Abstract
Inoculation of wild-type Arabidopsis plants with the fungus Alternaria brassicicola results in systemic induction of genes encoding a plant defensin (PDF1.2), a basic chitinase (PR-3), and an acidic hevein-like protein (PR-4). Pathogen-induced induction of these three genes is almost completely abolished in the ethylene-insensitive Arabidopsis mutant ein2-1. This indicates that a functional ethylene signal transduction component (EIN2) is required in this response. The ein2-1 mutants were found to be markedly more susceptible than wild-type plants to infection by two different strains of the gray mold fungus Botrytis cinerea. In contrast, no increased fungal colonization of ein2-1 mutants was observed after challenge with avirulent strains of either Peronospora parasitica or A. brassicicola. Our data support the conclusion that ethylene-controlled responses play a role in resistance of Arabidopsis to some but not all types of pathogens.
Ethylene is a gaseous plant hormone that has been implicated in a range of physiological processes including seed germination, organ senescence, organ abscission, fruit ripening, and morphological responses of organs (Abeles et al., 1992). It has been proposed that ethylene also plays an important role in controlling defense responses of plants to microbial pathogens. Pathogen challenge often causes an increase in ethylene production (Ross and Williamson, 1951; Van Loon, 1977; Mauch et al., 1984; Boller, 1991; Penninckx et al., 1998). Moreover, exogenous application of ethylene to plants can result in the activation of genes encoding antimicrobial pathogenesis-related (PR) proteins (Boller et al., 1983; Mauch and Staehelin, 1989; Memelink et al., 1990; Eyal et al., 1992; Beffa et al., 1995; Penninckx et al., 1996; Knoester et al., 1998), cell wall-strengthening Hyp-rich glycoproteins (Esquerré-Tugayé et al., 1979; Ecker and Davis, 1987; Tagu et al., 1992), or enzymes involved in the synthesis of phenylpropanoids (Ecker and Davis, 1987).
If ethylene plays a crucial role in plant defense mechanisms, one would predict that treatment of plants with exogenous ethylene would enhance resistance to subsequent challenge with microorganisms or, conversely, that treatment with ethylene inhibitors would adversely affect their resistance level. This has been demonstrated for a number of plant-pathogen interactions (Esquerré-Tugayé et al., 1979; El-Kazzaz et al., 1983a; Marte et al., 1993). However, for other plant-pathogen combinations, pretreatment with ethylene either had no effect on resistance or actually diminished the resistance level (El-Kazzaz et al., 1983b; Brown and Lee, 1993; Van Loon and Pennings, 1993). These contradictory results have made the role of ethylene in host defense a frequently debated matter of controversy.
Recently, however, conclusive evidence has been presented that ethylene is indeed involved in host resistance, albeit only to particular classes of pathogens and not to others, thus reconciling previous conflicting data (Knoester et al., 1998; Hoffman et al., 1999). In their experiments, Knoester et al. (1998) made use of transgenic tobacco plants transformed with a dominant-negative mutant allele of the Arabidopsis ethylene receptor gene ETR1. The transgenic plants with a disrupted ethylene response were more susceptible than wild-type plants to normally nonpathogenic soil-borne Pythium spp., whereas their level of resistance to tobacco mosaic virus was unaffected. Hoffman et al. (1999) found that some soybean mutants with reduced ethylene sensitivity had a tendency toward more severe symptoms compared with wild-type plants when challenged with virulent strains of the fungi Septoria glycines and Rhizoctonia solani and some but not all avirulent strains of Phytophthora sojae.
On the other hand, some of the ethylene-insensitive soybean mutants showed less-severe chlorotic symptoms relative to their wild-type parents upon inoculation with virulent strains of Pseudomonas syringae pv glycinea. Less-severe chlorosis was also observed in the ethylene-insensitive Never ripe tomato strain compared with wild-type plants when inoculated with either Xanthomonas campestris pv vesicatoria or Pseudomonas syringae pv tomato. In addition, the Never ripe tomato mutants also showed less-severe wilting symptoms upon challenge with the fungal vascular pathogen Fusarium oxysporum f. sp. lycopersici (Lund et al., 1998). Ethylene is known to promote events such as chlorophyll degradation (Stall and Hall, 1984) and xylem occlusion (VanderMolen et al., 1983), which are positively correlated with severity of disease symptoms such as chlorosis and wilting, respectively. In conclusion, it appears that ethylene controls both disease resistance responses and symptom expression. Therefore, this hormone can influence particular plant-pathogen interactions in different ways, depending on the offensive strategies of the pathogen, the efficacy of the defense genes it controls, and the nature of the physiological reactions that are triggered by the pathogen.
Although most of our current highly detailed knowledge on the process of ethylene perception and signal transduction comes from the study of Arabidopsis mutants (Kieber, 1997; McGrath and Ecker, 1998), the role of ethylene in the resistance of this plant to microbial pathogens has so far only been examined in a handful of cases. Bent et al. (1992) studied the interaction between Arabidopsis and the phytopathogenic bacteria Xanthomonas campestris pv campestris and Ps. syringae pv tomato. They observed that mutant ein2-1, a mutant affected in a membrane-associated signal transduction component of the ethylene response (McGrath and Ecker, 1998), showed less macroscopically visible chlorosis and less chlorophyll degradation compared with wild-type plants. However, when the bacteria multiplying in ein2-1 and wild-type plants were counted, no significant difference was found. It therefore appears that ethylene does not play a role in actual resistance to these bacteria but, rather, in the development of pathogen-induced chlorosis symptoms.
Suppression of chlorotic disease symptoms after challenge with these bacteria was not observed for the ethylene-insensitive mutant etr1-3 (Bent et al., 1992), which is affected in the ETR1 gene encoding an ethylene receptor (Chang et al., 1993). This result is apparently difficult to reconcile with the supposed role of ethylene in chlorotic symptom development. However, when testing alongside the allelic mutants etr1-1 and etr1-3 for their ability to induce PDF1.2 in response to challenge with Alternaria brassicicola, Penninckx et al. (1998) observed that etr1-3 is a very leaky allele in contrast to etr1-1, at least with respect to its impact on this pathogen-induced response in adult plants. Therefore, the observation that the etr1-3 mutation does not affect bacterially induced symptom development may well be due to leakiness of this allele. When the etr1-1 and the ein2-1 mutants were tested for susceptibility to the Oomycete Peronospora parasitica strain Noco, a strain that is virulent on the wild-type parental line Columbia (Col-0), no differences in susceptibility relative to wild-type plants were observed (Lawton et al., 1994).
