Abstract
Background
Cardiac contractility modulation (CCM) is an electrical‐device therapy for patients with heart failure with reduced ejection fraction (HFrEF). Patients with left ventricular ejection fraction (LVEF) ≤35% also have indication for an implantable cardioverter‐defibrillator (ICD), and in some cases subcutaneous ICD (S‐ICD) is selected.
Hypothesis
CCM and S‐ICD can be combined to work efficaciously and safely.
Methods
We report on 20 patients with HFrEF and LVEF ≤35% who received CCM and S‐ICD. To exclude device interference, patients received intraoperative crosstalk testing, S‐ICD testing, and bicycle exercise testing while CCM was activated. Clinical and QOL measures before CCM activation and at last follow‐up were analyzed. S‐ICD performance was evaluated while both CCM and S‐ICD were active.
Results
Mean follow‐up was 34.3 months. NYHA class improved from 2.9 ± 0.4 to 2.1 ± 0.7 (P < 0.0001), Minnesota Living With Heart Failure Questionnaire score improved from 50.2 ± 23.7 to 29.6 ± 22.8 points (P < 0.0001), and LVEF improved from 24.4% ± 8.1% to 30.9% ± 9.6% (P = 0.002). Mean follow‐up time with both devices active was 22 months. Three patients experienced a total of 6 episodes of sustained ventricular tachycardia, all successfully treated with first ICD shock. One case received an inappropriate shock unrelated to the concomitant CCM. One patient received an LVAD, so CCM and S‐ICD were discontinued.
Conclusions
CCM and S‐ICD can be successfully combined in patients with HFrEF. S‐ICD and CCM remain efficacious when used together, with no interference affecting their function.
Keywords: Cardiac Contractility Modulation, Heart Failure, Subcutaneous ICD
1. INTRODUCTION
Cardiac contractility modulation (CCM) is an electric device therapy that applies a nonactivating electrical impulse to the cardiac muscle during the absolute refractory period.1 Indications for CCM include patients with reduced left ventricular ejection fraction (LVEF) and normal or slightly prolonged QRS duration, thus filling a therapeutic gap among the two‐thirds of patients with heart failure (HF) who do not meet criteria for cardiac resynchronization therapy.2, 3
Two prospective, randomized, multicenter studies have demonstrated significant improvements of New York Heart Association (NYHA) functional class, quality of life indexed by Minnesota Living with Heart Failure Questionnaire (MLWHFQ), and peak oxygen uptake during cardiopulmonary exercise testing in patients with symptomatic HF with reduced LVEF (HFrEF).4, 5, 6 Although current data show improvements in symptoms and functional cardiopulmonary capacity, data on cardiovascular outcome are limited. Randomized controlled trials were not powered to detect statistically significant changes of cardiovascular mortality.4, 5 A recent meta‐analysis of published data found that CCM did not lower the risk of severe cardiovascular adverse events7; nevertheless, retrospective observations suggest that mortality rates in patients treated with CCM, especially in those with normal QRS and with moderate disease stage, were lower than estimated by the Meta‐analysis Global Group in Chronic Heart Failure (MAGGIC) model, by the Seattle Heart Failure Model (SHFM) model risk scores, or by a control group.8, 9, 10 Recently, in the European Society of Cardiology (ESC) Guidelines on Acute and Chronic Heart Failure (2016), it was stated that CCM may be considered in selected patients with HF.3
Because many patients receiving CCM have an LVEF ≤35%, an implantable cardioverter‐defibrillator (ICD) is also indicated. In most of these cases, a separate implantation procedure is performed using intracardiac defibrillation leads and a separate implantable pulse generator, as no device currently combines CCM and ICD capabilities into a single device. As a result, CCM has been extensively studied in combination with intracardiac ICDs, revealing little interference between devices. However, the need for 2 devices, both with intracardiac leads, poses the risk of additional adverse events because the cumulative risk of electrode complications, such as systemic infections or thrombosis of central venous lines, increases with the number of implanted intracardiac leads.11
The subcutaneous implantable cardioverter‐defibrillator (S‐ICD) was developed as an alternative to the transvenous ICD without the need to implant transvenous leads.12, 13 Its safety and effectiveness have been established,14, 15 and the therapy has been included in current guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death.15, 16
In this present study, we analyzed the long‐term clinical outcome of patients in whom both CCM and S‐ICD were implanted.
