Abstract
Background & Aims
Daclatasvir has achieved high sustained virologic response (SVR) rates in diverse hepatitis C virus (HCV) populations. This study evaluated the long‐term efficacy and safety of daclatasvir‐based regimens administered during clinical studies.
Methods
Patients enrolled within 6 months of parent study completion or protocol availability at the study sites. The primary objective was durability of SVR at follow‐up Week 12 (SVR12). Secondary objectives included analysing HCV sequences in non‐responders or responders who relapsed, and characterization of liver disease progression.
Results
Between 24 February 2012 and 17 July 2015, this study enrolled and began following 1503 recipients of daclatasvir‐based regimens (follow‐up cut‐off, 13 October 2015); 60% were male, 18% aged ≥65 years, 87% had genotype‐1a (42%) or ‐1b (45%) infection, and 18% had cirrhosis. Median follow‐up from parent study follow‐up Week 12 was 111 (range, 11‐246) weeks. 1329/1489 evaluable patients were SVR12 responders; 1316/1329 maintained SVR until their latest visit. Twelve responders relapsed by (n = 9) or after (n = 3) parent study follow‐up Week 24; one was reinfected. Relapse occurred in 3/842 (0.4%) and 9/487 (2%) responders treated with interferon‐free or interferon‐containing regimens, respectively. Hepatic disease progression and new hepatocellular carcinoma were diagnosed in 15 and 23 patients, respectively. Among non‐responders, emergent non‐structural protein‐5A (NS5A) and ‐3 (NS3) substitutions were replaced by wild‐type sequences in 27/157 (17%) and 35/47 (74%) patients, respectively.
Conclusions
SVR12 was durable in 99% of recipients of daclatasvir‐based regimens. Hepatic disease progression and new hepatocellular carcinoma were infrequent. Emergent NS5A substitutions persisted longer than NS3 substitutions among non‐responders.
Keywords: chronic hepatitis C virus, daclatasvir, hepatocellular carcinoma, long‐term follow‐up, sustained virologic response
Abbreviations
- AFP
alpha‐fetoprotein
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ASV
asunaprevir
- BCV
beclabuvir
- DAA
direct‐acting antiviral
- DCV
daclatasvir
- EOT
end‐of‐treatment
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- LLOQ
lower limit of quantification
- NS3
non‐structural protein‐3
- NS5A
non‐structural protein‐5A
- pegIFNα/RBV
peginterferon‐alfa+ribavirin
- SAE
serious adverse event
- SOF
sofosbuvir
- SVR
sustained virologic response
Key points.
This large, long‐term follow‐up study investigated efficacy and safety among 1503 patients with chronic HCV infection and a diverse range of disease backgrounds treated with daclatasvir‐based regimens
Among responders, SVR12 durability was 99% (n = 1316/1329), with most relapses occurring within 24 weeks of parent study EOT (n = 9) rather than during this long‐term follow‐up study (n = 3)
Hepatic disease progression or new HCC, while infrequent, was more common among patients treated in studies of DAA‐only regimens (n = 24/36), which could enroll patients with more advanced liver disease
Among non‐responders, emergent NS5A substitutions were more persistent than NS3 substitutions.
1. INTRODUCTION
Direct‐acting antiviral (DAA)‐only regimens have largely replaced peginterferon‐alfa plus ribavirin (pegIFNα/RBV)‐containing regimens as standard of care in many countries for chronic hepatitis C virus (HCV) infection. DAA‐only regimens are, by comparison, better tolerated and less susceptible to attenuation by factors including cirrhosis, older age and male gender.1, 2
Daclatasvir (DCV), a pangenotypic non‐structural protein‐5A (NS5A) inhibitor, exhibits in vitro and clinical activity against HCV genotypes 1‐6 and has a favourable safety profile.3, 4, 5, 6, 7 Multiple studies have evaluated DCV plus other DAAs and/or pegIFNα/RBV. DCV plus sofosbuvir (DCV+SOF) or asunaprevir (DCV+ASV) has achieved high sustained virologic response (SVR) rates in diverse and difficult‐to‐treat populations.8, 9, 10, 11 Compared with studies of pegIFNα/RBV‐containing regimens, studies of DAA‐only regimens have enrolled patients with more advanced disease due to less restrictive clinical and laboratory criteria, and DCV+SOF has achieved high SVR rates in patients with human immunodeficiency virus (HIV) coinfection, decompensated cirrhosis and post‐liver transplant recurrence.12, 13, 14, 15
While favourable outcomes have been reported with short follow‐ups, data describing SVR durability, long‐term safety and frequency of outcomes of liver failure and hepatocellular carcinoma (HCC), are limited in recipients of DAA‐only regimens. Patients with more advanced disease may remain at risk of hepatic disease progression and HCC despite SVR, and require long‐term surveillance of their liver disease.16, 17 This study followed patients treated with DCV‐based regimens for chronic HCV infection in phase 2 or 3 studies with the aim of evaluating long‐term efficacy and safety, including type and frequency of hepatic disease progression.
2. METHODS
2.1. Study design
This 144‐week, observational, multicenter study enrolled patients within 6 months of parent study completion or protocol availability at the clinical site (NCT01492504). The study protocol was approved by the institutional review board or independent ethics committee at each site (Table S1). Study conduct adhered to local laws and regulatory requirements, and was in accordance with Good Clinical Practice as defined by the International Conference on Harmonization and the principles of the Declaration of Helsinki. All patients provided written informed consent.
2.2. Patients
Eligible patients were aged ≥18 years and had received ≥1 DCV dose for chronic HCV. Enrolment was permitted regardless of cirrhosis status and virologic response. Patients in control arms could participate until unblinded treatment information was released for the parent study protocol, at which time they could choose to continue follow‐up in this study. Patients retreated for HCV infection post‐parent study completion were ineligible.
2.3. Study objectives
The primary objective was to determine SVR12 durability (time to loss of SVR achieved at parent study follow‐up Week 12; HCV‐RNA ≥lower limit of quantification [LLOQ]). Secondary objectives included analysing HCV sequences in non‐responders or responders who relapsed, and characterizing liver disease progression.
2.4. Assessments
Visits occurred at Screening/Day 1 and follow‐up Weeks 24, 48, 96 and 144 (cirrhotic patients had additional visits at follow‐up Weeks 72 and 120). Serum HCV‐RNA was centrally‐determined (COBAS TaqMan HCV Test, v2.0; LLOQ, 25 IU/mL; Roche Molecular Systems, Inc.) at each visit. Patients with HCV‐RNA <LLOQ upon entry underwent centrally‐performed reflex genotype tests (VERSANT HCV Genotype 2.0 Assay [LiPA]; Bayer Healthcare) if they developed HCV‐RNA ≥LLOQ to determine relapse or reinfection. Similarly, responders developing HCV‐RNA ≥LLOQ underwent centralized re‐testing for HCV‐RNA and genotype at unscheduled visits as soon as possible.
Patients were monitored for hepatic disease progression (bleeding and non‐bleeding oesophageal or gastric varices, hepatic encephalopathy, spontaneous bacterial peritonitis, hepatorenal syndrome, ascites and liver transplant requirement), HCC, cirrhosis, subsequent anti‐HCV therapy use, all‐cause and liver‐related mortality. Cirrhosis was diagnosed per the investigator's judgement (parent study criteria provided in Table S2). Safety was evaluated on deaths or serious adverse events (SAEs) related to parent treatment. Albumin, alpha‐fetoprotein (AFP), bilirubin, international normalized ratio and thyroid stimulating hormone were measured at each visit; alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine and platelets were measured in cirrhotic and post‐transplant patients enrolled from the ALLY‐1 study;12 ALT and AST were measured in responders who relapsed. Patients with AFP >50 ng/mL (>41.3 IU/mL) underwent liver ultrasonography to diagnose possible HCC, repeated at intervals indicated by the standard‐of‐care guidelines at study initiation, and per the investigator's judgment; cirrhotic patients also underwent liver ultrasonography on Day 1, and follow‐up Weeks 48, 96 and 144.
