Abstract
Sodium glucose co‐transporter 2 (SGLT2) inhibitors have been associated with increased serum ketone body levels in patients with type 2 diabetes mellitus (T2DM). In the present analysis we evaluated serum ketone body levels and variability in 1278 Japanese patients with T2DM treated with canagliflozin 100 or 200 mg. Similar mean increases in ketone body concentrations of ~2‐fold were seen with both canagliflozin doses. The median (interquartile range) percent change from baseline was 62% (0;180) for acetoacetate and 78% (2;236) for β‐hydroxybutyrate. Approximately two‐thirds of the variability in each ketone measure was attributed to intra‐subject variability. Intra‐subject variability was higher for serum ketones than other metabolites. Patients in the lowest response tertile exhibited no increase in ketones. Those in the highest response tertile tended to be male and have higher fasting plasma glucose levels, lower insulin levels, and longer T2DM duration at baseline. Moreover, changes in serum ketones were not fully explained by changes in plasma fatty acids, suggesting downstream effects of SGLT2 inhibition on hepatic metabolism that favour ketogenesis. In summary, increases in serum ketone bodies with canagliflozin were greater and more variable than changes in other metabolic measures in Japanese patients with T2DM.
Keywords: canagliflozin, type 2 diabetes, SGLT2 inhibitor
1. INTRODUCTION
The ketone bodies β‐hydroxybutyrate (BHB) and acetoacetate (AcAc) are produced primarily in the liver and are utilized by the heart, muscles, kidneys and brain for energy metabolism when carbohydrate supply is limited (eg, fasting, after exercise).1 Overnight‐fasted serum ketone body concentrations are typically ~50 to 200 μM, but dramatic increases can be seen during prolonged fasting (~1 mM) or diabetic ketoacidosis (DKA; up to ~20 mM).1
Ketogenesis is often viewed as a proxy for hepatic fat oxidation and as a spillover metabolic pathway in the liver.2 Under conditions of reduced insulin concentrations, adipose lipolysis and delivery of fatty acyl chains to hepatocytes is increased. After transport of fatty acyl chains across the mitochondrial membranes and β‐oxidation, the mitochondrial isoform of 3‐hydroxymethylglutaryl‐CoA synthase 2 (HMGCS2) catalyses the fate‐committing condensation reaction in ketogenesis. HMGCS2 enzyme activity is regulated through multiple allosteric and covalent post‐translational modifications, and an active area of research investigation is whether these inputs might be responsible for intra‐subject and/or inter‐subject variation in ketogenesis, beyond that which can be explained by free fatty acid (FFA) availability to the liver.2 In conditions of DKA, the glucagon/insulin ratio is often dramatically increased and leads to increased ketone production; in addition, the abundance and function of succinyl‐CoA oxoacid transferase, the key enzyme required for extrahepatic ketone body oxidation, become diminished, limiting ketone body disposal.1
Ketone bodies are a preferred energy substrate for the heart and their use is increased in failing hearts. It has been hypothesized that, in the setting of heart failure, the energy‐starved myocardium adapts its fuel metabolism from primarily fatty acid oxidation to ketone bodies to maintain adequate fuel supplies.3
Sodium glucose co‐transporter 2 (SGLT2) inhibitors lower the renal threshold for glucose, thereby increasing urinary glucose excretion (UGE) and lowering blood glucose levels in patients with type 2 diabetes mellitus (T2DM); increased UGE also results in mild osmotic diuresis and a net caloric loss, leading to reductions in blood pressure (BP) and body weight (BW).4 Increased serum concentrations of ketone bodies have also been observed after SGLT2 inhibitor treatment, with high between‐subject variability.5, 6, 7 SGLT2 inhibitors have been associated with rare cases of DKA,8 with potential risk factors including inappropriate reductions in insulin doses and low carbohydrate intake6; however, in light of the positive effects of the SGLT2 inhibitors on cardiovascular outcomes,9, 10 it has been hypothesized that SGLT2 inhibition shifts cardiac energy utilization away from glucose and towards fatty acids and ketone bodies, which may improve overall cardiac energetics, thereby leading to reductions in adverse cardiovascular and renal outcomes in patients with T2DM.11
In relatively small and short‐term studies, the increases in serum ketone bodies observed in response to SGLT2 inhibitor treatment were found to be highly variable.12, 13 The extent to which this variability is attributable to inter‐subject vs intra‐subject variability was not assessed in these studies. The aim of the present analysis was to characterize the inter‐ and intra‐subject variability in serum ketone body responses in patients with T2DM treated with canagliflozin.