2. METHODS
2.1. Patients
Twenty patients with symptomatic HF and reduced LVEF (≤35%) received CCM implantation in our tertiary university HF center between March 2009 and May 2016. Patients were required to be on stable guideline‐directed medical therapy for HF, including a β‐blocker, angiotensin‐converting enzyme inhibitor and/or angiotensin receptor blocker, and mineralocorticoid receptor antagonist. Informed consent was obtained from all subjects. This study was approved by the local ethics committee.
Each of these 20 patients also received an S‐ICD. In 14 cases, the S‐ICD was implanted as the first ICD device, with CCM implanted during follow‐up. In 6 cases, a formerly implanted transvenous ICD was replaced by the S‐ICD system. After implantation of CCM, patients were followed per standard clinical practice at 3‐ to 4‐month intervals. At each follow‐up visit, clinical assessments were obtained, including NYHA class, quality of life (MLWHFQ), 12‐vector electrocardiogram recordings, and NT‐proBNP levels. In addition, transthoracic echocardiograms were performed every 6 months. Frequency of procedural complications or revisions, programming changes, and incidence of appropriate or inadequate device detections as well as clinical data and therapies were recorded.
2.2. CCM device description and implantation procedure
In 14 patients, the Optimizer IVs system (Impulse Dynamics Inc., Orangeburg, NJ) was implanted. Six patients received the prior model, the Optimizer III. The implantation procedure has been described in detail.2 Briefly, the Optimizer device is implanted into the pectoral region in a minimally invasive procedure utilizing 3 bipolar pacemaker leads that are introduced into the right side of the heart via the subclavian vein (Tendril STS; St. Jude Medical/Abbott, St. Paul, MN). One of these leads is placed into the right atrium to detect the electrical activity as part of the algorithm for timing CCM delivery. CCM signal delivery occurs through the remaining 2 leads, positioned at the ventricular septum, after electrical activity is sensed in those leads. Active CCM treatment is typically programmed for 5 or 7 segments of 1 hour spread equally throughout the day.
2.3. S‐ICD device description, implantation procedure, and device testing
The S‐ICD system (Emblem; Boston Scientific, Marlborough, MA) and its implantation procedure have been described in detail.2, 17, 18 The lead is positioned parallel to the sternum (normally 1 to 2 cm to the left) and the pulse generator is positioned in the left axillary region at the level of the sixth rib. The 2 sensing electrodes of the subcutaneous lead and the IPG itself represent 3 vector projections of electrical conduction through the heart. The S‐ICD automatically selects an optimal vector for adequate rhythm sensing and to avoid T‐wave oversensing or double QRS counting. A conditional shock zone incorporating a feature‐extraction technique can be programmed between rates of 170 and 250 bpm. S‐ICD therapy within conditional shock zone and shock zone consists of an 80‐J shock, with the potential for temporary transthoracic backup pacing for 30 seconds.
At the end of each S‐ICD implantation procedure, device testing is performed. Ventricular fibrillation (VF) is induced by a high‐voltage 50‐Hz signal. During S‐ICD testing, VF is terminated by a 65‐J shock to ensure a margin of safety.
Every patient implanted with an S‐ICD receives routine bicycle ergometer testing 1 to 7 days postoperatively. Subsequently, different provocation maneuvers are performed, which have been described previously.17 The 3 sensing vectors of the S‐ICD are monitored in real time during these tests to exclude oversensing or double counting that occurs during exercise or sinus tachycardia. In case of an unclear signal, oversensing, or double counting during exercise or provocation maneuvers, the automatically selected sensing vector can be changed manually to select the clearest signal. Characteristics of a clear signal are a stable baseline, high signal amplitude, and a high QRS/T ratio.
