Abstract
We undertook a systematic review to assess 1) the level and quality of pharmacy and drug shop provision of medical abortion (MA) in low‐ and middle‐income countries (LMICs) and 2) interventions to improve quality of provision. We used standardized terms to search six databases for peer‐reviewed and grey literature. We double‐extracted data using a standardized template, and double‐graded studies for methodological quality. We identified 22 studies from 16 countries reporting on level and quality of MA provision through pharmacies and drug sellers, and three intervention studies. Despite widespread awareness and provision of MA drugs, even in legally restricted contexts, most studies found that pharmacy workers and drug sellers had poor knowledge of effective regimens. Evidence on interventions to improve pharmacy and drug shop provision of MA was limited and generally low quality, but indicated that training could be effective in improving knowledge. Programmatic attention should focus on the development and rigorous evaluation of innovative interventions to improve women's access to information about MA self‐management in low‐and middle‐income countries.
An estimated 7 million women in the developing world were treated for complications from unsafe abortion in 2012 (Singh and Maddow‐Zimet 2015), and deaths due to complications of unsafe abortion have been estimated to account for between 8 and 18 percent of all maternal deaths (Graham et al. 2017; Say et al. 2014). On the other hand, there have been global declines in case‐fatality from unsafe abortions since 1990, which have in part been attributed to a shift from higher‐ to lower‐risk methods of unsafe abortion, including self‐administered medical abortion (MA) pills (Fernandez, Coeytaux, Gomez Ponce de León, and Harrison 2009; World Health Organization 2011). Medical abortion is a non‐surgical method of abortion administered through the use of the pharmaceutical drugs mifepristone and misoprostol, or misoprostol alone. Misoprostol is a WHO‐approved drug for MA that is widely available in pharmacies in low‐ and middle‐income countries (LMICs) as it has other indications including treatment of gastric ulcers and postpartum hemorrhage. The availability of misoprostol in pharmacies and drug shops has increased women's access to a safer alternative to other methods of self‐induced abortion (Hyman et al. 2013), and increasing availability of misoprostol has also been linked to declining abortion‐related morbidity in some countries (Faúndes, Santos, Carvalho, and Gras 1996). Misoprostol is more effective for inducing a safe abortion when used in combination with mifepristone, although a misoprostol‐only regimen is recommended where mifepristone is unavailable (World Health Organization 2012). The combined regimen of mifepristone and misoprostol is also increasingly available in pharmacies in some countries, though mifepristone is much less widely available than misoprostol since its main use is for medical abortion and its registration is often therefore prevented by political sensitivities related to abortion (Culwell and Hurwitz 2013).
Pharmacies and drug shops are often the first, preferred source of health care in LMICs in light of their convenience, privacy, anonymity, and low cost (Ahmed and Hossain 2007; Chalker et al. 2000; Goel, Ross‐Degnan, Berman, and Soumerai 1996). Although a prescription is often legally required for selling misoprostol or mifepristone, sale of drugs without prescription is common in many LMICs (Goodman et al. 2007). Although studies show that MA is safe, effective, and easily self‐administered in low‐resource settings (Harper et al. 2007), effective use of MA drugs requires that women receive adequate information and counseling and high‐quality products (World Health Organization 2012).
A review of pharmacy provision of MA, published in 2012 (Sneeringer, Billings, Ganatra, and Baird 2012), found that pharmacy workers rarely offer adequate information to women buying MA. Since 2012, however, research on pharmacy and drug shop provision of MA has expanded rapidly. Moreover, the 2012 study did not take a systematic approach to the literature search, nor find evidence from intervention studies on how provision quality could be improved. The present study is a systematic review that builds on this previous work to meet two objectives: 1) to describe the level and quality of pharmacy and drug shop provision of MA in LMICs; and 2) to assess the effectiveness and cost‐effectiveness of interventions aiming to improve the level and/or quality of MA provision by pharmacy workers and drug sellers. We review the evidence both on the provision of misoprostol‐only and the mifepristone‐misoprostol combined regimen of medical abortion and on provision of other, ineffective drugs that may be sold to women seeking abortion. In addition, the term pharmacy can refer to a range of businesses of various sizes and legal status, and in this article we refer to pharmacies and drug shops as any outlet whose business is selling medicines, regardless of the training and qualifications of their staff or their legal status.
MATERIALS AND METHODS
Six online databases (MEDLINE, Web of Science, POPLINE, Embase, Global Health, and WHO Reproductive Health Library) were searched using standardized search terms (Table 1).1 For objective 1, search terms related to abortion and to pharmacies, drug sellers, or self‐medication were used (row 1 and 2, Table 1). For objective 2, additional search terms related to interventions were added (rows 1–3). Further studies (including peer‐reviewed papers and grey literature) were identified using snowball techniques applied to reference lists of identified studies.
Studies were screened for inclusion by title and abstract, were further screened by full text, and were included if they met the following criteria: outcomes met the review objectives; study design used quantitative primary data collection methods; published between 1 January 1990 and 1 April 2017; in English, Spanish, or French; LMIC setting (defined using the latest World Bank data http://data.worldbank.org/about/country-and-lending-groups); published peer‐reviewed articles or grey literature (published reports including a formal research element). Grey literature was considered for inclusion if a study was reported in sufficient detail to extract data and assess quality, and if there was a formal evaluation element for intervention studies. Conference abstracts were included if we could access full‐length papers with sufficient detail for quality assessment. Studies reporting provision of any type of medication intended to end a pregnancy were included, irrespective of whether the products provided were safe or effective abortifacients. Both quasi‐ and fully experimental intervention study designs were included. Abstracts and studies in French or Spanish were screened by colleagues with working proficiency in that language, and one Spanish‐language study was selected for inclusion following assessment by a co‐author fluent in the language.