Inoculation of leaves of wild-type plants with an avirulent Ps. syringae pv tomato strain was found to trigger a systemic defense response that protected the leaves against subsequent inoculation with either virulent strains of P. parasitica or Ps. syringae pv tomato (Lawton et al., 1995; Pieterse et al., 1998). This systemic response was equally effective in the ethylene-insensitive etr1-1 mutant (Lawton et al., 1995; Pieterse et al., 1998). On the other hand, Pieterse et al. (1998) observed that inoculating Arabidopsis roots with a nonpathogenic root-colonizing strain of Pseudomonas fluorescence conferred systemic resistance in wild-type plants but not etr1-1 mutants to subsequent inoculation of the leaves with a virulent Ps. syringae pv tomato strain. Therefore, a systemic resistance response triggered by leaf inoculation with an avirulent bacterium appears to be ethylene independent, while that induced by inoculating roots with a nonpathogenic bacterium is ethylene dependent. So far, however, no pathogens of Arabidopsis have been described for which ethylene plays a role in local resistance responses.
One complication in the study of the role of ethylene in disease resistance is that there appears to be an interrelationship with another stress hormone, jasmonate. Our previous studies on the expression of Arabidopsis gene PDF1.2, encoding an antifungal plant defensin peptide, have shown that this gene can be activated systemically upon pathogen challenge and that this activation requires both functional components of the ethylene response pathway, including ETR1 and EIN2, and the jasmonate response pathway, including COI1 (Penninckx et al., 1996). Both hormone response pathways need to be triggered concomitantly in order for pathogen-induced activation of PDF1.2 to occur (Penninckx et al., 1998). On the other hand, activation of PDF1.2 is independent of the salicylate response pathway (Penninckx et al., 1996), which controls pathogen-induced expression of other antimicrobial proteins such as PR-1, PR-2, and PR-5 (Uknes et al., 1992). When assessing the role of jasmonate in disease resistance, we observed that a jasmonate-insensitive mutant, coi1-1, showed enhanced disease susceptibility to the fungal pathogens A. brassicicola and Botrytis cinerea, but not to P. parasitica, whereas the opposite resistance responses were observed for the salicylate response mutant npr1-1 and the salicylate degrading transgenic line NahG (Thomma et al., 1998). The main objectives of the current study were to assess the effect of a mutation in the ethylene transduction gene EIN2 on the resistance response to the above-mentioned pathogens and the induction of some PR genes.
MATERIALS AND METHODS
Biological Material and Plant Inoculations
The mutant ein2-1 (Guzmán and Ecker, 1990) was obtained from the Arabidopsis Biological Resource Center (Columbus, OH). The Arabidopsis mutants coi1-1 (Feys et al., 1994), npr1-1 (Cao et al., 1994), and pad3-1 (Glazebrook and Ausubel, 1994) were obtained from Drs. J. Turner (University of East Anglia, Norwich, UK), X. Dong (Duke University, Durham, NC), and J. Glazebrook (University of Maryland, College Park), respectively. All of these mutants are derived from the Col-0 ecotype. Arabidopsis plants were essentially grown as described previously (Penninckx et al., 1996).
Growth and spore harvesting of the fungi Alternaria brassicicola (strain MUCL20297; Mycothèque Université Catholique de Louvain, Louvain-la-Neuve, Belgium), Botrytis cinerea (strains IMI169558, International Mycology Institute, Kew, UK; and MUCL30158, Mycothèque Université Catholique de Louvain) were done as described previously (Broekaert et al., 1990). The transgenic A. brassicicola strain (MUCL20297) containing a chimeric GUS-expressing transgene is described in Thomma et al. (1998). Peronospora parasitica strain Wela (Delaney et al., 1994) was maintained on living Arabidopsis plants of the Weiningen ecotype, and was kindly provided by Drs. R. Vogelsang and A. Slusarenko (Rheinisch-Westfälische Technischetlochschule Aachen, Germany).
Inoculation of 4-week-old soil-grown Arabidopsis plants with A. brassicicola, B. cinerea, and P. parasitica was performed as described previously (Thomma et al., 1998). For inoculation with A. brassicicola and B. cinerea, care was taken to place drops with inoculum on fixed positions left and right from the midvein.
Detection of Fungi in Inoculated Plants
A transgenic A. brassicicola strain containing a chimeric UidA (GUS) expressing transgene driven by a constitutive glyceraldehyde-3-P dehydrogenase promoter was used for quantifying fungal biomass in inoculated plants. Plants were inoculated with three 5-μL drops per leaf of a suspension in water of 5 × 105 conidial spores of this strain per milliliter. Inoculated plants were incubated at 100% RH. Quantification of fungal biomass was performed as described previously (Thomma et al., 1998), using a quantitative RNA dot-blot assay with UidA as a probe. The presence of P. parasitica in inoculated plants was detected by microscopic observation of leaves stained with lactophenol trypan blue as described by Mauch-Mani and Slusarenko (1996).
RNA Gel-Blot Analysis
RNA was extracted from tissues of Arabidopsis by the phenol-LiCl method according to the method of Eggermont et al. (1996). RNA gel-blot analysis was performed as described previously (Penninckx et al., 1996). Riboprobes for PDF1.2, PR-3, PR-4, and β-Tubulin 1 were synthesized as described previously (Penninckx et al., 1996; Thomma et al., 1998).