2.4. Combining CCM and S‐ICD
Three tests were performed upon insertion of the second device (either CCM or S‐ICD) to exclude device interactions (Table 1).
Table 1.
Safety tests for combination of Optimizer CCM and S‐ICD
| Test 1: Intraoperative Crosstalk Test |
| Activate both devices and monitor all 3 sensing configurations of S‐ICD. |
| Temporarily program CCM to various therapy timings (ie, test longer delay of CCM signal delivery within the QRS complex) to exclude potential double counting or oversensing by the S‐ICD even at extremes of CCM programming intervals. |
| Program S‐ICD vector with the clearest result as selected sensing vector. |
| In case CCM is implanted after S‐ICD, repositioning of CCM leads is possible during the implantation procedure to a location where the CCM signal shows fewer artifacts on the S‐ICD sensing vectors. |
| Test 2: Intraoperative S‐ICD Testing |
| Turn CCM signal delivery to “on” and induce VF. |
| CCM device contains a built‐in algorithm that inhibits delivery of a CCM signal when irregular electrical activity is detected (such as premature atrial or ventricular complexes or sensing defects). This is designed to eliminate the possibility of CCM signal delivery during a T‐wave. |
| When CCM detects ventricular arrhythmias, CCM signal delivery ceases. |
| S‐ICD can properly recognize the arrhythmia and VF is terminated through ICD shock delivery. |
| Test 3: Postoperative Bicycle Ergometer Testing and Provocation Maneuvers |
| Turn CCM signal delivery to “on” and monitor all 3 sensing configurations of S‐ICD. |
| Perform bicycle ergometer testing. |
| Perform provocation maneuvers (eg, aggregate manipulation, physical maneuvers, standing and supine posture). |
| Select sensing vector with the clearest signal, avoiding double counting or oversensing as well as noise that was produced by the CCM device. |
Abbreviations: CCM, cardiac contractility modulation; ICD, implantable cardioverter‐defibrillator; S‐ICD, subcutaneous implantable cardioverter‐defibrillator; VF, ventricular fibrillation.
2.5. Statistical analysis
Changes in each tested parameter were calculated for each patient comparing baseline to last follow‐up visit. Data are reported as mean ± SD, and the t test was used for the univariate analysis.
3. RESULTS
3.1. Patient population
Baseline characteristics of the 20 CCM patients are given in Table 2. Mean age at CCM implant was 54.3 ± 11.5 years, and mean baseline LVEF was 24.4% ± 8.1%. Thirty‐five percent of patients had ischemic cardiomyopathy.
Table 2.
Characteristics of the study population before CCM implantation
| Characteristic | Value |
|---|---|
| Age, y | 54.3 ± 11.5 |
| Male sex | 18 (90) |
| Weight, kg | 103.5 ± 23.7 |
| BMI, kg/m2 | 32.5 ± 7.2 |
| SBP, mm Hg | 118.2 ± 15.7 |
| LVEF, % | 24.4 ± 8.1 |
| DM | 4 (20) |
| CKD | 8 (40) |
| Current smoker | 8 (40) |
| Chronic lung disease | 7 (35) |
| QRS width, ms | 108.9 ± 19.4 |
| HF etiology | |
| Ischemic | 7 (35) |
| DCM | 13 (65) |
| NYHA class | |
| II | 3 (15) |
| III | 16 (80) |
| IV | 1 (5.0) |
| Medications | |
| ACEI/ARB | 20 (100) |
| β‐Blocker | 19 (95) |
| MRA | 17 (85) |
| Ivabradine | 4 (20) |
| Diuretic | 20 (100) |
| Amiodarone | 4 (20) |
| Cr, mg/dL | 1.37 ± 0.83 |
| NT‐proBNP, pg/mL | 2882.7 ± 5456.6 |
| ICD indication | |
| Primary preventive | 16 (80) |
| Secondary preventive | 4 (20) |
| Patients with former transvenous ICDs | 6 (30) |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CCM, cardiac contractility modulation; CKD, chronic kidney disease; Cr, creatinine; DCM, dilated cardiomyopathy; DM, diabetes mellitus; HF, heart failure; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro brain natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SD, standard deviation.