NS5A and non‐structural protein‐3 (NS3) sequencing was performed at each visit on plasma samples with HCV‐RNA ≥1000 IU/mL by Janssen Diagnostics (sensitivity, ≥20%; population‐based sequencing) in DCV+ASV, DCV+ASV+pegIFNα/RBV or DCV+pegIFNα/RBV recipients, and LabCorp (sensitivity, ≥10%; Ilumina next‐generation sequencing) in DCV+SOF±RBV or DCV+ASV+beclabuvir (BCV)±RBV recipients.
2.5. Statistical analysis
Enrolled patients had signed informed consent forms and were assigned patient identification numbers. Eligible patients had enrolled and met the eligibility criteria (primary analysis population).
Efficacy and liver disease progression endpoints were evaluated per parent treatment. Exploratory analyses included parent study baseline, end‐of‐treatment (EOT) and follow‐up data. Categorical variables were summarized with counts and percentages, continuous variables and changes from baseline with univariate statistics. Unless specified otherwise, longitudinal efficacy analyses used pre‐defined intervals from parent study EOT, and endpoints were presented at 24‐week intervals using the last available measurement up to and including the analysis time‐point. Patients without measurements were excluded for that interval. Laboratory data were summarized with US values and units using measurements taken centrally or locally, graded using the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events. For hepatic disease progression, HCC, cirrhosis, all‐cause and liver‐related mortality, imputed onset dates were used to calculate event durations.
3. RESULTS
3.1. Patient disposition
Between 24 February 2012 and 17 July 2015, this study enrolled 1503 patients from 21 phase 2/3 studies, treated with DCV+SOF±RBV (n = 237), DCV+ASV (n = 389), DCV+ASV+BCV±RBV (n = 267), DCV+ASV+pegIFNα/RBV (n = 199), or DCV+pegIFNα/RBV (n = 411) for 12 or 24 weeks (Table 1); DCV+pegIFNα/RBV recipients may have received 24 weeks of additional pegIFNα/RBV. Patients were followed until 13 October 2015, at which point follow‐up had been completed by 201 patients; 157 discontinued due to withdrawn consent (n = 52), death (n = 9) or other reason (n = 19), 34 were lost to follow‐up, and 43 no longer met study criteria due to HCV retreatment (n = 41) or incarceration (n = 2) (Table S3).
Table 1.
Parent studies
| Regimen (N)a | Study (phase) [n] | wk | HCV genotype | Prior treatment experience |
|---|---|---|---|---|
| DCV+SOF ±RBV (239) | 444‐04037 (2a) [72] | 12 or 24 | 1‐3 | Naive |
| 1 | Telaprevir or boceprevir failures | |||
| 444‐21512 (3) [65] | 12 | 1‐6 | Cirrhotic or post‐liver transplant | |
| 444‐21614 (3) [51] | 8 or 12 | 1‐4 | Naive or experienced with HIV coinfection | |
| 444‐21810 (3) [51] | 12 | 3 | Naive or experienced | |
| DCV+ASV (389) | 447‐01138 (2a) [22] | 24 | 1 | Null‐responder |
| 447‐01739 (2a) [37] | 24 | 1b | Null‐responder or IFN‐ineligible/‐intolerant | |
| 444‐026 (2b) [5] | 24 | 1b | Naive | |
| 447‐02640 (3) [201] | 24 | 1b | Non‐responder or IFN‐ineligible/‐intolerant | |
| 447‐0289 (3) [124] | 24 | 1b | Naive, non‐responder, or IFN‐ineligible/‐intolerant | |
|
DCV+ASV+BCV ±RBV (267) |
443‐01441, 42 (2a/b) [132] | 12 or 24 | 1 | Naive or null‐responder |
| 4 | Naive | |||
| 443‐10243 (3) [55] | 12 | 1 | Naive or experienced (non‐cirrhotic) | |
| 443‐11344 (3) [80] | 12 | 1 | Naive or experienced (cirrhotic) | |
|
DCV+ASV +pegIFNα/RBV (199) |
447‐01138 (2a) [36] | 24 | 1 | Null‐responder |
| 444‐026 (2b) [36] | 24 | 1, 4 | Non‐responder | |
| 447‐02945 (3) [127] | 24 | 1, 4 | Partial or null‐responder | |
|
DCV +pegIFNα/RBVa (411) |
444‐010,46 ‐011 ‐014,47 ‐026, ‐031,48 ‐038,49 ‐042,50 ‐043, ‐05251 (2a/b, 3) [419] |
24 or 48 | 1‐4 | Naive or non‐responder |
HIV, human immunodeficiency virus.
These patients may have received 24 additional weeks of pegIFNα/RBV.
3.2. Baseline characteristics
Most patients were male (60%), infected with genotype‐1a (42%) or ‐1b (45%), and received DAA‐only regimens (59%); 18% had cirrhosis and 3% were liver transplant recipients (Table 2). Of 269 cirrhotic patients, 183 (68%) and 86 (32%) received DAA‐only or IFN‐containing regimens, respectively. All liver transplant recipients received DAA‐only regimens.
Table 2.
Baseline characteristics
| Parameter, n (%)a | DCV+SOF±RBV | DCV+ASV | DCV+ASV+BCV±RBV | DCV+ASV+pegIFN/RBV | DCV+pegIFN/RBV |
|---|---|---|---|---|---|
| N = 237 | N = 389 | N = 267 | N = 199 | N = 411 | |
| Age, median, years (range) | 58 (22‐83) | 62 (22‐79) | 57 (25‐77) | 54 (21‐77) | 53 (23‐73) |
| Male | 157 (66) | 154 (40) | 181 (68) | 138 (69) | 268 (65) |
| Race | |||||
| White | 191 (81) | 103 (26) | 229 (86) | 162 (81) | 353 (86) |
| Black/African American | 34 (14) | 11 (3) | 30 (11) | 22 (11) | 30 (7) |
| Japanese | 0 | 238 (62) | 0 | 0 | 1 (<1) |
| Other Asian | 7 (3) | 33 (8) | 4 (1) | 14 (7) | 13 (3) |
| Other | 5 (2) | 4 (1) | 4 (1) | 1 (<1) | 14 (3) |
| HCV genotypeb | |||||
| 1 (not subtyped) | 0 | 0 | 0 | 1 (<1) | 3 (1) |
| 1a | 121 (51) | 1 (<1) | 200 (75) | 104 (52) | 202 (49) |
| 1b | 32 (14) | 388 (100) | 61 (23) | 70 (35) | 120 (29) |
| 2 | 15 (6) | 0 | 0 | 0 | 26 (6) |
| 3 | 68 (29) | 0 | 0 | 0 | 23 (6) |
| 4 | 1 (<1) | 0 | 5 (2) | 24 (12) | 37 (9) |
| 6 | 0 | 0 | 1 (<1) | 0 | 0 |
|
HCV RNA, median log10 IU/mL (range) |
6.51 (3.4‐7.9) | 6.60 (3.9‐7.7) | 6.62 (3.8‐7.7) | 6.52 (4.6‐7.6) | 6.53 (3.6‐7.8) |
| Cirrhoticc | 55 (23) | 56 (14) | 72 (27) | 44 (22) | 42 (10) |
| Post‐liver transplant | 39 (16) | 0 | 0 | 0 | 0 |
Unless otherwise stated.
Determined at parent study baseline.
Reported in the medical histories prior to parent study EOT (n = 261), or between parent study EOT and Day 1 of this study (n = 8).