2. METHODS
2.1. Study design and patient population
The present analysis was based on results from post hoc analyses that were performed using data from a 52‐week, multicentre, open‐label study of canagliflozin in 1299 Japanese patients with T2DM (http://clinicaltrials.gov identifier: NCT01387737).5 Patients on diet/exercise or diet/exercise plus an oral antihyperglycaemic drug were randomized to receive canagliflozin 100 or 200 mg while continuing prior therapy; the treatment period was 52 weeks and there was a follow‐up period of 2 weeks after the treatment period. The study was conducted in accordance with Good Clinical Practice guidelines and the Pharmaceutical Affairs Law in Japan and complied with the ethical principles of the Declaration of Helsinki. The present analyses included only patients with available ketone body data.
2.2. Endpoints and statistical analyses
Serum ketone bodies (BHB, AcAc and total ketone bodies [TKB]) and other metabolic variables were measured every 4 weeks for 52 weeks. All laboratory measurements were performed at a central laboratory (SRL Inc, Tokyo, Japan) according to standard operating procedures. Data from all patients (including those who discontinued at some point during the study) were included, and no imputation of missing values was performed.
Changes in ketone bodies and the other metabolic variables were modelled using a random effects model of the form:
where ΔKB ij is the change from baseline at the i th time in subject j, μ is the mean change for all subjects/visits, β j is a subject‐specific random effect (the difference between the mean change in subject j and the mean change in the population), and ε ij is a visit‐specific deviation for visit i in subject j. The population intraclass correlation coefficient (ICC),
was used to characterize intra‐ and inter‐subject variability for the different measures. As σ2 β provides a measure of inter‐subject variability and σ2 ɛ provides a measure of intra‐subject variability, high values of ICC (close to 1) occur when most of the variability in the response can be accounted for by between‐subject variability with relatively little variation over time within each subject, whereas lower values of ICC occur when there is more within‐subject variability in the response over time. The random effects models were used to divide subjects into tertiles of ketone response based on the β j parameters.
To assess the extent to which changes in serum ketone bodies could be directly related to changes in other measurements, stepwise regression was performed between the change from baseline in ΔTKB and the changes in other measurements (ΔFFA, fasting plasma glucose [ΔFPG], triglycerides [ΔTG], LDL cholesterol [ΔLDL‐C], HDL cholesterol [ΔHDL‐C], ΔBW, systolic BP [ΔSBP], and Δinsulin) using all post‐baseline measurements; gender was also included in the model. All calculations were performed in matlab version 9.0 (MathWorks®, Natick, Massachusetts).
3. RESULTS
3.1. Patient population
Of 1299 patients who participated in the study, 1278 had ketone body data; baseline characteristics were balanced between the canagliflozin 100 and 200 mg groups (Table S1). The median (interquartile range [IQR]) BHB and AcAc concentrations at baseline were 55 (34;97) μM and 28 (19;44) μM, respectively, for patients included in this analysis.
3.2. Ketone body changes
Similar median increases in BHB and AcAc concentrations were seen with both canagliflozin doses over 52 weeks; median concentrations were approximately doubled with canagliflozin treatment (Figure 1A). Canagliflozin 100 and 200 mg were associated with an initial increase in both BHB and AcAc from baseline to week 4; the increase was sustained over 52 weeks, with modestly greater mean increases seen at the earlier visits.
Figure 1.
