Abstract
The aim of this study was to evaluate cenicriviroc (CVC), a dual antagonist of C—C chemokine receptor types 2 and 5, for treatment of nonalcoholic steatohepatitis (NASH) with liver fibrosis (LF). A randomized, double‐blind, multinational phase 2b study enrolled subjects with NASH, a nonalcoholic fatty liver disease activity score (NAS) ≥4, and LF (stages 1‐3, NASH Clinical Research Network) at 81 clinical sites. Subjects (N = 289) were randomly assigned CVC 150 mg or placebo. Primary outcome was ≥2‐point improvement in NAS and no worsening of fibrosis at year 1. Key secondary outcomes were: resolution of steatohepatitis (SH) and no worsening of fibrosis; improvement in fibrosis by ≥1 stage and no worsening of SH. Biomarkers of inflammation and adverse events were assessed. Full study recruitment was achieved. The primary endpoint of NAS improvement in the intent‐to‐treat population and resolution of SH was achieved in a similar proportion of subjects on CVC (N = 145) and placebo (N = 144; 16% vs. 19%, P = 0.52 and 8% vs. 6%, P = 0.49, respectively). However, the fibrosis endpoint was met in significantly more subjects on CVC than placebo (20% vs. 10%; P = 0.02). Treatment benefits were greater in those with higher disease activity and fibrosis stage at baseline. Biomarkers of systemic inflammation were reduced with CVC. Safety and tolerability of CVC were comparable to placebo. Conclusion: After 1 year of CVC treatment, twice as many subjects achieved improvement in fibrosis and no worsening of SH compared with placebo. Given the urgent need to develop antifibrotic therapies in NASH, these findings warrant phase 3 evaluation. (Hepatology 2018;67:1754‐1767).
Abbreviations
- APRI
aspartate aminotransferase‐to‐platelet count ratio index
- BMI
body mass index
- CCL
chemokine (C‐C motif) ligand
- CCR
C‐C motif chemokine receptor
- CD
cluster of differentiation
- CI
confidence interval
- CONSORT
Consolidated Standards of Reporting Trials
- CRN
Clinical Research Network
- CVC
cenicriviroc
- CVD
cardiovascular disease
- ELF
enhanced liver fibrosis
- FIB‐4
fibrosis‐4
- HbA1c
hemoglobin A1c
- HF
hepatic fibrosis
- HIV
human immunodeficiency virus
- hs‐CRP
high‐sensitivity C‐reactive protein
- IL
interleukin
- ITT
intent‐to‐treat
- LF
liver fibrosis
- MetS
metabolic syndrome
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
nonalcoholic steatohepatitis
- NFS
NAFLD fibrosis score
- OR
odds ratio
- sCD
soluble CD
- SD
standard deviation
- SH
steatohepatitis
- T2DM
type 2 diabetes mellitus
Nonalcoholic fatty liver disease (NAFLD) is now the most common cause of liver disease, with a prevalence of 25% globally.1 Nonalcoholic steatohepatitis (NASH), the more severe form of the disease, is characterized by the presence of steatosis, lobular and/or portal inflammation, hepatocyte injury (i.e., ballooning), and fibrosis.2 The presence of liver fibrosis (LF) confers an increased risk of disease progression to cirrhosis, liver failure, and hepatocellular carcinoma, with a higher mortality.3, 4 Fibrosis stage is the only histological feature of NASH independently linked to an increased likelihood of liver‐related and all‐cause (e.g., cardiovascular disease [CVD]) mortality in recent studies.3, 4, 5 Therefore, reducing LF is expected to improve the long‐term clinical outcomes of patients with NASH.6 Among pharmacological treatments currently undergoing evaluation, a number have reported improvement in histological features of NASH,6, 7, 8, 9 but only obeticholic acid significantly improved fibrosis in a randomized clinical study in adults with noncirrhotic NASH.10
Cenicriviroc (CVC) is an oral, dual antagonist of C‐C motif chemokine receptor (CCR) types 2 and 5. Preclinical11, 12, 13, 14 and clinical evidence15, 16, 17 support its anti‐inflammatory and antifibrotic properties, which are mediated by CCR types 2 and 5 (CCR2/CCR5) blockade. CVC has demonstrated antifibrotic activity in animal models of LF and renal fibrosis.11 These findings are supported in patients by improvements in noninvasive markers of hepatic fibrosis (HF; aspartate aminotransferase‐to‐platelet ratio index [APRI], fibrosis‐4 [FIB‐4], and enhanced liver fibrosis [ELF] test) observed in post‐hoc analyses of a 48‐week phase 2b study in human immunodeficiency virus (HIV)‐infected subjects.18, 19 Furthermore, extensive clinical experience using CVC, with over 1,000 subjects treated to date, indicates a favorable safety profile including in subjects with cirrhosis and mild‐to‐moderate (Child‐Pugh A or B) hepatic impairment.17, 20
CVC‐mediated antagonism of CCR2 is expected to reduce the recruitment, migration, and infiltration of proinflammatory monocytes and macrophages at the site of liver injury.14, 15 CCR5 antagonism by CVC is expected to additionally impair the migration, activation, and proliferation of collagen‐producing activated hepatic stellate cells/myofibroblasts.21 We designed the phase 2 CENTAUR study to test the efficacy and safety of CVC in adults with NASH and LF; results from the year 1 primary analysis are reported here.
Materials and Methods
STUDY DESIGN
The study design, rationale, and procedures of CENTAUR (NCT02217475) have been reported previously.15 This is a phase 2b, randomized, double‐blinded, placebo‐controlled, and multinational study. The protocol was approved by the Institutional Review Board or Independent Ethics Committee for each center. The study is being conducted in accord with the Declaration of Helsinki and with all applicable laws/regulations of the study locations; all subjects gave written informed consent. Data were analyzed by Medpace, Inc., and the sponsor. Authors had access to the data and participated in drafting the manuscript; editorial support was funded by the sponsor and provided by independent medical writers under author guidance. All authors approved the manuscript and assume full responsibility for data accuracy and completeness.
Subjects were randomized to receive CVC 150 mg or a matched placebo once‐daily. After 1 year, half of the subjects receiving placebo crossed over to CVC, based on preplanned randomization, for a second year of treatment. At baseline, eligible subjects were assigned to the treatment arms using permuted block randomization stratified by NAFLD activity score (NAS) at screening (4 or ≥5) and fibrosis stage (≤2 or >2). Subjects were randomized 2:1:1 to arm A (CVC 150 mg once‐daily for 2 years), arm B (placebo for 1 year then CVC 150 mg for 1 year), or arm C (placebo for 2 years). Randomization was accomplished by interactive voice response system.