Of 1503 patients, 1489 (99%) were evaluable; 1329 (88%) were SVR12 responders (median age, 56 years), 160 (11%) were non‐responders (median age, 57 years). Male and cirrhotic patients were proportionally similar between responders (n = 792/1329, 60%; n = 238/1329, 18%) and non‐responders (n = 100/160, 63%; n = 24/160, 15%). However, genotype‐1a‐infected patients and recipients of IFN‐containing regimens were proportionally larger among non‐responders (n = 88/160, 55%; n = 114/160, 71%) vs responders (n = 537/1329, 40%; n = 487/1329, 37%).
Median (range) follow‐up from parent study follow‐up Week 12 was 111 (11‐246) weeks; 44 (11‐178) weeks in DCV+SOF±RBV recipients (n = 236), 114 (12‐239) weeks in DCV+ASV recipients (n = 384), 63 (12‐167) weeks in DCV+ASV+BCV±RBV recipients (n = 267), 113 (25‐225) weeks in DCV+ASV+pegIFNα/RBV recipients (n = 197), and 163 (12‐246) weeks in DCV+pegIFNα/RBV recipients (n = 402).
3.3. Durability of virologic response
SVR12 was maintained until the latest follow‐up visit by 1316/1329 (99%) responders treated with DCV+SOF±RBV (n = 232/232, 100%), DCV+ASV (n = 349/350, >99%), DCV+ASV+BCV±RBV (n = 257/260, 99%), DCV+ASV+pegIFNα/RBV (n = 187/190, 98%), or DCV+pegIFNα/RBV (n = 291/297, 98%) (Figure 1). Nine responders treated with DAA‐only (n = 2) or IFN‐containing (n = 7) regimens relapsed by parent study follow‐up Week 24.
Figure 1.
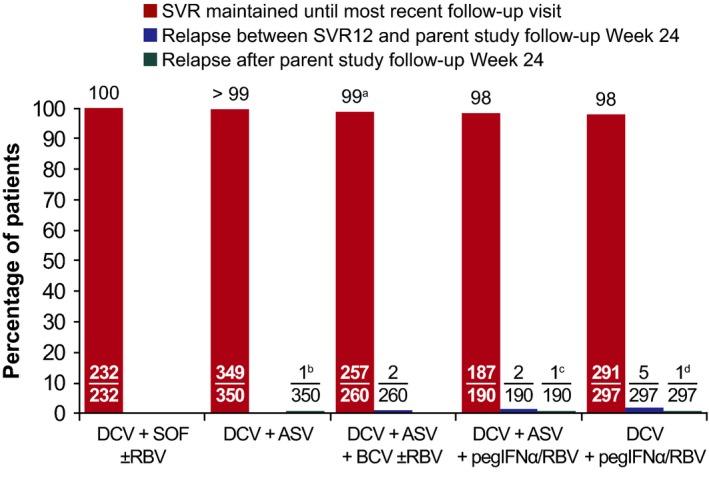
Durability of parent study SVR12. Median (range) follow‐up from parent study follow‐up Week 12: overall, 111 (11‐246) wk; DCV+SOF±RBV recipients, 44 (11‐178) wk; DCV+ASV recipients, 114 (12‐239) wk; DCV+ASV+BCV±RBV, 63 (12‐167) wk; DCV+ASV+pegIFNα/RBV, 113 (25‐225) wk; DCV+pegIFNα/RBV, 163 (12‐246) wk. aOne responder, treated for genotype‐1a infection, was re‐infected with genotype‐3a during this study; bResponder treated for genotype‐1b infection with NS5A‐Y93H at baseline relapsed at week 24 with emergent NS5A‐L31M; cResponder treated for genotype‐1b infection with NS5A‐L31V and ‐Y93H at baseline relapsed at week 24 with no emergent substitutions; dResponder treated for genotype‐1b infection relapsed on Day 1 (76 wk after parent study EOT) with emergent NS5A‐L31V and ‐Y93H
Three responders treated for genotype‐1b infection with DAA‐only (n = 1) or IFN‐containing (n = 2) regimens relapsed during this study. One DCV+ASV recipient, with NS5A‐Y93H at baseline, relapsed at week 24 with emergent NS5A‐L31M. One DCV+ASV+pegIFNα/RBV recipient, with NS5A‐L31V and ‐Y93H at baseline, relapsed at week 24 with no emergent substitutions. One DCV+pegIFNα/RBV recipient relapsed on Day 1, 76 weeks after parent study EOT, with emergent NS5A‐L31V and ‐Y93H.
One responder, treated for genotype‐1a infection, was re‐infected with genotype‐3a during this study.
3.4. Hepatic disease progression
Prior to parent study EOT, the medical histories reported hepatic disease progression in 88 recipients of DAA‐only (n = 75) or IFN‐containing (n = 13) regimens, HCC in 10 recipients of DAA‐only regimens, and cirrhosis in 261 recipients of DAA‐only (n = 181) or IFN‐containing (n = 80) regimens (Table 3). Between parent study EOT and the latest follow‐up visit, 20 hepatic disease progression events were diagnosed in 15 recipients of DAA‐only (n = 8) or IFN‐containing (n = 7) regimens, while new HCC was diagnosed in 23 recipients of DAA‐only (n = 18) or IFN‐containing (n = 5) regimens (Table 3; Kaplan‐Meier estimates on development of HCC provided in Figure 2); median time from parent study EOT to diagnosis was 70 (range, 0.4‐206) weeks.
Table 3.