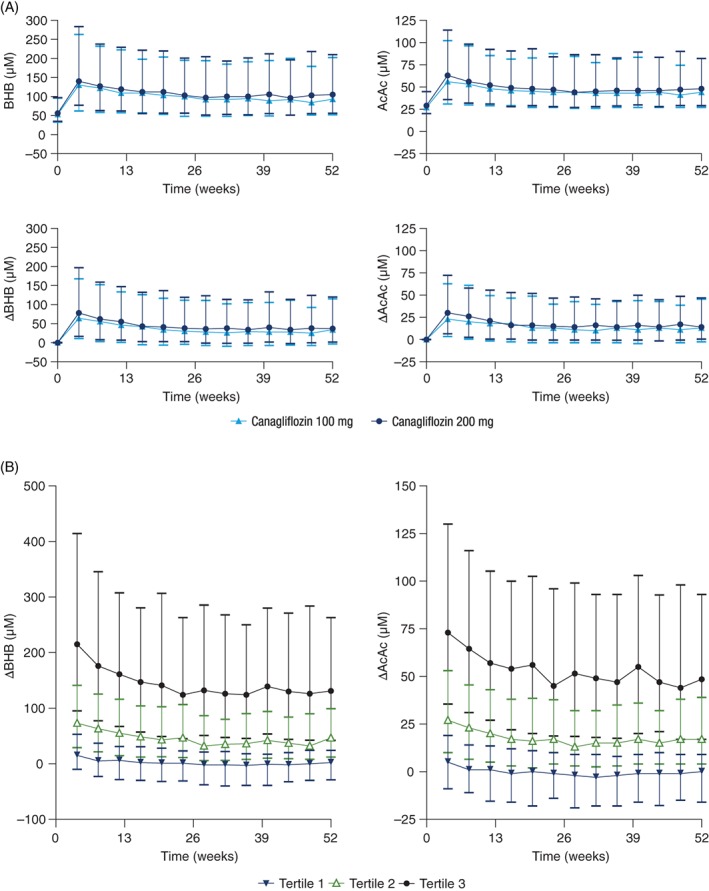
A, Serum ketone body concentrations (top) and change from baseline values (bottom) and B, change from baseline for ketone bodies, divided into tertiles of response. Values shown are median (interquartile range). AcAc, acetoacetate; BHB, β‐hydroxybutyrate
The median percent change from baseline in ketone body concentrations with canagliflozin (pooled 100 and 200 mg) was much greater than for the other measurements (Table 1); median percent increases were 78%, 62% and 73% with BHB, AcAc and TKB, respectively. Only one‐third of the variability in BHB, AcAc and TKB was attributed to inter‐subject variability; the remaining two‐thirds of the variability was attributed to intra‐subject variability. In contrast, variability in FPG, glycated haemoglobin (HbA1c) and insulin were mainly attributed to inter‐subject variability, while variability in FFA was nearly evenly split between inter‐ and intra‐subject variability.
Table 1.
Changes from baseline and intraclass correlation coefficient values for different measuresa
| Baseline Median (IQR) | Percent change from baseline Median (IQR) | ICC for change from baseline values | Inter‐subject variability, σβ b | Intra‐subject variability, σɛ b | |
|---|---|---|---|---|---|
| BHB, μM | 55 (34;97) | 78 (2;236) | 0.33 | 135 | 193 |
| AcAc, μM | 28 (19;44) | 62 (0;180) | 0.34 | 43 | 60 |
| TKB, μM | 82 (53;141) | 73 (1;216) | 0.33 | 177 | 251 |
| FPG, mmol/L | 8.5 (7.4;9.8) | −17 (−27;–9) | 0.83 | 1.5 | 0.66 |
| HbA1c, % | 7.9 (7.4;8.6) | −11 (−16;–6) | 0.91c | 0.68 | 0.22 |
| FFA, μM | 510 (380;660) | 10 (−15;46) | 0.47 | 149 | 157 |
| Insulin, pmol/L | 47 (30;74) | −22 (−40;2) | 0.73 | 26 | 16 |
| TG, mmol/L | 1.4 (1.0;2.0) | −15 (−33;9) | 0.53 | 0.76 | 0.72 |
| LDL cholesterol, mmol/L | 3.1 (2.6;3.6) | 4 (−6;15) | 0.55 | 0.39 | 0.36 |
| HDL cholesterol, mmol/L | 1.3 (1.1;1.6) | 9 (0;19) | 0.55 | 0.14 | 0.13 |
| UGE, g/g creatinine | 0.15 (0.08;0.66) | 29400 (7900;51500) | 0.68 | 17000 | 12000 |
Abbreviations: AcAc, acetoacetate; BHB, β‐hydroxybutyrate; FFA, free fatty acids; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; ICC, intraclass correlation coefficient; IQR, interquartile range; TG, triglycerides; TKB, total ketone bodies; UGE, urinary glucose excretion.