Subjects, the sponsor, investigators, and all site personnel involved with dispensing study medication, carrying out study procedures, evaluating subjects, entering study data, and/or evaluating study data remain blinded to individual treatment assignment until all subjects complete the 2‐year study and the database is locked for all study data. CVC and matching placebo provided by the sponsor were visually indistinguishable and the packaging identical except for a unique bottle identification number. We report herein the results at year 1 of treatment, comparing CVC to placebo.
Adult subjects with histological evidence of NASH, a NAS ≥4 with ≥1 in each component, and LF (NASH Clinical Research Network [CRN] stages 1‐3) were enrolled at 81 clinical sites in Australia, Belgium, France, Germany, Hong Kong, Italy, Poland, Spain, the UK, and the United States. Subjects had either type 2 diabetes mellitus (T2DM), a high body mass index (BMI; >25 kg/m2) with ≥1 criteria of metabolic syndrome (MetS; National Cholesterol Education Program's Adult Treatment Panel III (ATP III) definition), or bridging fibrosis (NASH CRN stage 3) and/or high disease activity (NAS ≥5). The screening and year 1 liver biopsies were read by a central pathologist, who remained blinded to individual subject treatment assignment. Screening biopsies were not reread at the time year 1 biopsies were assessed; however, biopsy sequence was not blinded, because of logistical challenges.
The study protocol instructed sites to provide and review patient education materials about NASH and LF by the National Institute of Diabetes and Digestive Kidney Diseases at the screening visit, but relied on local standard of care for implementing diet and lifestyle intervention in randomized subjects. Subjects were excluded from the study if they had bariatric surgery in the past 5 years, or planned bariatric surgery during the trial. Alcohol consumption (current drinker, former drinker, or never consumed) was recorded at baseline and at subsequent visits. Height of subjects was recorded at screening and month 12; body weight was monitored at regular intervals (screening, baseline, and at months 3, 6, and 12 during treatment period 1 [i.e., year 1]). Change in BMI was calculated for CVC and placebo recipients.
Treatment of T2DM and dyslipidemia was allowed with certain restrictions or precautions, depending on the coadministered drug and its drug‐drug interaction potential with CVC. The use of frequently administered concomitant medications, including biguanides, glucose‐lowering drugs (excluding insulin), hydroxymethylglutaryl CoA reductase inhibitors, and angiotensin II inhibitors, were noted throughout year 1 of the study and are listed in http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo. Pioglitazone and high‐dose vitamin E (>400 UI/day) were disallowed because of potential confounding effects on efficacy.
STUDY OUTCOMES
The primary outcome evaluated hepatic histological improvement at year 1 relative to the screening biopsy (defined by ≥2‐point improvement in NAS with ≥1‐point reduction in either lobular inflammation or hepatocellular ballooning) and no worsening of fibrosis stage (i.e., no progression of NASH CRN fibrosis stage). This endpoint was based on previously published phase 2b trials in NASH.9, 10 Two key secondary outcomes were prospectively selected based on regulatory guidance and were evaluated at year 1: (1) complete resolution of steatohepatitis (SH; histopathological interpretation of fatty liver disease, or simple or isolated steatosis and no SH) and no worsening of fibrosis stage; (2) improvement in fibrosis by ≥1 stage (NASH CRN system) and no worsening of SH (no worsening of lobular inflammation or hepatocellular ballooning grade).
Other secondary outcomes included: change in fibrosis stage (NASH CRN and Ishak systems); change in histological scores for steatosis, lobular inflammation, and hepatocellular ballooning; change in collagen morphometry on liver biopsy; safety and tolerability of CVC; and change in liver biochemistry and fasting metabolic parameters. Inflammatory biomarkers were also assessed.
A tertiary objective of the study was to evaluate the change from baseline in liver stiffness through noninvasive methods (e.g., ultrasound transient elastography, two‐dimensional magnetic resonance elastography, or acoustic radiation force impulse). Unfortunately, most sites did not have access to these methods at the time of initiation of the study; therefore, only a limited number of subjects had available data.
The http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo provides details on CENTAUR study objectives (http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo) and efficacy endpoints (http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo).
STATISTICAL ANALYSES
To assess CVC efficacy for the primary endpoint, the original required sample size was 252 subjects, assuming a 20% response rate for placebo and a 36% response rate for CVC at year 1. The study overenrolled by 15% but, because of an anticipated dropout rate of 15%, this sample size was still expected to provide at least an 80% power to demonstrate superiority (for a two‐sided type 1 error rate of 0.05) of CVC versus placebo.
The primary efficacy endpoint was analyzed using logistic regression, which included terms for treatment and the two stratification variables (i.e., baseline NAS of 4 or ≥5 and fibrosis stage ≤2 or >2). A preordered, step‐down approach was used (http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo). If statistical significance was achieved at α = 0.05, two‐sided for the primary endpoint, a composite analysis on the sum of the two key secondary endpoints was to be performed with an ordinal logistic regression model. If statistical significance was achieved for the summation, a parallel, simultaneous analysis for each key secondary endpoint was to be performed. The type I error rate was controlled for the key secondary endpoints by only testing the composite analysis if the primary endpoint was positive, and only testing each secondary endpoint if the composite analysis was also positive.
Supportive analyses were planned in the modified intent‐to‐treat (ITT) population, consisting of all subjects in the ITT population with evaluable biopsies, and with the full analysis set, compiling all subjects with evaluable biopsies at both baseline and year 1.
A post‐hoc analysis of various factors that might affect response (including baseline characteristics, demographics, laboratory tests, and histological features) was conducted without control of the type I error rate. In a logistic regression model, potential predictors were added to the model in a step‐wise selection process if the P value was less than or equal to 0.30 after adjustment for all previously included factors. When all such factors were found, those with resulting P values less than or equal to 0.05 were considered nominally significant after adjustment for all other potential predictors.
Results
SUBJECTS
This phase 2b, randomized, double‐blinded, placebo‐controlled, multinational study was initiated in September 2014 and fully enrolled by June 2015. It is currently ongoing and will be conducted over 2 years, with the primary analysis having been performed at year 1 (cut‐off date July 2016). A total of 812 subjects were screened; 610 underwent liver biopsy and 289 were randomized to treatment (Fig. 1). At the end of year 1, 252 subjects had available screening and year 1 biopsies. The primary efficacy analysis, reported in the ITT population, comprised all randomized subjects.
Figure 1.
