Abstract
Objectives
To explore assessment of supra‐annular structure for self‐expanding transcatheter heart valve (THV) size selection in patients with bicuspid aortic stenosis (AS).
Background
Annulus‐based device selection from CT measurement is the standard sizing strategy for tricuspid aortic valve before transcatheter aortic valve replacement (TAVR). Because of supra‐annular deformity, device selection for bicuspid AS has not been systemically studied.
Methods
Twelve patients with bicuspid AS who underwent TAVR with self‐expanding THVs were included in this study. To assess supra‐annular structure, sequential balloon aortic valvuloplasty was performed in every 2 mm increments until waist sign occurred with less than mild regurgitation. Procedural results and 30 day follow‐up outcomes were analyzed.
Results
Seven patients (58.3%) with 18 mm; three patients (25%) with sequential 18 mm, 20 mm; and only two patients (16.7%) with sequential 18 mm, 20 mm, and 22 mm balloon sizing were performed, respectively. According to the results of supra‐annular assessment, a smaller device size (91.7%) was selected in all but one patient compared with annulus based sizing strategy, and the outcomes were satisfactory with 100% procedural success. No mortality and 1 minor stroke were observed at 30 d follow‐up. The percentage of NYHA III/IV decreased from 83.3% (9/12) to 16.7% (2/12). No new permanent pacemaker implantation and no moderate or severe paravalvular leakage were found.
Conclusions
A supra‐annular structure based sizing strategy is feasible for TAVR in patients with bicuspid AS.
Keywords: balloon sizing, bicuspid aortic valve, supra‐annular structure, TAVR
1. INTRODUCTION
Transcatheter aortic valve replacement (TAVR) has emerged as a favorable alternative for severe symptomatic aortic stenosis (AS) patients who are at intermediate to high surgical risk or are inoperable 1, 2, 3, 4, 5. Compared to tricuspid AS, TAVR for patients with bicuspid AS is prone to specific adverse procedural outcomes, such as lower device success rate, more moderate or severe paravalvular leak (PVL), as well as TAV‐in‐TAV 6.
Multidetector computed tomography is now a standard imaging modality for device sizing in TAVR 7. Since the aortic valve annulus typically represents the tightest part of the aortic root, sizing of aortic valve annulus has been regarded as the “gold standard” in transcatheter heart valve (THV) size selection 8, 9. In the TAVR era, balloon aortic valvuloplasty is applied to provide additional information for THV size selection when encountering a borderline annulus 10, or as a bridge to TAVR procedure [11].
Clinical experience in China suggests bicuspid aortic valves and heavy calcium burden are more common among TAVR candidates 12. Morphological characteristics at supra‐annular structure (from annulus to the level of sinotubular junction) are quite complex in bicuspid AS, especially concomitant with heavily calcified leaflets. Because only two leaflet hinge points provide the definition of the annulus plane, current CT‐based annulus measurements might not be accurate under these circumstances. From our clinical practice, “waist sign” above the annulus during balloon aortic valvuloplasty in TAVR was often observed in patients with bicuspid AS, indicating that the supra‐annular structure may serve a key role in anchoring the THV. Therefore, we sought to investigate sizing strategies for self‐expanding device size selection in TAVR based on supra‐annular structure assessment for patients with bicuspid AS.
2. MATERIALS AND METHODS
2.1. Patients
From April 2016 to April 2017, 70 consecutive patients with severe AS underwent TAVR at Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China. Twelve patients with bicuspid AS implanted with self‐expanding THVs using supra‐annular assessment for device size selection were included in this retrospective study. TAVR appropriateness for each AS patient was determined by the dedicated heart team of our hospital. Clinical, procedural, and imaging data were prospectively included in our TAVR database.
