Abstract
Although metal‐ion‐binding interlocked molecules have been under intense investigation for over three decades, their application as scaffolds for the development of sensors for metal ions remains underexplored. In this work, we demonstrate the potential of simple rotaxanes as metal‐ion‐responsive ligand scaffolds through the development of a proof‐of‐concept selective sensor for Zn2+.
Keywords: fluorescent probes, rotaxanes, sensors, supramolecular chemistry, zinc
Small‐molecule fluorescent probes are powerful tools for visualizing metal ions in living systems due to their rapid response times and potential for non‐invasive, high resolution, and quantitative imaging.1 In particular, the development of small‐molecule sensors2, 3 for the detection and quantification of Zn2+ in vivo has attracted considerable recent attention due to the spectroscopically silent nature of the d10 Zn2+ ion, combined with the recognition that changes in zinc homeostasis are associated with high‐morbidity diseases such as Alzheimer's disease,4 Type II diabetes,5 and age‐related macular degeneration.6
Such small‐molecule probes are generally composed of a multidentate chelating ligand linked to a fluorophore whose output is modulated by the metal binding event. Mechanically interlocked molecules,7 particularly those synthesized using metal‐mediated approaches,8 often possess a well‐defined binding pocket containing multiple donor atoms for metal ions.9 Such multidentate “mechanically chelating” ligands10 seem ideal for the development of metal‐selective ligands and related metal‐ion sensors by exploiting the size and shape of the three dimensional cavity formed by the mechanical bond. However, almost all interlocked molecules that display a fluorescent response11 upon metal binding rely on large‐amplitude motion in relatively structurally complex molecular shuttles.12, 13, 14 Furthermore, in most cases, selectivity between competing analytes is not reported. Indeed, to our knowledge, only one example has been reported in which the mechanical bond is used to generate a metal binding pocket to report the binding of competing analytes; in 2004 Hiratani and co‐workers disclosed a [1]rotaxane that selectively binds Li+ over Na+ and K+ and reports metal binding through a “switch on” fluorescence response.15, 16
Given that the synthesis of mechanically chelating ligands is now relatively simple, it is perhaps surprising that these scaffolds have been overlooked in the development of cation sensors, particularly since a related strategy for the sensing of anions has been developed by Beer and co‐workers.17 We thus set out to demonstrate the potential of the mechanical bond as a structural motif in the development of selective metal‐ion sensors through the demonstration of a proof‐of‐concept selective sensor for Zn2+. Herein, we report not only that is this approach successful, but that relatively small structural changes in the axle component lead to large changes in the photophysical response to divalent metal ions.
We synthesized rotaxane 4 18 in excellent yield (88 %) using the active‐template19 Cu‐mediated alkyne–azide cycloaddition (AT‐CuAAC) reaction20, 21 between azido fluorophore 3 and acetylene 2 in the presence of readily available bipyridine macrocycle 1 22 and [Cu(MeCN)4]PF6. Addition of one equivalent of Zn(ClO4)2⋅6 H2O to a solution of 4 in CD3CN resulted in large changes in the 1H NMR spectrum (see Figure S39 in the Supporting Information), which is consistent with the binding of the metal ion into the cavity of the macrocycle, confirming that 4 is capable of acting as a ligand. The behavior of rotaxane 4 as a metal‐responsive sensor for Zn2+ was investigated by fluorescence titration with Zn(ClO4)2⋅6 H2O. Portion‐wise addition of Zn2+ to rotaxane 4 in MeCN led to monotonic quenching of the emission at 560 nm23 that plateaued once a full equivalent had been added (Figure 1 a). In contrast, titration of the non‐interlocked axle with Zn2+ revealed no change by UV/Vis or fluorescence spectroscopy (Figure S61).24
Figure 1.
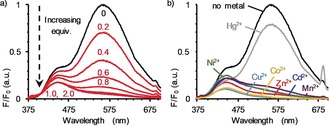
Emission profile of rotaxane 4 (MeCN, 100 μm, λ ex=343 nm) in the presence of varying amounts of Zn(ClO4)2⋅6 H2O (a), and in the presence of 5 equiv M(ClO4)2 (b). No emission was observed with Fe(ClO4)2.
Rotaxane 4 is a candidate “switch off” fluorescent sensor for Zn2+. However, examination of the selectivity of this response revealed a complete lack of discrimination between Zn2+ and selected divalent metal cations; addition of M(ClO4)2 (M=Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Cd2+, Hg2+) to a solution of 4 led to quenching of the emission to a greater or lesser extent than that observed with Zn2+ (Figure 1 b).23 Thus, 4 cannot be classed as a metal‐ion sensor since, although it responds to metal‐ion binding, it cannot discriminate between competing analytes.