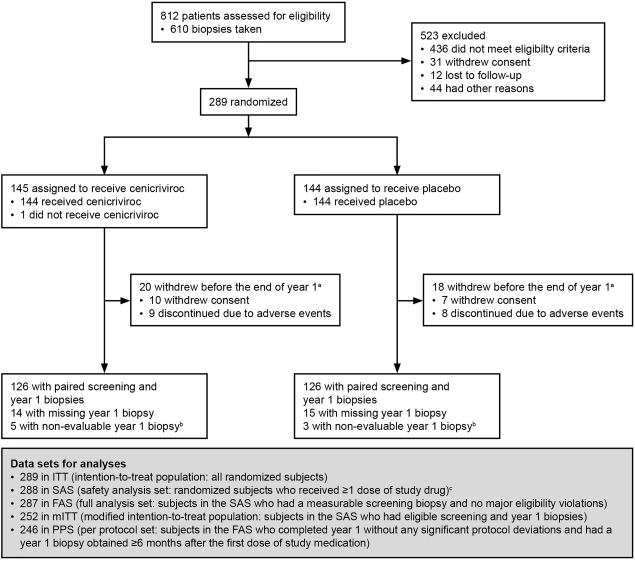
Subject disposition (CONSORT flow diagram). aThe disposition of 4 subjects who withdrew early (1 for protocol deviation, 1 lost to follow‐up, 1 because of physician's decision, 1 other) cannot be reported in specific treatment arm as the study is ongoing and remains blinded. bLiver biopsy sample too small or fragmented, therefore inadequate for assessment of efficacy endpoints. cA subject was randomized in error without an adequate screening biopsy. Abbreviation: CONSORT, Consolidated Standards of Reporting Trials.
Baseline demographics and disease characteristics are presented in Table 1. With the exception of T2DM, the treatment groups were well balanced. Overall, 51% (146 of 289) of subjects had T2DM, 95% (273 of 289) had BMI >25 kg/m2 with ≥1 criteria of MetS, and 38% (111 of 289) had bridging fibrosis (NASH CRN stage 3). A total of 72% (208 of 289) of subjects met ≥3 criteria of MetS. The majority of subjects had NAS ≥5 (74% [214 of 289]) and 67% (193 of 289) had fibrosis stage 2 or 3 at screening.
Table 1.
Baseline Demographics and Disease Characteristics of Randomized Subjects per Treatment Group
|
CVC 150 mg (N = 145) |
Placebo (N = 144) |
All (N = 289)a |
|
|---|---|---|---|
| Demographics | |||
| Mean age, year (SD) | 54.6 (10.2) | 53.7 (11.0) | 54.1 (10.6) |
| Female, no. (%) | 73 (50.3) | 79 (54.9) | 152 (52.6) |
| Race or ethnicity, no. (%) | |||
| White | 129 (89.0) | 121 (84.0) | 250 (86.5) |
| Black | 5 (3.4) | 3 (2.1) | 8 (2.8) |
| Asian | 6 (4.1) | 15 (10.4) | 21 (7.3) |
| Hispanic ethnicity | 23 (15.9) | 25 (17.4) | 48 (16.6) |
| Serum biochemistry | |||
| Mean alanine aminotransferase, U/L (SD) | 61.3 (35.2) | 65.5 (39.6) | 63.4 (37.5) |
| Mean aspartate aminotransferase, U/L (SD) | 43.7 (22.0) | 48.3 (24.0) | 46.0 (23.1) |
| Mean alkaline phosphatase, U/L (SD) | 79.0 (20.9) | 80.8 (27.8) | 79.9 (24.5) |
| Mean gamma‐glutamyl transferase, U/L (SD) | 69.6 (79.0) | 65.2 (43.5) | 67.4 (63.7) |
| Mean total bilirubin, mg/dL (SD) | 0.510 (0.531) | 0.483 (0.273) | 0.496 (0.422) |
| Lipids | |||
| Triglycerides | |||
| Mean, mg/dL (SD) | 180.3 (149.0) | 174.5 (110.1) | 177.4 (130.8) |
| >150 mg/dL, no. (%) | 70 (48.3) | 71 (49.3) | 141 (48.8) |
| Mean cholesterol, mg/dL (SD) | |||
| Total | 192.5 (48.9) | 187.9 (47.4) | 190.2 (48.1) |
| High‐density lipoprotein | 42.1 (12.2) | 40.9 (13.2) | 41.5 (12.7) |
| Low‐density lipoprotein | 121.9 (44.4) | 118.7 (42.8) | 120.3 (43.6) |
| Very‐low‐density lipoprotein | 36.1 (30.0) | 34.9 (22.0) | 35.5 (26.3) |
| Metabolic factors | |||
| Mean body weight, kg (SD) | 95.1 (20.4) | 97.1 (21.9) | 96.1 (21.1) |
| Mean BMI, kg/m2 (SD) | 33.6 (5.7) | 34.1 (7.2) | 33.9 (6.5) |
| Mean HbA1c, % (SD) | 6.71 (1.36) | 6.37 (1.15) | 6.54 (1.27) |
| T2DM, no. (%) | 82 (57.2) | 64 (44.4) | 146 (50.5) |
| ≥3 criteria of MetS, no. (%) | 104 (71.7) | 104 (72.2) | 208 (72.0) |
| Histological features | |||
| NAFLD activity score | |||
| Mean total (SD) | 5.3 (1.1) | 5.4 (1.0) | 5.3 (1.0) |
| Mean steatosis (SD) | 1.4 (0.6) | 1.4 (0.5) | 1.4 (0.6) |
| Mean lobular inflammation (SD) | 2.4 (0.6) | 2.4 (0.6) | 2.4 (0.6) |
| Mean hepatocellular ballooning (SD) | 1.5 (0.5) | 1.5 (0.5) | 1.5 (0.5) |
| NAS | |||
| = 4, no. (%) | 39 (26.9) | 35 (24.3) | 74 (25.6) |
| ≥5, no. (%) | 106 (73.1) | 108 (75.0) | 214 (74.0) |
| Fibrosis stage (NASH CRN) | |||
| 1, no. (%) | 47 (32.4) | 48 (33.3) | 95 (32.9) |
| 2, no. (%) | 42 (29.0) | 40 (27.8) | 82 (28.4) |
| 3, no. (%) | 56 (38.6) | 55 (38.2) | 111 (38.4) |
One subject was randomized in error without an adequate screening biopsy.
PRIMARY AND KEY SECONDARY OUTCOMES
At year 1, a similar proportion of subjects receiving CVC or placebo achieved the primary endpoint of hepatic histological improvement in NAS by ≥2 points and no worsening of fibrosis stage (16% vs. 19%; odds ratio [OR], 0.82 [95% confidence interval {CI}, 0.44‐1.52]; P = 0.52; Fig. 2A).