Among the identified studies, data were double‐extracted using a standardized template and results were summarized narratively. For objective 1, we extracted the following data: study setting, study design (including sampling and limitations), outlet type, population outcomes measured, and comparisons made. We considered various outcomes indicating level and quality of provision, including types of abortifacients offered/sold, knowledge of and counseling on effective regimen and complications, and prescription requirements. For objective 1, methodological quality was assessed by two reviewers using a standardized checklist of ten items on prevalence design and reporting (Munn, Moola, Riitano, and Lisy 2014). This checklist included recruitment, representativeness, sample size, measurement, analysis, and whether confounders and subgroups were considered (see full list and scores in Table 2). Differences in scoring were reconciled through discussion, and on the few occasions when an agreement could not be reached, a third opinion was sought. An overall index score was calculated for each study (0 points for each criteria not reported or met, 1 point if partially met, and 2 points if fully met). For studies with multiple outcomes, quality assessment of the various outcome measurements was averaged to produce an overall score. The quality of the study was considered to be low if the total score was less than 50 percent of the potential score, medium if 50–75 percent, and high if greater than 75 percent.
For objective 2, the following data were extracted: intervention description, study setting, population, study design (including any randomization procedure, sampling, and comparison group), outcome, analysis method, and results. The quality of the intervention studies for objective 2 was also assessed by two reviewers using a checklist of 11 items adapted from the Critical Appraisal Skills Programme (CASP) (http://www.casp-uk.net/casp-tools-checklists), amended to allow applicability to both randomized and quasi‐experimental studies (see Table 3). An overall score was calculated by giving 0 points for not meeting criteria/unknown, 1 point for partially meeting, and 2 points for meeting criteria. Study quality was graded with the following scores: 0–11, low quality; 12–17, medium quality; and 18–22, high quality. Scores were adjusted proportionally in the case of a lower denominator. Given the heterogeneity of outcomes and study designs, it was determined that meta‐analysis would not be appropriate.
RESULTS
Objective 1: Level of Provision and Provision Practices
Characteristics of Studies and Study Populations
Figure 1 describes the search process for objective 1. Table 4 describes the main characteristics of the 22 studies selected for inclusion for objective 1. Most (20) were peer‐reviewed journal articles, one was a conference paper (Reiss et al. 2015b), and the other was a grey literature research report (Zamberlin 2007). The earliest study was published in 1991 (conducted in 1985), but most were published since 2010; 13 of those had not been included in an earlier literature review on this topic (Sneeringer, Billings, Ganatra, and Baird 2012). The studies were conducted in various locations in Latin America (Billings et al. 2009; Bonnema and Dalebout 1992; de Oddone et al. 1991; Lara, Garcia, Wilson, and Paz 2011; Miller et al. 2005; Zamberlin 2007), sub‐Saharan Africa (Adinma and Adinma 2013; Akiode et al. 2010; Fetters et al. 2015; Hendrickson et al. 2015; Reiss et al. 2017) South Asia (Huda et al. 2014; Mishra et al. 2016; Powell‐Jackson, Acharya, Filippi, and Ronsmans 2015; Reiss et al. 2015b; Tamang, Puri, Lama, and Shrestha 2015; Tamang and Tamang 2005), and South East Asia (Ngo, Park, and Nguyen 2012). In two of the locations, legal restrictions on abortion did not make exceptions to save the mother's life at the time of study (Senegal and Dominican Republic); in the majority abortion was allowed only to save the mother's life or to preserve her health (Nigeria, Mexico, Peru, Paraguay, Bangladesh, Kenya, and Argentina); and in the remaining locations abortion was available on socioeconomic grounds or on request (Zambia, India, Vietnam, and Nepal).
Figure 1.
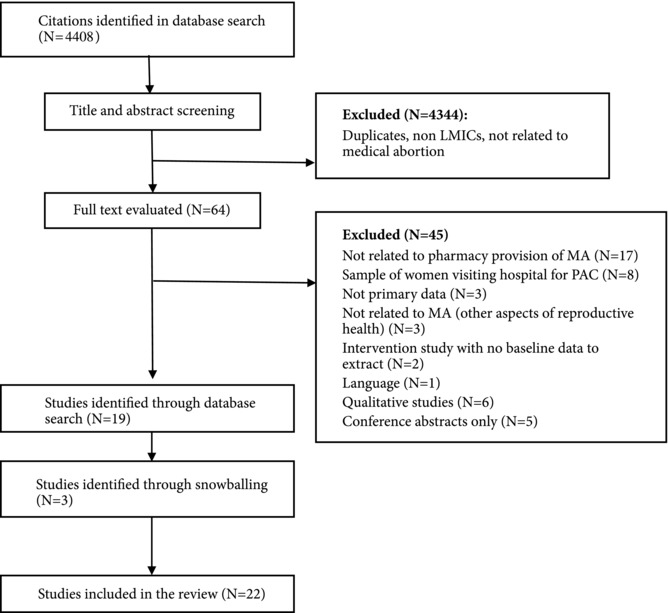
Summary of search results for objective 1
NOTE: MA = medical abortion. PAC = post‐abortion care.