Having confirmed that the binding of metal ions within the cavity of the rotaxane can, in principle, lead to an optical response, we extended our investigation to heteroatom‐substituted naphthalimide rotaxanes 5–7 (Figure 2), which were readily synthesized in good to excellent yield (62 %, 72 % and 86 % respectively, see the Supporting Information). These were selected because heteroatoms can potentially interact directly with the metal ion and are known to significantly alter the photophysical properties of naphthalimide fluorophores.25 1H NMR spectroscopy (Figures S39–41) confirmed that rotaxanes 5–7 act as ligands for Zn2+, and UV/Vis titration revealed excellent goodness of fit to a 1:1 binding isotherm; addition of Zn(ClO4)2⋅6 H2O resulted in the appearance of absorbance bands at 312 and 322 nm corresponding to the metal‐bound bipyridine moiety (Figures S46–48).26
Figure 2.
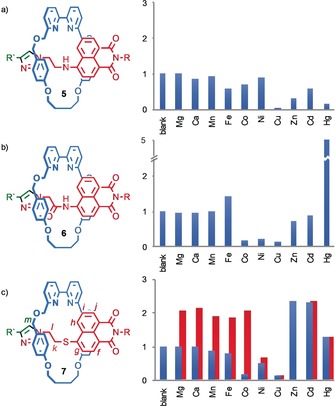
Rotaxanes a) 5 (λ ex=435 nm), b) 6 (λ ex=380 nm), and c) 7 (λ ex=379 nm) and their fluorescence response to 5 equiv M(ClO4)2 (MeCN, 100 μm; blue bars). Red bars in (c) refer to the fluorescence response upon sequential addition of M2+ followed by Zn2+ (5 equiv each). R=CH2C(H)Ph2, R′=3,5‐di‐tBu‐C6H3.
The fluorescence response of rotaxanes 5–7 to the binding of selected divalent metal ions revealed very different behavior. Similar to rotaxane 4, rotaxane 5 displays a simple switch‐off response to Zn2+ and the majority of other metal ions investigated (Figure 2 a), although the extent of quenching varied considerably depending on the metal ion. In contrast, rotaxane 6 (Figure 2 b) displays little or no response to Mg2+, Ca2+, or Mn2+, a switch‐off response of varying degree to Co2+, Ni2+, Cu2+ Zn2+, and Cd2+ and a weak switch‐on response to Fe2+. Strikingly, Hg2+ produced a significant switch‐on response, suggesting that rotaxane 6 is a good starting point for the development of a selective sensor for Hg2+. Pleasingly, addition of Zn2+ to rotaxane 7 triggers a switch‐on response with a concomitant blue shift in the emission of 15 nm. All other metal ions, with the exception of Cd2+, which produces a switch‐on response and a blue shift of 8 nm, produce a switch‐off or no response (Figure 2 c). Competition experiments demonstrate that in many cases, Zn2+ is also able to displace metal ions from ligand 7; addition of M2+ followed by Zn2+ to 7 resulted in recovery of fluorescence in the case of Mg2+, Ca2+, Mn2+, Fe2+, and Co2+.
Having identified rotaxane 7 as a switch‐on sensor for Zn2+, albeit with Cd2+ as a confounding analyte,27 we investigated its behavior in more detail. The 1H NMR spectrum of 7 (Figure 3 b and Scheme 1) displays a number of differences to the corresponding non‐interlocked axle (Figure 3 a). In particular triazole proton Hm appears significantly deshielded in the interlocked structure by Δδ=1.36 ppm, which is consistent with an expected C−H⋅⋅⋅N hydrogen bond with the bipyridine moiety,28 and alkyl protons Hk and Hl appear at lower ppm, suggesting that they engage in C−H⋅⋅⋅π contacts with the aromatic rings of the macrocycle. Crystals of 7 suitable for X‐ray analysis were grown by slow evaporation from MeCN. The solid‐state structure is largely consistent with the interactions proposed to be present in solution (Figure 4 a); triazole proton Hm is engaged in a C−H⋅⋅⋅N hydrogen bond with the bipyridine nitrogens, as is one of Hk, and one each of protons Hk and Hl are in close contact with the phenyl rings of the macrocycle.
Figure 3.