2.2. Echocardiography
The diagnosis of severe aortic stenosis was confirmed with transthoracic echocardiography (TTE) according to established guidelines 13, 14. Function of the THVs was evaluated by TTE at 30‐day follow‐up. TTE measurements, including aortic valve area, mean gradient, maximal velocity, and left ventricular ejection fraction (LVEF) were documented. Baseline aortic regurgitation and post‐TAVR paravalvular leakage grade were classified as none/trace (0), mild (1), moderate (2), or severe (3) 14.
2.3. Dual source computed tomography data acquisition and analysis
Dual source computed tomography (DSCT) was performed in all patients pre‐procedurally for aortic root measurement and access route selection. All DSCT examinations were performed with the second generation dual‐source CT (SOMATOM Definition Flash, Siemens Medical Solutions, Germany). The scan area was craniocaudal from the subclavian artery to the iliofemoral branches. Prospective ECG gating with a pitch of 2.4 was performed. Around 60–80 ml of iodine‐containing contrast agent (Omnipaque 370 mg I/ml, GE Healthcare, Shanghai, China) was injected with a dual head power injector (Mallinckrodt, American) at a flow rate of 4 ml/s followed by 60 ml 0.9% saline solution at the same flow rate. A bolus tracking method was used in the descending aorta with a pre‐set threshold of 180 Hounsfield Units (HU) to achieve optimal synchronization. The tube voltage was 100 kV, with a reference tube current‐time product of 280 mAs and a collimation of 38.4 mm (2 × 32 × 0.6 mm3) with double sampling by z‐axis flying focal spot.
DSCT datasets were analyzed using 3mensio 8.0 (3mensio Medical Imaging BV, the Netherlands) 15. Bicuspid aortic valve was diagnosed based on short‐axis images of the aortic valve on DSCT. Bicuspid aortic valve was classified by the number of raphes (type0, type1 and type2) 16. The orientation of raphe is defined in relation to the sinuses as left‐right (LR), right‐non (RN), and left‐non (LN). Maximal, minimal, mean, and perimeter‐derived diameter of annulus, mean diameter of sinotubular junction (STJ), and coronary ostium height were measured as previous described 8. Due to the deformity of bicuspid aortic valve, only the maximum and minimum diameter of the sinus of Valsalva were measured. The threshold for detecting aortic root calcification was set at 650 HU; then, calcium volume was measured within the region from left ventricular outflow tract (LVOT) to the leaflet tips. Distribution of calcification was classified as symmetrical or asymmetrical, and the specific distribution was described.
2.4. TAVR procedure
All TAVR procedures were performed by trans‐femoral access under general anesthesia or local anesthesia with sedation. Two domestic self‐expanding THVs, Venus A (Venus Medtech Inc., Hangzhou, China) and VitaFlow valve (Shanghai MicroPort CardioFlow Medtech Co., Ltd., Shanghai, China) were selected to this patient population. The design of both devices is similar to that of the CoreValve. According to the respective manufacturers, both Venus A and VitaFlow valves use perimeter‐derived annulus diameter as a sizing guide for THV in tricuspid AS. The 23 mm Venus A and 21 mm VitaFlow valves are designed for a perimeter‐derived annulus diameter of 18–20 mm, 26 mm Venus A and 24 mm VitaFlow valve for 20–23 mm, 29 mm Venus A and 27 mm VitaFlow valve for 23–26 mm, and 32 mm Venus A and 30 mm VitaFlow valve for 26–29 mm, respectively.
Clinical outcomes were evaluated by VARC‐2 criteria 14. Angiographic aortic regurgitation and gradients reduction immediately after TAVR were measured as previously described 17, 18. Implantation depth was defined as the distance from the native aortic annulus plane to the left ventricular edge of THV by fluoroscopy 19, 20. Mean implantation depth was defined as the average of the left and right side implantation depths.