The combined regimen of misoprostol and mifepristone was approved for use for MA in six of the locations at the time of study (Bangladesh, India, Zambia, Vietnam, Kenya, and Nepal) (Fetters et al. 2015; Ganatra, Manning, and Pallipamulla 2005; Hendrickson et al. 2015; Mishra et al. 2016; Ngo, Park, and Nguyen 2012; Powell‐Jackson, Acharya, Filippi, and Ronsmans 2015; Reiss et al. 2015b; Reiss et al. 2016; Tamang, Puri, Lama, and Shrestha 2015). In all other locations misoprostol was registered for use to treat other illnesses such as gastric ulcers, but not for MA. Most studies were conducted in urban settings and involved interviews with pharmacy workers (10 studies: Adinma and Adinma 2013; Akiode et al. 2010; de Oddone et al. 1991; Fetters et al. 2015; Ganatra, Manning, and Pallipamulla 2005; Mishra et al. 2016; Reiss et al. 2015b,; Reiss et al. 2016; Reiss et al. 2017; Tamang and Tamang 2005), mystery client surveys (where trained researchers pretend to be clients and record their experiences) (6 studies: Billings et al. 2009; Hendrickson et al. 2015; Huda et al. 2014; Lara, Garcia, Wilson, and Paz 2011; Miller et al. 2005; Zamberlin 2007), or a combination of the two (6 studies: Bonnema and Dalebout 1992; Lara, Abuabara, Grossman, and Díaz‐Olavarrieta 2006; Ngo, Park, and Nguyen 2012; Powell‐Jackson, Acharya, Filippi, and Ronsmans 2015; Reiss, Aung, Aung, and Ngo 2014; Reiss et al. 2016). Indicators used to report MA provision varied considerably between studies, including for example: “stocking” or “selling” MA drugs in pharmacy worker surveys, and “offering,” “recommending,” mentioning,” and “discussing” MA drugs in mystery client surveys. Distinctions were also made between prompted and spontaneous mentions of abortifacients in two mystery client surveys (Billings et al. 2009; Lara, Garcia, Wilson, and Paz 2011).
Studies were conducted in a range of outlet types: some sampling frames included all outlets that sold drugs (whether registered or not) (Ganatra, Manning, and Pallipamulla 2005; Huda et al. 2014; Powell‐Jackson, Acharya, Filippi, and Ronsmans 2015; Reiss, Aung, Aung, and Ngo 2014); some did not specify registration status (Bonnema and Dalebout 1992; Miller et al. 2005; Mishra et al. 2016; Zamberlin 2007); and the rest used lists of registered pharmacies. Respondents were not always specified, but included salespersons, owners, or managers. Of the 13 studies that reported respondents’ education or qualifications, a substantial proportion had higher education and/or specialist training. In 10 of the studies, the majority of pharmacy workers were qualified as pharmacists, had been trained to dispense medicines, or had professional training, a bachelor's degree, or a diploma in pharmacy. In 4 of the studies, the person invited for interview was the most senior, or the owner of the pharmacy, rather than the person most often working behind the counter.
Studies were of variable methodological quality: 7 were graded as high, 11 medium, and 4 low quality (see Table 2). Low‐quality studies typically had unrepresentative samples, inadequate recruitment methods, underpowered sample sizes, and inadequate statistical analysis.
Level of Abortifacient Provision
Table 5 shows data extracted on level and practice of abortifacient provision by pharmacy workers and drug sellers. The proportion of outlets offering abortifacients varied widely by setting, by legal status of abortion, and over time. High levels of provision were seen even in legally restrictive settings such as Latin America (Bonnema and Dalebout 1992; Huda et al. 2014; Reiss et al. 2015b). In most studies where both surveys and mystery clients were used, higher provision rates were reported by the latter (Powell‐Jackson, Acharya, Filippi, and Ronsmans 2015; Reiss, Aung, Aung, and Ngo 2014, 2016). Two studies found that provision was also more common when mystery clients asked directly about misoprostol or a specific brand of drug, rather than asking generally about a medication to induce abortion (Billings et al. 2009; Lara, Garcia, Wilson, and Paz 2011). Misoprostol‐only was the more commonly offered MA regimen, except in one recent Indian study (Powell‐Jackson, Acharya, Filippi, and Ronsmans 2015). In sub‐Saharan Africa, self‐reported provision of misoprostol for abortion was low: less than 1 percent of pharmacies in a study in Senegal in 2013 (Reiss et al. 2017) and 3 percent of pharmacies in Nigeria in 2006 (Akiode et al. 2010). One mystery client study in Kenya in 2013, however, reported higher provision levels (26 percent) (Reiss et al. 2016). In a repeated mystery client survey in Zambia , a significant increase in misoprostol provision was recorded, from 51 percent in 2009 to 72 percent in 2011 (Hendrickson et al. 2015). Misoprostol provision to mystery clients was common in legally restrictive settings such as Latin America (which studies were of mixed quality), ranging from 78–90 percent in Mexico (Billings et al. 2009; Lara, Garcia, Wilson, and Paz 2011) to 64 percent in the Dominican Republic (Miller et al. 2005) and 55 percent in Argentina (Zamberlin 2007). In Asia, two surveys in Bangladesh (Huda et al. 2014; Reiss et al. 2015b) and one Indian study (Powell‐Jackson, Acharya, Filippi, and Ronsmans 2015) also found that misoprostol provision in pharmacies and drug shops was relatively common. Provision of the mifepristone‐misoprostol combination to mystery clients was most commonly found in studies in South Asia where abortion was less legally restricted, particularly in India where 34 percent of chemists in Bihar and Jharkland reported selling the combination regimen in surveys in 2005 (Ganatra, Manning, and Pallipamulla 2005), and 67 percent reported selling the combination regimen in a 2015 study (Powell‐Jackson, Acharya, Filippi, and Ronsmans 2015).
Provision of other types of abortifacients was also reported and observed. A 2014 study in an unnamed South East Asian city, where safe, legal abortion is highly restricted, found provision of misoprostol was uncommon, and almost half of mystery clients were offered an ineffective drug: either oral or emergency contraceptives, traditional medicines, or Penorit (a hormone preparation for secondary amenorrhea designed to restore menstruation) (Reiss, Aung, Aung, and Ngo 2014). In India, Nepal, and Bangladesh provision of ayurvedic and herbal abortion preparations was also commonly observed in pharmacy and drug seller surveys (Tamang and Tamang 2005; Huda et al. 2014; Powell‐Jackson, Acharya, Filippi, and Ronsmans 2015). Sales of high‐dose contraceptives or hormonal injections were commonly reported by pharmacy workers and mystery clients in surveys in Latin America during the early 1990s (de Oddone et al. 1991; Bonnema and Dalebout 1992).