Data for both canagliflozin doses were pooled in this analysis.
The σβ and σɛ values provide measures of inter‐ and intra‐subject variability, respectively, as described in the methods section.
For HbA1c, only changes after week 12 were included in the analysis to avoid variability associated with the time lag required for equilibration of HbA1c.
To further assess variability in ketone body concentrations and explore which baseline factors were associated with the largest changes in ketone bodies, patients were stratified into tertiles based on average ketone body response. In the lowest tertile of ketone response, no notable changes in mean ketone concentrations were observed at any time point (Figure 1B). Patients in the intermediate tertile of ketone response had changes in ketone body concentrations that were generally steady over time. Among patients in the highest tertile of ketone response, changes in ketone body concentrations were highest at 4 weeks then modestly decreased at subsequent visits before stabilizing at week 24. Patients in the tertile with the greatest BHB increase tended to be male and have higher baseline FPG levels, lower baseline insulin levels, and longer duration of T2DM (Table S2). There were no significant differences in background oral antihyperglycaemic agents across the different tertiles of ketone response.
In regression analyses relating ΔTKB to changes in other variables individually (ΔFFA, ΔFPG, ΔTG, ΔLDL‐C, ΔHDL‐C, ΔBW, ΔSBP and Δinsulin), the highest correlation was seen with ΔFFA (r 2 = 0.16; P <10−10), with smaller correlations seen with other variables (all r 2 ≤0.03; all P <.01, except ΔSBP, which was not statistically significant; Table S3). A joint model that included gender and all statistically significant terms for change from baseline values in the combined model (ΔFFA, ΔFPG, ΔTG, ΔHDL‐C and ΔBW) gave r 2 = 0.22 (Table S3). Thus, while changes in several variables were significantly correlated with the changes in ketone bodies, only a modest fraction of the variability observed in the ketone body changes was explained by the changes in these other variables.
Because ΔFFA was most correlated with ΔTKB, the ratio of TKB/FFA was calculated to assess whether the mean ΔTKB could be largely explained by ΔFFA. As shown in Figure S1, the ratio of TKB/FFA increased, suggesting that the increases in ketone bodies were more than proportional to increases in FFA.
4. DISCUSSION
The results of the present study showed that treatment with canagliflozin 100 and 200 mg increases serum ketone bodies in Japanese patients with T2DM. The approximate doubling of median serum ketone body concentrations is similar to what has been reported in studies with other SGLT2 inhibitors.13, 14 The changes in ketone bodies were considerably greater and more variable than changes in other metabolic measures, such as glucose, insulin, HbA1c or FFA. Changes in serum ketones were not fully explained by changes in plasma fatty acids, suggesting downstream effects of SGLT2 inhibitors on hepatic metabolism that favour ketogenesis.
Intra‐subject variability accounted for nearly two‐thirds of the overall variability in ketone responses, which was much higher than for the other metabolic measures, while inter‐subject variability accounted for one‐third of overall variability. Patients in the lowest tertile of ketone response had no notable mean change in ketones at any time between week 4 and week 52. Patients in the highest tertile of ketone response tended to be male and to have longer duration of T2DM, higher baseline FPG, and lower baseline insulin, which suggests that patients with more advanced T2DM have the highest ketone body response with canagliflozin.
The pre‐treatment variables associated with the greatest increase in serum ketone body concentrations were higher FPG levels, lower plasma insulin levels, and greater duration of T2DM. Increases in ketone bodies were also greater in men than women in this study. Post‐treatment, changes in ketone bodies had the greatest correlations with changes in FFA, while weaker correlations were observed with other variables, including FPG, BW and insulin. In addition, there were no significant correlations between the ketone body concentrations in the present study and the ketogenic index proposed by Al Jobori et al.12 (P = .28); the ketogenic index in the present study was calculated using the measured values of insulin, glucose and FFA at each visit rather than after the first treatment day as in Al Jobori et al.
