Abstract
Purpose
To assess efficacy and safety of intravitreal ranibizumab 0.5 mg plus laser (COMBI) versus laser monotherapy (LASER) in patients with visual impairment due to diabetic macular oedema (DME) in either nonproliferative diabetic retinopathy (NPDR) or proliferative diabetic retinopathy (PDR) and to analyse the relevance of inner versus outer retinal thickness.
Methods
In this double‐masked, multicentre phase IIIb study, patients (N = 128) were randomized (2:1) to receive COMBI (n = 85) versus LASER (n = 43). Patients received four initial monthly injections of ranibizumab 0.5 mg (COMBI) or sham (LASER) followed by pro re nata (PRN) injections. In both groups, patients received laser at baseline and additional laser at 3 monthly intervals, as needed. The study was started in 2010 and was prematurely terminated due to approval of ranibizumab for DME.
Results
The least squares (LS) mean change in mean best‐corrected visual acuity (BCVA) from baseline to month 12 was higher in the COMBI (6.5) versus LASER (2.3) group (LS mean difference: 4.2 [95% CI 0.9; 7.4] letters, p = 0.01, primary end‐point). There was also a tendency in the same direction for the subgroup of 26 patients with PDR (LS mean difference 14.7, p = 0.11). Mean central retinal thickness decreased by 107.3 μm in the COMBI group and by 80.3 μm in the LASER group from baseline to month 12 (p = 0.28). Ranibizumab was well tolerated.
Conclusion
This study showed that ranibizumab plus laser is a valuable treatment option for the management of DME. Patients with DME in PDR might also benefit from combined therapy compared to laser alone.
Keywords: diabetic macular oedema, intravitreal injections, laser monotherapy, proliferative diabetic retinopathy, ranibizumab, visual acuity
Introduction
Diabetic retinopathy (DR) and DME (Klein et al. 1995, 2009) are major complications of diabetes mellitus, and the most common causes of blindness in people of working age with diabetes (Klein et al. 1995, 2009). In Germany, the prevalence of DR and DME was estimated at 10.6% and 0.85%, respectively (Blum et al. 2007).
The phase IIIb RELATION study was designed to assess the efficacy and safety of intravitreal ranibizumab 0.5 mg plus focal laser compared with laser monotherapy in patients with visual impairment due to DME in either NPDR or PDR. When the study was designed, laser photocoagulation was considered the standard of care for the treatment of DME and DR (Fong 2002), while ranibizumab was only approved for the treatment of age‐related macular degeneration. It was hypothesized that a combination of focal laser and ranibizumab might optimize the benefits of both treatments with fewer applications of ranibizumab.
However, after study start, ranibizumab received European and German approval for the treatment of DME based on the favourable results from global phase II and phase III studies demonstrating superiority of ranibizumab to laser monotherapy in terms of vision gain (Nguyen et al. 2009, 2010; Elman et al. 2010; Massin et al. 2010; Mitchell et al. 2011; Lang et al. 2013). Hence, the RELATION study was terminated prematurely, because, with an approved and superior treatment available, further randomization of patients into the laser monotherapy group or continuation of laser monotherapy treatment was considered inappropriate for ethical reasons. Nevertheless, this prematurely terminated study did yield relevant information for the management of DME using a modified PRN regime, especially in patients with PDR, who had been excluded from previous studies.
The primary objective of the study was to demonstrate superiority of combination therapy compared to laser monotherapy with respect to the mean change in BCVA from baseline to month 12. Secondary objectives were to evaluate BCVA outcome in a subgroup of patients with PDR, retinal thickness and volume outcomes as well as safety. A post hoc exploratory objective was to assess the correlation between inner and outer retinal thickness (measured with optical coherence tomography [OCT]) and BCVA as well as severity of macular ischaemia and type of DME (focal, intermediate, diffuse).
Patients and Methods
Study design
The two‐armed, randomized, double‐masked, multicentre, phase IIIb RELATION study was conducted in 37 study centres in Germany from 22 July 2010 to 20 July 2011. Eligible patients were randomized in a 2:1 ratio to combination therapy with ranibizumab plus laser (COMBI group) or to laser monotherapy with sham injections (LASER group).
The study was conducted in accordance with the Declaration of Helsinki and ICH‐GCP guidelines. Approval was obtained from the ethics committee or institutional review board as well as from health authorities. Patients provided written informed consent before entering the study. The study is registered with clinicaltrials.gov as NCT01131585.
Patients
The study population consisted of patients aged ≥18 years. Inclusion criteria included visual impairment due to DME in at least one eye, BCVA scores between 78 and 39 early treatment diabetic retinopathy study (ETDRS) letters, HbA1c ≤10.0% as well as stable medication for the management of diabetes within 3 months before randomization and expected to remain stable during the study course. Type 1 diabetes and type 2 diabetes as well as NPDR and PDR were allowed.
Key exclusion criteria were decrease in vision due to other causes, vitreous haemorrhage, concomitant conditions in the study eye or other ocular disorders that may confound interpretation of study results. Additional exclusion criteria were focal/grid laser photocoagulation 3 months before, and panretinal photocoagulation 6 months before randomization; treatment with anti‐angiogenic drugs or intraocular surgery in the study eye 3 months before randomization; vitrectomy; intravitreal corticosteroid application in phakic study eyes or chronic treatment with topical ocular or systemic corticosteroids.
Treatment
Initial treatment (baseline to month 3)
Patients in the COMBI group received four initial consecutive monthly injections of ranibizumab at baseline and months 1–3 (Fig. 1). Focal laser treatment was mandatorily applied at baseline and reapplied if needed at month 3, based on the investigator's judgement. Focal laser and ranibizumab injections were administered on the same day with a minimum interval of 30 min between the two treatments.
Figure 1.