2.5. Supra‐annular structure assessment by sequential balloon sizing
In severe AS patients, bicuspid AS is often encountered with heavy calcification 12. THV size selection is still largely unknown in clinical practice for this patient population. To assess supra‐annular structure, we developed a sequential balloon sizing strategy for bicuspid AS to select THV size. Unlike the traditional balloon sizing strategy focusing on the borderline annulus size in tricuspid AS, the strategy of sequential balloon sizing started from an 18‐mm Z‐Med balloon (NuMED, Hopkinton, NY) (the minimum aortic diameter requirement for prostheses used in this study). Waist sign on the balloon and regurgitation were checked with a simultaneous contrast injection during balloon inflation. Sequential balloon sizing in every 2mm increments was performed until waist sign occurred with less than mild regurgitation. Importantly, if the next size of balloon is larger than the annulus, measurement should be stopped and the device size should be selected based on annulus size. Then, we took the calculated average diameter instead of perimeter‐derived annulus diameter as the reference for device size selection, and the following equation was used: calculated average diameter (mm) = (diameter of the final balloon + perimeter derived diameter based on DSCT)/2. Step by step illustration of device size selection based on supra‐annular assessment was showed on Figure 1.
Figure 1.
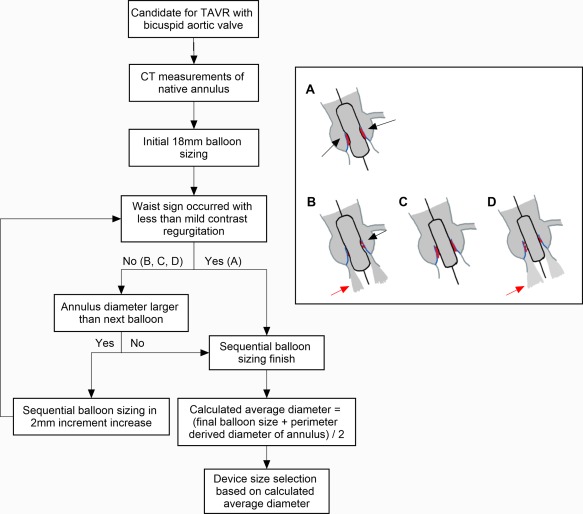
Schematic illustration of device size selection based on supra‐annular assessment using sequential balloon sizing. A: Waist sign with less than mild contrast regurgitation; B: Waist sign with mild or more contrast regurgitation; C: No waist sign with less than mild contrast regurgitation; D: No waist sign with mild or more contrast regurgitation; Black arrow: waist sign; Red arrow: contrast regurgitation; Red dots: calcification [Color figure can be viewed at http://wileyonlinelibrary.com]
2.6. Follow‐up
Clinical and TTE follow up were performed at 30d at our center. Indexed effective orifice area was calculated to quantify prosthesis‐patient mismatch (PPM). Clinical improvement was evaluated by New York Heart Association (NYHA) class. All outcomes were defined according to VARC‐2 criteria 14.
2.7. Statistical analysis
Data are expressed as mean ± SD or as median (interquartile range). The data were analyzed using SPSS statistics 21.0 (SPSS Inc., Chicago, Illinois, USA).
3. RESULTS
3.1. Baseline characteristics
Among the 70 patients that underwent TAVR from April 2016 to April 2017, 22 patients had bicuspid AS. Twelve patients with bicuspid AS underwent TAVR with self‐expanding THVs using the sequential balloon sizing strategy included in this study, excluding six with Lotus valve and four with no sequential balloon sizing strategy (Figure 2). Patients' clinical characteristics at baseline are listed in Table 1. The mean age was 76 ± 4 years and the Society of Thoracic Surgeons (STS) score was (6.86 ± 4.27)%. Baseline TTE showed that aortic valve area was 0.61 ± 0.17 cm2, mean gradient 47 ± 11 mm Hg, maximal velocity 4.54 ± 0.57 m/s, and LVEF (53 ± 20)%. NYHA III/IV was demonstrated in 83.3% (9/12) of the patients. Concomitant moderate aortic regurgitation (AR) was found in 3 patients, and mild AR in 4 patients. DSCT revealed that 8 patients were type 0 bicuspid AS and 4 were type 1 bicuspid AS. Measurements from DSCT analysis were listed in Table 2. The calcium volume measured at the threshold of 650U was 1052.0 ± 726.2 mm3 and distribution of calcification in 7 patients was asymmetric.