Figure 2.
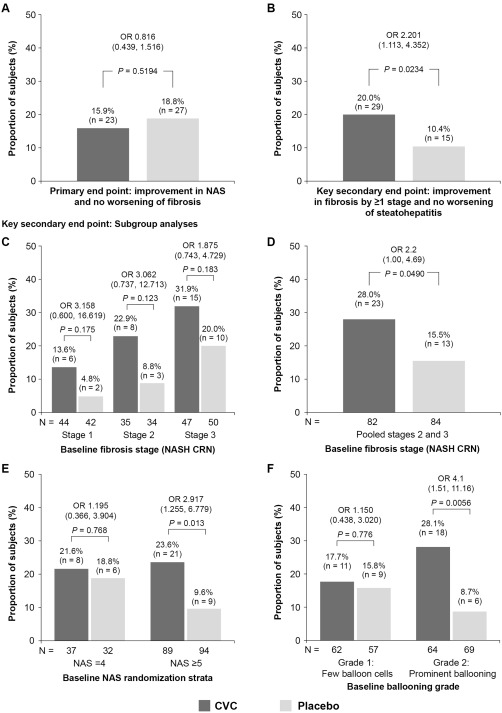
Primary endpoint and key secondary endpoint of improvement in fibrosis by ≥1 stage and no worsening of SH at year 1 (ITT analysis), with subgroup analyses for the key secondary endpoint (mITT population). (A) Subjects meeting the primary endpoint (improvement in NAS and no worsening of fibrosis). (B) Subjects meeting the key secondary endpoint of improvement in fibrosis by ≥1 stage and no worsening of SH. Missing biopsies were counted as treatment failure. (C,D,E,F) Response for the key secondary endpoint by baseline: (C) fibrosis stage (NASH CRN system); (D) fibrosis stages 2 and 3 pooled (NASH CRN system); (E) NAS stratification; and (F) hepatocellular ballooning grade. OR are presented with 95% CI and P values and were calculated using a logistic regression model with factors for randomized treatment group, NAS at screening (4 or ≥5), and fibrosis stage (≤2 or >2). Abbreviation: mITT, modified intent‐to‐treat.
Analysis of the key secondary endpoints was conducted as prespecified and is presented for full disclosure of data, although the primary endpoint was not met. The composite secondary endpoint (summation of “complete resolution of SH and no worsening of fibrosis stage” and “improvement in fibrosis stage by ≥1 stage and no worsening of SH”) was achieved by significantly more subjects receiving CVC than those receiving placebo (18% vs. 10%; OR, 1.93 [95% CI, 1.04‐3.61]; P = 0.04). When the two key secondary endpoints were analyzed separately, a similar proportion of subjects achieved complete resolution of SH and no worsening of fibrosis stage (8% vs. 6%; OR, 1.40 [95% CI, 0.54‐3.63]; P = 0.49), whereas twice as many subjects on CVC achieved improvement in fibrosis stage by ≥1 stage and no worsening of SH compared to those on placebo (20% vs. 10%; OR, 2.20 [95% CI, 1.11‐4.35]; P = 0.02; Fig. 2B).
SUBGROUP ANALYSES FOR KEY SECONDARY FIBROSIS ENDPOINT
CVC provided antifibrotic benefits in both fibrosis strata (stages ≤2 and >2; Figs. 2C and 3). When subjects with baseline fibrosis stages 2 and 3 were pooled, CVC benefits were significant (P < 0.05; Figs. 2D and 3). CVC treatment benefits were consistent across prespecified subgroups; the greatest treatment benefits were in subjects with baseline NAS ≥5 and those with prominent hepatocellular ballooning, relative to those with baseline NAS =4 and few ballooned cells (Figs. 2E,F and 3).
Figure 3.
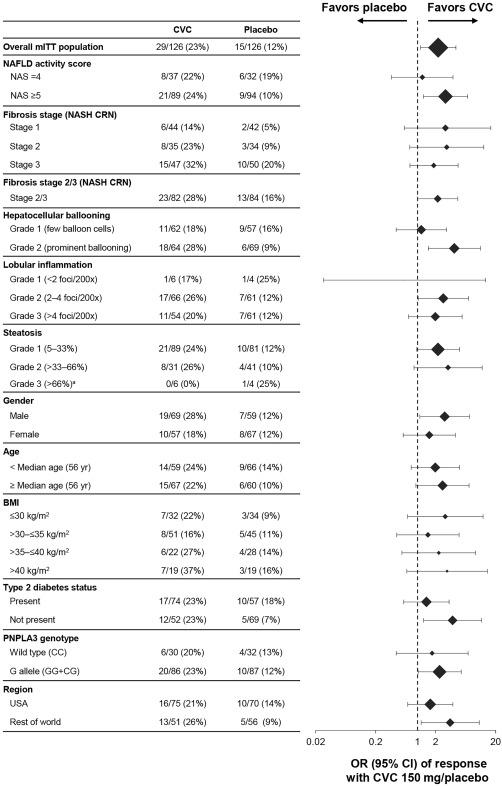
Subgroup analyses for the key secondary endpoint of improvement in fibrosis by ≥1 stage and no worsening of SH (mITT population). Response by baseline NAS stratification, fibrosis stage (NASH CRN system), hepatocellular ballooning grade, lobular inflammation, steatosis, sex, age, BMI, T2DM, PNPLA3 genotype, and region. aOR and 95% CI not calculable. Abbreviations: mITT, modified intent‐to‐treat; PNPLA3, patain‐like phospholipase domain‐containing protein 3.
A post‐hoc analysis was conducted to evaluate the effect of biopsy length (<15 or ≥15 mm), in the modified ITT population (http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo). The majority of liver biopsies collected at baseline (78%‐83%) and year 1 (79%‐82%) had a length of ≥15 mm, a length above which sampling variability is expected to be lower.
Findings from this post‐hoc analysis were generally consistent with the main results, except for the smaller subset of subjects with a year 1 liver biopsy length of <15 mm, where placebo response was higher than in other placebo subgroups (22%; 6 of 27 placebo‐treated subjects). In contrast, the most pronounced treatment benefits were observed in the larger subset of subjects with a year 1 liver biopsy of ≥15 mm; improvement in fibrosis by ≥1 stage and no worsening of SH was achieved by 24% (78 of 103) of CVC‐treated subjects compared to 9% (9 of 99) of placebo‐treated subjects. In this subgroup, the OR (CVC/placebo) was 3.21 (95% CI, 1.41‐7.28).
