 5‐year period. QJM 2009; 102: 29–34. [DOI] [PubMed] [Google Scholar]
- 18. National Health Service . Health Survey for England – 2011, Health, Social Care and Lifestyles, 2012. Available at http://content.digital.nhs.uk/catalogue/PUB09300 Last accessed 17 March 2017.
- 19. UK Hypoglycaemia Study Group . Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007; 50: 1140–1147. [DOI] [PubMed] [Google Scholar]
- 20. Public Health England . Diabetes Prevalence Model for Local Authorities and CCGs; Diabetes Prevalence Estimates by CCG: Registered Populations, 2016. Available at http://www.yhpho.org.uk/resource/view.aspx?RID=154049 Last accessed 17 March 2017.
- 21. StatsWales . Patients on Quality and Outcomes Framework (QOF) Disease Registers by Local Health Board, 2016. Available at https://statswales.gov.wales/Catalogue/Health-and-Social-Care/NHS-Primary-and-Community-Activity/GMS-Contract/patientsonqualityandoutcomesframework-by-localhealthboard-diseaseregister Last accessed 17 March 2017.
- 22. NHS Scotland . Scottish Diabetes Survey 2015, 2015. Available at http://diabetesinscotland.org.uk/Publications/SDS2015.pdf Last accessed 17 March 2017.
- 23. Department of Health . 2015/16 Raw disease prevalence data for Northern Ireland, 2016. Available at https://www.health-ni.gov.uk/publications/201516-raw-disease-prevalence-trend-data-northern-ireland Last accessed 17 March 2017.
- 24. National Health Service . National Diabetes Audit – 2013–2014 and 2014–2015: Report 1, Care Processes and Treatment Targets. 2016. Available at http://content.digital.nhs.uk/catalogue/PUB19900 Last accessed 17 March 2017.
- 25. McEwan P, Evans M, Kan H, Bergenheim K. Understanding the inter‐relationship between improved glycaemic control, hypoglycaemia and weight change within a long‐term economic model. Diabetes Obes Metab 2010; 12: 431–436. [DOI] [PubMed] [Google Scholar]
- 26. National Institute for Health and Care Excellence . Continuous Subcutaneous Insulin Infusion for the Treatment of Diabetes Mellitus. Technology appraisal guidance [TA151]. 2008. Available at https://www.nice.org.uk/guidance/ta151 Last accessed 13 October 2017.
- 27. Garg S, Moser E, Dain MP, Rodionova A. Clinical experience with insulin glargine in type 1 diabetes. Diabetes Technol Ther 2010; 12: 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morales J, Schneider D. Hypoglycemia. Am J Med 2014; 127(Suppl. 10): S17–S24. [DOI] [PubMed] [Google Scholar]
- 29. Chen J, Fan F, Wang J, Long Y, Gao C, Stanton R, Xu Y. The efficacy and safety of SGLT2 inhibitors for adjunctive treatment of type 1 diabetes: a systematic review and meta‐analysis. Sci Rep 2017; 7: 44128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM et al The CORE Diabetes Model: projecting long‐term clinical outcomes, costs and cost effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision‐making. Curr Med Res Opin 2004; 20(Suppl. 1): S5–S26. [DOI] [PubMed] [Google Scholar]
- 31. Kruger J, Brennan A, Thokala P, Basarir H, Jacques R, Elliott J et al The cost‐effectiveness of the Dose Adjustment for Normal Eating (DAFNE) structured education programme: an update using the Sheffield Type 1 Diabetes Policy Model. Diabet Med 2013; 30: 1236–1244. [DOI] [PubMed] [Google Scholar]
- 32. Mueller E, Maxion‐Bergemann S, Gultyaev D, Walzer S, Freemantle N, Mathieu C et al Development and validation of the Economic Assessment of Glycemic Control and Long‐Term Effects of diabetes (EAGLE) model. Diabetes Technol Ther 2006; 8: 219–236. [DOI] [PubMed] [Google Scholar]
- 33. Mount Hood 4 Modeling Group . Computer modeling of diabetes and its complications: A report on the Fourth Mount Hood Challenge Meeting. Diabetes Care 2007; 30: 1638–1646. [DOI] [PubMed] [Google Scholar]
- 34. Palmer AJ. Mount Hood 5 Modeling Group. Computer modeling of diabetes and its complications: a report on the Fifth Mount Hood Challenge Meeting. Value Health 2013; 16: 670–685. [DOI] [PubMed] [Google Scholar]
- 35. Mount Hood Diabetes Challenge Network . Cardiff Model, 2017. Available at https://www.mthooddiabeteschallenge.com/cardiff Last accessed 12 October 2017.
- 36. Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, Paul SK. Hypoglycemia and risk of cardiovascular disease and all‐cause mortality in insulin‐treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care 2015; 38: 316–322. [DOI] [PubMed] [Google Scholar]
- 37. Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. JAMA 1998; 280: 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khazai NB, Beck GR, Umpierrez GE. Diabetes and fractures: an overshadowed association. Curr Opin Endocrinol Diabetes Obes 2009; 16: 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow diagram of the Cardiff Type 1 Diabetes Model.
Figure S2. Breakdown of discounted per‐person costs related to Type 1 diabetes (average UK profile) at different HbA1c levels.
Figure S3. Breakdown of discounted per‐person costs related to Type 1 diabetes (younger UK profile) at different HbA1c levels.
Figure S4. Discounted per‐person and national level costs related to Type 1 diabetes at different HbA1c levels.
Figure S5. Discounted per‐person and national level life‐years related to Type 1 diabetes at different HbA1c levels.
Figure S6. Discounted per‐person and national level QALYs related to Type 1 diabetes at different HbA1c levels.
Figure S7. Discounted value associated with reduced severe hypoglycaemia event (SHE) incidence in people with Type 1 diabetes (net monetary benefit at £20 000 per QALY gained).
Table S1. Summary of approaches to model Type 1 diabetes related complications in the Cardiff Type 1 Diabetes Model.
Table S2. Costs applied within the Cardiff Type 1 Diabetes Model.
Table S3. Derivation of minor and major amputation costs.
Table S4. Utilities applied within the Cardiff Type 1 Diabetes Model.