Hepatic disease progression
| Parameter, n (%) | DCV+SOF±RBV | DCV+ASV | DCV+ASV+BCV±RBV | DCV+ASV +pegIFNα/RBV | DCV +pegIFNα/RBV |
|---|---|---|---|---|---|
| N = 237 | N = 389 | N = 267 | N = 199 | N = 411 | |
| Parent study | |||||
| Pre‐EOT | |||||
| Cirrhosis | 53/237 (22) | 56/389 (14) | 72/267 (27) | 41/199 (21) | 39/411 (9) |
| HCC | 10/237 (4) | 0/389 (0) | 0/267 (0) | 0/199 (0) | 0/411 (0) |
| Non‐bleeding oesophageal varices | 7/237 (3) | 3/389 (1) | 12/267 (4) | 6/199 (3) | 7/411 (2) |
| Bleeding oesophageal varices | 2/237 (1) | 0/389 (0) | 1/267 (<1) | 0/199 (0) | 0/411 (0) |
| Ascites | 19/237 (8) | 0/389 (0) | 0/267 (0) | 0/199 (0) | 0/411 (0) |
| Hepatic encephalopathy | 13/237 (5) | 0/389 (0) | 0/267 (0) | 0/199 (0) | 1/411 (<1) |
| Non‐bleeding gastric varices | 6/237 (3) | 0/389 (0) | 2/267 (1) | 0/199 (0) | 0/411 (0) |
| Bleeding gastric varices | 2/237 (1) | 0/389 (0) | 1/267 (<1) | 0/199 (0) | 0/411 (0) |
| Liver transplant | 39/237 (16) | 0/389 (0) | 0/267 (0) | 0/199 (0) | 0/411 (0) |
| Post‐EOT follow‐up | |||||
| EOTa‐<FU Week 24 | |||||
| Cirrhosis | 0/237 (0) | 0 (0) | 0/267 (0) | 2/199 (1) | 0/411 (0) |
| HCC | 0/237 (0) | 1/389 (<1) | 1/267 (<1) | 0/199 (0) | 0/411 (0) |
| Non‐bleeding oesophageal varices | 0/237 (0) | 2/389 (1) | 0/267 (0) | 0/199 (0) | 1/411 (<1) |
| Bleeding oesophageal varices | 0/237 (0) | 0/389 (0) | 0/267 (0) | 0/199 (0) | 1/411 (<1) |
| Ascites | 1/237 (<1) | 0/389 (0) | 1/267 (<1) | 0/199 (0) | 0/411 (0) |
| Non‐bleeding gastric varices | 0/237 (0) | 1/389 (<1) | 0/267 (0) | 0/199 (0) | 1/411 (<1) |
| FU Weeks 24‐<48 | |||||
| Cirrhosis | 3/237 (1) | 2/389 (1) | 1/267 (<1) | 2/199 (1) | 4/411 (1) |
| HCC | 0/237 (0) | 1/389 (<1) | 3/267 (1) | 0/199 (0) | 0/411 (0) |
| Ascites | 0/237 (0) | 0/389 (0) | 1/267 (<1) | 0/199 (0) | 0/411 (0) |
| Liver transplant | 1/237 (<1) | 0/389 (0) | 0/267 (0) | 0/199 (0) | 0/411 (0) |
| FU Weeks 48‐<72 | |||||
| Cirrhosis | 0/235 (0) | 0/388 (0) | 0/267 (0) | 0/198 (0) | 1/411 (<1) |
| HCC | 0/235 (0) | 1/388 (<1) | 2/267 (1) | 0/198 (0) | 2/411 (<1) |
| Non‐bleeding oesophageal varices | 0/235 (0) | 1/388 (<1) | 0/267 (0) | 0/198 (0) | 0/411 (0) |
| Liver transplant | 0/235 (0) | 0/388 (0) | 1/267 (<1) | 0/198 (0) | 0/411 (0) |
| FU Weeks 72‐<96 | |||||
| Cirrhosis | 0/100 (0) | 0/382 (0) | 0/263 (0) | 1/195 (1) | 0/409 (0) |
| HCC | 0/100 (0) | 3/382 (1) | 1/263 (<1) | 0/195 (0) | 0/409 (0) |
| Non‐bleeding oesophageal varices | 0/100 (0) | 0/382 (0) | 0/263 (0) | 1/195 (1) | 0/409 (0) |
| Ascites | 0/100 (0) | 0/382 (0) | 0/263 (0) | 0/195 (0) | 1/409 (<1) |
| FU Weeks 96‐<120 | |||||
| Cirrhosis | 0/72 (0) | 0/374 (0) | 0/126 (0) | 1/189 (1) | 1/403 (<1) |
| HCC | 0/72 (0) | 0/374 (0) | 0/126 (0) | 2/189 (1) | 0/403 (0) |
| Non‐bleeding oesophageal varices | 0/72 (0) | 0/374 (0) | 0/126 (0) | 0/189 (0) | 1/403 (<1) |
| ≥FU Week 120 | |||||
| Cirrhosis | 0/72 (0) | 0/363 (0) | 0/114 (0) | 0/179 (0) | 3/385 (1) |
| HCC | 0/72 (0) | 5/363 (1) | 0/114 (0) | 0/179 (0) | 1/385 (<1) |
| Non‐bleeding oesophageal varices | 0/72 (0) | 0/363 (0) | 0/114 (0) | 0/179 (0) | 1/385 (<1) |
| Ascites | 0/72 (0) | 0/363 (0) | 0/114 (0) | 0/179 (0) | 2/385 (<1) |
| Hepatic encephalopathy | 0/72 (0) | 0/363 (0) | 0/114 (0) | 0/179 (0) | 1/385 (<1) |
| Non‐bleeding gastric varices | 0/72 (0) | 0/363 (0) | 0/114 (0) | 0/179 (0) | 1/385 (<1) |
FU, follow‐up.
Results derived from the hepatic‐related diagnoses CRF pages. Pre‐EOT implies that diagnoses came prior to parent study EOT.
Parent study.
Figure 2.
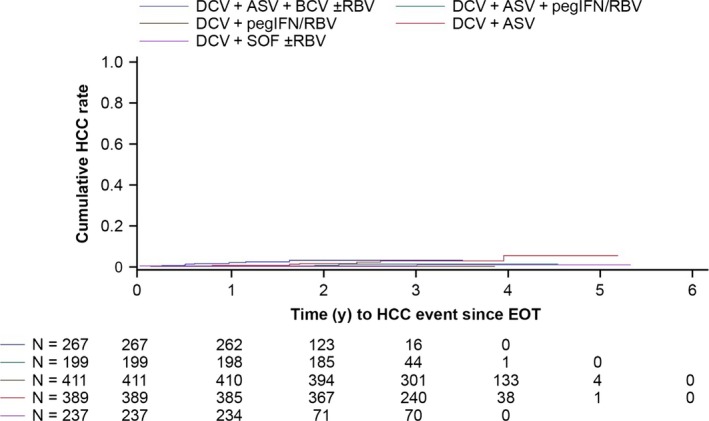
Kaplan‐Meier‐estimated cumulative HCC rate since parent study EOT
The 36 patients with hepatic disease progression or new HCC (2 had both) had a median age of 61 (range, 43‐78) years, 50% had cirrhosis, 97% had genotype‐1 infection, and 67% received DAA‐only regimens (Table 4); 20 had histories of alcohol use, obesity, HCC, diabetes, oesophageal or gastric varices, fatty‐liver disease, bleeding gastric ulcer, cirrhosis, or liver transplantation. Treatment and virologic response duration (until diagnosis) in patients with new HCC are shown in Figure 3; 20 were responders, 2 had detectable HCV‐RNA at parent study EOT, and 1 relapsed during parent study follow‐up. New HCC was comparable between responders (n = 20/1329, 2%) and non‐responders (n = 3/160, 2%).
Table 4.
Baseline characteristics in patients with hepatic disease progression or new HCC
| Parameter, n (%)a | All patients | Hepatic disease progressionc | HCC | ||
|---|---|---|---|---|---|
| SVR | Non‐SVR | SVR | Non‐SVR | ||
| N = 1503b | N = 8 | N = 7 | N = 20 | N = 3 | |
| Age, median, years (range) | 56 (21‐83) | 61 (50‐72) | 56 (43‐71) | 66 (52‐78) | 62 (58‐71) |
| Male | 898 (60) | 6 (75) | 4 (57) | 14 (70) | 1 (33) |
| HCV genotype | |||||
| 1 (not subtyped) | 4 (<1) | 0 | 0 | 0 | 0 |
| 1a | 628 (42) | 3 (38) | 4 (57) | 6 (30) | 1 (33) |
| 1b | 671 (45) | 4 (50) | 3 (43) | 14 (70) | 2 (67) |
| 2 | 41 (3) | 0 | 0 | 0 | 0 |
| 3 | 91 (6) | 1 (13) | 0 | 0 | 0 |
| 4 | 67 (4) | 0 | 0 | 0 | 0 |
| 6 | 1 (<1) | 0 | 0 | 0 | 0 |
| Regimen | |||||
| DAA‐only | 893 (59) | 7 (88) | 1 (14) | 16 (80) | 2 (67) |
| IFN‐containing | 610 (41) | 1 (13) | 6 (86) | 4 (20) | 1 (33) |
| Cirrhoticd | 269 (18) | 5 (63) | 5 (71) | 9 (45) | 1 (33) |
| Laboratory data, mean | |||||
| Total bilirubine (mg/dL) | 0.55 | 0.54 | 0.89 | 0.65 | 0.63 |
| INRe (fraction) | 1.09 | 1.44 | 1.15 | 1.21 | 1.13 |
| Plateletsf (×109 cells/L) | 192 | 115 | 127 | 145 | 130 |
| Creatininef (mg/dL) | 0.83 | 1.06 | 0.68 | 0.86 | 0.76 |
INR, international normalized ratio.
Unless otherwise stated.