A post‐hoc analysis of predictors of response determined that the factors most strongly associated with improvement in fibrosis stage and no worsening of SH at year 1 were treatment (i.e., receiving CVC), a higher fibrosis stage at baseline, mild or no portal inflammation at baseline, and a higher baseline BMI (P < 0.050 for each, after adjustment for the other predictors). Although differences were observed in subgroups for sex, region, and presence of T2DM, these factors were not associated with response to CVC.
OTHER SECONDARY ENDPOINTS
Change in Fibrosis Stage
The shift in fibrosis stage from baseline to year 1 was assessed using the NASH CRN and Ishak systems (http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo). The proportion of subjects with a decrease in fibrosis stage was 29% for CVC and 19% for placebo using the NASH CRN system; and 35% and 22%, respectively, using the Ishak system. A total of 27 and 20 subjects in the CVC and placebo groups, respectively, improved by one NASH CRN fibrosis stage (33 and 23 subjects, respectively, improved by one Ishak fibrosis stage). Eight and 3 subjects in the CVC and placebo groups, respectively, improved by two fibrosis stages on the NASH CRN system; 10 and 4 subjects, respectively, improved by two stages on the Ishak system. Ten subjects achieved resolution of fibrosis with CVC compared to 5 subjects on placebo (both systems). Two subjects progressed to cirrhosis with CVC compared to 5 subjects on placebo (both systems).
Collagen Area by Morphometry on Liver Biopsy
Change from baseline to year 1 in collagen area by morphometry was analyzed as prespecified in the study protocol. A post‐hoc analysis was then performed to evaluate the change in collagen area from baseline to year 1, according to histological response (i.e., improvement in NASH CRN or Ishak stage) in subjects with paired liver biopsies. At baseline, the mean (SD) collagen area was relatively low in both groups: 2.37 (1.827) for CVC and 2.49 (2.389) for placebo. Although mean (SD) changes from baseline to year 1 were small in both groups (0.02 [2.357] for CVC and –0.14 [2.389] for placebo), a larger proportion of subjects receiving CVC had a reduction in collagen and improvement in fibrosis by at least one stage compared to those receiving placebo (NASH CRN: CVC = 28 of 121 [23%], placebo = 18 of 123 [15%]; Ishak: CVC = 36 of 121 [30%], placebo = 22 of 123 [18%]). Moreover, there was good correspondence between improvement in fibrosis stage and reduction in collagen area by morphometry; of those subjects who achieved an improvement in fibrosis stage (whether in the CVC or placebo groups), the majority (80% for CVC group, 75% for placebo) had a concordant reduction in collagen area. When assessed similarly by Ishak, 84% of CVC and 79% of placebo subjects had a concordant improvement in both fibrosis stage and collagen area.
Another post‐hoc analysis was conducted using only slides with liver biopsy tissue surface of ≥5 mm2, where sampling variability is expected to be lower. In subjects with collagen morphometry of ≥2% at baseline, all subjects achieving at least one stage improvement in fibrosis (NASH CRN) had concordant reduction in collagen at year 1. However, there was substantial variability in changes in collagen between baseline and year 1 in subjects with <2% collagen morphometry at baseline, which represents a sizeable portion of all CENTAUR subjects (http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo).
Improvement in NAS
Changes in histological scores at the end of year 1 for CVC versus placebo for steatosis, lobular inflammation, and hepatocellular ballooning are reported in http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo. No notable differences in the individual components of NAS were observed.
Body weight, Liver and Fasting Metabolic Parameters, and Noninvasive HF Markers
There were no meaningful differences in body weight or BMI (mean change [SD] from baseline to year 1) between groups (–0.24 [4.177] kg for CVC and –0.08 [4.301] for placebo for body weight; –0.13 [1.493] kg/m2 for CVC and –0.01 [1.751] kg/m2 for placebo for BMI). Changes from baseline to year 1 in liver transaminases, fasting metabolic parameters, and noninvasive HF markers (NAFLD fibrosis score [NFS], FIB‐4, APRI, and ELF test) were modest, and similar between the CVC and the placebo groups (Table 2 and http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo).
Table 2.
Change from Baseline to Year 1 in Biomarkers of Systemic Inflammation, Monocyte/Macrophage Activation, CCR2 and CCR5 Blockade, and Hepatocellular Apoptosis (PP Population)
| CVC 150 mg (N = 144) | Placebo (N = 143) | |||||
|---|---|---|---|---|---|---|
| Biomarker | Baseline | Year 1 | Change | Baseline | Year 1 | Change |
| hs‐CRP | ||||||
| no. | 110 | 110 | 110 | 110 | 110 | 110 |
| Median (min, max), mg/L | 2.35 (0.2, 24.0) | 1.70 (0.2, 35.1) | –0.40 (–16.4, 29.1) | 2.45 (0.2, 31.7) | 2.55 (0.3, 34.8) | 0.30 (–10.8, 28.2) |
| 95% CI for difference in change from baseline (CVC 150 mg—placebo) | (–1.3, –0.4) | |||||
| Fibrinogen | ||||||
| no. | 94 | 94 | 94 | 102 | 102 | 102 |
| Median (min, max), mg/dL | 376.5 (145, 607) | 355.5 (20, 536) | –36.5 (–439, 154) | 382.5 (20, 724) | 392.5 (235, 760) | 7.0 (–272, 569) |
| 95% CI for difference in change from baseline (CVC 150 mg—placebo) | (–58, –14) | |||||
| IL‐1β | ||||||
| no. | 95 | 95 | 95 | 102 | 102 | 102 |
| Median (min, max), pg/mL | 0.090 (0.00, 2.69) | 0.050 (0.00, 0.92) | –0.020 (–2.65, 0.76) | 0.030 (0.00, 0.83) | 0.050 (0.00, 1.05) | 0.005 (–0.81, 1.02) |
| 95% CI for difference in change from baseline (CVC 150 mg—placebo) | (–0.06, 0) | |||||
| IL‐6 | ||||||
| no. | 95 | 95 | 95 | 102 | 102 | 102 |
| Median (min, max), pg/mL | 4.30 (1.4, 475.6) | 2.60 (0.9, 521.6) | –1.50 (–13.1, 46.0) | 4.50 (1.5, 22.7) | 3.65 (1.0, 24.4) | –0.55 (–8.8, 12.0) |
| 95% CI for difference in change from baseline (CVC 150 mg—placebo) | (–1.5, –0.2) | |||||
| sCD14 | ||||||
| no. | 97 | 97 | 97 | 103 | 103 | 103 |
| Median (min, max), μg/L | 1,731.0 (138, 3,601) | 1,628.0 (768, 2,635) | –115.0 (–1,306, 1,337) | 1,808.0 (1,030, 3,137) | 1,803.0 (927, 3,562) | –45.0 (–1,199, 1,646) |
| 95% CI for difference in change from baseline (CVC 150 mg—placebo) | (–204, 19) | |||||
| sCD163 | ||||||
| no. | 97 | 97 | 97 | 103 | 103 | 103 |
| Median (min, max), μg/L | 615.0 (263, 1,486) | 615.0 (189, 1,410) | 3.0 (–736, 532) | 679.0 (278, 1,738) | 642.0 (237, 1,927) | –41.0 (–527, 624) |