Figure 2.
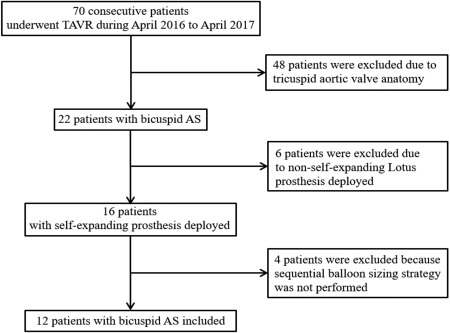
Flow chart of patient inclusion. AS: aortic stenosis; TAVR: transcatheter aortic valve replacement
Table 1.
Clinical characteristics at baseline
| TTE measurements | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age (years) | Gender | BMI (kg/m2) | Comorbidities | NYHA class | STS score (%) | AVA (cm2) | PGmean (mm Hg) | V max (m/s) | LVEF (%) | AR grade (0–3) |
| 1 | 81 | F | 17.1 | PH, CKD | IV | 17.35 | 0.36 | 47 | 4.49 | 20 | 0 |
| 2 | 67 | M | 26.9 | DM, HTN, Af, COPD | IV | 8.61 | 0.65 | 50 | 4.69 | 54 | 1 |
| 3 | 77 | M | 19.3 | HTN, COPD, CKD | IV | 12.99 | 0.64 | 42 | 4.18 | 20 | 0 |
| 4 | 75 | M | 22.0 | Af, COPD, CKD | III | 4.54 | 0.50 | 32 | 3.90 | 36 | 2 |
| 5 | 80 | M | 21.3 | CKD | II | 4.67 | 0.44 | 46 | 4.37 | 52 | 1 |
| 6 | 71 | F | 23.7 | HTN | IV | 3.69 | 0.77 | 40 | 4.06 | 72 | 2 |
| 7 | 79 | M | 22.4 | DM, HTN | III | 4.53 | 0.67 | 42 | 4.10 | 63 | 0 |
| 8 | 72 | F | 23.1 | Anemia | IV | 4.29 | 0.81 | 59 | 5.31 | 41 | 0 |
| 9 | 74 | M | 24.3 | DM, HTN, Prior PCI, PVD, COPD | IV | 7.02 | 0.83 | 41 | 4.50 | 69 | 1 |
| 10 | 77 | M | 24.0 | DM, HTN | III | 5.03 | 0.60 | 43 | 4.40 | 61 | 1 |
| 11 | 81 | F | 16.7 | CKD | II | 6.49 | 0.33 | 77 | 5.93 | 77 | 2 |
| 12 | 72 | M | 24.0 | HTN | III | 3.08 | 0.70 | 46 | 4.50 | 66 | 0 |
Abbreviations: Af, atrial fibrillation; AR, aortic regurgitation; AVA, aortic valve area; PG, pressure gradient; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; LVEF, left ventricle ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PH, pulmonary hypertension; PVD, peripheral vascular disease; STS, Society of Thoracic Surgeons; TTE, transthoracic echocardiography; V max, maximum velocity.
Table 2.