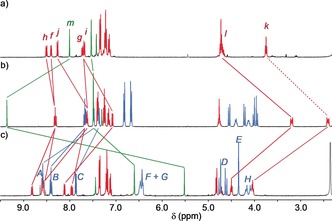
Partial 1H NMR spectra (CD3CN, 400 MHz, 298 K) of a) the non‐interlocked axle of rotaxane 7, b) rotaxane 7, and c) rotaxane 7+Zn(ClO4)2⋅6 H2O. For labelling scheme, see Scheme 1 (macrocycle) and Figure 2 (axle).
Scheme 1.
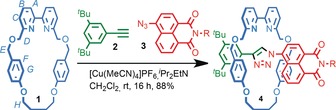
Synthesis of fluorescent rotaxane 4 using the AT‐CuAAC reaction. R=CH2C(H)Ph2.
Figure 4.
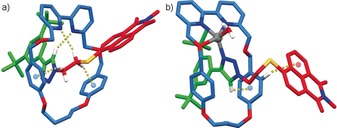
a) Solid‐state structure of rotaxane 7 (selected distances in Å: C−Hm⋅⋅⋅N=2.46, C‐Hk⋅⋅⋅N=2.71, C−Hk⋅⋅⋅π=2.66, C‐Hl⋅⋅⋅π=2.79; dihedral angle Ck‐S‐C‐Cipso=8.2°). b) Solid‐state structure of [Zn(7)]2+ (selected distances: C−O⋅⋅⋅Zn=2.28, C−Hm⋅⋅⋅π=2.74, C−HF⋅⋅⋅π=2.86; dihedral angle Ck‐S‐C‐Cipso=29.9°).
Portion‐wise addition of Zn(ClO4)2⋅6 H2O to 7 in CD3CN led to broadening of the 1H NMR resonances corresponding to 7 and the appearance of a new set of resonances assigned to [Zn(7)]2+ (Figure S42). Once one equivalent of Zn2+ had been added (Figure 3 c), no further change was observed. Strikingly, in addition to the expected deshielding of the bipyridine resonances HA, HB, and HC, the triazole and alkyl resonances shift significantly upon Zn2+ binding. Hm is observed at 5.52 ppm in the metal complex (Δδ=−3.84 ppm), suggesting it is considerably shielded relative to the non‐interlocked axle, while Hk and Hl shift to higher ppm, suggesting that the shielding C−H⋅⋅⋅π interactions are interrupted.
The solid‐state structure of [Zn(7)](OTf)2 obtained by slow evaporation of a MeCN solution confirms coordination of Zn2+ within the macrocycle cavity and exhibits a number of features consistent with the solution‐state 1H NMR data. The Zn2+ ion is coordinated by the bipyridine and triazole N‐donors, alongside one of the aliphatic ether O‐donors and a water molecule.29 This coordination interrupts the triazole C−H⋅⋅⋅N hydrogen bond and this proton is now engaged in a shielding C−H⋅⋅⋅π interaction with one of the macrocycle phenyl rings. The shielding C−H⋅⋅⋅π interactions of Hk and Hl are also interrupted. Interestingly, in the solid state, coordination leads to the appearance of a C−H⋅⋅⋅π interaction between one of the aromatic phenyl ring protons and the naphthalene rings and a large change in the dihedral angle about the S‐naphthyl bond. With the obvious caveat that the solid‐state structure of [Zn(7)]2+ is not necessarily representative of the solution‐state (co)conformation, the observed changes in the interactions between the macrocycle and fluorophore component, along with the changes in the conjugation between the S‐donor and the naphthalene core and the altered dihedral angle, suggest that the changes in emission properties of 7 on metal binding may be due to changes in the (co)conformation of the ensemble.
The binding of rotaxane 7 with Zn2+ was determined to be extremely strong (K d<10−8 m −1) by UV/Vis titration30 with non‐linear regression analysis (Figure 5 a), stronger than that of macrocycle 1 alone (K d=8.9×10−8 m −1; Figure S57), suggesting that the mechanically chelating triazole ligand significantly enhances Zn2+ binding in 7.31 Conversely, the binding of rotaxane 7 to Cd2+ (K d=3.6×10−5 m −1; Figure 5 b) is about three orders of magnitude weaker than that of macrocycle 1 alone (7.5×10−8 m −1; Figure S59). The difference in binding strength for 7 with Zn2+ and Cd2+ was further corroborated by 1H NMR titration. In the presence of 1 equiv of both Zn2+ and Cd2+, [Zn(7)]2+ is observed to form selectively (Figure S43), whereas the same experiment with macrocycle 1 produced a 4:1 mixture of Zn2+ and Cd2+ complexes (Figure S45). These results demonstrate that the mechanical bond imparts a significant degree of selectivity to the binding of otherwise similar metal ions, perhaps due to the different sizes of the Zn2+ and Cd2+ ions (88 pm vs. 109 pm, respectively)32 or by sterically excluding additional ligands from the coordination sphere of the metal ion.