Provision Practices and Knowledge
Pharmacy worker/drug seller knowledge of or counseling on an effective misoprostol‐only or mifepristone‐misoprostol regimen was poor, ranging from 3 to 17 percent in Latin America (Billings et al. 2009; Lara, Abuabara, Grossman, and Díaz‐Olavarrieta 2006; Lara, Garcia, Wilson, and Paz 2011), from 9 to 41 percent in Asia (Ganatra, Manning, and Pallipamulla 2005; Huda et al. 2014; Mishra et al. 2016; Powell‐Jackson, Acharya, Filippi, and Ronsmans 2015; Reiss et al. 2015b, Reiss, Aung, Aung, and Ngo 2014), and from 0 to 21 percent in Africa (Hendrickson et al. 2015; Reiss et al. 2016; Reiss et al. 2017). A recent high‐quality study in India comparing data from a mystery client study and a drug seller survey found that knowledge did not translate into accurate advice to clients: while 51 percent of pharmacy workers had correct knowledge of the dosage and timing of the combined regimen for MA, only 35 percent provided correct information to clients (Powell‐Jackson, Acharya, Filippi, and Ronsmans 2015).
In the 8 mystery client studies reporting whether a prescription was requested, less than half of clients were asked for a prescription, ranging from none in a restrictive South East Asian city (Reiss, Aung, Aung, and Ngo 2014) to 40 percent in Zambia (Hendrickson et al. 2015) and Argentina (Zamberlin 2007). There was also wide variation in advice given regarding potential complications in the 10 mystery client studies reporting on this indicator, ranging from 0 percent in Peru (Bonnema and Dalebout 1992) to 78–100 percent in Zambia (Hendrickson et al. 2015). Referral to other providers in the case of complications was low in studies from Peru and Argentina (Bonnema and Dalebout 1992; Zamberlin 2007) but relatively high (above 50 percent) in several studies from India (Ganatra, Manning, and Pallipamulla 2005; Mishra et al. 2016), Mexico (Billings et al. 2009), Vietnam (Ngo, Park, and Nguyen 2012), and Bangladesh (Reiss et al. 2015b).
Objective 2: Interventions to Improve the Level or Quality of MA Pharmacy Provision
We found three studies evaluating interventions to improve the quality of pharmacy and drug shop provision of MA (see Figure 2 and Table 6).
Figure 2.
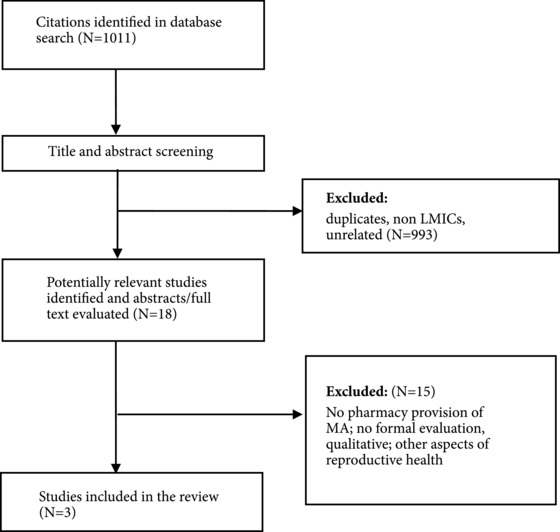
Summary of search results for objective 2: MA through pharmacy intervention studies
Characteristics of Studies
The three studies were conducted in Zambia, Bangladesh, and Nepal. Two were reported in peer‐reviewed journals (Fetters et al. 2015; Tamang, Puri, Lama, and Shrestha 2015) and one was a conference abstract for which we could access the full paper (Reiss et al. 2015a). The interventions evaluated included a call center (Reiss et al. 2015a), training courses (Fetters et al. 2015; Reiss et al. 2015a; Tamang, Puri, Lama, and Shrestha 2015), printed materials (Tamang, Puri, Lama, and Shrestha 2015), and one‐to‐one education visits (Reiss et al. 2015a). All of the interventions aimed to improve the knowledge of pharmacy workers and were graded as low quality, commonly lacking random allocation to the intervention, blinding of participants, and adequate comparisons between groups or over time (see Table 3 for full scores). The study from Bangladesh was a cross‐sectional post‐intervention analytical study. The studies conducted in Zambia and Nepal were pre‐ and post‐intervention evaluations. Only one of the studies included a comparison group of pharmacies from a non‐intervention area (Tamang, Puri, Lama, and Shrestha 2015). The cross‐sectional study was the only one to use probabilistic sampling and to adjust for potential confounding factors (Reiss et al. 2015a). A wide range of outcomes were included in the studies (Table 6), but no studies included data on cost‐effectiveness.
Results of Interventions
Training interventions were evaluated in all three studies, and reported results were positive. A one‐day training course in Zambia was associated with self‐reported increases in referrals, provision of information about MA to clients, and sales of misoprostol when participants were interviewed 12–24 months later (Fetters et al. 2015). As there was no control group, however, the evidence that these increases are related to training, rather than reflecting general improvements in awareness and knowledge, is weak, and self‐reported outcomes may reflect courtesy bias. A two‐day training intervention was conducted in Nepal, augmented by printed materials, referral vouchers, and interactive meetings between pharmacy workers and abortion providers. The study also included refresher training after 10 months and an end‐line evaluation at one year. At baseline, knowledge of MA in both the intervention and control groups was high, which the authors speculated was due to recent NGO training in the area, but knowledge fell among the control group during the study, highlighting the need for training to be periodic (Tamang, Puri, Lama, and Shrestha 2015).