| 95% CI for difference in change from baseline (CVC 150 mg—placebo) | (–18, 88) | |||||
| CCL2 | ||||||
| no. | 95 | 95 | 95 | 102 | 102 | 102 |
| Median (min, max), pg/mL | 499.00 (166.1, 1,497.4) | 2,115.20 (305.9, 6,725.5) | 1,674.90 (–49.8, 6,351.4) | 464.50 (264.3, 763.6) | 445.40 (240.3, 1,023.4) | –6.20 (–320.7, 452.3) |
| 95% CI for difference in change from baseline (CVC 150 mg—placebo) | (1,454, 1,878) | |||||
| CCL4 | ||||||
| no. | 95 | 95 | 95 | 102 | 102 | 102 |
| Median (min, max), pg/mL | 90.80 (2.6, 2,432.9) | 241.30 (2.5, 36,238.8) | 126.00 (–227.2, 36,190.0) | 92.85 (31.9, 881.0) | 102.70 (5.4, 2,746.4) | 5.00 (–118.0, 2,697.8) |
| 95% CI for difference in change from baseline (CVC 150 mg—placebo) | (103.9, 140.9) | |||||
| CK‐18 (caspase‐cleaved [M30]) | ||||||
| no. | 97 | 97 | 97 | 103 | 103 | 103 |
| Median (min, max) | 624.0 (125, 2,353) | 433.0 (107, 2,562) | –77.0 (–1,600, 1,365) | 704.0 (98, 3,564) | 472.0 (37, 2,426) | –155.0 (–2,240, 1,368) |
| 95% CI for difference in change from baseline (CVC 150 mg—placebo) | (–25, 228) | |||||
| CK‐18 (total [M65]) | ||||||
| no. | 97 | 97 | 97 | 103 | 103 | 103 |
| Median (min, max) | 421.0 (104, 3,673) | 438.0 (84, 7,031) | 1.0 (–1,273, 6,296) | 448.0 (113, 2,149) | 415.0 (100, 6,023) | –22.0 (–1,156, 5,119) |
| 95% CI for difference in change from baseline (CVC 150 mg – placebo) | (–45, 151) | |||||
Abbreviations: CK‐18, cytokeratin 18; PP, per protocol.
A post‐hoc analysis was conducted to explore the relationship between change in fibrosis indices and improvement in liver histology. Changes from baseline to year 1 in fibrosis indices were calculated for subjects who improved in fibrosis by ≥1 stage at year 1 (NASH CRN) and for subjects who did not (http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo). This post‐hoc analysis was not powered to demonstrate a difference for treatment (CVC or placebo) and/or subgroup (histological improvement or not). In general, more favorable changes (i.e., smaller mean increases or larger mean decreases) in fibrosis indices (NFS, FIB‐4, APRI, and ELF) were observed in subjects in whom fibrosis improved by ≥1 stage at year 1 relative to subjects in whom fibrosis did not improve. These observations were noted in subjects who received CVC and in those who received placebo.
Biomarkers of Inflammation
Marked reductions in circulating biomarkers of systemic inflammation (high‐sensitivity C‐reactive protein [hs‐CRP], interleukin [IL]‐6, fibrinogen, and IL‐1ß) and of monocyte activation (sCD14) were observed with CVC (vs. placebo; Table 2). Reciprocal increases in chemokine (C‐C motif) ligands (CCL) 2 and 4 were observed in CVC‐treated subjects only, confirming potent CCR2 and CCR5 blockade, as previously described.16, 17, 22
A post‐hoc analysis was conducted to evaluate correlations between change in markers of inflammation, where pronounced treatment differences were observed (i.e., hs‐CRP, IL‐6, fibrinogen, IL‐1ß, and sCD14), and change in markers of insulin sensitivity (i.e., hemoglobin A1c [HbA1c], homeostatic model of assessment of insulin resistance, and adipose tissue insulin resistance). The results showed limited, if any, relationship (Spearman's rank correlation of 0.20 or less for almost all comparisons; data not shown).
SAFETY AND TOLERABILITY
The safety population comprised all 288 subjects who were randomized and received at least one dose of study drug. The incidence of treatment‐emergent adverse events was similar in both groups, and in general mild or moderate in severity (http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo).
Twenty‐six treatment‐emergent serious adverse events were reported (CVC, n = 16; placebo, n = 10). All serious adverse events but one (grade 2 arrhythmia; subject remained on blinded treatment) were considered not related to treatment. The incidence of treatment‐emergent grade 3 or 4 laboratory abnormalities was generally similar between groups. Grade 4 uric acid elevations, which occurred in subjects with increased baseline values, and asymptomatic grade 3 amylase elevations were observed more frequently in the CVC than placebo group (7.6% vs. 4.2% and 4.2% vs. 0.7%, respectively; http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo). No treatment‐emergent adverse events of pancreatitis were reported in subjects with grade 3 amylase elevations.
Changes from baseline in liver biochemistry and fasting metabolic parameters are reported in http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo.
Discussion
NASH is highly prevalent globally and represents an unmet medical need, based on related morbidity and mortality burdens, and the lack of approved therapies.1 CENTAUR prospectively analyzed and reported on composite clinical efficacy endpoints currently being evaluated in phase 3 NASH studies (NCT02548351, NCT02704403, and NCT030287 40; https://clinicaltrials.gov), and demonstrated a benefit on fibrosis in subjects with NASH after only 1 year of treatment. Although the primary outcome was not met, twice as many subjects on CVC than placebo achieved the clinically important key secondary outcome of improvement in fibrosis by ≥1 stage and no worsening of SH. Fibrosis is the only histological feature that has been independently associated with clinical outcomes in longitudinal cohorts.3, 4, 5 CENTAUR exclusively enrolled subjects with NASH and LF; additionally, subjects were required to have active metabolic dysfunction (T2DM or MetS), a well‐known risk factor for disease progression. The primary outcome was chosen based on the standard established in past phase 2 studies that assessed the efficacy of NASH therapies.9, 10 Improvement in fibrosis by ≥1 stage and no worsening of SH was selected as one of the two key secondary outcomes, both because of its association with clinical outcomes and to inform the phase 3 program. Greater CVC treatment benefits were observed in subjects with higher disease activity and fibrosis stage (i.e., NAS ≥5, prominent hepatocellular ballooning, and moderate‐to‐severe fibrosis); these observations help identify which patients are most likely to benefit from CVC treatment and are aligned with known risk factors of disease progression. The majority of subjects who achieved an improvement in fibrosis stage also achieved a reduction in collagen area by morphometry, supporting findings from secondary efficacy endpoints related to improvement in fibrosis.