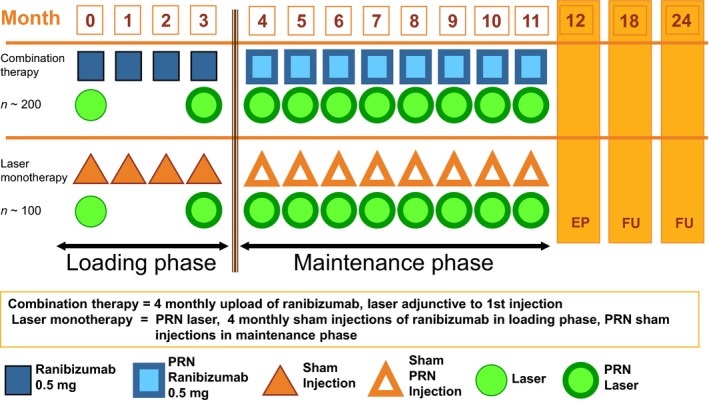
Study design. PRN = pro re nata, on demand.
Patients in the control group (LASER) received laser treatment at baseline and if needed at month 3, based on the investigator's judgement. In addition, they received sham injections at baseline and months 1–3. In both groups, panretinal laser photocoagulation (PRP) treatment was to be used for PDR.
Retreatment criteria during maintenance phase (month 4 to study end)
As of month 4, the treatment in either group was based on a PRN regimen. Criteria for retreatment included (1) further reduction in BCVA due to DME persistence or progression in the opinion of the evaluating physician, or (2) central retinal thickness gain by ≥20% as compared to best value ever, or (3) central retinal thickness >250 μm (unless evaluating physician deemed no further reduction in thickness would occur under treatment continuation). In the COMBI group, patients always received both ranibizumab and focal laser on the same day.
Treatment masking
Patients, visual acuity (VA) assessors performing the BCVA examination and evaluating physicians, who were responsible for treatment decision, were masked to treatment assignment and injection procedures. A further treating physician was unmasked to the treatment assignment and performed injection procedures.
Efficacy and safety assessments
Early treatment diabetic retinopathy study (ETDRS) BCVA measurements as well as OCT scans were performed at every study visit. Optical coherence tomography (OCT) scans were reviewed by a central reading centre to ensure error‐corrected measurements of foveal centre point (FCP) thickness, as well as central subfield mean (FCS) thickness and total retinal volume within the ETDRS grid for the combined thickness of neurosensory retina plus subretinal fluid. Foveal central subfield (FCS) thickness was additionally calculated for the neurosensory retina, as well as for the outer retina (outer nuclear layer and photoreceptors) and the inner retina (inner limiting membrane to outer retina) separately.
Colour fundus photography (FP) and fluorescein angiography (FA) were performed at baseline, month 4 (FP only) and month 12. Staging of DR was performed by clinical sites as well as the reading centre. Fluorescein angiography (FA) images were evaluated by the reading centre for classification of DME (focal: ≥67% of leakage associated with microaneurysms; diffuse: ≥67% of leakage associated with telangiectatic capillaries, or intermediate). Safety assessments consisted of ongoing monitoring and recording of all treatment emergent adverse events (TEAEs) and serious adverse events (SAEs) at each visit.
Statistical analysis
The planned sample size was 300 patients, and at the time of premature termination of the trial, 128 patients had been enrolled. Thus, the statistical power as envisaged with the original sample size estimation was not achieved. Moreover, follow‐up per patient was up to 11 months at a maximum as a consequence of premature termination. We present the available data in terms of the originally planned safety and efficacy analyses referring to 12 months of treatment – using the last observation carried forward (LOCF) approach for the imputation of missing data.
An analysis of covariance (ancova) model with the factors, ‘centre’, ‘treatment’, ‘type of DME’ (focal/intermediate/diffuse), ‘PDR present’ (yes/no) and the covariate ‘baseline BCVA’, was used for the primary end‐point analysis. Adjusted least‐square (LS‐) means with corresponding 95% confidence intervals (CI) and p‐values were calculated as point estimates for the treatment contrasts. In the primary analysis, the presence of PDR was assessed by the local investigator. An additional post hoc sensitivity analysis of the primary end‐point was carried out using the PDR evaluation of the reading centre.
For the binary secondary end‐points (VA > 73 letters, VA gain ≥15 letters and any VA gain), the difference in proportions and the odds ratios were calculated, including respective CIs and p‐values. Retinal thickness and progression in PDR were descriptively analysed by providing the mean (±[standard deviation (SD)])/median and range values per visit where applicable. Inner and outer retinal thickness values were correlated with BCVA, severity of macular ischaemia and type of DME on FA, using Pearson correlation coefficients.
Results
Patient disposition and demographics
A total of 179 patients were screened for eligibility, and 128 patients were randomized (COMBI [N = 85], LASER group [N = 43]). The most common reason for study discontinuation was early termination of the study (Fig. 2). The full analysis set (FAS) and safety set included all 128 randomized patients. Spectral domain OCT images were available from 112 patients at baseline, 76 at month 4 and 112 at month 12. Stratus OCT was used for 16 eyes at baseline, 11 at month 4 and 15 at month 12. Subanalysis for inner versus outer retinal thickness measurements was available for 109 eyes.
Figure 2.