The use of a call center to improve pharmacy worker knowledge was assessed in Bangladesh. Knowledge was greater among pharmacy workers who had contact with a call center (set up by an international NGO to provide information on MA) after adjusting for potential confounding factors (Reiss et al. 2015a). The Bangladesh study also assessed whether knowledge was higher among pharmacy workers who reported receiving pharmaceutical company visits or training from an NGO; there was some evidence that NGO training had a positive effect. However, since this study did not collect baseline data, the associations cannot be assumed to be causal.
DISCUSSION
The studies in this systematic review suggest that provision of abortifacients by pharmacy workers and drug sellers in LMICs is common, as is provision of medications to induce abortion without a prescription (including effective and ineffective drugs). The highest quality evidence of these findings relates to Mexico, India, Bangladesh, and Nepal, but there were studies of reasonable methodological quality from sub‐Saharan Africa (Nigeria, Zambia, Senegal, Kenya), Latin America (Peru and Dominican Republic), and Asia (India, Bangladesh, Vietnam, Nepal). However, in Latin America, there was a paucity of high‐quality recent studies (published in the last 5 years) from countries other than Mexico, and some studies from the 1990s are of limited relevance today. Use of standardized indicators and repeated cross‐sectional studies would improve comparability between regions and over time to assess trends in MA availability in pharmacies and drug shops.
In general, the review found that the information provided by individuals working in pharmacies and drug shops was poor, with many recommending ineffective drugs, few advising an effective regimen, and few giving information on potential complications and what to do if they occur. Poor‐quality products and information provision by pharmacies is not a problem unique to MA. Incorrect advice and inappropriate supply of medicines in LMIC pharmacies have been documented for health services ranging from childhood diarrhea to tuberculosis (Smith 2009). However, stigma and legal restrictions related to abortion may create additional challenges for quality of pharmacy provision of MA, not least because drugs are often used off‐label. In Africa the reported level of provision (proportion of pharmacy workers/drug sellers who offered MA drugs/ mystery clients who were offered MA) was higher in less restrictive settings (i.e. Zambia), but this was not the case in other regions such as Latin America, where provision was relatively high despite legal restrictions. The highest levels of knowledge of the MA regimen were also found in settings where abortion was less legally restricted (India, Nepal).
We found only three studies that evaluated interventions to improve pharmacy worker knowledge or practice of MA, despite the search including unpublished grey literature. All three studies were in settings where abortion/menstrual regulation had few legal restrictions, suggesting that legal issues may be hindering interventions and research on this topic in other regions. The studies did not have rigorous evaluation designs, and two of them lacked a control group. The studies found that pharmacy worker training, with some form of follow up, can improve knowledge. One study suggested that call centers, but not pharmaceutical one‐to‐one education visits, may be effective in improving knowledge among pharmacy workers. The evidence for training is supported by two other systematic reviews which found that educational initiatives have positive impacts on pharmacy provision of general health services (Smith 2009; Wafula and Goodman 2010). However, the studies did not assess whether improved knowledge resulted in higher quality provision practice. Research suggests that increased knowledge of MA regimens may not result in improved counseling (Powell‐Jackson, Acharya, Filippi, and Ronsmans 2015). Additionally, none of the studies in this review looked at the costs or cost‐effectiveness of the interventions; there are an estimated 210,000 registered and unregistered drug sellers in Bangladesh (Uzzal 2014), and 40,000 in Vietnam (Minh, Huong, Byrkit, and Murray 2013), so scaling up training initiatives to the national level may not be feasible in many LMICs. High mobility of pharmacies (either moving location or closing down) (Hendrickson et al. 2015), high turnover of staff, and high numbers of informal drug sellers are also challenges to the feasibility of scaling up training initiatives. In this review, as in others (Smith 2009; Wafula and Goodman 2010), there was no evaluation of more innovative approaches to improving pharmacy performance, such as incentive structures, feedback, or regulatory mechanisms. Further evidence is needed on efficient ways of strengthening the information pharmacy workers receive about MA, such as one‐to‐one education meetings and telemedicine.
Most of the interventions in this review were targeted at changing pharmacy worker behavior. Evidence is also needed on the effectiveness of interventions to provide information directly to women who purchase MA drugs from pharmacies/drug shops and self‐administer the medications. The WHO task‐sharing guidelines for abortion recommend against pharmacy provision of MA, because of a lack of comparative evidence (World Health Organization 2012), but recommend self‐management for sub‐tasks of MA in circumstances where women have access to appropriate information and emergency care. Directly providing women with the information they need to self‐manage a medical abortion, for example through telephone hotlines, may improve the outcomes of women who purchase MA from drug shops and reduce the potential harm of unsafe abortion. However, women may continue to purchase ineffective medicines sold as abortifacients from pharmacies and drug shops if effective MA drugs are not available or if they cannot afford the most effective medications and are sold an inferior product. Evaluations of strategies to reduce sales of unsafe, ineffective, and incorrect medications are also needed.