SVR (n = 1329, 88%); non‐SVR (n = 160, 11%); missing data (n = 14, 1%).
Bleeding and non‐bleeding oesophageal or gastric varices, ascites, hepatic encephalopathy, spontaneous bacteria peritonitis, hepatorenal syndrome, and liver transplant.
Reported in the medical histories prior to parent study EOT (n = 261), or between parent study EOT and Day 1 of this study (n = 8).
Measured upon entry to this study.
Last available parent study measurements (measurements during this study only taken in cirrhotic and post‐transplant patients enrolled from the ALLY‐1 study12).
Figure 3.
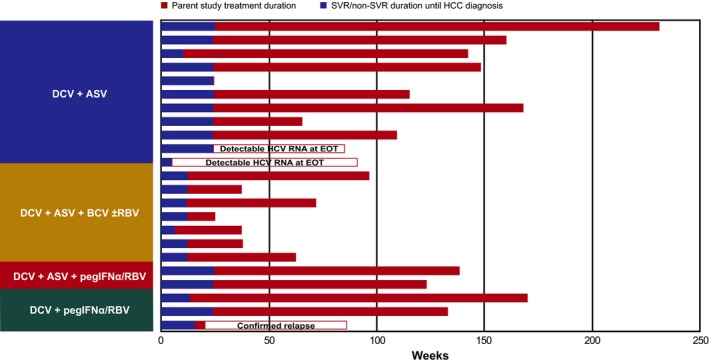
Treatment and response duration in patients with new HCC
3.5. Safety
Eleven deaths, all unrelated to parent treatment, were reported (Table 5). Three recipients of DCV+ASV (n = 2) or DCV+pegIFNα/RBV (n = 1) died from liver disease. Eight patients died from events unrelated to the liver; 1 DCV+SOF±RBV recipient had a suspected chronic obstructive pulmonary embolism, 3 DCV+ASV recipients had septic shock (n = 1), upper gastrointestinal bleeding (n = 1) or cholangiocellular carcinoma (n = 1), 1 DCV+ASV+pegIFNα/RBV recipient had sudden cardiac arrest, and 3 DCV+pegIFNα/RBV recipients had heart attack (n = 1), chronic kidney failure (n = 1) or carcinoma of the cervix (n = 1).
Table 5.
Safety outcomes
| Parameter, n (%) | DCV+SOF±RBV | DCV+ASV | DCV+ASV+BCV ±RBV | DCV+ASV+pegIFNα/RBV | DCV+pegIFNα/RBV |
|---|---|---|---|---|---|
| N = 237 | N = 389 | N = 267 | N = 199 | N = 411 | |
| Death | 1 | 5 | 0 | 1 | 4 |
| Liver‐related | 0 | 2a | 0 | 0 | 1b |
| Other | 1c | 3d | 0 | 1e | 3f |
| SAEsg | 0 | 0 | 0 | 0 | 0 |
Adenocarcinoma liver after post‐HCV cirrhosis (n = 1); liver disease (n = 1).
Liver disease.
Suspected chronic obstructive pulmonary disease.
Cholangiocellular carcinoma (n = 1); septic shock (n = 1); upper gastrointestinal bleeding (n = 1).
Sudden cardiac arrest.
Heart attack (n = 1); chronic kidney failure (n = 1); carcinoma of the cervix (n = 1).
Treatment‐related.
No SAEs related to parent treatment were reported.
3.6. Clinical resistance
Emergent NS5A substitutions were replaced with wild‐type sequences in 27/157 non‐responders with genotype‐1a (n = 21/92; M28T [n = 1], Q30E/H/R [n = 16], Y93C/H/N [n = 4]), ‐1b (n = 3/57; L31M/V [n = 1], Y93H/N [n = 2]), ‐3 (n = 1/5; Y93H), or ‐4 (n = 2/3; L28S [n = 1], Y93H [n = 1]) infection; median (range) time to replacement was 94 (8‐233) weeks overall, 109 (8‐233) and 48 (23‐156) weeks among those with genotype‐1a or ‐1b infection, respectively, and 56 and 60 (26‐94) weeks among those with genotype‐3 or ‐4 infection respectively (Table 6; Figure 4A,B). The most commonly replaced NS5A substitution among non‐responders with genotype‐1a infection was Q30E (n = 8); median (range) time to replacement was 131 (54‐194) weeks. Among non‐responders with emergent Y93H (n = 4), replacement was observed at weeks 124 (genotype‐1a), 23 (genotype‐1b), 56 (genotype‐3), and 26 (genotype‐4). Replacement of L31M/V was observed in one non‐responder with genotype‐1b infection at week 156.
Table 6.
Replacement of emergent NS5A and NS3 substitutions since parent study EOT among non‐responders
| HCV genotypea (N) | DCV+SOF±RBVb | DCV+ASVc | DCV+ASV+BCV ±RBVb | DCV+ASV+pegIFNα/RBVc | DCV+pegIFNα/RBVc | All | Median (range), weeks since parent study EOT | ||
|---|---|---|---|---|---|---|---|---|---|
| Duration of monitoring | Time to replacementd | Duration of persistencee | |||||||
| Emergent NS5A substitutions | |||||||||
| 1a (92) | ‐ | 1/1 | 3/9 | 2/7 | 15/75 | 21/92 | 158 (51‐242) | 109 (8‐233) | 154 (51‐243) |
| 1b (57) | ‐ | 3f/31 | ‐ | 0/1 | 0/25g | 3/57 | 144 (36‐257) | 48 (23‐156) | 147 (36‐257) |
| 3 (5) | 0/1 | ‐ | ‐ | ‐ | 1/4 | 1/5 | 162 (58‐191) | 56 | 149 (58‐192) |
| 4 (3) | ‐ | ‐ | ‐ | 0/1 | 2/2 | 2/3 | 94 (90‐113) | 60 (26‐94) | 90 |
| Total | 0/1 | 4/32 | 3/9 | 2/9 | 18/106 | 27/157 | 149 (36‐257) | 94 (8‐233) | 150 (36‐257) |
| Emergent NS3 substitutions | |||||||||
| 1a (16) | ‐ | 0/1 | 6/7 | 6/8 | ‐ | 12/16 | 80 (54‐235) | 52 (21‐62) | 104 (56‐235) |
| 1b (31) | ‐ | 22/30 | ‐ | 1/1 | ‐ | 23/31 | 134 (29‐228) | 24 (4‐146) | 132 (70‐228) |
| Total | 22/31 | 6/7 | 7/9 | 35/47 | 121 (29‐235) | 32 (4‐146) | 131 (56‐235) | ||
Identified using the VERSANT HCV genotype 2.0 Assay (LiPA; Bayer Healthcare).
Sensitivity, ≥10% (Labcorp).
Sensitivity, ≥20%.
Pertaining to emergent substitutions that were replaced.
Pertaining to emergent substitutions that were not replaced.
Also detected using next‐generation sequencing (sensitivity, ≥1%).
Excludes two patients initially designated as having genotype‐1b infection, but later shown using population‐based sequencing of the NS5A region to have genotype‐1a infection.
Figure 4.