Data are presented as n (%) or mean ± SD.
In the course of their treatment all 20 patients received an S‐ICD (Figure, A). In 14 patients, the S‐ICD was the first implanted ICD device. Thirteen of these 14 patients had a primary preventive ICD indication because LVEF was ≤35% for ≥3 months of optimal medical treatment. One of these 14 patients had a secondary preventive ICD indication because he had a history of life‐threatening sustained ventricular tachyarrhythmias in addition to LVEF ≤35%. These 14 patients received their S‐ICDs before CCM implantation.
Figure 1.
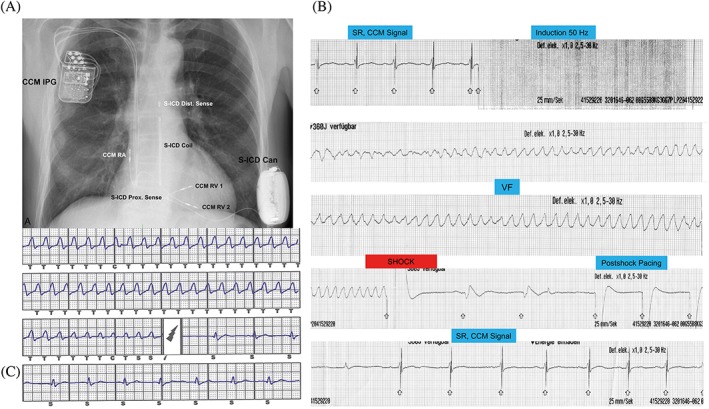
(A) Chest x‐ray of patient with CCM and S‐ICD. (B) Intraoperative S‐ICD testing with activated CCM: rhythm strip ECG. (C) S‐ICD report of the same test from the same patient. Abbreviations: CCM, cardiac contractility modulation; ECG, electrocardiogram; IPG, implantable pulse generator; RA, right atrium; RV, right ventricle; S‐ICD, subcutaneous implantable cardioverter‐defibrillator; VF, ventricular fibrillation
In 6 patients, a formerly implanted transvenous ICD was replaced by an S‐ICD due to ICD lead defects. In 3 of these 6 patients, the first transvenous device was implanted for primary prevention and in the other 3 patients for secondary prevention.
Taken together, 80% of the study subjects underwent implantation of their first ICD for primary prevention and 20% for secondary prevention (Table 2).
3.2. Operation results
S‐ICD and CCM implantations were successfully performed in all patients.
At the time of implantation of the second device (either CCM or S‐ICD), intraoperative crosstalk testing was performed. No patient had double counting due to a concomitant CCM. In 9 patients, ≥1 of the 3 S‐ICD vectors showed “noise‐free” ventricular sensing during active CCM therapy. In 11 patients, “noise” was recognized in all 3 S‐ICD sensing vectors when CCM was activated. Nevertheless, during the S‐ICD testing with CCM activated, the CCM signal delivery stopped immediately during ventricular tachycardia (VT)/VF, and the S‐ICD properly recognized the arrhythmia with no undersensing or undue delay (Figure, B,C). In all cases VF was terminated by the first ICD shock and within an adequate time window (time to shocks, 12 to 20 seconds).
During the ergometric testing with activated CCM therapy, none of the 20 patients showed double counting or T‐wave oversensing. Therefore, both devices could be activated appropriately in all 20 patients.
Other than postimplantation and postergometry configuration, no reprogramming of the S‐ICD device was required. Later follow‐up processes were routinely done as with any other ICD and/or CCM device cases.