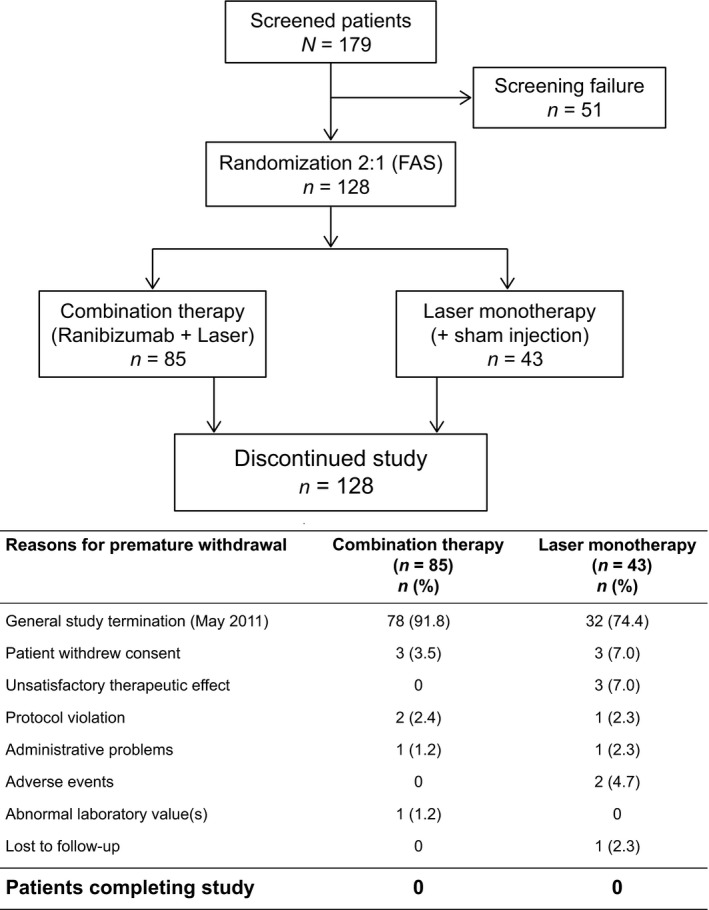
Patient disposition. FAS = Full analysis set.
Baseline demographics and disease characteristics were comparable across the two treatment groups (Table 1). The mean follow‐up time was similar in the COMBI (6.2 ± 2.8 months [range 1.0–11.1 months]) and LASER groups (6.2 ± 2.5 months [range 0.9–10.8 months]). Most patients in both groups received the planned complete set of 4 initial monthly injections of active or sham (Table 2). Most patients in both groups had received laser treatment at baseline. The mean and median number of laser treatments, and the time to first laser retreatment was similar between the COMBI and LASER groups (Table 2).
Table 1.
Baseline demographic and disease characteristics (all randomized patients)
| Variable (N) | Combination therapy (ranibizumab + laser) (N = 85) | Laser monotherapy (+ sham injection) (N = 43) |
|---|---|---|
| Mean age ± SD, years | 63.5 ± 9.3 | 63.5 ± 10.5 |
| Sex, n (%) | ||
| Male | 53 (62.4) | 27 (62.8) |
| Female | 32 (37.6) | 16 (37.2) |
| HbA1c | ||
| Mean SD ± SD | 7.5 ± 1.0 | 7.5 ± 1.2 |
| Median (range) | 7.2 (5.6–10.0) | 7.3 (5.8–9.6) |
| DME type, n (%) | ||
| Focal | 13 (15.3) | 6 (14.3) |
| Intermediate | 33 (38.8) | 18 (42.9) |
| Diffuse | 36 (42.4) | 18 (42.9) |
| Confounding factors† | 1 (1.2) | 0 (0.0) |
| Cannot grade | 2 (2.4) | 0 (0.0) |
| Missing | 0 (0.0) | 1 (2.3) |
| Time since first diagnosis of DME, median (range), years | 1.0 (0.0–8.8) | 1.3 (0.0–20.6) |
| Patients with PDR*, n (%) | 19 (22.4) | 7 (16.3) |
| Time since first PDR diagnosis, median (range), years | 1.4 (0.0–9.8) | 2.5 (0.0–14.7) |
| Mean VA at baseline (BCVA letter score), mean ± SD | 62.0 ± 11.7 | 64.6 ± 9.7 |
| Previous treatment | ||
| Laser (any specifications), n (%) | 62 (72.9) | 27 (62.8) |
DME = diabetic macular oedema, HbA1c = glycosylated haemoglobin, PDR = proliferative diabetic retinopathy, SD = standard deviation, VA = visual acuity. *Assessed by local study centres in 26 patients, one patient with missing data. †≥50% of leakage associated with neovascularization or other confounding factors.
Table 2.
Treatment exposure during the study (safety set)
| Combination therapy (ranibizumab + laser) (N = 85) | Laser monotherapy (+ sham injection) (N = 43) | |
|---|---|---|
| Received injections at baseline, n (%) | 85 (100.0) | 43 (100.0) |
| Received all 4 initial monthly injections, n (%) | 69 (81.2) | 34 (79.1) |
| Mean (SD) injections during the first 3 months | 3.7 (0.7) | 3.7 (0.6) |
| Median time to first reinjection in the maintenance phase, days | 55 | 35 |
| No injections during the maintenance phase, n (%) | 30 (42.9) | 15 (41.7) |
| Mean (SD) injections during the entire study | 5.0 (2.1) | 5.2 (2.3) |
| Laser treatment at baseline, n (%) | 83 (97.6) | 43 (100.0) |
| Total number of laser treatments, mean (SD) | 1.4 (0.9) | 1.7 (1.2) |
| Median | 1.0 | 1.0 |
| Time to first laser retreatment, 1st quartile (days) | 125 | 121 |
SD = standard deviation.
Efficacy analysis
In the COMBI arm, there was a rapid and clinically relevant increase from baseline in mean BCVA as early as month 1, which continued up to month 4, and was sustained at the month 4 level until month 12. The increase from baseline in mean BCVA was smaller in the LASER arm, Fig. 3.
Figure 3.