Baseline DSCT measurements
| Annulus measurements | Distribution of calcification | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Valve type | Max diameter (mm) | Min diameter (mm) | Mean diameter (mm) | Perimeter derived diameter (mm) | Calcium volume (mm3) | Asymmetry | Location |
| 1 | type 0 | 24.7 | 21.7 | 23.2 | 23.4 | 985.8 | No | Annulus, Free edge |
| 2 | type 0 | 31.3 | 22.7 | 27.0 | 27.8 | 2857.4 | Yes | Annulus, Free edge, LVOT |
| 3 | type 0 | 28.9 | 18.3 | 23.6 | 23.9 | 804.1 | Yes | Annulus, Free edge |
| 4 | type 1 (LR) | 28.8 | 16.8 | 22.8 | 24.2 | 1407.1 | No | Annulus, Free edge, Raphe,LVOT |
| 5 | type 1 (LR) | 31.2 | 24.1 | 27.7 | 27.5 | 1057.7 | No | Annulus, Free edge |
| 6 | type 0 | 24.1 | 22.7 | 23.4 | 23.6 | 261.4 | Yes | Free edge |
| 7 | type 0 | 29.9 | 23.1 | 26.5 | 26.6 | 577.8 | No | Annulus, Free edge |
| 8 | type 0 | 28.0 | 19.0 | 23.5 | 23.9 | 670.4 | Yes | Annulus, Free edge |
| 9 | type 0 | 27.1 | 25.5 | 26.3 | 26.7 | 431.6 | Yes | Free edge |
| 10 | type 1 (LR) | 29.2 | 23.0 | 26.1 | 26.3 | 1459.1 | Yes | Annulus, Free edge, Raphe |
| 11 | type 1 (LR) | 25.3 | 20.4 | 22.9 | 22.7 | 1689.8 | No | Annulus, Free edge, Raphe |
| 12 | type 0 | 25.6 | 21.4 | 23.5 | 24.2 | 422.2 | Yes | Free edge |
Abbreviations: LVOT, left ventricular outflow tract; SOV, sinus of Valsalva.
3.2. Supra‐annular structure assessment by sequential balloon sizing, device size selection, and procedural outcomes
Supra‐annular structure was assessed by sequential balloon sizing that started with an 18 mm balloon and was successfully performed in all 12 patients. Among them, seven patients (58.3%) had obvious waist sign with less than mild regurgitation after 18 mm Z‐Med balloon predilation, three patients (25%) with sequential 18 mm and 20 mm balloon aortic valvuloplasty, and only two patients (16.7%) needed balloon inflation three times with sequential 18, 20, and 22 mm balloon sizing. Balloon aortic valvuloplasty was well‐tolerated in all patients during TAVR procedure.
Compared with an annulus‐based sizing strategy, the final selected device was one size smaller in nine patients, two sizes smaller in two patients, and the same in one patient Devices were successfully deployed in all 12 patients, and post‐dilation was performed in eight patients. No severe complications, including mortality, moderate to severe PVL, TAV‐in‐TAV and coronary obstruction were found. Mean implantation depth was 4.6 ± 3.1mm (Table 3).
Table 3.