3.3. Complications
One patient had postoperative wound‐healing delay of the S‐ICD pocket that required a prolonged hospital stay. One patient suffered from thrombosis of the subclavian vein after implantation of CCM, requiring oral anticoagulation for 3 months.
With regard to long‐term complications, 3 patients required lead‐revision procedures of CCM ventricular leads at a mean follow‐up of 30 months. One patient with a body mass index of 40 kg/m2 and a history of recurrent skin infections had a skin abscess on the thoracic wall that affected the tip of the subcutaneous lead, requiring temporary removal of the subcutaneous ICD at 22 months. The device was successfully re‐implanted 3 months later.
3.4. Efficacy of CCM therapy
The mean duration of follow‐up from CCM implantation was 34.3 ± 30.6 months (range, 7–94 months; median, 19 months).
There were significant improvements of NYHA class as well as MLWHFQ (Table 3). LVEF also improved significantly, by 6.5%, and there was a trend toward a decrease in left ventricular end‐diastolic diameter. Additionally, there were significant decreases of left ventricular end‐systolic diameter, and left ventricular end‐diastolic and end‐systolic volumes. There were no significant changes seen in N‐terminal pro brain natriuretic peptide or creatinine levels. Furthermore, QRS duration remained unchanged.
Table 3.
CCM efficacy results
| Before CCM Implantation | At Last Follow‐up | P Value | |
|---|---|---|---|
| NYHA class | 2.9 ± 0.4 | 2.1 ± 0.7 | <0.0001 |
| MLWHFQ score | 50.2 ± 23.7 | 29.6 ± 22.8 | <0.0001 |
| LVEF, % | 24.4 ± 8.1 | 30.9 ± 9.6 | 0.002 |
| LVEDD, mm | 66.6 ± 7.1 | 64.4 ± 6.5 | 0.087 |
| LVESD, mm | 59.2 ± 7.2 | 54.8 ± 7.3 | 0.012 |
| LVEDV, mL | 230.0 ± 49.2 | 207.8 ± 65.0 | 0.029 |
| LVESV, mL | 175.8 ± 46.1 | 147.2 ± 60.1 | 0.011 |
| QRS duration, ms | 108.9 ± 19.4 | 110.0 ± 22.0 | 0.663 |
| NT‐proBNP, pg/mL | 2882.7 ± 5456.6 | 2460.7 ± 7575.6 | 0.561 |
| Cr, mg/dL | 1.37 ± 0.83 | 1.32 ± 0.51 | 0.278 |
| Paroxysmal AF events, % | 5 | 15 | 0.163 |
Abbreviations: AF, atrial fibrillation; CCM, cardiac contractility modulation; Cr, creatinine; LVEDD, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; LVESV, left ventricular end‐systolic volume; MLWHFQ, Minnesota Living With Heart Failure Questionnaire; NT‐proBNP, N‐terminal pro brain natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation.
Data are presented as mean ± SD unless otherwise noted.
3.5. Arrhythmias
Mean follow‐up after dual device implantation was 22.0 ± 15.3 months (range, 5–61 months; median, 17 months). During this time, 3 patients experienced a total of 6 episodes of sustained VT at a mean follow‐up of 7.2 ± 2.5 months with both devices active. Each episode was adequately treated with a single ICD shock. Patients' electrophysiological characteristics are described in Table 4.
Table 4.
Details of appropriate S‐ICD therapies
| Patient | VT Date | VT Cycle Length, ms | Shock | Shock Successful? | Time to Shock, s |
|---|---|---|---|---|---|
| Male, 70 years, ICM | January 9, 2015 | 320 | 1 × 80 J | Yes | 19 |
| Male, 75 years, ICM | December 13, 2015 | 260 | 1 × 80 J | Yes | 24 |
| Male, 48 years, DCM | July 8, 2016 | 300 | 1 × 80 J | Yes | 30 |
| August 21, 2016 | 300 | 1 × 80 J | Yes | 53 | |
| August 22, 2016 | 260 | 1 × 80 J | Yes | 53 | |
| August 22, 2016 | 300 | 1 × 80 J | Yes | 19 |
Abbreviations: DCM, dilated cardiomyopathy; ICM, ischemic cardiomyopathy; S‐ICD, subcutaneous implantable cardioverter‐defibrillator; VT, ventricular tachycardia.