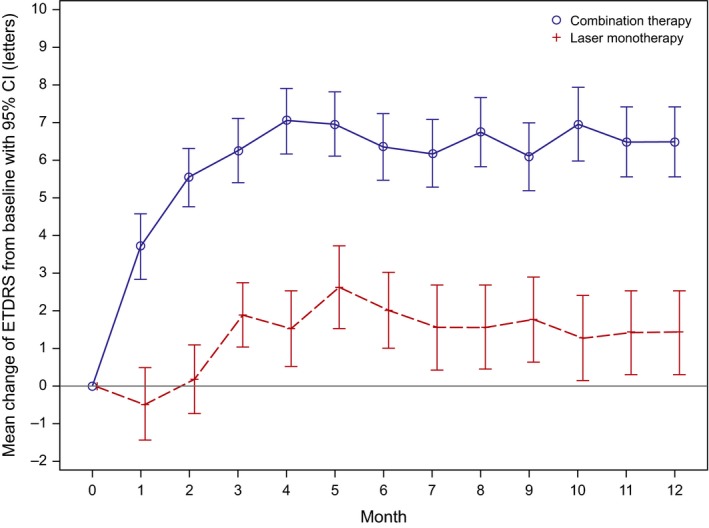
Mean change in BCVA values over time from baseline (LOCF, FAS). Note: error bars indicate 95% confidence intervals; CI = confidence interval; ETDRS = Early treatment diabetic retinopathy study, FAS = full analysis set, LOCF = last observation carried forward. Follow‐up per patient was up to only 11 months as a maximum, but the number of patients decreased as a consequence of premature termination.
The LS mean change in mean BCVA from baseline to month 12 was higher in the COMBI (6.5) versus LASER (2.3) group (LS mean difference: 4.2 [95% CI 0.9; 7.4] letters, p = 0.0143). The post hoc sensitivity analysis yielded similar results, confirming the robustness of the primary results. Of the various factors included in the ancova for primary analysis, only baseline BCVA had a notable effect on the BCVA outcome at month 12 (p = 0.001), while clinical site centre, type of DME and presence of PDR at baseline had no effect (all p > 0.05).
The categorical gains in BCVA from baseline at month 12 were higher in the COMBI versus LASER group; however, there was no notable difference (Table 3). Only one patient in each treatment group had a BCVA loss of ≥15 letters at month 12.
Table 3.
Proportions of patients achieving various dichotomous functional end‐points associated with BCVA (FAS, LOCF)
| Combination therapy (ranibizumab + laser) (N = 85) | Laser monotherapy (+ sham injection) (N = 43) | Difference [95% CI] | p‐value* | |
|---|---|---|---|---|
| BCVA > 73 letters, n (%)† | 35 (41.2) | 11 (25.6) | 15.6 [−2.9;34.1] | 0.084 |
| BCVA gain ≥15 letters, n (%)† | 13 (15.3) | 2 (4.7) | 10.6 [−1.0;22.3] | 0.078 |
| Any letter gain, n (%)† | 64 (75.3) | 23 (53.5) | 21.8 [2.6;41.4] | 0.013 |
| Loss of ≥15 letters, n (%)† | 1 (1.2) | 1 (2.3) | −1.1 [−8.0;5.7] | 0.662 |
BCVA = best‐corrected visual acuity, CI = confidence interval, FAS = full analysis set, LOCF = last observation carried forward. The FAS consisted of all randomized patients who received at least one application of study treatment ([sham] injection and/or laser) and had at least one postbaseline assessment for BCVA. *Wald′s chi square test; significance level p = 0.05; †at month 12 (LOCF method) compared to baseline.
In patients with PDR at baseline (COMBI: n = 19, LASER: n = 7), a trend towards a numerically higher BCVA change from baseline to month 12 in favour of COMBI treatment was observed (LS mean change [95% CI]: COMBI 7.35 [6.81; 21.52]; LASER −7.35 [−33.71; 19.01]; LS mean difference 14.7 [−7.93; 37.33], p = 0.1077). The low number of PRP treatments at or near baseline (four treatments [two in the COMBI and two in the LASER group]) suggests that PDR patients may not have received sufficient treatment. Similar numerically higher BCVA gains were also observed in the subgroups of patients with different types of DME and in those without PDR.
Retinal thickness
There was no difference in FCS, FCP and total volume measurements between both groups at baseline. There was a significant decrease in thickness from baseline to month 4 for FCS, FCP and total volume measurements, and a significantly greater difference between baseline and month 12 regarding total volume in the COMBI group compared to the LASER group (Table 4, Fig. 4).
Table 4.
Optical coherence tomography (OCT) results
| OCT parameters assessed by reading centre (neurosensory retina + subretinal fluid) | Combination n/N = 48/85 | Laser n/N = 26/43 | p‐value† |
|---|---|---|---|
| Foveal centre point thickness [μm (SD)] | |||
| Baseline | |||
| Mean (SD) | 405.0 (152.1) | 450.9 (157.9) | 0.139 |
| Mean change from baseline | |||
| Month 4 | −134.5 (153.9) | −31.4 (109.5) | 0.003* |
| Month 12 | −111.6 (152.5) | −59.3 (108.2) | 0.068 |
| Central subfield mean thickness [μm (SD)] | |||
| Baseline | |||
| Mean | 421.5 (130.1) | 470.1 (123.6) | 0.061 |
| Mean change from baseline | |||
| Month 4 | −118.7 (130.9) | −41.5 (86.1) | 0.007* |
| Month 12 | −96.7 (120.9) | −54.0 (89.9) | 0.062 |
| Total volume within 6 mm ETDRS grid, [mm³ (SD)] | |||
| Baseline | |||
| Mean | 9.6 (1.9) | 10.2 (2.0) | 0.204 |
| Mean change from baseline | |||
| Month 4 | −1.4 (1.4) | −0.4 (0.8) | 0.001* |
| Month 12 | −1.2 (1.1) | −0.5 (1.0) | 0.004* |
ETDRS = Early treatment diabetic retinopathy study, SD = standard deviation. †Paired t‐test p‐value, significance level *p < 0.05.