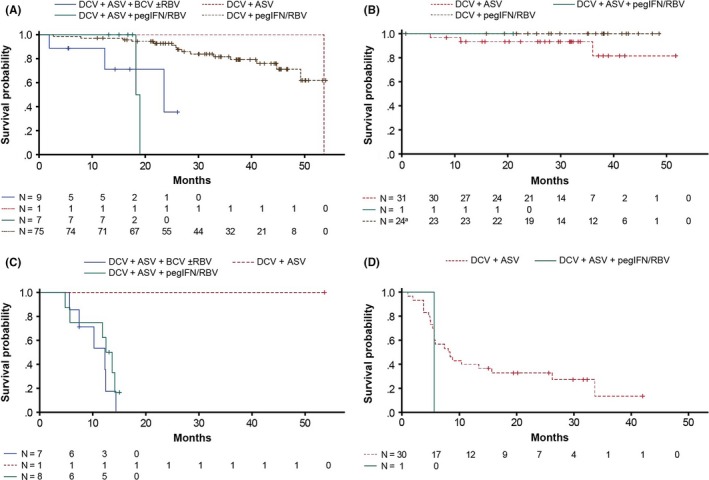
Replacement of emergent NS5A and NS3 substitutions with wild‐type sequences since parent study EOT among non‐responders. Time to replacement of emergent NS5A substitutions among patients infected with (A) genotype‐1a, or (B) genotype‐1b; time to replacement of emergent NS3 substitutions among patients infected with (C) genotype‐1a, or (D) genotype‐1b. aOne DCV+pegIFNα/RBV recipient infected with genotype‐1b is excluded due to replacement of their emergent NS5A substitution beyond the study's observational window
Emergent NS3 substitutions were replaced with wild‐type sequences in 35/47 non‐responders with genotype‐1a (n = 12/16; R155K/S [n = 10], D168E/H [n = 2]) or ‐1b (n = 23/31; D168A/E/T/V/Y [n = 22], R155Q [n = 1]) infection; median (range) time to replacement was 32 (4‐146) weeks overall, and 52 (21‐62) and 24 (4‐146) weeks in those with genotype‐1a or ‐1b infection, respectively (Table 6; Figure 4C,D). The most commonly replaced NS3 substitutions among non‐responders with genotype‐1a and ‐1b infection were R155K (n = 9) and D168V (n = 11), respectively; median (range) times to replacement were 53 (21‐62) and 24 (4‐114) weeks, respectively.
4. DISCUSSION
The emergence of DAAs has resulted in diverse and difficult‐to‐treat HCV populations achieving high SVR rates. Patients who achieve SVR are generally considered to be cured and at reduced risk of HCV‐related complications;18, 19, 20, 21 however, those with more advanced disease remain at risk of hepatic disease progression and HCC, and require long‐term surveillance of their liver disease.17, 22
This study, the largest conducted in the context of DAA‐only regimens, establishes the long‐term outcomes of DAA‐based treatment in patients with chronic HCV and a diverse range of disease backgrounds, including difficult‐to‐treat characteristics such as decompensated cirrhosis. A large number of patients (n = 1503) treated with DAA‐only or IFN‐containing regimens were enrolled and followed, 1489 of whom were evaluable. Most were responders (n = 1329), 99% of whom maintained SVR until their latest follow‐up visit, confirming the long‐term durability of SVR achieved with DCV‐based regimens. This is consistent with limited follow‐up data available for pegIFNα/RBV with or without DAAs.23, 24, 25, 26 Only 3 responders treated with DAA‐only (n = 1) or IFN‐containing regimens (n = 2) relapsed post‐parent study completion; 9, treated with DAA‐only (n = 2) or IFN‐containing (n = 7) regimens, relapsed by parent study follow‐up Week 24. Relapse appeared less frequently in DAA‐only recipients (n = 3), despite their more advanced disease, which included decompensated cirrhosis.
Long‐term safety appears favou\rable. All‐cause and liver‐related mortalities, all considered unrelated to parent treatment, were infrequent, consistent with reports showing improved overall survival in patients with advanced disease achieving SVR with IFN‐containing regimens.27, 28
Patients were evaluated for indicators of hepatic disease progression or HCC. Although it is generally expected that HCV eradication will prevent development of HCV‐related outcomes, high recurrence rates (28‐29%) observed in Spanish and cirrhotic Italian patients with prior HCC prompted speculation that DAAs may promote HCC recurrence.29, 30 However, reports elsewhere suggest that new or recurrent HCC is not promoted by DAAs. Patients with prior HCC from 3 cohorts of the France REcherche Nord&sud Sida‐vih Hépatites study showed no elevated HCC recurrence risk after DAA treatment; HCC recurred in 13% of DAA‐treated patients vs 21% of untreated patients, 8% of cirrhotic DAA‐treated patients vs 47% of untreated patients, and 2.2% of liver transplant recipients.31 Similarly, a retrospective Japanese study in a similar population found that early tumour recurrence was no higher after treatment with DAAs compared with IFN or control agents.32 As well as HCC recurrence, a retrospective cohort study of data from the Veterans Affairs HCV Clinical Case Registry identified elevated risks of new HCC in patients aged ≥65 years, or with cirrhosis, diabetes or genotype‐3 infection at the time of SVR.17 Such characteristics, while detrimental to pegIFNα/RBV treatment outcome, are readily overcome with DAA‐based regimens; persistence of such characteristics, however, may contribute to new HCC incidence several years post‐SVR.16, 17
During this study, hepatic disease progression (n = 15, 1%) or cirrhosis (n = 21, 1%) diagnoses were infrequent and evenly distributed among responders and non‐responders, despite 269 and 39 patients being cirrhotic or liver transplant recipients, respectively, upon entry. New HCC (n = 23, 2%) was equally infrequent and evenly distributed among responders (n = 20/1329, 2%) and non‐responders (n = 3/160, 2%). All diagnoses were non‐recurrent, although only 10 patients with prior HCC were enrolled; this population was excluded by the majority of parent studies. Nonetheless, the 2% incidence is low and comparable with incidences in similar populations of the aforementioned Italian study (3%) and a retrospective study of Japanese responders treated with DAAs (2.6%) or pegIFN/RBV (2.3%).30, 33 Among these 23 patients, 18 received DAA‐only regimens, most of whom were male (n = 12), cirrhotic (n = 10) or aged ≥65 years (n = 12) upon entry; all were treated for genotype‐1 infection, another HCC risk factor (Table S4).34, 35 Furthermore, platelet counts were low in many of these patients, including those without confirmed cirrhosis, meaning they may have had portal hypertension and thus misclassification of their liver disease.
Among the 269 patients with cirrhosis upon entry, 183 received DAA‐only regimens, 97% of whom were responders; in contrast, only 65/86 cirrhotic patients treated with IFN‐containing regimens (76%) were responders. While the larger number of cirrhotic patients responding with DAA‐only regimens likely reflects differences in the respective study eligibility criteria, they also highlight the advantages of DAA‐only regimens for patients with advanced disease, plus clinical and laboratory factors that would likely attenuate pegIFNα/RBV‐containing regimens.16 Indeed, parent studies of DAA‐only regimens enrolled patients with lower platelet counts (50 vs 90 × 109 cells/L in parent studies of IFN‐containing regimens), while three enrolled patients who were IFN‐intolerant/‐ineligible. Consequently, these studies could enroll patients with severe portal hypertension, which is considered an independent HCC predictor.36
Determining SVR12 durability was the primary objective of this study. However, non‐responders were also enrolled and their HCV sequences determined with the aim of identifying patterns of resistance that may guide retreatment. Persistence of emergent NS5A substitutions was high, particularly among non‐responders with genotype‐1b infection. Overall, 27/157 (17%) were replaced with wild‐type sequences during a median follow‐up of 149 (range, 36‐257) weeks. By contrast, emergent NS3 substitutions were less persistent, with replacement rates similar between non‐responders with genotype‐1a and ‐1b infection. Overall, 35/47 (74%) were replaced with wild‐type sequences during a median follow‐up of 121 (range, 29‐235) weeks. This information, alongside existing guidelines, should assist retreatment decisions.1, 2
All five DCV‐based regimens evaluated in this study are currently approved in various countries worldwide. DCV+SOF is recommended in many guidelines for patients with genotype‐3 infection and/or HIV coinfection.1, 2 DCV+ASV is approved in several countries across Asia and Latin America, as well as Russia and Israel, and was the first DAA regimen approved in Japan, and is currently the only DAA regimen approved in China. Co‐formulated DCV+ASV+BCV was recently approved in Japan and has proven effective against genotype‐1b NS5A polymorphisms known to attenuate response to dual‐drug NS5A inhibitor combinations.52
In summary, the results of this large, unique follow‐up study indicate that SVR achieved with DCV‐based regimens is durable in the long‐term, with no safety sequelae related to parent treatment. Hepatic disease progression was infrequent, and no increased risk for new or recurrent HCC was observed in patients with more advanced disease treated with DAA‐only regimens.