One patient (male, age 75 years, ischemic cardiomyopathy) received an inappropriate shock (see Supporting Information, Figure S1, in the online version of this article). The patient had nonsustained VT for 11 seconds that was then followed by a phase of T‐wave oversensing for 15 seconds. The T‐wave oversensing led to the inappropriate shock. During the inappropriate shock event, CCM therapy was in an “off” phase, and therefore the inappropriate shock is unrelated to CCM. The patient received antiarrhythmic therapy with amiodarone and had no further ventricular tachyarrhythmias.
During the 22 months of follow‐up with both devices active, none of the 20 patients had syncope.
3.6. Survival
For this patient population at the time of CCM implantation, the MAGGIC score estimated mean 1‐year and 3‐year death rates of 13.2% and 30.2%, respectively. The SHFM score predicted mean 1‐year, 2‐year, and 5‐year death rates of 5.1%, 10.1%, and 24.6%, respectively, for this same group of subjects. At the time of last follow‐up, 19 of the 20 patients were alive. No patient died while being treated with CCM. One 54‐year‐old female patient had received a left ventricular assist device after 14 months of follow‐up due to progressive HF; thus, CCM and S‐ICD were switched off. That patient died subsequently of postoperative complications.
4. DISCUSSION
The major new finding from this study is that combination of CCM with S‐ICD in patients with an indication for both is feasible and that it was safe and successful in this study cohort during long‐term follow‐up. The benefit of CCM therapy, as demonstrated by improvements of NYHA class, quality‐of‐life scores, and echocardiographic parameters, seems consistent with prior publications in larger populations.5, 8, 19
Recent retrospective single‐center observational studies have suggested prolonged survival of HF patients treated with CCM therapy.8, 9, 10 In most of these studies, patients receiving CCM therapy had LVEF ≤35%; therefore, they also had ICD devices with intracardiac leads in place. To date there is no device combining CCM with ICD functions; thus, multiple intracardiac leads are required (for the CCM and for the ICD), raising the risk of lead‐ and implantation‐related adverse events. Although CCM has been studied with patients receiving intravenous ICD in multiple studies,4, 5, 6, 8 and its safety, functionality, and efficacy were demonstrated, it is clearly also desired to have a future combined device to address this population with an integrated solution. The recent introduction of S‐ICD eliminates the need for intracardiac leads to deliver defibrillation shocks, and thus reduces the risk of lead‐related events.
Prior studies have reported successful combination of the S‐ICD with transvenous pacemakers14 in patients with need for pacing after implantation of an S‐ICD. Recently, Tjong et al. reported that combined leadless pacing and S‐ICD therapy appeared feasible in animal experiments and in 1 human subject.20 In a recent case series, we demonstrated that the S‐ICD can be combined with a variety of cardiac implantable electronic devices that require intracardiac or epicardial leads, including CCM, and that the devices can be programmed and tested to achieve efficacy and avoid interference when used in the same patient.17 This testing includes postprocedural ergometry and provocative maneuvers with the concomitant device active, while monitoring sensing vectors in real time. This enables observation of interference or malfunction that might appear only during exercise or tachycardia.
In this study, we present the first long‐term results of combined CCM and S‐ICD devices. Using our established algorithm, the chances for detrimental crosstalk between CCM and S‐ICD can be minimized, allowing both devices to function properly and safely. All device implantations were successfully performed, even in those 6 patients who had suffered complications with prior transvenous ICDs. Postprocedural and long‐term complications were successfully handled. This group of patients showed significant improvements in HF symptoms and LVEF. S‐ICD shock delivery was effective during device testing. In 3 patients, 6 ventricular arrhythmias that occurred during follow‐up were terminated properly. No patient died of arrhythmia or of unknown reasons during a mean follow‐up of 22 months of combined therapy.