Figure 5.
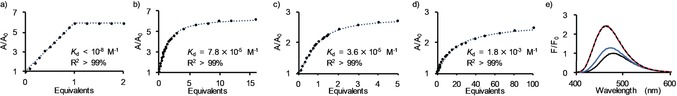
UV/Vis (λ=322 nm) titrations of 7 (100 μm) with M(ClO4)2 as a function of a) Zn2+, b) Zn2+ (2 % H2O/MeCN), c) Cd2+ (MeCN), and d) Cd2+ (2 % H2O‐MeCN). e) Emission spectra (λ ex=380 nm) of 7 (black), 7+5 equiv Cd2+ (blue), 7+5 equiv Zn2+ (red), 7+5 equiv each Zn2+ and Cd2+ (black dashed) in 2 % H2O/MeCN.
The stronger binding of Zn2+ compared with Cd2+ allowed us to use a more competitive solvent mixture (2 % water in MeCN) to impart selectivity to sensor 7. In the presence of H2O (Figures 5 c,d), the binding of both Zn2+ and Cd2+ to 7 was diminished (K d=7.8×10−5 and 1.8×10−3 m −1 respectively). As a result, whereas 1 equiv of Zn2+ achieved 50 % of F max and saturation was achieved at approximately 12 equiv, Cd2+ required around 20 equiv to achieve 50 % switch on and around 100 equiv to achieve saturation, demonstrating that under these conditions, Cd2+ is not bound by 7 to a significant extent. Thus, whereas addition of 5 equivalents of Cd2+ to 7 in MeCN/H2O leads to a weak response, when Zn2+ is added to the same solution, the expected switching on of luminescence is observed, thus demonstrating selectivity for Zn2+ over Cd2+ (Figure 5 e).
In conclusion, we have demonstrated that relatively simple interlocked molecules can provide an excellent scaffold for the design of metal ion sensors. Importantly we show that the binding pocket provided by the mechanical bond can impart not only an optical response but also a degree of binding selectivity, as in the case of rotaxane 7. It is also noteworthy that, in addition to sensor 7, which shows the desired Zn2+‐selective response in MeCN/H2O, rotaxane 6 also appears to show a selective switch‐on response, in this case to Hg2+.33 The mode of switching, at least in the case of 7, appears to be reorientation of the components upon metal binding, thereby altering the relative positions of the fluorophore and the macrocycle and leading to an enhancement of fluorescence, but this requires more detailed investigation. The origin of the different behaviors of 4–6 also requires further investigation; since it appears that the different linker units are not directly involved in the binding event, it seems likely that the specific photophysical properties of the fluorophore unit are important.25 From a practical viewpoint, the next step in the development of interlocked hosts for the detection of metal ions in biological systems is to render them water soluble, a task that is ongoing in our laboratories and is greatly facilitated by the synthetic flexibility of the AT‐CuAAC reaction. More generally, the results presented here suggest that, although interlocked molecular machines remain an exciting and important direction for the field, the use of the mechanical bond as a structural feature, for instance in the design of mechanically chelating ligands, has the potential to lead to new developments in a range of areas.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
K.J. and J.P. contributed equally. M.D. thanks the University of Southampton and EPSRC (EP/L505067/1) for financial support. K.J. thanks QMUL for financial support. J.P. thanks the EPSRC for financial support. S.M.G acknowledges funding from the European Research Council (Consolidator Grant Agreement no. 724987). S.M.G thanks the Royal Society for a Research Fellowship. M.W. acknowledges COST Action TD1304 Zinc‐Net. We are grateful to the EPSRC UK National Mass Spectrometry Facility (NMSF) for high resolution mass spectrometry.
M. Denis, J. Pancholi, K. Jobe, M. Watkinson, S. M. Goldup, Angew. Chem. Int. Ed. 2018, 57, 5310.
Contributor Information
Prof. Dr. Michael Watkinson, Email: m.watkinson@qmul.ac.uk
Prof. Dr. Stephen M. Goldup, Email: s.goldup@soton.ac.uk.