Figure 4.
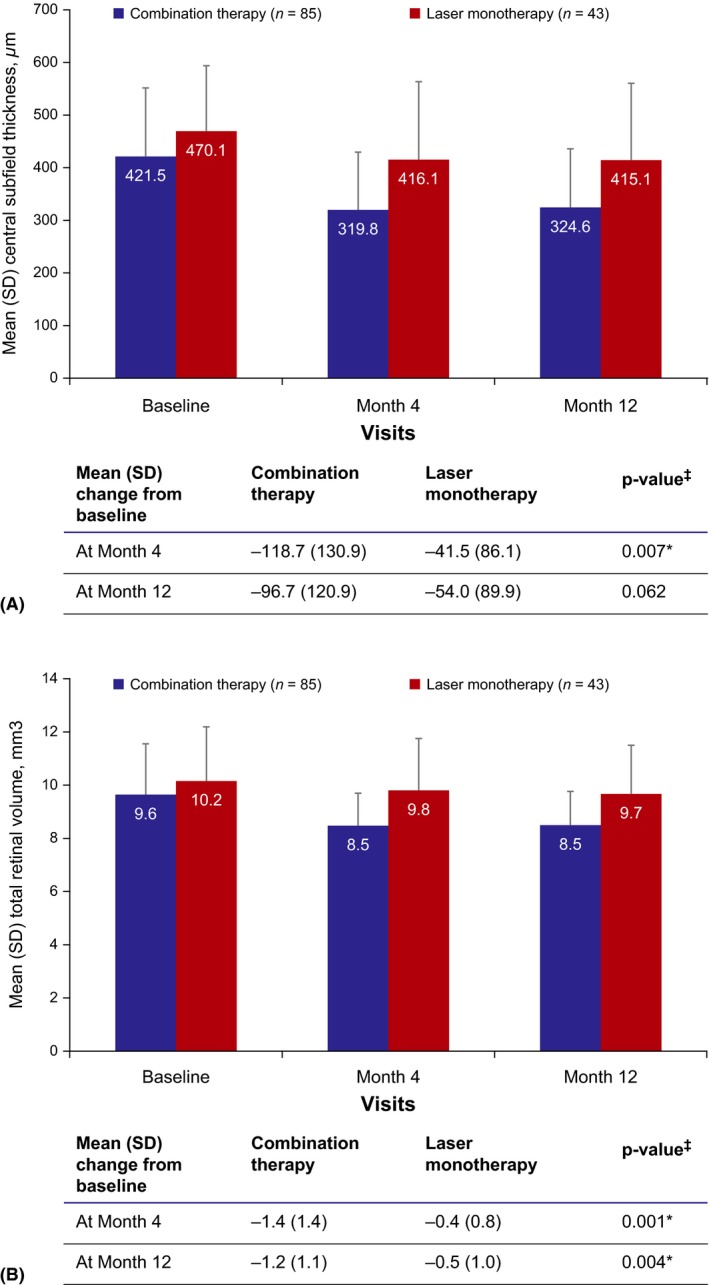
(A) Mean central subfield thickness (μm) and (B) mean total retinal volumea over time (FAS). ‡Paired t‐test p‐value, significance level *p < 0.05. aTotal volume within 6‐mm ETDRS grid. FAS = Full analysis set, SD = standard deviation. Follow‐up per patient was up to only 11 months as a maximum, but the number of patients decreased as a consequence of premature termination.
Subanalysis of inner and outer retinal thickness values
Correlation between inner and outer retinal thickness with BCVA, macular ischaemia severity and type of DME was performed in 109 eyes (91.7% spectral domain and 8.3% time domain OCT). The mean follow‐up in the overall group was 6.2 ± 2.7 months. Best‐corrected visual acuity (BCVA) at baseline negatively correlated with FCS thickness for neurosensory retina (r = −0.44, p < 0.0001), inner retina (r = −0.22, p < 0.05) and outer retina (r = −0.28, p < 0.01) at baseline. Best‐corrected visual acuity (BCVA) at month 12 negatively correlated with inner retinal thickness values at baseline (r = −0.32, p < 0.001), and no statistically significant correlation could be shown with outer retinal thickness at baseline. Eyes with diffuse DME showed greater inner retinal thickness values than eyes with focal DME (p < 0.01), and outer retinal thickness values showed no difference between groups. No difference in FCS thickness regarding severity of macular ischaemia was determined. At month 12, eyes in the COMBI group showed stronger decrease in inner retinal thickness than eyes in the LASER group (r = 0.34, p < 0.001), but there was no difference in reduction in outer retinal thickness values (Table 5).
Table 5.