A major requirement for successfully combining CCM and S‐ICD is the absence of significant bradycardia requiring cardiac pacing (neither of the 2 devices has a pacing function). Furthermore, the S‐ICD has no antitachycardia pacing functions. Patients with HFrEF requiring ventricular pacing or patients with a wide QRS complex should receive cardiac resynchronization therapy.3
The new‐generation Optimizer, the Optimizer Smart, includes an algorithm that does not require the implantation of an atrial lead (keeping the 2 ventricular leads only), thereby further simplifying the implantation procedure. The new mode also allows the delivery of CCM therapy in patients with permanent atrial fibrillation, which was considered a contraindication for the prior‐generation Optimizer device.21 In a recent study, it was demonstrated that efficacy and safety of CCM were similar when the signal was delivered through either 1 or 2 ventricular leads.22 These results support the potential future use of a single ventricular lead for delivery of CCM, further reducing device implantation–associated risk.
4.1. Study limitations
This study presents experience with the combination of CCM and S‐ICD in a small cohort of patients from a single site. It presents limited data on clinical outcome in a nonrandomized, noncontrolled manner. Further multicenter studies are needed to evaluate the long‐term impact of combining these 2 technologies in support of patients with HFrEF and LVEF ≤35%, who comprise a large segment of the chronic HF population.
5. CONCLUSION
S‐ICD and CCM can be successfully combined to work efficaciously and safely in HFrEF patients who do not require cardiac pacing. A careful intraprocedural crosstalk test and postoperative exercise testing with both devices activated is recommended to identify and abate any functional interactions between the 2 devices.
With the long‐term follow‐up, it can be concluded that S‐ICD therapy and CCM therapy can be safely used together, thereby decreasing risk by reducing the number of intracardiac leads implanted. A future device that combines CCM and ICD functions is desirable.
Conflicts of interest
Susanne Röger has received speaker fees from Impulse Dynamics. Jürgen Kuschyk has received speaker fees from Impulse Dynamics and serves on the international advisory board of Boston Scientific. Martin Borggrefe has received speaker fees from Impulse Dynamics and serves on their international advisory board. The authors declare no other potential conflicts of interest.
Supporting information
Figure S1 S‐ICD report of patient with one inappropriate shock: non‐sustained VT for 11 s, followed by a phase of T‐Wave oversensing for 15 s. T‐Wave oversensing leads to the inappropriate shock.
Röger S, Rudic B, Akin I, et al. Long‐term results of combined cardiac contractility modulation and subcutaneous defibrillator therapy in patients with heart failure and reduced ejection fraction. Clin Cardiol. 2018;41:518–524. 10.1002/clc.22919
REFERENCES
- 1. Abi‐Samra F, Gutterman D. Cardiac contractility modulation: a novel approach for the treatment of heart failure. Heart Fail Rev. 2016;21:645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuck KH, Bordachar P, Borggrefe M, et al. New devices in heart failure: an European Heart Rhythm Association report: developed by the European Heart Rhythm Association; endorsed by the Heart Failure Association. Europace. 2014;16:109–128. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, et al; ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC [published correction appears in Eur Heart J 2018;39:860]. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 4. Borggrefe MM, Lawo T, Butter C, et al. Randomized, double blind study of non‐excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur Heart J. 2008;29:1019–1028. [DOI] [PubMed] [Google Scholar]