This review has a number of limitations. First, in the search process we found several relevant study abstracts but could not access full‐text reports, so these are necessarily excluded, as were some relevant studies that used only qualitative methods. We also found studies evaluating innovative interventions such as telemedicine for reducing risk from unsafe MA, but we could not include these studies as they were not directed exclusively at pharmacy or drug shop provision. The research field is small and we necessarily had to include studies that some of us had authored ourselves. To avoid potential ethical bias, the quality scoring was conducted only by non‐authors of the paper being reviewed, and were checked by a second external reviewer. The heterogeneity of the study designs and outcomes posed challenges for selecting an appropriate methodological appraisal tool, particularly for the intervention studies. The tool we used to assess the intervention studies—CASP—was designed to evaluate randomized controlled trials. Although we adapted the question wording, our use of this tool meant that the intervention studies (only one of which had a control group) were appraised relatively harshly. Trends over time, between countries, and across regions could not be clearly assessed given the variability of methods used, indicators measured, and locations where the studies took place. Because most studies included in the review were cross‐sectional, we could not assess within‐country temporal trends. We also were unable to assess the quality of products offered or sold because none of the studies tested products, and we were unable to compare product costs across studies given the lack of data. The issue of MA product quality has been addressed only to a limited degree and warrants further attention (Hall 2016). The quality of studies selected was mixed, and intervention studies were not methodologically strong, often relying on small or convenience samples and using inadequate designs. This may reflect the challenge of conducting interventions and evaluations on a legally restricted practice. Finally, we included studies that reported on a number of different outcomes, making comparisons difficult.
CONCLUSION
Availability of MA in pharmacies and drug shops in LMICs is common, and evidence suggests that women seek assistance from such outlets when faced with an unwanted pregnancy. Pharmacies can expand access to medications for MA, but they do not always provide the right products or effective regimens, and the quality of their knowledge and information provision is often poor. Despite increasing research, there is a lack of high‐quality evidence in many settings where abortion is more highly restricted. The use of pharmacies and drug shops as a first port of call for health care, and the poor quality of health care provided by pharmacy workers and drug sellers, are not limited to abortion. However, leveraging the benefits of pharmacies and drug shops, such as their perceived accessibility, affordability, and confidentiality, has great potential to reduce the harm caused by unsafe abortion. More innovative interventions and rigorous evaluations that include cost‐effectiveness are needed to build the evidence base on what works to improve provision through pharmacy workers and drug sellers and to increase women's access to information. To reduce the consequences of unsafe abortion, interventions cannot rely solely on pharmacy workers and drug sellers providing higher quality information, but should seek to ensure that women have direct access to the information they need to self‐administer medical abortion.
Supporting information
Table 1 List of search terms used
Table 2 Individual item quality scores for methodological quality, objective 1
Table 3 Individual item quality scores for intervention studies, objective 2
Table 4 Summary of studies meeting inclusion criteria for objective 1
Table 5 Extent of MA provision and provision practices of pharmacy workers and drug sellers: data extracted from 22 studies meeting inclusion criteria for objective 1
Table 6 Intervention studies aimed at improving access to and knowledge of pharmacy/drug shop provision of MA: data extracted from 3 studies meeting inclusion criteria for objective 2
ACKNOWLEDGMENTS
This study was funded by the Strengthening Evidence for Programming on Unintended Pregnancy (STEP‐UP) Research Consortium, which is funded by UKaid from the Department for International Development. The authors are grateful to Suzanne Penfold‐Taylor and Rachel Scott for reviewing the quality grading of the studies.
Katharine Footman is Research Portfolio Manager, and Barbara Reichwein is Director, Research, Monitoring and Evaluation Team, Health Systems Department, Marie Stopes International, London. E‐mail: katy.footman@mariestopes.org. Katherine Keenan is Lecturer in Demography, Department of Geography and Sustainable Development, University of St Andrews, UK. Kate Reiss is a Research Degree Student, Department of Population Health, London School of Hygiene and Tropical Medicine, London. Pritha Biswas is Senior Advisor, Medical Development Team, Marie Stopes International, India. Kathryn Church is Senior Strategic Research Manager, Research, Monitoring and Evaluation Team, Health Systems Department, Marie Stopes International, London; and Honorary Assistant Professor, Department of Population Health, London School of Hygiene and Tropical Medicine, London.
Footnotes
Tables are available at the supporting information tab at wileyonlinelibrary.com/journal/sfp.
REFERENCES
- Adinma, E. and Adinma J.. 2013. “Knowledge and inventory management of misoprostol for reproductive health services amongst community pharmacists in Anambra and Delta states of Nigeria,” Afrimedic Journal 2(1): 13–18. [Google Scholar]
- Ahmed, S.M. and Hossain M.A.. 2007. “Knowledge and practice of unqualified and semi‐qualified allopathic providers in rural Bangladesh: Implications for the HRH problem,” Health Policy 84(2‐3): 332–343. https://doi.org/10.1016/j.healthpol.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Akiode, A. et al. 2010. “The availability of misoprostol in pharmacies and patent medicine stores in two Nigerian cities,” Ebonyi Medical Journal 9(2). [Google Scholar]