Mean FCS thickness values at baseline and last follow‐up
| Neurosensory retina Baseline (μm) | Neurosensory retina Month 12* (μm) | Inner retina Baseline (μm) | Inner retina Month 12* (μm) | Outer retina Baseline (μm) | Outer retina Month 12* (μm) | |
|---|---|---|---|---|---|---|
| DME type on FA at baseline | ||||||
| Focal (n = 16) | 378.6 ± 102.8 | 326.2 ± 116.6 | 129.9 ± 46.5 | 114.8 ± 46.3 | 248.7 ± 76.3 | 146.3 ± 42.1 |
| Intermediate (n = 44) | 427.4 ± 127.8 | 356.0 ± 117.2 | 179.1 ± 88.9 | 149.0 ± 75.3 | 240.1 ± 89.4 | 154.0 ± 52.0 |
| Diffuse (n = 46) | 473.4 ± 131.6 | 377.5 ± 155.6 | 200.6 ± 112.1 | 171.4 ± 117.0 | 272.9 ± 128.7 | 145.0 ± 44.2 |
| Macular ischaemia at baseline | ||||||
| Absent (n = 1) | 390.8 ± 0 | 271.0 ± 0 | 221.1 ± 0 | 117.0 ± 0 | 169.7 ± 0 | 128.1 ± 0 |
| Questionable (n = 12) | 483.0 ± 153.5 | 361.0 ± 121.7 | 193.7 ± 76.9 | 126.3 ± 44.2 | 262.3 ± 91.7 | 167.6 ± 62.3 |
| Definite (n = 31) | 445.6 ± 131.3 | 357.4 ± 154.9 | 193.3 ± 117.3 | 168.4 ± 126.5 | 252.3 ± 115.0 | 141.6 ± 50.5 |
| Moderate (n = 21) | 460.8 ± 141.2 | 344.6 ± 122.1 | 210.5 ± 120.7 | 165.4 ± 109.2 | 250.3 ± 110.1 | 147.9 ± 31.5 |
| Severe (n = 7) | 434.3 ± 91.8 | 385.7 ± 155.4 | 125.6 ± 42.0 | 151.6 ± 48.8 | 308.7 ± 96.4 | 155.2 ± 76.9 |
| Treatment | ||||||
| COMBI Group (n = 74) | 422.2. ± 131.3 | 325.9 ± 112.5 | 165.3 ± 72.7 | 131.2 ± 53.3 | 252.0 ± 109.0 | 140.3 ± 45.3 |
| LASER Group (n = 35) | 465.4 ± 123.5 | 419.9 ± 148.2 | 210.2 ± 129.2 | 201.3 ± 135.8 | 255.3 ± 104.3 | 164.3 ± 48.0 |
COMBI = ranibizumab plus laser, DME = diabetic macular oedema, FA = florescein angiography, FCS = foveal central subfield, SD = standard deviation. All values are mean ± SD. Data presented is for 109 patients for whom OCT images were available for analysis. *For month 12 values LOCF (last observation carried forward) method was used as study was terminated early.
Safety
There was only one ocular SAE reported by the clinical sites in the study eye during the study (diabetic retinal oedema in one patient in the COMBI group); this event was considered not causatively connected to the study drug or injection procedure. The incidence of nonocular SAEs was higher in the COMBI than in the LASER group (15.3% [n = 13] versus 7.0% [n = 3]), but all events in either treatment group were reported in one patient each, except for hypoglycaemia (n = 2) in the COMBI group. The nonocular SAEs in the COMBI group included four vascular disorder events (preferred terms: hypertension, hypertensive crisis, peripheral arterial occlusive disease and thrombophlebitis in one patient each) and one cerebrovascular accident event. In the COMBI group, two nonocular SAEs, peripheral arterial occlusive disease in one patient (with type 1 diabetes mellitus and history of arterial hypertension with multiple vessel disorders) and congestive heart failure in another patient (with type 2 diabetes mellitus, and history of arterial hypertension, hypercholesterolaemia and arterial occlusive disease), were considered study drug‐related; neither event resulted in treatment discontinuation. No patient died during the course of the study.
The incidences of ocular TEAEs were numerically higher in the COMBI than in the LASER group, while nonocular TEAEs were reported in similar proportion of patients in either group (Table 6). Most of the ocular and nonocular events in both groups were of mild severity. No cases of endophthalmitis or other infections of the study eye were reported. The incidence of study discontinuation due to TEAEs was low (COMBI group [n = 1, blood potassium increased]; LASER group [n = 2, diabetic retinal oedema and macular oedema each in one patient]). There were no clinically relevant differences in laboratory parameters, vital signs and intraocular pressure analysis between the treatment groups.
Table 6.
Most frequent ocular and nonocular treatment emergent adverse events (SOC and preferred term in the safety set)
| Most frequent TEAEs (in ≥2.0% of study patients) | Combination therapy (ranibizumab + laser) (N = 85) | Laser monotherapy (+ sham injection) (N = 43) |
|---|---|---|
| Any ocular TEAE in the study eye, n (%) | 35 (41.2) | 11 (25.6) |
| Eye disorders | 34 (40.0) | 11 (25.6) |
| Eye pain | 13 (15.3) | 4 (9.3) |
| Lacrimation increased | 5 (5.9) | 1 (2.3) |
| Eye irritation | 5 (5.9) | 0 (0.0) |
| Eye swelling | 4 (4.7) | 1 (2.3) |
| Conjunctival haemorrhage | 4 (4.7) | 0 (0.0) |
| Eye pruritus | 4 (4.7) | 0 (0.0) |
| Foreign body sensation in eyes | 1 (1.2) | 2 (4.7) |
| Ocular hyperaemia | 3 (3.5) | 0 (0.0) |
| Vitreous floaters | 3 (3.5) | 0 (0.0) |
| Investigations | ||
| Intraocular pressure increased | 3 (3.5) | 0 (0.0) |
| Any nonocular TEAE, n (%) | 48 (56.6) | 22 (51.2) |
| Infections and infestations | 19 (22.4) | 8 (18.6) |
| Nasopharyngitis | 13 (15.3) | 6 (14.0) |
| Vascular disorders | ||
| Hypertension | 9 (10.6%) | 5 (11.6%) |
| Hypertensive crisis | 5 (5.9%) | 4 (9.3%) |
| Haematoma | 2 (2.4%) | 0 (0.0%) |
| Venous thrombosis limb | 0 (0.0) | 1 (2.3%) |
| Nervous system disorders | 11 (12.9) | 2 (4.7) |
| Headache | 5 (5.9) | 1 (2.3) |
| Metabolism and nutrition disorders | 6 (7.1) | 1 (2.3) |
| Hypoglycaemia | 4 (4.7) | 0 (0.0) |
MedDRA = Medical Dictionary for Regulatory Activities, SOC = system organ class (according to MedDRA), TEAE = treatment emergent adverse event.