- 5. Kadish A, Nademanee K, Volosin K, et al. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure [published correction appears in Am Heart J, 2011;161:1220]. Am Heart J. 2011;161:329–337.e1–e2. [DOI] [PubMed] [Google Scholar]
- 6. Abraham WT, Nademanee K, Volosin K, et al; FIX‐HF‐5 Investigators and Coordinators . Subgroup analysis of a randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. J Card Fail. 2011;17:710–717. [DOI] [PubMed] [Google Scholar]
- 7. Liu X, Yang HJ, Ping HQ, et al. The safety and efficacy of cardiac contractility modulation in heart failure: a meta‐analysis of clinical trials. Herz. 2017;42:766–775. [DOI] [PubMed] [Google Scholar]
- 8. Kuschyk J, Röger S, Schneider R, et al. Efficacy and survival in patients with cardiac contractility modulation: long‐term single‐center experience in 81 patients. Int J Cardiol. 2015;183:76–81. [DOI] [PubMed] [Google Scholar]
- 9. Kloppe A, Lawo T, Mijic D, et al. Long‐term survival with cardiac contractility modulation in patients with NYHA II or III symptoms and normal QRS duration. Int J Cardiol. 2016;209:291–295. [DOI] [PubMed] [Google Scholar]
- 10. Liu M, Fang F, Luo XX, et al. Improvement of long‐term survival by cardiac contractility modulation in heart failure patients: a case‐control study. Int J Cardiol. 2016;206:122–126. [DOI] [PubMed] [Google Scholar]
- 11. Kirkfeldt RE, Johansen JB, Nohr EA, et al. Risk factors for lead complications in cardiac pacing: a population‐based cohort study of 28 860 Danish patients. Heart Rhythm. 2011;8:1622–1628. [DOI] [PubMed] [Google Scholar]
- 12. Weiss R, Knight BP, Gold MR, et al. Safety and efficacy of a totally subcutaneous implantable cardioverter‐defibrillator. Circulation. 2013;128:944–953. [DOI] [PubMed] [Google Scholar]
- 13. Lambiase PD, Barr C, Theuns DA, et al; EFFORTLESS Investigators . Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S‐ICD Registry. Eur Heart J. 2014;35:1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burke MC, Gold MR, Knight BP, et al. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2‐year results from a pooled analysis of the IDE Study and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65:1605–1615. [DOI] [PubMed] [Google Scholar]
- 15. Priori SG, Blomström‐Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 16. Al‐Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017. doi: 10.1161/CIR.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 17. Kuschyk J, Stach K, Tülümen E, et al. Subcutaneous implantable cardioverter‐defibrillator: first single‐center experience with other cardiac implantable electronic devices. Heart Rhythm. 2015;12:2230–2238. [DOI] [PubMed] [Google Scholar]
- 18. Knops RE, Olde Nordkamp LR, de Groot JR, et al. Two‐incision technique for implantation of the subcutaneous implantable cardioverter‐defibrillator. Heart Rhythm. 2013;10:1240–1243. [DOI] [PubMed] [Google Scholar]
- 19. Yu CM, Chan JY, Zhang Q, et al. Impact of cardiac contractility modulation on left ventricular global and regional function and remodeling. JACC Cardiovasc Imaging. 2009;2:1341–1349. [DOI] [PubMed] [Google Scholar]
- 20. Tjong FV, Brouwer TF, Smeding L, et al. Combined leadless pacemaker and subcutaneous implantable defibrillator therapy: feasibility, safety, and performance. Europace. 2016;18:1740–1747. [DOI] [PubMed] [Google Scholar]
- 21. Röger S, Schneider R, Rudic B, et al. Cardiac contractility modulation: first experience in heart failure patients with reduced ejection fraction and permanent atrial fibrillation. Europace. 2014;16:1205–1209. [DOI] [PubMed] [Google Scholar]
- 22. Röger S, Said S, Kloppe A, et al. Cardiac contractility modulation in heart failure patients: randomized comparison of signal delivery through one vs. two ventricular leads. J Cardiol. 2017;69:326–332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 S‐ICD report of patient with one inappropriate shock: non‐sustained VT for 11 s, followed by a phase of T‐Wave oversensing for 15 s. T‐Wave oversensing leads to the inappropriate shock.