References
- 1.Reviews:
- 1a. Domaille D. W., Que E. L., Chang C. J., Nat. Chem. Biol. 2008, 4, 168; [DOI] [PubMed] [Google Scholar]
- 1b. Chan J., Dodani S. C., Chang C. J., Nat. Chem. 2012, 4, 973; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1c. Carter K. P., Young A. M., Palmer A. E., Chem. Rev. 2014, 114, 4564; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1d. Pak Y. L., Swamy K. M. K., Yoon J., Sensors 2015, 15, 24374; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1e. Qian X. H., Xu Z. C., Chem. Soc. Rev. 2015, 44, 4487; [DOI] [PubMed] [Google Scholar]
- 1f. Li J., Yim D., Jang W.-D., Yoon J., Chem. Soc. Rev. 2017, 46, 2437. [DOI] [PubMed] [Google Scholar]
- 2.Review of Zn2+ sensing in biology: Maret W., Metallomics 2015, 7, 202. [DOI] [PubMed] [Google Scholar]
- 3.Review of genetically encoded sensors: Aper S. J. A., Dieriekx P., Merkx M., ACS Chem. Biol. 2016, 11, 2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnham K. J., Bush A. I., Curr. Opin. Chem. Biol. 2008, 12, 222. [DOI] [PubMed] [Google Scholar]
- 5. Rutter G. A., Islets 2010, 2, 49. [DOI] [PubMed] [Google Scholar]
- 6. Lengyel I., Flinn J. M., Peto T., Linkous D. H., Cano K., Bird A. C., Lanzirotti A., Frederickson C. J., van Kuijk F. J. G. M., Exp. Eye Res. 2007, 84, 772. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a. Neal E. A., Goldup S. M., Chem. Commun. 2014, 50, 5128; [DOI] [PubMed] [Google Scholar]
- 7b. Xue M., Yang Y., Chi X., Yan X., Huang F., Chem. Rev. 2015, 115, 7398; [DOI] [PubMed] [Google Scholar]
- 7c. Bruns C. J., Stoddart J. F., The Nature of the Mechanical Bond: From Molecules to Machines, Wiley, Hoboken, 2016. [Google Scholar]
- 8.
- 8a. Beves J. E., Blight B. A., Campbell C. J., Leigh D. A., McBurney R. T., Angew. Chem. Int. Ed. 2011, 50, 9260; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 9428; [Google Scholar]
- 8b. Lewis J. E. M., Beer P. D., Loeb S. J., Goldup S. M., Chem. Soc. Rev. 2017, 46, 2577. [DOI] [PubMed] [Google Scholar]
- 9.
- 9a. Albrecht-Gary A. M., Saad Z., Dietrich-Buchecker C. O., Sauvage J.-P., J. Am. Chem. Soc. 1985, 107, 3205; [Google Scholar]
- 9b. Blake A. J., Dietrich-Buchecker C. O., Hyde T. I., Sauvage J.-P., Schröder M., Chem. Commun. 1989, 1663; [Google Scholar]
- 9c. Dietrich-Buchecker C. O., Kern J.-M., Sauvage J.-P., Chem. Commun. 1985, 760; [Google Scholar]
- 9d. Dietrich-Buchecker C., Sauvage J.-P., Kern J. M., J. Am. Chem. Soc. 1989, 111, 7791; [Google Scholar]
- 9e. Masood A., Zacharias P. S., Polyhedron 1991, 10, 811; [Google Scholar]
- 9f. Leigh D. A., Lusby P. J., Slawin A. M. Z., Walker D. B., Angew. Chem. Int. Ed. 2005, 44, 4557; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2005, 117, 4633; [Google Scholar]
- 9g. Baggi G., Loeb S. J., Angew. Chem. Int. Ed. 2016, 55, 12533; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 12721; [Google Scholar]
- 9h. Baggi G., Loeb S. J., Chem. Eur. J. 2017, 23, 14163; [DOI] [PubMed] [Google Scholar]
- 9i. Ngo T. H., Labuta J., Lim G. N., Webre W. A., D'Souza F., Karr P. A., Lewis J. E. M., Hill J. P., Ariga K., Goldup S. M., Chem. Sci. 2017, 8, 6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.“Mechanically chelating” is used to describe ligands in which donor atoms bridge the mechanical bond: Lewis J. E. M., Galli M., Goldup S. M., Chem. Commun. 2017, 53, 298. [Google Scholar]
- 11.