Discussion
In the RELATION study, combination treatment with ranibizumab plus laser was shown to be more efficacious than laser monotherapy in a patient population with visual impairment due to DME with NPDR or PDR. Although direct comparisons cannot be made considering the differences in study design and patient population, the results are nevertheless consistent with the findings from previous studies comparing the combination of ranibizumab plus laser versus laser monotherapy (Elman et al. 2010, 2011; Mitchell et al. 2011; Nguyen et al. 2012; Lang et al. 2013; Brown et al. 2013; Schmidt‐Erfurth et al. 2014). DRCR.net studies have shown the combination to be noninferior to PRP alone in improvement of VA after 2 years of treatment (Writing Committee for the Diabetic Retinopathy Clinical Research Network et al. 2015). Although numerically higher improvement in BCVA was noted in the COMBI versus LASER group in our study, the sample size was too low to draw any robust conclusions. Because mean BCVA continued to steadily improve over the entire treatment duration in previous studies (Nguyen et al. 2012; Brown et al. 2013; Writing Committee for the Diabetic Retinopathy Clinical Research Network et al. 2015), it may be hypothesized that higher mean BCVA gains might have been observed in the RELATION study if it had completed the planned full 12 months. The United States Food and Drug Administration has recently approved ranibizumab for the treatment of all forms of DR (Genentech Press Release 2017).
The reduction in mean FCS thickness from baseline to month 12 was higher in the COMBI group versus the LASER group, consistent with findings from previous studies (Mitchell et al. 2011; Brown et al. 2013; Lang et al. 2013). Others have shown that the reduction in central retinal thickness is dependent on the baseline value with stronger reduction in eyes with higher retinal thickness values at baseline (Bressler et al. 2012). In the RELATION study, higher reductions in retinal thickness were seen in the COMBI group in spite of the baseline values being lower than those in the LASER group. Foveal central subfield (FCS) thickness values for the inner and the outer retina showed differences regarding the type of DME on FA, BCVA outcome as well as the response to treatment. These results suggest that subanalysis of various retinal layers on OCT may be relevant as outcome parameters in DME clinical trials.
It has been reported previously in DME patients that higher inner retinal thickness values correlate with more severe visual impairment (Murakami et al. 2012). This is in agreement with findings from our study, confirming lower visual acuity at baseline in eyes with greater FCS thickness values for inner and outer retina as well as total neurosensory retina. Lower outer retinal thickness values are reported to be associated with poor visual prognosis presumably due to photoreceptor degeneration (Murakami et al.2012). In our study, lower BCVA at month 12 was associated with greater inner retinal thickness at baseline; however, outer retinal thickness had no prognostic value in our study population.
The severity of outer retinal thickening and cystoid changes in the inner and outer retina were reported to correlate with the severity of leakage on FA, and loss of the inner retinal layers on OCT was associated with capillary nonperfusion on FA (Bodnar et al. 2017). In our study population, no correlation between inner or outer retinal thickness and severity of macular ischaemia could be observed. In agreement with Bodnar et al., eyes with diffuse DME in our study showed greater inner retinal thickness values than eyes with focal DME; however, outer retinal thickness values showed no difference between groups. The difference between the COMBI and LASER group regarding the decrease in retinal thickness during follow‐up was significant for inner retinal thickness values; however, there was no difference in reduction of outer retinal thickness values although the percentage decrease in thickness was much stronger for the outer compared to the inner retina.
Previous studies have shown focal laser photocoagulation to be effective for focal DME (Romero‐Aroca et al. 2012; Gonzalez‐Cortes, 2015), and anti‐VEGF agents to be effective in both focal and diffuse DME (Arevalo et al. 2007). However, our subgroup analyses did not show obvious and robust differences among the DME subgroups, which might be due to the small sample sizes.
In general, ranibizumab in combination with laser treatment was well tolerated in patients with DME. As expected, the incidences of adverse events were numerically higher in the COMBI group than in the LASER group and were mainly associated with the IVT route of administration. Rates of safety events were in line with incidence rates reported in other trials with ranibizumab in DME (Yanagida & Ueta 2014), and no new safety signals were identified.
Major limitation of the study is the low sample size due to premature termination of the study. However, the analysis was performed according to the intent‐to‐treat principle, as prespecified in the study protocol.
Conclusion
The study results showed that the combination of ranibizumab plus laser was more efficacious than laser alone in patients with visual impairment due to DME in PDR as well as NPDR, although definitive conclusions cannot be drawn considering the premature termination of the study. Results suggest that the combination might benefit patients with DME and PDR; in these patients, the combination of antioedematous and antiproliferative properties of ranibizumab seems to be helpful. Further studies are needed to confirm the beneficial effect of ranibizumab in PDR patients.
Financial support for medical editorial assistance was provided by Novartis Pharma GmbH, Germany. We thank Birgit Eschweiler, PhD, Medical Writing Services, Oerlinghausen, Germany, for medical editorial assistance with this manuscript, and Lakshmi Venkatraman, PhD (Scientific Services Practice, PLS, Novartis Healthcare Pvt. Ltd., Hyderabad, India) for editorial assistance and resubmission support. The authors wish to thank RPS Research Germany GmbH, Nuremberg for data entry, Winicker & Norimed GmbH, for planning and conduct of statistical analysis of this study, Labor Dr. Spranger & Partner for laboratory analysis, and the Cologne Image Reading Center (CIRCL) for reading the imaging data. Further thanks are extended to the investigators who participated in the RELATION study in Germany: B Kirchhof (Köln), K‐H Emmerich (Darmstadt), K Engelmann (Chemnitz), R Guthoff (Würzburg), F Holz (Bonn), C Lohmann (München), B Lorenz (Gießen), G Scharioth (Recklinghausen), S Dithmar (Heidelberg), U Kellner (Siegburg), K‐M Kreusel (Berlin), U Ritzau‐Tondrow (Göttingen), J Zurdel (Stuttgart), S Biester (Karlsruhe), H Dave (Hamburg), N Eter (Münster), S Mennel (Marburg), D Sandner (Dresden), W Schrader (Nürnberg), J Carstens (Dessau), A Mohr (Bremen), A Ringwald (Dortmund), J Ugi (Landshut), W Wolf (München), N Bornfeld (Essen), L Hansen (Freiburg), R Krist (Frankfurt), A Liekfeld (Potsdam), F‐H Mietz (Aschaffenburg), J Roider (Kiel), P Sendtner (Eichstätt), J Wachtlin (Berlin), E Fabian (Rosenheim). Research was funded by Novartis Pharma GmbH, Germany.