Procedural information and outcomes
| Supra‐annular assessment | Device selection | Implantation depth (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Final balloon diameter (mm) | Calculated average diameter (mm) | Annulus based device selection | Final device choice | Balloon post‐dilation | Procedural success | Procedural complicationsa | Angiographic aortic regurgitation grade (0–4) | Gradient reduction (mm Hg) | Left | Right | Mean |
| 1 | 18 | 20.7 | 29mm Venus A | 26mm Venus A | 22mm | Yes | None | 0 | 92 | 10 | 8 | 9.0 |
| 2 | 18 | 22.9 | 32mm Venus A | 26mm Venus A | 20mm | Yes | None | 1 | 82 | 0 | −2 | −1.0 |
| 3 | 18 | 21.0 | 29mm Venus A | 26mm Venus A | 20mm | Yes | None | 1 | 47 | 9 | 6 | 7.5 |
| 4 | 20 | 22.1 | 29mm Venus A | 26mm Venus A | None | Yes | None | 1 | 47 | 2 | 0 | 1.0 |
| 5 | 22 | 24.8 | 32mm Venus A | 29mm Venus A | 26mm | Yes | None | 1 | 48 | 10 | 7 | 8.5 |
| 6 | 20 | 21.8 | 29mm VitaFlow | 24mm Vita Flow | None | Yes | Minor bleeding | 0 | 65 | 5 | 4 | 4.5 |
| 7 | 18 | 22.3 | 27mm VitaFlow | 24mm Vita Flow | 20mm | Yes | Minor bleeding | 1 | 72 | 6 | 4 | 5.0 |
| 8 | 18 | 21.0 | 27mm VitaFlow | 24mm Vita Flow | 20mm | Yes | None | 0 | 68 | 4 | 6 | 5.0 |
| 9 | 20 | 23.4 | 30mm VitaFlow | 27mm Vita Flow | None | Yes | None | 1 | 34 | 4 | 3 | 3.5 |
| 10 | 18 | 22.1 | 30mm VitaFlow | 24mm Vita Flow | None | Yes | None | 0 | 78 | 3 | 2 | 2.5 |
| 11 | 18 | 20.4 | 24mm VitaFlow | 21mm Vita Flow | 18mm | Yes | AKI, minor vascular complication | 1 | 115 | 3 | 1 | 2.0 |
| 12 | 22 | 23.1 | 27mm VitaFlow | 27mm Vita Flow | 22mm | Yes | None | 1 | 64 | 9 | 6 | 7.5 |
Including mortality, valve malpositioning, aortic root rupture, tamponade, conversion to open surgery, coronary obstruction, TAV‐in‐TAV deployment, bleeding, acute kidney injury (AKI), vascular complications, etc.
Patient 2 was a typical aortic stenosis patient with type 0 bicuspid AS (Figure 3A) and 32 mm VENUS A would be recommended according to annulus based sizing strategy (Figure 3B). However, waist sign was obvious during balloon sizing with 18 mm Z‐Med balloon (Figure 3C), and calculated average diameter was 22.9 mm, so a 26 mm VENUS A was selected. A prosthesis was successfully deployed above the annulus and the implantation depth was 0 and −2 mm in the left and right side (Figure 3D). DSCT follow up showed the VENUS A was anchored by the supra‐annular structure while not even attached to the annulus (Figure 3E,F), indicating the important role of supra‐annular structure for the device anchoring and sizing.
Figure 3.
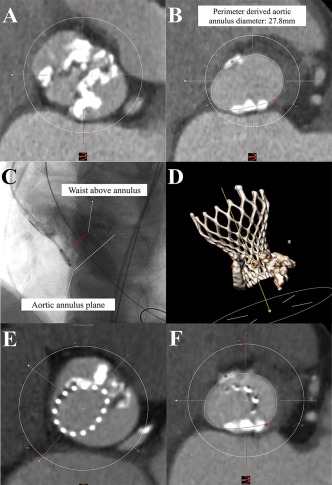
A typical case (A) heavily calcified bicuspid aortic valve (type 0); (B) perimeter‐derived diameter of 27.8mm; (C) 18 mm balloon sizing showing obvious waist sign above the annulus without regurgitation; (D) pre‐discharge CT follow‐up of the 26 mm Venus A valve; (E) short axis of the device at the level of bioprosthetic leaflet's nadirs; (F) short axis showing no attachment of device with the native annulus [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Outcome of 30d follow‐up
All 12 patients finished 30d follow‐up and there were no mortalities, myocardial infarctions, or new pacemaker implantations observed. Only one patient with non‐disabling stroke was observed and symptom was fully recovered before discharge. The heart function status of the patients was improved, as the percentage of NYHA III/IV decreased from 83.3% (9/12) to 16.7% (2/12). Aortic valve area increased from 0.61 ± 0.17 cm2 to 1.63 ± 0.34 cm2, mean pressure gradient reduced from 47 ± 11 mm Hg to 11 ± 4 mm Hg, and maximum velocity decreased from 4.54 ± 0.57 m/s to 2.35 ± 0.40 m/s. No moderate or severe PVL was found in any of the 12 patients. Importantly, there were 3 patients with moderate PPM and no patients with severe PPM because of the selection of downsized THVs (Table 4). Both the rate and severity of PPM are less than that in the CoreValve US High Risk Pivotal Trial 21.