While most patients in the present study had observed increases in ketone bodies, nearly all (>99%) of the values were <2 mM. This is consistent with the infrequent observations of DKA across the canagliflozin clinical programme, which were more likely to occur in patients who were on insulin or had other precipitating factors (eg, recent illness, diagnosis of T1DM or latent autoimmune diabetes of adulthood).10, 15 Notably, 1 patient in this study who was treated with canagliflozin 100 mg added to a sulphonylurea had a severe episode of DKA shortly after discontinuing study treatment.5 This was the only case of DKA during the study, and the event was preceded by infectious gastroenteritis and was suspected to be the result of fulminant T1DM after viral infection; the patient's highest TKB level was 13 263 μM.5
In the CANVAS Program, canagliflozin significantly reduced the risk of major adverse cardiovascular events (MACE; 3‐point: cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke) compared with placebo in patients with T2DM and a history or high risk of cardiovascular disease.10 These findings were consistent with those from the EMPA‐REG OUTCOME trial with empagliflozin.9 These data, along with data from recent meta‐analyses and real‐world studies, suggest that SGLT2 inhibitors as a class may be associated with a reduction in cardiovascular risk.9, 10, 16, 17 Consequently, elucidating the potential mechanism of cardioprotection with SGLT2 inhibitors is an active area of research. One possible explanation is that under conditions of mild hyperketonaemia, such as those caused by SGLT2 inhibitors, the heart preferentially metabolizes ketone bodies instead of glucose, thereby improving myocardial work efficiency and function.11 Alternatively, haemodynamic changes associated with SGLT2 inhibition (ie, BP reduction and reduced intravascular volume) may decrease the cardiac workload and allow the heart to work more efficiently.18 Another possibility is that cardioprotection could be attributable to direct enhancement of myocardial function via increased levels of glucagon and the natriuretic effect.19
There have been limited data on ketone body changes with canagliflozin to date. In the present study, canagliflozin treatment was associated with increases in serum ketone bodies that were greater and more variable than other metabolic measures in Japanese patients with T2DM. One limitation of the study is that, while day‐to‐day changes in diet and exercise probably contribute to the high within‐subject variability in plasma ketone levels, data on within‐subject changes in diet and exercise over the course of the study were not collected and thus the relationships between changes in diet and exercise and changes in ketone body concentrations cannot be determined from this study. Another limitation is that this study was performed in Japanese patients with a mean body mass index (BMI) of 26 kg/m2, which is lower than values commonly found in Western patients with T2DM, and it is possible that individuals with higher BMI may have different ketone body responses. Future investigations may help to clarify the sources of variability in serum ketone bodies and the implications of ketone body elevations in patients with T2DM treated with SGLT2 inhibitors. In conclusion, increases in serum ketone bodies with canagliflozin were greater and more variable than changes in other metabolic measures in Japanese patients with T2DM.
Supporting information
Table S1. Baseline demographic and disease characteristics.
Table S2. Baseline characteristics and demographic factors in patients in the different tertiles of BHB response*.
Table S3. Results from univariate and multivariate regression analyses for relationship between ΔTKB and changes in values of other measures*.
Figure S1. Ratio of TKB/FFA over time. Values shown are mean ± s.e.
ACKNOWLEDGMENTS
This study was based on data from a study supported by Mitsubishi Tanabe Pharma Corporation, and this analysis was supported by Janssen Research & Development, LLC. Medical writing support was provided by Dana Tabor, PhD, of MedErgy, and was funded by Janssen Global Services, LLC.
Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
Conflict of interest
D.P. is a full‐time employee of Janssen Research & Development, LLC. H.I., M.G. and N.M. are full‐time employees of Mitsubishi Tanabe Pharma Corporation. N.I. has received consulting fees and/or speakers bureau fees from Astellas Pharma Inc., MSD K.K., Nippon Boehringer Ingelheim Co., Ltd, Sanofi K.K. and Takeda Pharmaceutical Co., Ltd; has received research support from AstraZeneca K.K., Daiichi Sankyo Co., Ltd, Eli Lilly Japan K.K., MSD K.K. and Mitsubishi Tanabe Pharma Corporation; and received scholarship grants from Astellas Pharma Inc., AstraZeneca K.K., Daiichi Sankyo Co., Ltd, Japan Tobacco Inc., Kissei Pharmaceutical Co., Ltd, Kyowa Hakko Kirin Co., Ltd, MSD K.K., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd, Novartis Pharma K.K., Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, Pfizer Japan Inc., Sanwa Kagaku Kenkyusho Co., Ltd, Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd, Takeda Pharmaceutical Co., Ltd, and Taisho Toyama Pharmaceutical Co., Ltd. P.A.C. has no relevant disclosures.