Ethylene and Methyl Jasmonate Treatments
For testing the protective effect on Arabidopsis plants of ethylene against A. brassicicola, 4-week-old soil-grown pad3-1 plants were placed in a gastight translucent chamber. Ethylene was applied by injecting the appropriate amount of ethylene gas with a syringe through a rubber septum in the chamber. Methyl jasmonate was applied by pipeting an appropriate amount of 1% (v/v) liquid methyl jasmonate in ethanol on a cotton plug inside the chamber. After 48 h of treatment, the chambers were opened and the plants were inoculated with either A. brassicicola or B. cinerea as described above, except that for B. cinerea inoculation only one inoculation spot per leaf was applied. Six days after inoculation, infections were analyzed macroscopically by measuring lesion diameters (for A. brassicicola-inoculated plants) or by counting the ratio of inoculated leaves showing spreading necrosis versus total amount of inoculated leaves (for B. cinerea-inoculated plants).
RESULTS
Requirement of EIN2 for Pathogen-Induced Expression of PR-3 and PR-4
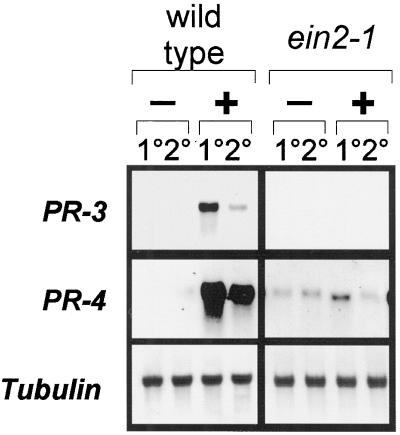
The Arabidopsis genes encoding the plant defensin PDF1.2, basic PR-3-type chitinase (also called ChitB), and the basic PR-4 protein (also called hevein-like protein or Hel) have all been shown previously to be inducible by exogenous application of ethylene (Samac et al., 1990; Potter et al., 1993; Chen and Bleecker, 1995; Penninckx et al., 1996), as well as by methyl jasmonate (Thomma et al., 1998). Pathogen-induced expression of all of these genes is known to require a functional jasmonate response pathway, as expression of these genes is abolished in the coi1-1 mutant (Thomma et al., 1998), whereas requirement of a functional ethylene response pathway for pathogen-induced expression has so far only been demonstrated for PDF1.2 (Penninckx et al., 1996, 1998). We now show that the expression of both PR-3 and PR-4 is, like that of PDF1.2, severely reduced in A. brassicicola-inoculated leaves of the ethylene-insensitive mutant ein2-1 compared with similarly treated leaves of wild-type (Col-0) plants (Fig. 1). In noninoculated leaves of A. brassicicola-inoculated wild-type plants, systemic induction was clearly observed for PDF1.2, PR-3, and PR-4 genes, but this response was completely abolished in the ein2-1 mutants (Fig. 1). These results indicate that functional EIN2 and COI1 (Thomma et al., 1998) are required for pathogen-induced expression of PDF1.2, PR-3, and PR-4, suggesting that these genes are controlled by a similar jasmonate/ethylene-dependent signal transduction pathway.
Figure 1.
Induction of the PR genes in Arabidopsis in response to infection with A. brassicicola. Four-week-old soil-grown wild-type (Col-0) and ein2-1 plants were infected with A. brassicicola and harvested 48 h following treatment. RNA blots were hybridized with the various probes indicated on the left. Symbols on top of the lanes are as follows: −, Mock-inoculated with water; +, inoculated with A. brassicicola spore suspension; 1°, treated lower rosette leaves; 2°, untreated upper rosette leaves.
Requirement of EIN2 for Resistance to Particular Fungi
Thomma et al. (1998) have previously shown that the jasmonate-insensitive Arabidopsis mutant coi1-1 is more susceptible than wild-type plants to infection by the fungi B. cinerea strain IMI169558 and A. brassicicola strain MUCL20297, but not by the Oomycete P. parasitica strain Wela. To investigate whether the ethylene-insensitive ein2-1 mutant shares the same defects in disease resistance as the coi1-1 mutant, the ein2-1 mutants were challenged with these three different fungal pathogens under the same conditions described in Thomma et al. (1998). All of these tests were performed on 4-week-old plants.
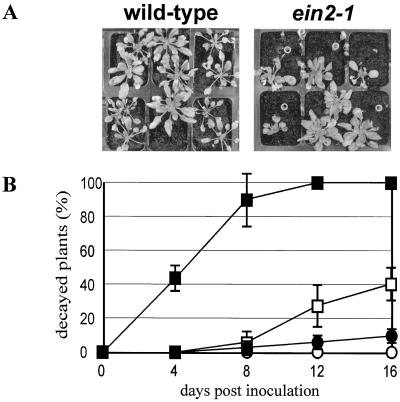
Strain IMI169558 of the gray mold fungus B. cinerea did not cause any single case of complete plant decay among 60 inoculated wild-type plants. In contrast, 42% of the inoculated ein2-1 plants were completely macerated by this strain over a 16-d period following inoculation (Fig. 2). B. cinerea strain MUCL30158, which was apparently more aggressive than strain IMI169558, caused decay of 9% and 100% of the inoculated wild-type and ein2-1 plants, respectively, within 16 d (Fig. 2). Therefore, ein2-1 mutants are more susceptible than wild-type plants to infection by either of two different strains of B. cinerea, which is in line with the observations made for the jasmonate-insensitive mutant coi1-1.
Figure 2.
Disease development on Arabidopsis inoculated with B. cinerea. A, Four-week-old Arabidopsis plants were drop-inoculated with B. cinerea strain IMI169558, and photographs were taken 12 d later. Circles (heads of pipet tips) indicate positions of completely decayed plants. B, Decay of Arabidopsis plants drop-inoculated with B. cinerea strains IMI169558 and MUCL30158. The percentage of dead plants is expressed as a function of time after inoculation. Plants were considered dead when their hearts were completely rotten. Data represent averages ± se of three different experiments performed with 20 plants per genotype. Circles, Wild-type (Col-0) plants; squares, the mutant ein2-1; white symbols, plants inoculated with strain IMI169558; black symbols, plants inoculated with strain MUCL30158.