Table 4.
Outcomes of 30d follow‐up
| TTE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Mortality | MI | Stroke | New pacemaker | Indexed EOA (cm2/m2) | PPM | NYHA class | AVA (cm2) | PGmean (mm Hg) | V max (m/s) | LVEF (%) | PVL grade (0–3) |
| 1 | 0 | 0 | 0 | 0 | 1.02 | Insignificant | III | 1.35 | 8 | 1.97 | 40.0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0.98 | Insignificant | I | 1.89 | 18 | 2.97 | 65.0 | 1 |
| 3 | 0 | 0 | 0 | 0 | 1.46 | Insignificant | III | 2.3 | 10 | 2.30 | 29.2 | 1 |
| 4 | 0 | 0 | 0 | 0 | 0.78 | Moderate | II | 1.35 | 16 | 2.70 | 48.0 | 1 |
| 5 | 0 | 0 | 0 | 0 | 0.94 | Insignificant | II | 1.57 | 9 | 2.22 | 59.7 | 1 |
| 6 | 0 | 0 | 1 (non‐disabling) | 0 | 1.17 | Insignificant | II | 1.98 | 9 | 2.21 | 60.1 | 0 |
| 7 | 0 | 0 | 0 | 0 | 1.08 | Insignificant | II | 1.84 | 6 | 1.65 | 59.8 | 1 |
| 8 | 0 | 0 | 0 | 0 | 0.81 | Moderate | I | 1.20 | 16 | 3.00 | 69.1 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0.90 | Insignificant | II | 1.56 | 8 | 2.10 | 62.1 | 1 |
| 10 | 0 | 0 | 0 | 0 | 0.82 | Moderate | I | 1.76 | 10 | 2.15 | 66.1 | 0 |
| 11 | 0 | 0 | 0 | 0 | 0.85 | Insignificant | II | 1.20 | 12 | 2.50 | 68.1 | 1 |
| 12 | 0 | 0 | 0 | 0 | 0.87 | Insignificant | II | 1.60 | 11 | 2.40 | 58.0 | 1 |
Abbreviations: AVA, aortic valve area; EOA, effective orifice area; LVEF, left ventricle ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PGmean, mean pressure gradient; PPM, prosthesis‐patient mismatch; PVL, paravalvular leakage; TTE, transthoracic echocardiography; Vmax, maximum velocity.
4. DISCUSSION
We report Hangzhou's experience, with supra‐annular structure assessments by sequential balloon sizing for device size selection, in TAVR patients with bicuspid AS for the first time. Sequential balloon sizing was successfully performed to assess the supra‐annular structure in all 12 patients. A smaller device size was selected in all but one patient, and the outcomes were satisfactory with 100% procedural success, no 30d mortality, good hemodynamic results, and heart function recovery.
Bicuspid aortic valve deformity is a heritable disease with an estimated prevalence of 0.5%–2% 22. Unfavorable morphological characteristics of bicuspid aortic valve patients, such as annular eccentricity, asymmetrical leaflet calcification, unequally‐sized leaflets, and concomitant aortopathy 23 increase possibility of deeper implantation, PVL, TAV‐in‐TAV, annulus rupture, aortic dissection, etc. during TAVR procedures. Thus, early TAVR clinical trials and guidelines regarded bicuspid AS as a relative contraindication 24, 25, which resulted in a lack of data on TAVR for patients with bicuspid AS. Recently, a few studies showed encouraging short‐ and mid‐term clinical outcomes in bicuspid AS patients undergoing TAVR 6, 26. It is reported that the proportion of bicuspid AS is from 37.5% to 47.5% in Chinese TAVR patients 12, 27. Therefore, it is especially important for Chinese interventionalists to improve the outcomes with the only available first‐generation domestic self‐expanding THVs, VENUS A and VitaFlow Valve at the present time.