The safety and tolerability of NASH therapies are paramount, because the condition is typically asymptomatic and patients are often being treated for comorbidities including T2DM and CVD. In CENTAUR, the incidence of treatment‐emergent adverse events and laboratory abnormalities was comparable between CVC and placebo. The most frequently reported treatment‐emergent adverse events of at least moderate severity (i.e., fatigue, diarrhea, and headache) were consistent with the extensive clinical experience with CVC in past studies.16, 17, 22 Changes in fasting metabolic parameters from baseline were relatively small and comparable between groups, indicating that CVC is not likely to worsen pre‐existing metabolic disease in NASH patients.
The results of CENTAUR are potentially paradigm‐shifting, given that they challenge the common assumption that the antifibrotic effects of NASH agents can only be observed by improving the underlying metabolic liver disease. Instead, the beneficial impact of CVC on fibrosis without affecting the histological features of SH at year 1 reinforces the rationale for directly targeting inflammatory and fibrotic mechanisms. The antifibrotic activity of CVC observed here is consistent with findings in several animal models of chronic liver injury.11 Although the study did not meet the primary endpoint at year 1, it nonetheless underscores the evolving principles of clinical‐trial design that increasingly look to assign endpoints that are aligned with the mechanism of action.
Based on its mechanism of action, the lack of effect of CVC on lobular inflammation was unexpected and will need to be further explored. One possible explanation may be that the impact of CVC on the composition of immune cells in the inflamed lobule, as well as the noncellular components of inflammation (i.e., chemokines and soluble mediators), cannot be fully characterized by the hematoxylin‐eosin stain alone (used to grade the degree of lobular inflammation). Detailed characterization of immune cell subsets will be valuable in the future to further clarify the impact of CVC on hepatic inflammation. Although the NAS has been widely used to evaluate early treatment effects in phase 2 studies, it does not distinguish targeted effects of CVC on CCR2‐expressing monocyte‐derived macrophages, as demonstrated in models of liver injury.12, 14 Specifically, activities of chemokine signaling, including intrahepatic monocyte and macrophage recruitment and fibrogenesis, occur downstream of liver‐cell injury and metabolic dysregulation in the pathophysiology of NASH; therefore, they may not be reflected in the traditional histological features of the NAS, including steatosis, lobular inflammation, and hepatocellular ballooning. Therefore, further evaluation using cell‐specific markers will be required to elucidate the effects of CVC on immune cells in patients.
Importantly, a broad mechanistic impact of CVC on inflammatory signaling is underscored by reductions in circulating markers of systemic inflammation (ie, hs‐CRP, IL‐6, fibrinogen) and sCD14 (a marker of monocyte activation), which is consistent with previous studies in subjects with HIV infection.17, 22
In this study in subjects with NASH, a large histological data set of 252 paired biopsies was available for year 1 evaluation in the modified ITT population. All liver biopsies were read centrally by a single pathologist, thereby reducing reader variability. Limitations of our study include: differences in responses among subgroups (e.g., region, sex, and T2DM) that may reflect the multifactorial nature of the disease or be associated with the sample size of the subgroups; and the inherent variability of liver biopsy sampling,23 which will require further investigation in subsequent studies.
Improvement in fibrosis stage has been reported in phase 2 NASH randomized clinical trials, in some studies as early as 24 weeks.10, 24, 25 These and similar studies have also demonstrated that a small, but significant, proportion of subjects, up to approximately 20%,8, 9, 10 will have spontaneous improvement on placebo. This improvement has often been attributed to increased clinical monitoring, motivation, and compliance to diet and lifestyle changes of subjects participating in such trials. Therefore, the observation that some placebo subjects improved in the CENTAUR study is neither unexpected nor out of line with other reported results.
In conclusion, CVC showed a significant antifibrotic benefit at year 1 and was well tolerated. Although the primary endpoint of the study was not met, the fact that the CENTAUR year 1 study results showed that CVC provided clinically meaningful benefits and resulted in twice as many subjects achieving “improvement in fibrosis by ≥1 stage and no worsening of SH” as compared to placebo suggests that the study did, in fact, show proof of concept, warranting phase 3 development of CVC. If this benefit is corroborated by the continued follow‐up over the planned second year of treatment, and subsequent confirmatory trials, CVC will represent an important advance in the treatment of LF in patients with NASH.
Author names in bold designate shared co‐first authorship.
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo.
Supporting Information 1
Acknowledgments
The investigators thank the study subjects for their participation, and the CENTAUR study team. Editorial support was provided by Catrina Milgate, Sandra Whitelaw, Heather Bromby and Camille Bonomelli of Alpharmaxim Healthcare Communications. The full clinical trial protocol can be accessed as a http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo.
Potential conflict of interest: Dr. Friedman consults for, advises for, and received grants from Tobira and Allergan. Dr. Goodman received grants from Allergan, Gilead, Conatus, Galectin, Exalenz, and Alexion. Dr. Kowdley consults for, advises for, is on the speakers' bureau for, and received grants from Gilead. He consults for, advises for, and is on the speakers' bureau for Intercept. He consults and received grants from NGM Biopharma. He consults for Enanta and Verylx. He advises for Allergan and Conatus. He received grants from Galectin, Immuron, and Tobira. Dr. Tacke advises for and received grants from Tobira and Allergan. Dr. Ratziu consults for Allergan, Genfit, Intercept, Novartis, Boehringer Ingelheim, and Pfizer. He received grants from Gilead. Dr. Wong advises for and received lecture fees from Gilead. He consults for Allergan and Pfizer. He advises AbbVie, Janssen, and Perspectum. He received lecture fees from Bristol‐Myers Squibb, Echosens, and Merck. Dr. Sanyal is President of Sanyal Biotechnology and has stock options in Genfit, Akarna, Tiziana, Indalo, Durect. He has served as a consultant to AbbVie, Astra Zeneca, Allergan, Nitto Denko, Ardelyx, Conatus, Nimbus, Amarin, Salix, Tobira, Takeda, Sanofi, General Electric, Fibrogen, Jannsen, Gilead, Boehringer, Lilly, Zafgen, Novartis, Pfizer, Immuron, Exhalenz and Genfit. He has been an unpaid consultant to Intercept, Echosens, Immuron, Galectin, Fractyl, Syntlogic, Novo Nordisk, Affimune, Chemomab, Nordic Bioscience and Bristol Myers Squibb. His institution has received grant support from Gilead, Salix, Tobira, Bristol Myers, Shire, Intercept, Merck, Astra Zeneca, Malinckrodt, Cumberland and Novartis. . He receives royalties from Elsevier and UptoDate. Dr. Harrison consults and received grants from Genfit. He consults for Allergan, Cirius, NGM, Madrigal, and Perspectum. He advises for Intercept, Conatus, Echosens, Galmed, Gilead, Pfizer, and Fibrogen. He is on the speakers' bureau for Alexion. Dr. Abdelmalek advises and has received grants from Allergan. Dr. Fischer is employed by Allergan. Mrs. Melchor‐Khan is employed by Allergan. Dr. Wiens is employed by Allergan. Dr. Lefebvre is employed by Allergan. Dr. Vig is employed by Allergan. Dr. Seyedkazemi is employed by Allergan.