- 11a. Ma X., Tian H., Chem. Soc. Rev. 2010, 39, 70; [DOI] [PubMed] [Google Scholar]
- 11b. Langton M. J., Beer P. D., Acc. Chem. Res. 2014, 47, 1935. [DOI] [PubMed] [Google Scholar]
- 12.
- 12a. Zhou W., Li J., He X., Li C., Lv J., Li Y., Wang S., Liu H., Zhu D., Chem. Eur. J. 2008, 14, 754; [DOI] [PubMed] [Google Scholar]
- 12b. Hsueh S. Y., Lai C. C., Chiu S. H., Chem. Eur. J. 2010, 16, 2997. [DOI] [PubMed] [Google Scholar]
- 13.Examples where the response is not due to shuttling:
- 13a. Armaroli N., De Cola L., Balzani V., Sauvage J.-P., Dietrich-Buchecker C. O., Kern J.-M., Bailalb A., J. Chem. Soc. Dalton Trans. 1993, 3241; [Google Scholar]
- 13b. Armaroli N., De Cola L., Balzani V., Barigelletti F., Flamigni L., Sauvage J. P., Hemmert C., J. Am. Chem. Soc. 1994, 116, 5211; [Google Scholar]
- 13c. MacLachlan M. J., Rose A., Swager T. M., J. Am. Chem. Soc. 2001, 123, 9180; [DOI] [PubMed] [Google Scholar]
- 13d. Kwan P. H., MacLachlan M. J., Swager T. M., J. Am. Chem. Soc. 2004, 126, 8638; [DOI] [PubMed] [Google Scholar]
- 13e. Kwan P. H., Swager T. M., J. Am. Chem. Soc. 2005, 127, 5902; [DOI] [PubMed] [Google Scholar]
- 13f. Nakatani Y., Furusho Y., Yashima E., Angew. Chem. Int. Ed. 2010, 49, 5463; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 5595. [Google Scholar]
- 14. Erbas-Cakmak S., Leigh D. A., McTernan C. T., Nussbaumer A. L., Chem. Rev. 2015, 115, 10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hiratani K., Kaneyama M., Nagawa Y., Koyama E., Kanesato M., J. Am. Chem. Soc. 2004, 126, 13568. [DOI] [PubMed] [Google Scholar]
- 16.An example of selective discrimination of alkali metal ions by a rotaxane host using 1H NMR: Chen N.-C., Huang P.-Y., Lai C.-C., Liu Y.-H., Wang Y., Peng S.-M., Chiu S.-H., Chem. Commun. 2007, 4122. [DOI] [PubMed] [Google Scholar]
- 17.See Ref. [11b] and:
- 17a. Curiel D., Beer P. D., Chem. Commun. 2005, 1909; [DOI] [PubMed] [Google Scholar]
- 17b. Bayly S. R., Gray T. M., Chmielewski M. J., Davis J. J., Beer P. D., Chem. Commun. 2007, 2234; [DOI] [PubMed] [Google Scholar]
- 17c. Chmielewski M. J., Davis J. J., Beer P. D., Org. Biomol. Chem. 2009, 7, 415; [DOI] [PubMed] [Google Scholar]
- 17d. Evans N. H., Beer P. D., Org. Biomol. Chem. 2011, 9, 92; [DOI] [PubMed] [Google Scholar]
- 17e. Evans N. H., Serpell C. J., White N. G., Beer P. D., Chem. Eur. J. 2011, 17, 12347; [DOI] [PubMed] [Google Scholar]
- 17f. Evans N. H., Serpell C. J., Beer P. D., Chem. Commun. 2011, 47, 8775; [DOI] [PubMed] [Google Scholar]
- 17g. Hancock L. M., Marchi E., Ceroni P., Beer P. D., Chem. Eur. J. 2012, 18, 11277; [DOI] [PubMed] [Google Scholar]
- 17h. Lehr J., Lang T., Blackburn O. A., Barendt T. A., Faulkner S., Davis J. J., Beer P. D., Chem. Eur. J. 2013, 19, 15898; ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17i. Langton M. J., Robinson S. W., Marques I., Félix V., Beer P. D., Nat. Chem. 2014, 6, 1039; [DOI] [PubMed] [Google Scholar]
- 17j. Langton M. J., Marques I., Robinson S. W., Félix V., Beer P. D., Chem. Eur. J. 2016, 22, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotaxane 4 is a derivative of previously reported fluorescent rotaxane S15 (R=Et, Ref. [22a]). The metal binding behaviors of 4 and S15 are identical (see the Supporting Information). However, whereas S15 is stable, analogues of rotaxanes 5–7 with R=Et de-threaded on standing at RT in MeCN. Presumably due to the increased axle flexibility of 5–7.