CONFLICTS OF INTEREST
KRR (advisory boards/research support: BMS, Gilead, Merck, Abbvie, Janssen); SP (grants/advisory boards: BMS, Gilead, MSD, AbbVie; advisory boards: Janssen, Novartis); PJT (grants/advisory boards: BMS); KC (grants/speaking: Ajinomoto, Astellas, Asuka, Bayer, Daiichi Sankyo, Dainippon Sumitomo, Eisai, MSD, Nihon Kayaku, Nihon Shinyaku; speaking: AbbVie, Abbott, AstraZeneca, BMS, Chugai, J&J, Jimro, Miyarisan, Olympus; grants: Janssen, Kowa, Mitsubishi Tanabe, Nippon Eli Lily, Otsuka, Roche, Takeda, Toray, Torii, Tsumura, Zeria); EJL (grants/personal fees: BMS, AbbVie, Achillion Pharmaceuticals, BMS, Enanta Pharmaceuticals, Janssen, Merck, Santaris Pharmaceuticals, Theravance, Gilead; grants: Boehringer Ingelheim, GSK, Roche, Salix, Tacere; personal fees: Idenix Pharmaceuticals, Novartis, Regulus); AG (speaking/investigator for: BMS); GG (consulting/speaking: AbbVie, BMS, Gilead, MSD, Roche); MB (speaking: BMS, Gilead, Janssen; advisory boards: Roche, AbbVie, Gilead, Janssen, MSD); CYP (advisory boards: AbbVie, BMS, Gilead, MSD, Roche); MS (speaking/advisory boards: BMS); SS (speaking/advisory boards/travel support: BMS, Gilead, AbbVie, MSD); FMP, ZL, ML (BMS employees); RY (BMS employee/stockholder); SN (BMS employee/stockholder; former Merck/Schering‐Plough employee/stockholder; J&J stockholder); HK, JT, JL, WG, JH (nothing to disclose).
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1111/liv.13596/suppinfo
ACKNOWLEDGEMENTS
Editorial assistance was provided by Matthew Young, DPhil, of Articulate Science Ltd., funded by Bristol‐Myers Squibb.
Reddy KR, Pol S, Thuluvath PJ, et al. Long‐term follow‐up of clinical trial patients treated for chronic HCV infection with daclatasvir‐based regimens. Liver Int. 2018;38:821–833. https://doi.org/10.1111/liv.13596
Funding information
This study was funded by Bristol‐Myers Squibb.
Clinical trial number: NCT01492504
Handling Editor: Alessio Aghemo
REFERENCES
- 1. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America . Recommendations for testing, managing, and treating hepatitis C. http://www hcvguidelines org/full‐report‐view. Accessed September 1, 2017.
- 2. European Association for Study of Liver . EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63:199‐236. [DOI] [PubMed] [Google Scholar]
- 3. Gao M. Antiviral activity and resistance of HCV NS5A replication complex inhibitors. Curr Opin Virol. 2013;3:514‐520. [DOI] [PubMed] [Google Scholar]
- 4. Hezode C, Abergel A, Chas J, et al. Sustained virologic response to daclatasvir and sofosbuvir, with or without ribavirin, among patients in the french daclatasvir ATU programme infected with HCV genotypes‐4, 5, and 6. J Hepatol. 2016;64(suppl):S755. [Google Scholar]
- 5. European Medicines Agency . Daklinza (daclatasvir) summary of product characteristics (updated, 19‐September‐2016).
- 6. Bunchorntavakul C, Reddy KR. The efficacy and safety of daclatasvir in the treatment of chronic HCV infection. Aliment Pharmacol Ther. 2015;42:258‐272. [DOI] [PubMed] [Google Scholar]
- 7. Herbst DA, Reddy KR. NS5A inhibitor, daclatasvir, for the treatment of chronic HCV infection. Expert Opin Investig Drugs. 2013;22:1337‐1346. [DOI] [PubMed] [Google Scholar]
- 8. Wei L, Zhang M, Xu M, et al. A phase 3, open‐label study of daclatasvir plus asunaprevir in asian patients with chronic HCV genotype‐1b infection who are ineligible for or intolerant to interferon alfa therapies with or without ribavirin. J Gastroenterol Hepatol. 2016;31:1860‐1867. [DOI] [PubMed] [Google Scholar]
- 9. Manns M, Pol S, Jacobson IM, et al. All‐oral daclatasvir plus asunaprevir for HCV genotype‐1b: a multinational, phase 3, multicohort study. Lancet. 2014;384:1597‐1605. [DOI] [PubMed] [Google Scholar]
- 10. Nelson DR, Cooper JN, Lalezari JP, et al. All‐oral 12‐week treatment with daclatasvir plus sofosbuvir in patients with HCV genotype‐3 infection: ALLY‐3 phase 3 study. Hepatology. 2015;61:1127‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leroy V, Angus P, Bronowicki JP, et al. Daclatasvir, sofosbuvir, and ribavirin for HCV genotype‐3 and advanced liver disease: a randomized phase III study (ALLY‐3 + ). Hepatology. 2016;63:1430‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poordad F, Schiff ER, Vierling JM, et al. Daclatasvir with sofosbuvir and ribavirin for HCV infection with advanced cirrhosis or post‐liver transplant recurrence. Hepatology. 2016;63:1493‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Welzel TM, Petersen J, Herzer K, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virologic response rates in patients with HCV infection and advanced liver disease in a real‐world cohort. Gut. 2016;65:1861‐1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV‐1. NEJM. 2015;373:714‐725. [DOI] [PubMed] [Google Scholar]
- 15. Hezode C, de Ledinghen V, Fontaine H, et al. Daclatasvir plus sofosbuvir with or without ribavirin in genotype‐3 patients from a large French multicenter compassionate use program. Hepatology. 2015;62(suppl 1):314A.25914200 [Google Scholar]
- 16. Toyoda H, Kumada T, Tada T. Changes in patient backgrounds may increase the incidence of HCC after SVR in the era of IFN‐free therapy for HCV. Hepatology. 2016;64:1818‐1819. [DOI] [PubMed] [Google Scholar]
- 17. El‐Serag HB, Kanwal F, Richardson P, et al. Risk of hepatocellular carcinoma after sustained virological response in veterans with HCV infection. Hepatology. 2016;64:130‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morgan RL, Baack B, Smith BD, et al. Eradication of HCV infection and the development of hepatocellular carcinoma: a meta‐analysis of observational studies. Ann Intern Med 2013;1(5‐Pt‐1):329‐337. [DOI] [PubMed] [Google Scholar]
- 19. Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic HCV infection: a cure and so much more. Clin Infect Dis. 2011;52:889‐900. [DOI] [PubMed] [Google Scholar]
- 20. Foster GR, Irving WL, Cheung MC, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224‐1231. [DOI] [PubMed] [Google Scholar]
- 21. Cheung MC, Walker AJ, Hudson BE, et al. Outcomes after successful direct‐acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741‐747. [DOI] [PubMed] [Google Scholar]
- 22. Nault JC, Colombo M. Hepatocellular carcinoma and direct acting antiviral treatments: controversy after the revolution. J Hepatol. 2016;65:663‐665. [DOI] [PubMed] [Google Scholar]
- 23. Howe AY, Long J, Nickle D, et al. Long‐term follow‐up of patients receiving boceprevir for treatment of chronic hepatitis C. Antiviral Res. 2015;113:71‐78. [DOI] [PubMed] [Google Scholar]