- Billings, D.L. , Walker D., Mainero del Paso G., Clark K.A., and Dayananda I.. 2009. “Pharmacy worker practices related to use of misoprostol for abortion in one Mexican state,” Contraception 79(6): 445–451. https://doi.org/10.1016/j.contraception.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Bonnema, J. and Dalebout J.A.. 1992. “The abuse of high dose estrogen/progestin combination drugs in delay of menstruation: The assumptions and practices of doctors, midwives and pharmacists in a peruvian city,” Social Science & Medicine 34(3): 281–289. https://doi.org/10.1016/0277-9536(92)90270-Z. [DOI] [PubMed] [Google Scholar]
- Chalker, J. , Chuc N.T.K., Falkenberg T., Do N.T., and Tomson G.. 2000. “STD management by private pharmacies in Hanoi: Practice and knowledge of drug sellers,” Sexually Transmitted Infections 76(4): 299–302. https://doi.org/10.1136/sti.76.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culwell, K.R. and Hurwitz M.. 2013. “Addressing barriers to safe abortion,” International Journal of Gynecology and Obstetrics 121(S1): S16–S19. https://doi.org/10.1016/j.ijgo.2013.02.003. [DOI] [PubMed] [Google Scholar]
- de Oddone, N.K. , Shedlin M.G., Welsh M., Potts M., and Feldblum P.. 1991. “Paraguayan pharmacies and the sale of pseudo‐abortifacients,” Journal of Biosocial Science 23(2): 201–209. https://doi.org/doi:10.1017/S0021932000019210. [DOI] [PubMed] [Google Scholar]
- Faúndes, A. , Santos L.C., Carvalho M., and Gras C.. 1996. “Post‐abortion complications after interruption of pregnancy with misoprostol,” Advances in Contraception 12(1): 1–9. https://doi.org/10.1007/bf01849540 [DOI] [PubMed] [Google Scholar]
- Fernandez, M.M. , Coeytaux F., Gomez Ponce de León R., and Harrison D.L.. 2009. “Assessing the global availability of misoprostol,” International Journal of Gynecology & Obstetrics 105(2): 180–186. https://doi.org/10.1016/j.ijgo.2008.12.016 [DOI] [PubMed] [Google Scholar]
- Fetters, T. et al. 2015. “Using a harm reduction lens to examine post‐intervention results of medical abortion training among Zambian pharmacists,” Reproductive Health Matters 22(44): 116–124. [DOI] [PubMed] [Google Scholar]
- Ganatra, B. , Manning V., and Pallipamulla S.P.. 2005. “Availability of medical abortion pills and the role of chemists: A Study from Bihar and Jharkhand, India,” Reproductive Health Matters 13(26): 65–74. https://doi.org/10.1016/S0968-8080(05)26215-8. [DOI] [PubMed] [Google Scholar]
- Goel, P. , Ross‐Degnan D., Berman P.. and Soumerai S.. 1996. “Retail pharmacies in developing countries: A behavior and intervention framework,” Social Science & Medicine 42(8): 1155–1161. 1155–1161. https://doi.org/10.1016/0277-9536(95)00388-6. [DOI] [PubMed] [Google Scholar]
- Goodman, C. , Brieger W., Unwin A., Mills A., Meek S., and Greer G.. 2007. “Medicine sellers and malaria treatment in sub‐Saharan Africa: What do they do and how can their practice be improved?,” The American Journal of Tropical Medicine and Hygiene 77(6 Suppl.): 203–218. http://www.ajtmh.org/content/77/6_Suppl/203.abstract. [PMC free article] [PubMed] [Google Scholar]
- Graham, W. et al. 2017. “Diversity and divergence: the dynamic burden of poor maternal health,” The Lancet 388(10056): 2164–2175. https://doi.org/10.1016/S0140-6736(16)31533-1. [DOI] [PubMed] [Google Scholar]
- Hall, P.E. 2016. “Quality of Misoprostol products,” WHO Drug Information 30(1). [Google Scholar]
- Harper, C.C. , Blanchard K., Grossman D., Henderson J.T., and Darney P.D.. 2007. “Reducing maternal mortality due to elective abortion: Potential impact of misoprostol in low‐resource settings,” International Journal of Gynecology & Obstetrics 98(1): 66–69. https://doi.org/10.1016/j.ijgo.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Hendrickson, C. , Fetters T., Mupeta S., Vwallika B., Djemo P., and Raisanen K.. 2015. “Client–pharmacy worker interactions regarding medical abortion in Zambia in 2009 and 2011,” International Journal of Gynecology and Obstetrics. https://doi.org/10.1016/j.ijgo.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Huda, F.A. , Ngo T.D., Ahmed A., Alam A., and Reichenbach L.. 2014. “Availability and provision of misoprostol and other medicines for menstrual regulation among pharmacies in Bangladesh via mystery client survey,” International Journal of Gynecology & Obstetrics 124(2): 164–168. [DOI] [PubMed] [Google Scholar]
- Hyman, A. , Blanchard K., Coeytaux F., Grossman D., and Teixeira A.. 2013. “Misoprostol in women's hands: A harm reduction strategy for unsafe abortion,” Contraception 87(2): 128–130. [DOI] [PubMed] [Google Scholar]
- Lara, D. , Abuabara K., Grossman D., and Díaz‐Olavarrieta C.. 2006. “Pharmacy provision of medical abortifacients in a Latin American city,” Contraception 74(5): 394–399. [DOI] [PubMed] [Google Scholar]
- Lara, D. , Garcia S. G., Wilson K.S., and Paz F.. 2011. “How often and under which circumstances do Mexican pharmacy vendors recommend misoprostol to induce an abortion?,” International Perspectives on Sexual and Reproductive Health 37(2): 75–83. http://www.jstor.org/stable/41228998. [DOI] [PubMed] [Google Scholar]
- Miller, S. et al. 2005. “Misoprostol and declining abortion‐related morbidity in Santo Domingo, Dominican Republic: A temporal association,” BJOG: An International Journal of Obstetrics & Gynaecology 112(9): 1291–1296. https://doi.org/10.1111/j.1471-0528.2005.00704.x. [DOI] [PubMed] [Google Scholar]
- Minh, P.D. , Huong D.T.M., Byrkit R., and Murray M.. 2013. “Strengthening pharmacy practice in vietnam: Findings of a training intervention study,” Tropical Medicine & International Health 18(4): 426–434. https://doi.org/10.1111/tmi.12062. [DOI] [PubMed] [Google Scholar]
- Mishra, A. , Yadav A., Malik S., Purwar R., and Kumari S.. 2016. “Over the counter sale of drugs for medical abortion‐ Knowledge, Attitude, and Practices of pharmacists of Delhi, India,” International Journal of Pharmacological Research 6(3): 92–96. [Google Scholar]