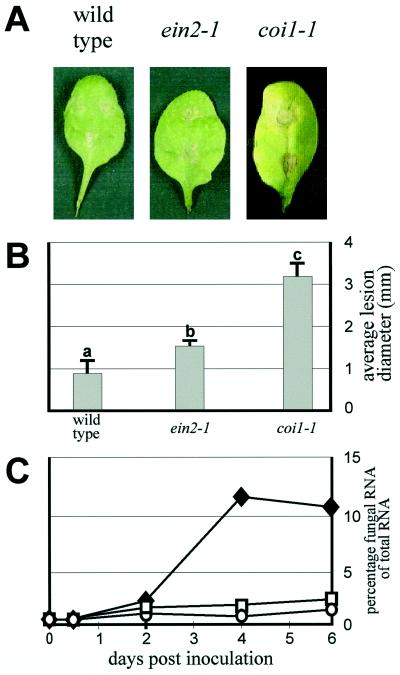
When challenged with A. brassicicola strain MUCL20297, the ein2-1 mutant produced restricted necrosis symptoms indicative of an incompatible interaction (Fig. 3A). The necrotic lesions formed on A. brassicicola-inoculated ein2-1 plants had an average diameter that was about 2-fold higher compared with the diameter of lesions on wild-type plants (Fig. 3B). However, measurements of fungal biomass in the infected zones by hybridization of RNA dot blots with a fungus-specific probe did not reveal increased colonization of ein2-1 plants by A. brassicicola compared with wild-type plants (Fig. 3C). In contrast, inoculation of the jasmonate-insensitive coi1-1 mutant with A. brassicicola yielded spreading lesions with markedly enhanced fungal colonization (Fig. 3; Thomma et al., 1998). Therefore, ein2-1 does not respond in the same way as coi1-1 to this particular fungus.
Figure 3.
Disease development on Arabidopsis inoculated with A. brassicicola. A, Necrotic lesions on leaves of 4-week-old Arabidopsis wild-type (Col-0), ein2-1, and coi1-1 plants drop-inoculated with spores of A. brassicicola. B, Average diameter of lesions formed after 6 d on 4-week-old Arabidopsis plants inoculated with a spore suspension of A. brassicicola. Data points represent averages ± se of measurements from 60 lesions on 15 different plants. Bars with different letter labels indicate that the corresponding data are significantly different (P > 0.95) according to Tukey's studentized range test (Neter et al., 1996). C, Percentage fungal RNA of total RNA in infection sites at different times after inoculation of leaves with A. brassicicola. Data points represent measurements on RNA extracted from 30 leaf discs. ○, Col-0; □, ein2-1; and ♦, coi1-1. The experiment was repeated twice with similar results.
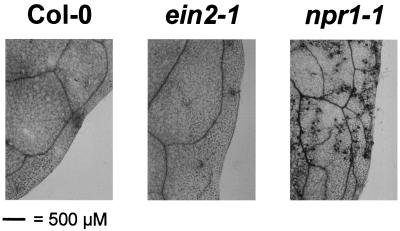
P. parasitica strain Wela has previously been shown to be avirulent on Arabidopsis Col-0 wild-type plants and on the jasmonate-insensitive mutant coi1-1, whereas Arabidopsis lines showing a defect in the salicylate-dependent defense pathway (NahG and npr1-1) were found to be susceptible to infection by this pathogen (Delaney et al., 1994; Thomma et al., 1998). When ein2-1 plants were challenged with P. parasitica strain Wela, a fully incompatible interaction was observed (Fig. 4). No intercellularly growing hyphae or oospores could be detected in any of 20 ein2-1 leaf samples analyzed under the microscope. In contrast, npr1-1 plants subjected to the same treatment showed an abundance of intercellularly growing hyphae and oospores (Fig. 4). This indicates that the ethylene response pathway is, unlike the salicylate response pathway, not implicated in the resistance of wild-type plants to an avirulent P. parasitica strain. Previous work established that Arabidopsis mutants affected in the ethylene-response pathway (etr1-1, ein2-1) do not show enhanced disease susceptibility relative to wild-type Col-0 plants to the virulent P. parasitica strain Noco (Lawton et al., 1994).
Figure 4.
Disease development on Arabidopsis inoculated with P. parasitica. Microscopic view of leaves of 4-week-old Arabidopsis wild-type (Col-0), ein2-1, and npr1-1 plants spray-inoculated with conidiospores of P. parasitica strain Wela. Eleven days after inoculation, inoculated leaves were stained with lactophenol trypan blue prior to microscopic examination. Leaves of the npr1-1 mutant reveal the presence of intracellular hyphae and oospores.
Protection against A. brassicicola and B. cinerea by Ethylene and Methyl Jasmonate Pretreatment
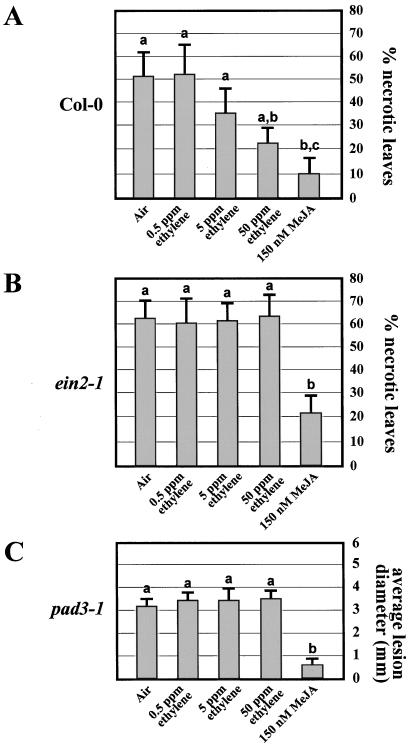
The remarkable susceptibility to the fungus B. cinerea of the ethylene-insensitive ein2-1 mutant (Fig. 2) and the jasmonate-insensitive coi1-1 mutant (Thomma et al., 1998) suggests that ethylene- and jasmonate- dependent pathogen-inducible effector molecules contribute to resistance against this pathogen. Based on these observations, one would expect that increased production of such effector molecules prior to infection attempts by B. cinerea would enhance the resistance level to this pathogen. To test this prediction, wild-type Col-0 plants were placed for 2 d in airtight chambers containing either air or air supplemented with 0.5, 5.0, or 50 μL L−1 ethylene or 150 nm methyl jasmonate, whereafter plants were inoculated with B. cinerea. The number of leaves showing soft rot symptoms was reduced by 57% in plants pretreated with 50 μL L−1 ethylene, while pretreatment with 150 nm methyl jasmonate reduced the number of leaves showing soft rot symptoms by 80% (Fig. 5A).