- 24. Uyanikoglu A, Kaymakoglu S, Danalioglu A, et al. Durability of sustained virologic response in chronic hepatitis C. Gut Liv. 2013;7:458‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rutter K, Hofer H, Beinhardt S, et al. Durability of SVR in chronic hepatitis C patients treated with peginterferon‐alpha2a/ribavirin in combination with a direct‐acting anti‐viral. Aliment Pharmacol Ther. 2013;38:118‐123. [DOI] [PubMed] [Google Scholar]
- 26. George SL, Bacon BR, Brunt EM, et al. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5‐year follow‐up of 150 patients. Hepatology. 2009;49:729‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between SVR and all‐cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584‐2593. [DOI] [PubMed] [Google Scholar]
- 28. Bruno S, Di Marco V, Iavarone M, et al. Survival of patients with HCV cirrhosis and sustained virologic response is similar to the general population. J Hepatol. 2016;64:1217‐1223. [DOI] [PubMed] [Google Scholar]
- 29. Reig M, Marino Z, Perello C, et al. Unexpected high rate of early tumor recurrence in patients with HCV‐related HCC undergoing interferon‐free therapy. J Hepatol. 2016;65:719‐726. [DOI] [PubMed] [Google Scholar]
- 30. Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV‐related cirrhosis treated with direct‐acting antivirals. J Hepatol. 2016;65:727‐733. [DOI] [PubMed] [Google Scholar]
- 31. Pol S. Lack of evidence of an effect of direct acting antivirals on the recurrence of hepatocellular carcinoma: the ANRS collaborative study group on hepatocellular carcinoma. J Hepatol. 2016;65:734‐740. [DOI] [PubMed] [Google Scholar]
- 32. Minami T, Tateishi R, Nakagomi R, et al. The impact of direct‐acting antivirals on early tumor recurrence after radiofrequency ablation in hepatitis C‐related hepatocellular carcinoma. J Hepatol. 2016;65:1272‐1273. [DOI] [PubMed] [Google Scholar]
- 33. Kobayashi M, Suzuki F, Fujiyama S, et al. Sustained virologic response by direct antiviral agents reduces the incidence of hepatocellular carcinoma in patients with HCV infection. J Med Virol. 2016;89:476‐483. [DOI] [PubMed] [Google Scholar]
- 34. Raimondi S, Bruno S, Mondelli MU, et al. HCV genotype‐1b as a risk factor for hepatocellular carcinoma development: a meta‐analysis. J Hepatol. 2009;50:1142‐1154. [DOI] [PubMed] [Google Scholar]
- 35. Lee MH, Yang HI, Lu SN, et al. HCV seromarkers and subsequent risk of hepatocellular carcinoma: long‐term predictors from a community‐based cohort study. J Clin Oncol. 2010;28:4587‐4593. [DOI] [PubMed] [Google Scholar]
- 36. Ripoll C, Groszmann RJ, Garcia‐Tsao G, et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50:923‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sulkowski MS, Gardiner DF, Rodriguez‐Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. NEJM. 2014;370:211‐221. [DOI] [PubMed] [Google Scholar]
- 38. Lok AS, Gardiner DF, Lawitz E, et al. Preliminary study of two antiviral agents for hepatitis C genotype‐1. N Engl J Med. 2012;366:216‐224. [DOI] [PubMed] [Google Scholar]
- 39. Suzuki Y, Ikeda K, Suzuki F, et al. Dual oral therapy with daclatasvir and asunaprevir for patients with HCV genotype‐1b infection and limited treatment options. J Hepatol. 2013;58:655‐662. [DOI] [PubMed] [Google Scholar]
- 40. Kumada H, Suzuki Y, Ikeda K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype‐1b infection. Hepatology. 2014;59:2083‐2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Everson GT, Sims KD, Rodriguez‐Torres M, et al. Efficacy of an interferon‐ and ribavirin‐free regimen of daclatasvir, asunaprevir, and BMS‐791325 in treatment‐naive patients with HCV genotype‐1 infection. Gastroenterology. 2014;146:420‐429. [DOI] [PubMed] [Google Scholar]
- 42. Everson GT, Sims KD, Thuluvath PJ, et al. Daclatasvir+asunaprevir+beclabuvir+/‐ribavirin for chronic HCV genotype‐1‐infected treatment‐naive patients. Liver Int. 2016;36:189‐197. [DOI] [PubMed] [Google Scholar]
- 43. Poordad F, Sievert W, Mollison L, et al. Fixed‐dose combination therapy with daclatasvir, asunaprevir, and beclabuvir for noncirrhotic patients with HCV genotype‐1 infection. JAMA. 2015;313:1728‐1735. [DOI] [PubMed] [Google Scholar]
- 44. Muir AJ, Poordad F, Lalezari J, et al. Daclatasvir in combination with asunaprevir and beclabuvir for HCV genotype‐1 infection with compensated cirrhosis. JAMA. 2015;313:1736‐1744. [DOI] [PubMed] [Google Scholar]
- 45. Jensen D, Sherman KE, Hezode C, et al. Daclatasvir and asunaprevir plus peginterferon alfa and ribavirin in HCV genotype‐1 or ‐4 non‐responders. J Hepatol. 2015;63:30‐37. [DOI] [PubMed] [Google Scholar]
- 46. Hezode C, Hirschfield GM, Ghesquiere W, et al. Daclatasvir plus peginterferon alfa and ribavirin for treatment‐naive chronic hepatitis C genotype‐1 or ‐4 infection: a randomised study. Gut. 2015;64:948‐956. [DOI] [PubMed] [Google Scholar]
- 47. Pol S, Ghalib RH, Rustgi VK, et al. Daclatasvir for previously untreated chronic hepatitis C genotype‐1 infection: a randomised, parallel‐group, double‐blind, placebo‐controlled, dose‐finding, phase‐2a trial. Lancet Infect Dis. 2012;12:671‐677. [DOI] [PubMed] [Google Scholar]
- 48. Dore GJ, Lawitz E, Hezode C, et al. Daclatasvir plus peginterferon and ribavirin is noninferior to peginterferon and ribavirin alone, and reduces the duration of treatment for HCV genotype‐2 or ‐3 infection. Gastroenterology. 2015;148:355‐366. e1. [DOI] [PubMed] [Google Scholar]
- 49. Rodriguez‐Torres M, Lawitz E, Yangco B, et al. Daclatasvir and peginterferon/ribavirin for black/african‐american and latino patients with HCV infection. Ann Hepatol. 2016;15:834‐845. [DOI] [PubMed] [Google Scholar]
- 50. Hezode C, Alric L, Brown A, et al. Randomized controlled trial of the NS5A inhibitor daclatasvir plus pegylated interferon and ribavirin for HCV genotype‐4. Antivir Ther. 2015;21:195‐205. [DOI] [PubMed] [Google Scholar]
- 51. Jacobson I, Zeuzem S, Flisiak R, et al. Daclatasvir vs telaprevir plus peginterferon alfa/ribavirin for HCV genotype‐1. World J Gastroenterol. 2016;22:3418‐3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McPhee F, Hernandez D, Zhou N, et al. Pooled analysis of HCV genotype 1 resistance‐associated substitutions in NS5A, NS3 and NS5B pre‐ and post‐treatment with 12 weeks of daclatasvir, asunaprevir and beclabuvir. Antivir Ther. 2017. [Epub ahead of print] https://doi.org/10.3851/imp3177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1111/liv.13596/suppinfo