- Munn, Z. , Moola S., Riitano D., and Lisy K.. 2014. “The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence,” International Journal of Health Policy and Management 3: 123–128 https://doi.org/10.15171/ijhpm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo, T.D. , Park M.H., and Nguyen T.H.. 2012. “Pharmacy workers’ knowledge and provision of abortifacients in Ho Chi Minh City, Vietnam,” International Journal of Gynecology & Obstetrics 117(2): 187–188. https://doi.org/10.1016/j.ijgo.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Powell‐Jackson, T. , Acharya R., Filippi V., and Ronsmans C.. 2015. “Delivering medical abortion at scale: A study of the retail market for medical abortion in Madhya Pradesh, India,” PLoS ONE 10(3): e0120637 https://doi.org/10.1371/journal.pone.0120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss, K. , Aung O., Aung M.M., and Ngo T.D.. 2014. “Availability and provision of abortifacients among pharmacies in a restricted Southeast Asian city,” Journal of Pharmaceutical Care & Health Systems S1(5). [Google Scholar]
- Reiss, K. , Keenan K., Dijkerman S., Choudhury P., Mitu S., Nuremowla S., and Ngo T.. 2015a. “FCS35.8 Improving pharmacy workers’ knowledge of misoprostol for menstrual regulation in Bangladesh: The effectiveness of training, detailing and call centre interventions,” International Journal of Gynecology and Obstetrics 131 Suppl (E72–E313: Free Communication (Oral) Presentations at XXI FIGO World Congress of Gynecology and Obstetrics held in Vancouver, CA).29644647 [Google Scholar]
- Reiss, K. , Keenan K., Dijkerman S., Choudhury P., Mitu S., Nuremowla S., and Ngo T.. 2015b. P0277: Pharmacy provision of medication for menstrual regulation in Bangladesh: a national survey of knowledge and practice, in XXI FIGO World Congress of Gynecology and Obstetrics Vancouver: International Journal of Gynocology and Obstetrics 131, Suppl 5 E314‐E607, pp. E393–E394.
- Reiss, K. , Footman K., Akora V., Liambila W., and Ngo T. D.. 2016. “Pharmacy workers’ knowledge and provision of medication for termination of pregnancy in Kenya,” Journal of Family Planning and Reproductive Health Care. https://doi.org/10.1136/jfprhc-2013-100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss, K. , Footman K., Burke E., Diop N., Ndao R., Mane B., … Ngo T.D.. 2017. “Knowledge and provision of misoprostol among pharmacy workers in Senegal: a cross sectional study,” BMC Pregnancy and Childbirth 17(1): 211 https://doi.org/10.1186/s12884-017-1394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Say, L. et al. 2014. “Global causes of maternal death: A WHO systematic analysis,” The Lancet Global Health 2(6): e323–e333. https://doi.org/10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- Singh, S. and Maddow‐Zimet I.. 2015. “Facility‐based treatment for medical complications resulting from unsafe pregnancy termination in the developing world, 2012: A review of evidence from 26 countries,” BJOG: An International Journal of Obstetrics & Gynaecology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, F. 2009. “The quality of private pharmacy services in low and middle‐income countries: A systematic review,” Pharmacy World & Science 31(3): 351–361. https://doi.org/10.1007/s11096-009-9294-z. [DOI] [PubMed] [Google Scholar]
- Sneeringer, R. K. , Billings D. L., Ganatra B., and Baird T.L.. 2012. “Roles of pharmacists in expanding access to safe and effective medical abortion in developing countries: A review of the literature,” Journal of Public Health Policy 33(2): 218–229. https://doi.org/10.1057/jphp.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamang, A. , Puri M., Lama K., and Shrestha P.. 2015. “Pharmacy workers in Nepal can provide the correct information about using mifepristone and misoprostol to women seeking medication to induce abortion,” Reproductive Health Matters 22(44): 104–115. [DOI] [PubMed] [Google Scholar]
- Tamang, A. and Tamang J.. 2005. “Availability and acceptability of medical abortion in Nepal: Health care providers' perspectives,” Reproductive Health Matters 13(26): 110–119. https://doi.org/10.1016/S0968-8080(05)26194-3 [DOI] [PubMed] [Google Scholar]
- Uzzal, M. 2014, “50% pharmacies unregistered,” Dhaka Tribune, July 5. http://archive.dhakatribune.com/safety/2014/jul/05/50-pharmacies-unregistered. [Google Scholar]
- Wafula, F.N. and Goodman C.A.. 2010. “Are interventions for improving the quality of services provided by specialized drug shops effective in sub‐Saharan Africa? A systematic review of the literature,” International Journal for Quality in Health Care 22(4): 316–323. https://doi.org/10.1093/intqhc/mzq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2011. Unsafe Abortion: Global and Regional Estimates of the Incidence of Unsafe Abortion and Associated Mortality in 2008. Geneva. [Google Scholar]
- World Health Organization . 2012. Safe Abortion: Technical and Policy Guidance for Health Systems (2nd edition). Geneva. [PubMed] [Google Scholar]
- Zamberlin, N. 2007. “Acceso, saberes y experiencias acerca del aborto con medicamentos: el circuito del misoprostol en la Ciudad de Buenos Aires” [Knowledge and experience of accessing medical abortion: The circulation of misoprostol in the city of Buenos Aires]. Buenos Aires:s Ministerio de Salud, Argentina
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1 List of search terms used
Table 2 Individual item quality scores for methodological quality, objective 1
Table 3 Individual item quality scores for intervention studies, objective 2
Table 4 Summary of studies meeting inclusion criteria for objective 1
Table 5 Extent of MA provision and provision practices of pharmacy workers and drug sellers: data extracted from 22 studies meeting inclusion criteria for objective 1
Table 6 Intervention studies aimed at improving access to and knowledge of pharmacy/drug shop provision of MA: data extracted from 3 studies meeting inclusion criteria for objective 2