Figure 5.
Protective effect of exogenously applied ethylene and methyl jasmonate on infection by B. cinerea strain MUCL30158 and A. brassicicola. A, Percentage of inoculated leaves showing spreading necrosis symptoms 6 d after inoculation of Arabidopsis wild-type (Col-0) plants with a spore suspension of B. cinerea. Prior to inoculation, separate sets of plants were placed for 48 h in gastight translucent chambers with an atmosphere containing the gaseous compounds as indicated below the bars. Data points represent averages ± se of seven series of inoculations on 16 leaves from two plants. Bars with different letter labels indicate that the corresponding data are significantly different (P > 0.95) according to Tukey's studentized range test (Neter et al., 1996). B, Percentage of inoculated leaves showing spreading necrosis symptoms 6 d after inoculation of Arabidopsis ein2-1 plants with a spore suspension of B. cinerea. Specifications are as in the legend to A. C, Average diameter of lesions formed after 6 d on 4-week-old Arabidopsis pad3-1 mutants inoculated with a spore suspension of A. brassicicola. Prior to inoculation, separate sets of plants were placed for 48 h in gastight translucent chambers with an atmosphere containing the gaseous compounds as indicated below the bars. Data points represent averages ± se of measurements from 40 lesions on 10 different plants. Bars with different letter labels indicate that the corresponding data are significantly different (P > 0.95) according to Tukey's studentized range test (Neter et al., 1996). MeJA, Methyl jasmonate.
In contrast, pretreatment of ein2-1 plants with 50 μL L−1 ethylene did not reduce the disease incidence (Fig. 5B), indicating that the events causing protection in ethylene-treated wild-type plants are indeed dependent on a functional ethylene-response pathway. Pretreatment of ein2-1 plants with methyl jasmonate, on the other hand, still caused a reduction of the disease incidence by 64% (Fig. 5B). Similar experiments were also performed using A. brassicicola as a pathogen. In this case, however, wild-type Col-0 plants could not be used because A. brassicicola causes highly restricted, non-spreading lesions on this genotype. Instead, the pad3-1 mutant was used in these experiments. The pad3-1 mutant is deficient in an enzyme involved in the biosynthesis of camalexin (Glazebrook and Ausubel, 1994; N. Zhou and J. Glazebrook, personal communication), an antimicrobial metabolite that is an important determinant for resistance to A. brassicicola (Thomma et al., 1999). Previous work certified that ethylene- and jasmonate-dependent defense responses are still fully operative in the pad3-1 mutant (Thomma et al., 1999). Pretreatment of this mutant with 0.5, 5.0, or 50 μL L−1 ethylene in the atmosphere for 2 d prior to inoculation failed to confer any protection against A. brassicicola (Fig. 5C). In contrast, pretreatment of the plants with 150 nm gaseous methyl jasmonate reduced the average lesion diameter by 80% (Fig. 5C).
DISCUSSION
The results presented here confirm that Arabidopsis possesses a jasmonate/ethylene-dependent pathway for the induction of a particular subset of PR genes, including a plant defensin gene (PDF1.2), a basic chitinase gene (PR-3), and a hevein-like gene (PR-4). The involvement of both ethylene and jasmonate in this pathway is based on the observations that PDF1.2, PR-3, and PR-4 can be activated by exogenous treatment with either methyl jasmonate (Thomma et al., 1998) or ethylene (Samac et al., 1990; Potter et al., 1993; Penninckx et al., 1996; B.P.H.J. Thomma, unpublished results), while they are not or very weakly induced by exogenous application of salicylic acid (Thomma et al., 1998). Moreover, induction of this set of genes upon challenge of Arabidopsis plants with the fungus A. brassicicola is largely abolished in a mutant (coi1-1; Thomma et al., 1998) affected in the COI1 gene, a gene encoding a signal transduction component of the jasmonate response (Xie et al., 1998). We have now shown that A. brassicicola-induced expression of these genes is also dramatically reduced in an ethylene-insensitive mutant (ein2-1) with a dysfunctional EIN2 gene encoding a membrane-associated signal transduction component of the ethylene response (McGrath and Ecker, 1998). Therefore, we consider PDF1.2, PR-3, and PR-4 as a class of co-regulated jasmonate/ethylene-dependent PR-genes whose regulation is clearly distinct from that of the salicylate-dependent PR-genes such as PR-1, PR-2, and PR-5 (Uknes et al., 1992; Cao et al., 1994; Delaney et al., 1994).
The occurrence of two subsets of differentially regulated PR-genes has also been demonstrated in tobacco. The genes encoding extracellular isoforms such as acidic PR-1, acidic β-1,3-glucanase, and acidic chitinase are efficiently induced by salicylic acid but less so by ethylene (Memelink et al., 1990; Ohshima et al., 1990; Ward et al., 1991). Pathogen-induced activation of these genes is abolished in a transgenic line expressing the salicylate-degrading NahG gene (Gaffney et al., 1993). Another subset of PR genes, those encoding vacuolar PR proteins such as basic PR-1, basic β-1,3-glucanase, and basic chitinase, are more efficiently induced by ethylene than by salicylate (Memelink et al., 1990; Eyal et al., 1992; Beffa et al., 1995) and their pathogen-induced expression is down-regulated in transgenic tobacco plants expressing a dominant-negative mutant form of the Arabidopsis ethylene receptor ETR1 (Knoester et al., 1998). The role of jasmonate in the induction of the latter subset of PR genes has not yet been intensively studied, but Niki et al. (1998) recently reported that these genes can be induced by floating tobacco leaf discs on a jasmonate-containing solution. Therefore, a jasmonate/ethylene-dependent pathway for induction of particular PR genes also appears to be operative in tobacco.