- 19.
- 19a. Crowley J. D., Goldup S. M., Lee A.-L., Leigh D. A., McBurney R. T., Chem. Soc. Rev. 2009, 38, 1530; [DOI] [PubMed] [Google Scholar]
- 19b. Saito S., J. Inclusion Phenom. Macrocyclic Chem. 2015, 82, 437; [Google Scholar]
- 19c. Denis M., Goldup S. M., Nat. Rev. Chem. 2017, 1, 0061. [Google Scholar]
- 20.
- 20a. Aucagne V., Hänni K. D., Leigh D. A., Lusby P. J., Walker D. B., J. Am. Chem. Soc. 2006, 128, 2186; [DOI] [PubMed] [Google Scholar]
- 20b. Aucagne V., Berna J., Crowley J. D., Goldup S. M., Hänni K. D., Leigh D. A., Lusby P. J., Ronaldson V. E., Slawin A. M. Z., Viterisi A., Walker D. B., J. Am. Chem. Soc. 2007, 129, 11950. [DOI] [PubMed] [Google Scholar]
- 21.Selected applications of the AT-CuAAC reaction:
- 21a. Goldup S. M., Leigh D. A., Long T., McGonigal P. R., Symes M. D., Wu J., J. Am. Chem. Soc. 2009, 131, 15924; [DOI] [PubMed] [Google Scholar]
- 21b. Goldup S. M., Leigh D. A., McGonigal P. R., Ronaldson V. E., Slawin A. M. Z., J. Am. Chem. Soc. 2010, 132, 315; [DOI] [PubMed] [Google Scholar]
- 21c. Barran P. E., Cole H. L., Goldup S. M., Leigh D. A., McGonigal P. R., Symes M. D., Wu J., Zengerle M., Angew. Chem. Int. Ed. 2011, 50, 12280; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 12488; [Google Scholar]
- 21d. Fernandes A., Viterisi A., Aucagne V., Leigh D. A., Papot S., Chem. Commun. 2012, 48, 2083; [DOI] [PubMed] [Google Scholar]
- 21e. Noor A., Moratti S. C., Crowley J. D., Chem. Sci. 2014, 5, 4283; [Google Scholar]
- 21f. Noor A., Lo W. K. C., Moratti S. C., Crowley J. D., Chem. Commun. 2014, 50, 7044; [DOI] [PubMed] [Google Scholar]
- 21g. Denis M., Qin L., Turner P., Jolliffe K. A., Goldup S. M., Angew. Chem. Int. Ed. 2018, https://doi.org/10.1002/anie.201713105; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2018, https://doi.org/10.1002/ange.201713105. [Google Scholar]
- 22.
- 22a. Lahlali H., Jobe K., Watkinson M., Goldup S. M., Angew. Chem. Int. Ed. 2011, 50, 4151; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 4237; [Google Scholar]
- 22b. Neal E. A., Goldup S. M., Chem. Sci. 2015, 6, 2398; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22c. Lewis J. E. M., Bordoli R. J., Denis M., Fletcher C. J., Galli M., Neal E. A., Rochette E. M., Goldup S. M., Chem. Sci. 2016, 7, 3154; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22d. Neal E. A., Goldup S. M., Angew. Chem. Int. Ed. 2016, 55, 12488; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 12676; [Google Scholar]
- 22e. Lewis J. E. M., Winn J., Cera L., Goldup S. M., J. Am. Chem. Soc. 2016, 138, 16329; [DOI] [PubMed] [Google Scholar]
- 22f. Lewis J. E. M., Winn J., Goldup S. M., Molecules 2017, 22, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The second emission of 4 is tentatively assigned to intramolecular exciplex formation. A similar effect was observed previously, including metal-induced quenching of the exciplex emission (Refs [13c–f]). This unexpected effect is the subject of ongoing investigations.