References
- Arevalo JF, Fromow‐Guerra J, Quiroz‐Mercado H et al. (2007): Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan‐American Collaborative Retina Study Group at 6‐month follow‐up. Ophthalmology 114: 743–750. [DOI] [PubMed] [Google Scholar]
- Blum M, Kloos C, Müller N, Mandecka A, Berner R, Bertram B & Müller UA (2007): Prävalenz der diabetischen Retinopathy, Studie der Versicherten der Deutschen Betriebskrankenkasse 2002–2004. Ophthalmologe 104: 499–504. [DOI] [PubMed] [Google Scholar]
- Bodnar ZM, Desai A, Akduman L (2017): Diabetic macular edema In: Meyer CH, Saxena S, Sadda SR. (eds). Spectral domain optical coherence tomography in macular diseases (ebook). New Delhi, India: Springer; 124. [Google Scholar]
- Bressler SB, Qin H, Beck RW, Chalam KV, Kim JE, Melia M, Wells JA 3rd & Diabetic Retinopathy Clinical Research Network (2012): Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol 130: 1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Nguyen QD, Marcus DM et al. (2013): Long‐term outcomes of ranibizumab therapy for diabetic macular edema: the 36‐month results from two phase III trials: RISE and RIDE. Ophthalmology 120: 2013–2022. [DOI] [PubMed] [Google Scholar]
- Elman MJ, Aiello LP, Beck RW et al. (2010): Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 117: 1064–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman MJ, Bressler NM, Qin H et al. (2011): Expanded 2‐year follow‐up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 118: 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong DS (2002): Changing times for the management of diabetic retinopathy. Surv Ophthalmol 47(Suppl 2): S238–S245. [DOI] [PubMed] [Google Scholar]
- Genentech Press Release (2017): FDA approves Genentech's Lucentis (Ranibizumab injection) for diabetic retinopathy, the leading cause of blindness among working age adults in the United States. April 17, 2017. Available at: https://www.gene.com/media/press-releases/14661/2017-04-17/fda-approves-genentechs-lucentis-ranibiz. (Accessed on 28 June 2017).
- Gonzalez‐Cortes JH (2015): Treatment of diabetic macular edema (DME). Shifting paradigms. Medicina Universitaria 17: 243–247. [Google Scholar]
- Klein R, Klein BE, Moss SE & Cruickshanks KJ (1995): The Wisconsin epidemiologic study of diabetic retinopathy XV: the long‐term incidence of macular edema. Ophthalmology 102: 7–16. [DOI] [PubMed] [Google Scholar]
- Klein R, Knudtson MD, Lee KE, Gangnon R & Klein BE (2009): The Wisconsin epidemiologic study of diabetic retinopathy XXIII: the twenty‐five‐year incidence of macular edema in persons with type 1 diabetes. Ophthalmology 116: 497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang GE, Berta A, Eldem BM et al. (2013): Two‐year safety and efficacy of ranibizumab 0.5 mg in diabetic macular edema: interim analysis of the RESTORE extension study. Ophthalmology 120: 2004–2012. [DOI] [PubMed] [Google Scholar]
- Massin P, Bandello F, Garweg JG et al. (2010): Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12‐month, randomized, controlled, double‐masked, multicenter phase II study. Diabetes Care 33: 2399–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Bandello F, Schmidt‐Erfurth U et al. (2011): The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 118: 615–625. [DOI] [PubMed] [Google Scholar]
- Murakami T, Nishijima K, Akagi T, Uji A, Horii T, Ueda‐Arakawa N, Muraoka Y, & Yoshimura N (2012): Segmentational analysis of retinal thickness after vitrectomy in diabetic macular edema. Invest Ophthalmol Vis Sci 53: 6668–6674. [DOI] [PubMed] [Google Scholar]
- Nguyen QD, Shah SM, Heier JS et al. (2009): Primary end point (six months) results of the ranibizumab for edema of the mAcula in diabetes (READ‐2) study. Ophthalmology 116: 2175–2181. [DOI] [PubMed] [Google Scholar]
- Nguyen QD, Shah SM, Khwaja AA et al. (2010): Two‐year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ‐2) study. Ophthalmology 117: 2146–2151. [DOI] [PubMed] [Google Scholar]
- Nguyen QD, Brown DM, Marcus DM et al. (2012): Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 119: 789–801. [DOI] [PubMed] [Google Scholar]
- Romero‐Aroca P, Reyes‐Torres J, Baget‐Bernaldiz M, Blasco‐Suñe C (2014): Laser treatment for diabetic macular edema in the 21st century. Current Diabetes Reviews 10: 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt‐Erfurth U, Lang GE, Holz FG et al. (2014): Three‐year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology 121: 1045–1053. [DOI] [PubMed] [Google Scholar]
- Writing Committee for the Diabetic Retinopathy Clinical Research Network , Gross JG, Glassman AR et al. (2015): Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA 314: 2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida Y & Ueta T (2014): Systemic safety of ranibizumab for diabetic macular edema: meta‐analysis of randomized trials. Retina 34: 629–635. [DOI] [PubMed] [Google Scholar]