Arabidopsis PDF1.2 and PR-3 have previously been purified and shown to possess antifungal activity in vitro (Verburg and Huynh, 1991; Penninckx et al., 1996). Arabidopsis PR-4, on the other hand, has not yet been isolated, but it is known to be highly homologous to CBP-20, a tobacco PR protein with proven antifungal properties (Ponstein et al., 1994). PDF1.2, PR-3, and PR-4 are therefore likely to contribute to the defensive capacity of Arabidopsis plants directed against fungal organisms.
Our results clearly show that the ein2-1 mutation in Arabidopsis entails markedly enhanced susceptibility to at least two different strains of the pathogenic fungus B. cinerea (Fig. 2). On the other hand, the ein2-1 mutation had no impact on either resistance to an avirulent strain of A. brassicicola (Fig. 3) or to an avirulent or a virulent strain of P. parasitica (Fig. 4 and Lawton et al., 1994, respectively). This is in line with the data obtained by Knoester et al. (1998) on ethylene-insensitive tobacco plants that were more susceptible than control plants to soil-borne Pythium spp. but not to tobacco mosaic virus.
The ein2-1 mutation in Arabidopsis results in a lack of pathogen-inducible expression of a subset of PR genes (Penninckx et al., 1996; Fig. 1), as does expression of a dominant-negative mutant ETR1 gene in tobacco (Knoester et al., 1998). However, these observations by themselves do not prove that such PR proteins are responsible for the control of particular pathogens. Ethylene insensitivity is likely to have pleiotropic effects, which would therefore affect the expression of other effector molecules as well. It is conceivable that such ethylene-controlled effector events are effective at controlling particular pathogens but have no effect on others.
The data in the present study indicate that necrotrophic pathogens (e.g. B. cinerea in the case of Arabidopsis or Pythium spp. in the case of tobacco) are among those that are effectively contained by ethylene-controlled effector molecules, whereas biotrophic pathogens (e.g. P. parasitica in the case of Arabidopsis and tobacco mosaic virus in the case of tobacco) are more efficiently countered by other defense mechanisms, including salicylate-controlled effector events. However, this may be a matter of coincidence and at the present time, it is more cautious not to speculate beyond the observation that some pathogens are kept in check by ethylene-controlled effector events while others are not.
Both the Arabidopsis ein2-1 and coi1-1 mutants are more susceptible than wild-type plants to B. cinerea (Fig. 2; Thomma et al., 1998), although coi1-1 is more susceptible than ein2-1 in comparative assays (B.P.H.J. Thomma, unpublished results). In addition, a jasmonate-deficient mutant (fad3/fad7/fad8), a jasmonate-insensitive mutant (jar1) of Arabidopsis, and ethylene-insensitive tobacco plants are more susceptible than their respective control lines to soil-borne Pythium spp. (Knoester et al., 1998; Staswick et al., 1998; Vijayan et al., 1998). This may be seen as an additional argument for the involvement of jasmonate/ethylene-dependent PR genes in resistance against these pathogens, as expression of jasmonate/ethylene-dependent PR genes depends on both ethylene and jasmonate signal response pathways. Consistent with this notion we found that treatment of Arabidopsis plants with either methyl jasmonate or ethylene, both of which increase the levels of jasmonate/ethylene-dependent PR proteins, resulted in enhanced protection to B. cinerea. On the other hand, neither ein2-1 nor coi1-1 Arabidopsis mutants were more susceptible to P. parasitica (Fig. 4; Lawton et al., 1994; Thomma et al., 1998), excluding a role for jasmonate/ethylene-dependent PR genes in resistance against this pathogen.
One interesting observation was that the coi1-1 and ein2-1 mutants differed in their response to challenge by A. brassicicola. The coi1-1 mutant showed enhanced tissue colonization by this fungus relative to wild-type plants (Thomma et al., 1998), while the ein2-1 mutant did not (Fig. 3). The most likely explanation for these results is that the jasmonate/ethylene-dependent PR genes are not effective or are only very marginally effective against this fungus, while a presumed jasmonate-dependent/ethylene-independent effector molecule may contribute much more effectively. The fact that the camalexin-deficient pad3-1 mutant is also more susceptible to A. brassicicola compared with wild-type plants suggests that this hypothetical effector molecule might be camalexin, the major Arabidopsis phytoalexin. However, camalexin production is not induced by treatment with jasmonate (Thomma et al., 1999), so we believe the hypothetical effector molecule to be different from camalexin. Consistent with the presumed existence of a jasmonate-inducible yet ethylene-independent effector molecule, we observed that treatment of pad3-1 mutants with methyl jasmonate increased the level of resistance to A. brassicicola, whereas pretreatment with ethylene failed to do so (Fig. 5). The jasmonate-inducible yet ethylene-independent effectors may also be effective against B. cinerea, as inferred from the observation that the ein2-1 mutant can be protected against this fungus by pretreatment with methyl jasmonate but not by ethylene (Fig. 5).
A full range of pathogens are now available for future research that either cause less-severe symptoms on ethylene-insensitive versus ethylene-sensitive Arabidopsis genotypes (Ps. syringae and X. campestris, Bent et al., 1992), no or weak differences in symptoms or multiplication (A. brassicicola and P. parasitica, this study; Lawton et al., 1994), or more severe symptoms and increased multiplication (B. cinerea, this study). These data provide strong support to the notion that ethylene can play a balanced role in mounting disease resistance responses as well as in aggravation of disease symptoms, the outcome of which is dependent on the nature of the pathogen.
ACKNOWLEDGMENTS
The authors thank Drs. J. Turner, X. Dong, and J. Glazebrook for providing the mutants coi1-1, npr1-1, and pad3-1, respectively. The authors also thank Drs. R. Vogelsang and A. Slusarenko for providing P. parasitica strain Wela.