- 24.1,2,3-Triazole ligands are known as ligands for metal ions: Struthers H., Mindt T. L., Schibli R., Dalton Trans. 2010, 39, 675; [DOI] [PubMed] [Google Scholar]; Schulze B., Schubert U. S., Chem. Soc. Rev. 2014, 43, 2522; [DOI] [PubMed] [Google Scholar]; Vasdev R. A. S., Preston D., Crowley J. D., Dalton Trans. 2017, 46, 2402 However, the lack of photophysical response of the non-interlocked axle to Zn2+ was confirmed by 1H NMR to be due to a lack of coordination (Figure S44).28121321 [Google Scholar]
- 25. Banerjee S., Veale E. B., Phelan C. M., Murphy S. A., Tocci G. M., Gillespie L. J., Frimannsson D. O., Kelly J. M., Gunnlaugsson T., Chem. Soc. Rev. 2013, 42, 1601. [DOI] [PubMed] [Google Scholar]
- 26.Titration of 1 with Zn(ClO4)2⋅6 H2O (Figure S55) gave a similar change, confirming that this is the result of Zn2+ coordination to the bipyridine.
- 27.Selected examples in which the selectivity of sensors for Cd2+ over Zn2+ is investigated:
- 27a. Yuasa H., Miyagawa N., Izumi T., Nakatani M., Izumi M., Hashimoto H., Org. Lett. 2004, 6, 1489; [DOI] [PubMed] [Google Scholar]
- 27b. Zhong Z., Zhao Y., Org. Lett. 2007, 9, 2891; [DOI] [PubMed] [Google Scholar]
- 27c. Huang S., Clark R. J., Zhu L., Org. Lett. 2007, 9, 4999; [DOI] [PubMed] [Google Scholar]
- 27d. Park S. Y., Yoon J. H., Hong C. S., Souane R., Kim J. S., Matthews S. E., Vicens J., J. Org. Chem. 2008, 73, 8212; [DOI] [PubMed] [Google Scholar]
- 27e. Xu Z., Baek K.-H., Kim H. N., Cui J., Qian X., Spring D. R., Shin I., Yoon J., J. Am. Chem. Soc. 2010, 132, 601; [DOI] [PubMed] [Google Scholar]
- 27f. Jobe K., Brennan C. H., Motevalli M., Goldup S. M., Watkinson M., Chem. Commun. 2011, 47, 6036; [DOI] [PubMed] [Google Scholar]
- 27g. Zhou X., Li P., Shi Z., Tang X., Chen C., Liu W., Inorg. Chem. 2012, 51, 9226; [DOI] [PubMed] [Google Scholar]
- 27h. Mehdi H., Gong W., Guo H., Watkinson M., Ma H., Wajahat A., Ning G., Chem. Eur. J. 2017, 23, 13067. [DOI] [PubMed] [Google Scholar]
- 28.C−H⋅⋅⋅N interactions are commonly observed in rotaxanes synthesized using the AT-CuAAC reaction with bipyridine macrocycles. See Refs [22].
- 29.Similar coordination mode with Zn2+: Crowley J. D., Hänni K. D., Leigh D. A., Slawin A. M. Z., J. Am. Chem. Soc. 2010, 132, 5309. [DOI] [PubMed] [Google Scholar]
- 30.UV/Vis values allow comparison with macrocycle 1 Fluorescence titration gave K d=1.09×10−7 m −1 with a lower goodness of fit (R2=98.2 %; Figures S49,50). The discrepancy may be due to the water associated with the metal salt; the same procedure in the presence of 2 % H2O gave comparable values for K d (Figures S51-2).
- 31. 4, 5, and 6 exhibit similar or weaker binding with Zn2+ than 1 In the case of 4, this be caused by the sterically crowded binding motif. The weaker binding of 5 and 6 probably reflects the N−H⋅⋅⋅N H-bond interactions (implied by 1H NMR) to be overcome in binding the metal ion.
- 32.Radii given are for 6 coordinate metal ions: Cotton F. A., Wilkinson G., Advanced Inorganic Chemistry, 5th ed., Wiley, New York, 1988. [Google Scholar]
- 33.
- 33a. Lau Y. H., Rutledge P. J., Watkinson M., Todd M. H., Chem. Soc. Rev. 2011, 40, 2848; [DOI] [PubMed] [Google Scholar]
- 33b. Neupane L. N., Kim J. M., Lohani C. R., Lee K.-H., J. Mater. Chem. 2012, 22, 4003; [Google Scholar]
- 33c. Wu Y., Dong Y., Li J., Huang X., Cheng Y., Zhu C., Chem. Asian J. 2011, 6, 2725; [DOI] [PubMed] [Google Scholar]
- 33d. Wang H. F., Wu S. P., Tetrahedron 2013, 69, 1965. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