Author contributions
D.P. and P.A.C. contributed to the design and conduct of the study, the analysis and interpretation of the data, and the development of the manuscript. N.M. and N.I. contributed to the design and conduct of the study and the acquisition of the data. H.I., M.G., N.M. and N.I. contributed to the interpretation of the data and the development of the manuscript. All authors approved the final version of the submitted manuscript.
Polidori D, Iijima H, Goda M, Maruyama N, Inagaki N, Crawford PA. Intra‐ and inter‐subject variability for increases in serum ketone bodies in patients with type 2 diabetes treated with the sodium glucose co‐transporter 2 inhibitor canagliflozin. Diabetes Obes Metab. 2018;20:1321–1326. https://doi.org/10.1111/dom.13224
Funding information Mitsubishi Tanabe Pharma Corporation; Janssen Research & Development, LLC
REFERENCES
- 1. Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304(8):H1060‐H1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Puchalska P, Crawford PA. Multi‐dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25(2):262‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aubert G, Martin OJ, Horton JL, et al. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133(8):698‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mudaliar S, Polidori D, Zambrowicz B, Henry RR. Sodium‐glucose cotransporter inhibitors: effects on renal and intestinal glucose transport: from bench to bedside. Diabetes Care. 2015;38(12):2344‐2353. [DOI] [PubMed] [Google Scholar]
- 5. Inagaki N, Kondo K, Yoshinari T, Kuki H. Efficacy and safety of canagliflozin alone or as add‐on to other oral antihyperglycemic drugs in Japanese patients with type 2 diabetes: a 52‐week open‐label study. J Diabetes Investig. 2015;6(2):210‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yabe D, Iwasaki M, Kuwata H, et al. Sodium‐glucose co‐transporter‐2 inhibitor use and dietary carbohydrate intake in Japanese individuals with type 2 diabetes: a randomized, open‐label, 3‐arm parallel comparative, exploratory study. Diabetes Obes Metab. 2017;19(5):739‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Briand F, Mayoux E, Brousseau E, et al. Empagliflozin, via switching metabolism toward lipid utilization, moderately increases LDL cholesterol levels through reduced LDL catabolism. Diabetes. 2016;65(7):2032‐2038. [DOI] [PubMed] [Google Scholar]
- 8. US Food and Drug Administration . FDA drug safety communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. 2015. http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Accessed December 13, 2017.
- 9. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 10. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 11. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA‐REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39(7):1115‐1122. [DOI] [PubMed] [Google Scholar]
- 12. Al Jobori H, Daniele G, Adams J, et al. Determinants of the increase in ketone concentration during SGLT2 inhibition in NGT, IFG and T2DM patients. Diabetes Obes Metab. 2017;19(6):809‐813. [DOI] [PubMed] [Google Scholar]
- 13. Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium‐glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65(5):1190‐1195. [DOI] [PubMed] [Google Scholar]
- 14. Okamoto A, Yokokawa H, Sanada H, Naito T. Changes in levels of biomarkers associated with adipocyte function and insulin and glucagon kinetics during treatment with dapagliflozin among obese type 2 diabetes mellitus patients. Drugs R D. 2016;16(3):255‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015;38(9):1680‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu JH, Foote C, Blomster J, et al. Effects of sodium‐glucose cotransporter‐2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2016;4(5):411‐419. [DOI] [PubMed] [Google Scholar]
- 17. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on SGLT‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL Study. Circulation. 2017;136(3):249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdul‐Ghani M, Del Prato S, Chilton R, Defronzo RA. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA‐REG OUTCOME study. Diabetes Care. 2016;39(5):717‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ceriello A, Genovese S, Mannucci E, Gronda E. Glucagon and heart in type 2 diabetes: new perspectives. Cardiovasc Diabetol. 2016;15(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline demographic and disease characteristics.
Table S2. Baseline characteristics and demographic factors in patients in the different tertiles of BHB response*.
Table S3. Results from univariate and multivariate regression analyses for relationship between ΔTKB and changes in values of other measures*.
Figure S1. Ratio of TKB/FFA over time. Values shown are mean ± s.e.