Footnotes
This research was partially supported by a grant (no. G0218.97) from the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen. B.P.H.J.T. is research assistant of this fund. K.T. is the recipient of a predoctoral fellowship of the Vlaams Instituut voor Bevordering van het Wetenschappelijk-Technologisch Onderzoek in de Industrie.
LITERATURE CITED
- Abeles FB, Morgan PW, Salveit ME., Jr . Ethylene in Plant Biology. Ed 2. San Diego: Academic Press; 1992. [Google Scholar]
- Beffa R, Szell M, Meuwly P, Pay A, Vögeli-Lange R, Métraux J-P, Neuhaus G, Meins F, Nagy F. Cholera toxin elevates pathogen resistance and induces pathogenesis-related gene expression in tobacco. EMBO J. 1995;14:5753–5761. doi: 10.1002/j.1460-2075.1995.tb00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent AF, Innes RW, Ecker JR, Staskawicz BJ. Disease development in ethylene-insensitive Arabidopsis thaliana infected with virulent and avirulent Pseudomonas and Xanthomonas pathogens. Mol Plant-Microbe Interact. 1992;5:372–378. doi: 10.1094/mpmi-5-372. [DOI] [PubMed] [Google Scholar]
- Boller T. Ethylene in pathogenesis and disease resistance. In: Mattoo AK, Suttle JC, editors. The Plant Hormone Ethylene. Boca Raton, FL: CRC Press; 1991. pp. 293–314. [Google Scholar]
- Boller T, Gehri A, Mauch F, Vögeli U. Chitinase in bean leaves: induction by ethylene, purification, properties, and possible function. Planta. 1983;157:22–31. doi: 10.1007/BF00394536. [DOI] [PubMed] [Google Scholar]
- Broekaert WF, Terras FRG, Cammue BPA, Vanderleyden J. An automated quantitative assay for fungal growth. FEMS Microbiol Lett. 1990;69:55–60. [Google Scholar]
- Brown GE, Lee HS. Interaction of ethylene with citrus stem-end rot caused by Diplodia natalensis. Phytopathology. 1993;83:1204–1208. [Google Scholar]
- Cao H, Bowling SA, Gordon AS, Dong X. Characterization of an Arabidopsis mutant that is non-responsive to inducers of systemic acquired resistance. Plant Cell. 1994;6:1583–1592. doi: 10.1105/tpc.6.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene etr1: similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Chen QG, Bleecker AB. Analysis of ethylene signal-transduction kinetics associated with seedling-growth responses and chitinase induction in wild-type and mutant Arabidopsis. Plant Physiol. 1995;108:597–607. doi: 10.1104/pp.108.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, Ryals J. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- Ecker JR, Davis RW. Plant defense genes are regulated by ethylene. Proc Natl Acad Sci USA. 1987;84:5202–5206. doi: 10.1073/pnas.84.15.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont K, Goderis IJ, Broekaert WF. High-throughput RNA extraction from plant samples based on homogenisation by reciprocal shaking in the presence of a mixture of sand and glass beads. Plant Mol Biol Rep. 1996;14:273–279. [Google Scholar]
- El-Kazzaz MK, Chordas A, Kader AA. Physiological and compositional changes in orange fruit in relation to modification of their susceptibility to Penicillium italicum by ethylene treatments. J Am Soc Hortic Sci. 1983a;108:618–622. [Google Scholar]
- El-Kazzaz MK, Sommer NF, Fortlage RJ. Effects of different atmospheres on postharvest decay and quality of fresh strawberries. Phytopathology. 1983b;73:282–285. [Google Scholar]
- Esquerré-Tugayé MT, Lafitte C, Mazau D, Toppan A, Touze A. Cell surfaces in plant-microorganism interactions. II. Evidence for the accumulation of hydroxyproline-rich glycoproteins in the cell wall of diseased plants as a defense mechanism. Plant Physiol. 1979;64:320–326. doi: 10.1104/pp.64.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyal Y, Sagee O, Fluhr R. Dark-induced accumulation of a basic pathogenesis-related (PR-1) transcript and a light requirement for its induction by ethylene. Plant Mol Biol. 1992;19:589–599. doi: 10.1007/BF00026785. [DOI] [PubMed] [Google Scholar]
- Feys BJF, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessman H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Ausubel FM. Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc Natl Acad Sci USA. 1994;91:8955–8959. doi: 10.1073/pnas.91.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman T, Schmidt JS, Zheng X, Bent A. Isolation of ethylene-insensitive soybean mutants that are altered in pathogen susceptibility and gene-for-gene disease resistance. Plant Physiol. 1999;119:935–949. doi: 10.1104/pp.119.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ. The ethylene response pathway in Arabidopsis. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:277–296. doi: 10.1146/annurev.arplant.48.1.277. [DOI] [PubMed] [Google Scholar]
- Knoester M, Van Loon LC, Van Den Heuvel J, Hennig J, Bol JF, Linthorst HJM. Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc Natl Acad Sci USA. 1998;95:1933–1937. doi: 10.1073/pnas.95.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton KA, Potter SL, Uknes S, Ryals J. Acquired resistance signal transduction in Arabidopsis is ethylene independent. Plant Cell. 1994;6:581–588. doi: 10.1105/tpc.6.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton KA, Weymann K, Friedrich L, Vernooij B, Uknes S, Ryals J. Systemic acquired resistance in Arabidopsis requires salicylic acid but not ethylene. Mol Plant-Microbe Interact. 1995;8:863–870. doi: 10.1094/mpmi-8-0863. [DOI] [PubMed] [Google Scholar]
- Lund ST, Stall RE, Klee HJ. Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell. 1998;10:371–382. doi: 10.1105/tpc.10.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marte M, Buonaurio R, Dellatorre G. Induction of systemic resistance to tobacco powdery mildew by tobacco mosaic virus, tobacco necrosis virus or ethephon. J Phytopathol. 1993;138:137–144. [Google Scholar]