The CENTAUR study was sponsored by Tobira Therapeutics, a subsidiary of Allergan plc. The sponsor provided funding for the study and provided the study drug. Authors who were employees of Tobira Therapeutics were involved in study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analyses; obtained funding; administrative, technical or material support; and study supervision.
http://ClinicalTrials.gov no: NCT02217475 (CENTAUR).
REFERENCES
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease–meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 2. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol 2012;107:811‐826. [DOI] [PubMed] [Google Scholar]
- 3. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology 2015;61:1547‐1554. [DOI] [PubMed] [Google Scholar]
- 5. Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver‐related mortality. Hepatology 2011;53:1874‐1882. [DOI] [PubMed] [Google Scholar]
- 6. Ratziu V, Goodman Z, Sanyal A. Current efforts and trends in the treatment of NASH. J Hepatol 2015;62(1 Suppl):S65‐S75. [DOI] [PubMed] [Google Scholar]
- 7. Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz‐Lopez C, et al. Long‐term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized, controlled trial. Ann Intern Med 2016;165:305‐315. [DOI] [PubMed] [Google Scholar]
- 8. Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, et al. Elafibranor, an agonist of the peroxisome proliferator‐activated receptor‐α and ‐δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 2016;150:1147‐1159. [DOI] [PubMed] [Google Scholar]
- 9. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neuschwander‐Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo‐controlled trial. Lancet 2015;385:956‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lefebvre E, Moyle G, Reshef R, Richman LP, Thompson M, Hong F, et al. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS One 2016;11:e0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Puengel T, Krenkel O, Mossanen J, Longerich E, Lefebvre E, Trautwein C, et al. The dual CCR2/CCR5 antagonist cenicriviroc ameliorates steatohepatitis and fibrosis in vivo by inhibiting the infiltration of inflammatory monocytes into injured liver. J Hepatol 2016;64:s159‐s182. [Google Scholar]
- 13. Tacke F, Poulin D, Jenkins H, Wolfgang G, Lefebvre E. Oral, dual CCR2/CCR5 antagonist, cenicriviroc, leads to dose‐dependent decreases in monocyte recruitment in a thioglycollate‐induced model of peritonitis. Poster number 8 presented at AASLD and Industry Colloquium: Novel Targets and Therapies in Liver Disease, 20 March 2015, Durham, NC.
- 14. Mossanen JC, Krenkel O, Ergen C, Govaere O, Liepelt A, Puengel T, et al. Chemokine (C‐C motif) receptor 2‐positive monocytes aggravate the early phase of acetaminophen‐induced acute liver injury. Hepatology 2016;64:1667‐1682. [DOI] [PubMed] [Google Scholar]
- 15. Friedman SL, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, Fischer L, et al. Efficacy and safety study of cenicriviroc for the treatment of non‐alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp Clin Trials 2016;47:356‐365. [DOI] [PubMed] [Google Scholar]
- 16. Lefebvre E, Gottwald M, Lasseter K, Chang W, Willett M, Smith PF, et al. Pharmacokinetics, safety, and CCR2/CCR5 antagonist activity of cenicriviroc in participants with mild or moderate hepatic impairment. Clin Transl Sci 2016;9:139‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thompson M, Saag M, Dejesus E, Gathe J, Lalezari J, Landay AL, et al. A 48‐week randomized Phase 2b study evaluating cenicriviroc vs. efavirenz in treatment‐naive HIV‐infected adults with CCR5‐tropic virus. AIDS 2016;30:869‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sherman KE, Abdel‐Hameed E, Rouster SD. CCR2/CCR5 antagonism with cenicriviroc decreases fibrosis scores in HIV‐infected patients. Presented at HEP DART 2015, Frontiers in Drug Development for Viral Hepatitis, 6 December 2015, Wailea, Hawaii.
- 19. Thompson M, Chang W, Jenkins H, Flynt A, Gottwald M, Lefebvre E. Improvements in APRI and FIB‐4 fibrosis scores correlate with decreases in sCD14 in HIV‐1 infected adults receiving cenicriviroc over 48 weeks. Hepatology 2014;60(Suppl 1):424A. [Google Scholar]
- 20. Lefebvre E, Smith P, Willett MS, Lasseter KC, Chang W, Gottwald MD. Pharmacokinetics and safety of multiple‐dose cenicriviroc, a novel, oral, once‐daily CCR2 and CCR5 antagonist, in adults with mild or moderate hepatic impairment. Hepatology 2014;60(1 Suppl):23‐24. [Google Scholar]
- 21. Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology 2014;147:577‐594. [DOI] [PubMed] [Google Scholar]
- 22. Lalezari J, Gathe J, Brinson C, Thompson M, Cohen C, Dejesus E, et al. Safety, efficacy, and pharmacokinetics of TBR‐652, a CCR5/CCR2 antagonist, in HIV‐1‐infected, treatment‐experienced, CCR5 antagonist‐naive subjects. J Acquir Immune Defic Syndr 2011;57:118‐125. [DOI] [PubMed] [Google Scholar]
- 23. Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005;128:1898‐1906. [DOI] [PubMed] [Google Scholar]
- 24. Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo‐controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;355:2297‐2307. [DOI] [PubMed] [Google Scholar]
- 25. Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, et al. GS‐4997, an inhibitor of apoptosis signal‐regulating kinase (ASK1), alone or in combination with simtuzumab for the treatment of nonalcoholic steatohepatitis (NASH): a randomized, phase 2 trial [abstract]. Hepatology 2016;64:1119A‐1120A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep.29477/suppinfo.
Supporting Information 1