- Mauch F, Hadwiger LA, Boller T. Ethylene: symptom, not signal for the induction of chitinase and β-1,3-glucanase in pea pods by pathogens and elicitor. Plant Physiol. 1984;76:607–611. doi: 10.1104/pp.76.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch F, Staehelin LA. Functional implications of the subcellular localization of ethylene-induced chitinase and β-1,3-glucanase in bean leaves. Plant Cell. 1989;1:447–457. doi: 10.1105/tpc.1.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch-Mani B, Slusarenko AJ. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell. 1996;8:203–212. doi: 10.1105/tpc.8.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath RB, Ecker JR. Ethylene signalling in Arabidopsis: events from the membrane to the nucleus. Plant Physiol Biochem. 1998;36:103–113. [Google Scholar]
- Memelink J, Linthorst HJM, Schilperoort RA, Hoge JHCD. Tobacco genes encoding acidic and basic isoforms of pathogenesis-related proteins display different expression patterns. Plant Mol Biol. 1990;14:119–126. doi: 10.1007/BF00018553. [DOI] [PubMed] [Google Scholar]
- Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied Linear Statistical Models. Ed 4. Boston: WCB/McGraw-Hill; 1996. [Google Scholar]
- Niki T, Mitsuhara I, Seo S, Ohtsubo N, Ohashi Y. Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis-related (PR) protein genes in wounded mature tobacco leaves. Plant Cell Physiol. 1998;39:500–507. [Google Scholar]
- Ohshima M, Itoh H, Matsuoka M, Murakami T, Ohashi Y. Analysis of stress-induced or salicylic acid-induced expression of the pathogenesis-related 1a protein gene in transgenic tobacco. Plant Cell. 1990;2:95–106. doi: 10.1105/tpc.2.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IAMA, Eggermont K, Terras FRG, Thomma BPHJ, De Samblanx GW, Buchala A, Métraux J-P, Manners JM, Broekaert WF. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell. 1996;8:2309–2323. doi: 10.1105/tpc.8.12.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IAMA, Thomma BPHJ, Buchala A, Métraux J-P, Broekaert WF. Cooperative activation of jasmonate and ethylene response pathways in parallel is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;10:2103–2114. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Van Wees SCM, Van Pelt JA, Knoester M, Laan R, Gerrits H, Weisbeek PJ, Van Loon LC. A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell. 1998;10:1571–1586. doi: 10.1105/tpc.10.9.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponstein AS, Bres-Vloemans SA, Sela-Buurlage MB, Van Den Elzen PJM, Melchers LS, Cornelissen BJC. A novel pathogen- and wound-inducible tobacco (Nicotiana tabacum) protein with antifungal activity. Plant Physiol. 1994;104:109–118. doi: 10.1104/pp.104.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S, Uknes S, Lawton K, Winter AM, Chandler D, Dimaio J, Novitzky R, Ward E, Ryals J. Regulation of a hevein-like protein in Arabidopsis. Mol Plant-Microbe Interact. 1993;6:680–681. doi: 10.1094/mpmi-6-680. [DOI] [PubMed] [Google Scholar]
- Ross AF, Williamson CE. Physiologically active emanations from virus-infected plants. Phytopathology. 1951;41:431–438. [Google Scholar]
- Samac DA, Hironaka CM, Yallaly PE, Shah DM. Isolation and characterization of the genes encoding basic and acidic chitinase in Arabidopsis thaliana. Plant Physiol. 1990;93:907–914. doi: 10.1104/pp.93.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stall RE, Hall CB. Chlorosis and ethylene production in pepper leaves infected with Xanthomonas campestris pv vesicatoria. Phytopathology. 1984;74:373–375. [Google Scholar]
- Staswick PE, Yuen GY, Lehman CC. Jasmonate signalling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J. 1998;15:747–754. doi: 10.1046/j.1365-313x.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- Tagu D, Walker N, Ruiz-Avila L, Burgess S, Martínez-Izquierdo JA, Leguay JJ, Netter P, Puigdomènech P. Regulation of the maize HRGP gene expression by ethylene and wounding mRNA accumulation and qualitative expression analysis of the promoter by microprojectile bombardment. Plant Mol Biol. 1992;20:529–538. doi: 10.1007/BF00040611. [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF. Separate jasmonate-dependent and salicylate-dependent defense response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Nelissen I, Eggermont K, Broekaert WF. Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J. 1999;19:163–171. doi: 10.1046/j.1365-313x.1999.00513.x. [DOI] [PubMed] [Google Scholar]
- Uknes S, Mauch-Mani B, Moyer M, Potter S, Williams S, Dincher S, Chandler D, Slusarenko A, Ward E, Ryals J. Acquired resistance in Arabidopsis. Plant Cell. 1992;4:645–656. doi: 10.1105/tpc.4.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon LC. Induction by 2-chloroethylphosphonic acid of viral-like lesions, associated proteins, and systemic resistance in tobacco. Virology. 1977;80:417–420. doi: 10.1016/s0042-6822(77)80016-0. [DOI] [PubMed] [Google Scholar]
- Van Loon LC, Pennings GGH. Involvement of ethylene in the induction of systemic acquired resistance in tobacco. In: Fritig B, Legrand M, editors. Mechanisms of Plant Defense Responses. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 156–159. [Google Scholar]
- VanderMolen GE, Labavitch JM, Strand LL, DeVay JE. Pathogen-induced vascular gels: ethylene as a host intermediate. Physiol Plant. 1983;59:573–580. [Google Scholar]
- Verburg JG, Huynh QK. Purification and characterization of an antifungal chitinase from Arabidopsis thaliana. Plant Physiol. 1991;95:450–455. doi: 10.1104/pp.95.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan P, Shockey J, Levesque CA, Cook RJ, Browse J. A role for jasmonate in pathogen defense of Arabidopsis. Proc Natl Acad Sci USA. 1998;95:7209–7214. doi: 10.1073/pnas.95.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward ER, Uknes SJ, Williams SC, Dincher SS, Wiederhold DL, Alexander DC, Ahl-Goy P, Métraux J-P, Ryals JA. Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell. 1991;3:1085–1094. doi: 10.1105/tpc.3.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–1094. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]