Annulus‐based device selection from CT measurement is the standard sizing strategy for tricuspid AS; however, no standard sizing for bicuspid AS has been developed so far. Even though CT provides precise anatomic aortic root information, it is insufficient in revealing the mechanical characteristics of the annulus or supra‐annular structure for THV anchoring. Previously, balloon sizing was performed in patients with borderline annulus or bicuspid AS 28. However, the purpose of previous balloon sizing was focused on annulus instead of supra‐annular structure 28, 29. Balloon sizing provides information of supra‐annular mechanical characteristics by observation of the balloon waist sign in conjunction with contrast aortogram and AR evaluation, which has not been descripted before. Interestingly, THVs were deployed above the annulus in some patients at our center, indicating that the supra‐annular structure provides enough anchoring force. Our data demonstrated an advantage strategy for selection of a THV in bicuspid AS patient population.
Base on the principle of supra‐annular structure assessment, downsizing of the self‐expanding prosthesis was used in 91.7% of our bicuspid AS patient population. Good procedural outcomes demonstrated that our strategy avoided inadequate oversizing which may lead to deep implantation, paravalvular leak, conduction abnormality, and prosthesis under‐expansion. Compared with the CoreValve US High Risk Pivotal Trial, our strategy did not increase the rate or severity of PPM 21. Therefore, our strategy is both feasible and safe based on the experience of initial 12 cases.
5. STUDY LIMITATIONS
Admittedly, there are some limitations in our study. Firstly, repeated rapid ventricular pacing during sequential balloon sizing may have unfavorable impact on hemodynamic stability, although only one balloon was used in majority of the cases, and heart function deterioration was not observed in our entire study cohort. Secondly, balloon valvuloplasty may induce more native valve debris, which is a probable cause of ischemic stroke. One patient suffered from a non‐disabling stroke in our study; however previous published data suggests that pre‐dilation is not associated with stroke 30. The impact of sequential balloon sizing on stroke may need further research. Thirdly, the sample size and following up of the current study is small and short. A prospective randomized controlled trial to test the efficacy of supra‐annular structure based sizing strategy by sequential balloon sizing, as well as long‐term follow‐up study is currently ongoing in our center.
6. CONCLUSIONS
A supra‐annular assessment based sizing strategy by sequential balloon sizing is feasible for patients with bicuspid AS during TAVR procedure.
DISCLOSURES
Nothing to report.
ACKNOWLEDGEMENTS
The authors thank Cody R. Hou for his assistance in manuscript preparation. This work was supported by the Advanced Technique Research of Valvular Heart Disease Treatment Project (2015C03028).
Liu X, He Y, Zhu Q, et al. Supra‐annular structure assessment for self‐expanding transcatheter heart valve size selection in patients with bicuspid aortic valve. Catheter Cardiovasc Interv. 2018;91:986–994. https://doi.org/10.1002/ccd.27467
Funding information The Advanced Technique Research of Valvular Heart Disease Treatment Project, Grant number: 2015C03028.
REFERENCES
- 1. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin Iii JP, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'gara PT, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017,70:252–289. [DOI] [PubMed] [Google Scholar]
- 2. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 3. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J, Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK. Transcatheter aortic‐valve replacement with a self‐expanding prosthesis. N Engl J Med 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 4. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 5. Thyregod HGH, Steinbrüchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, Chang Y, Franzen OW, Engstrøm T, Clemmensen P, Hansen PB, Andersen LW, Olsen PS, Søndergaard L. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1‐Year results from the all‐comers NOTION Randomized Clinical Trial. J Am Coll Cardiol 2015;65:2184–2194. [DOI] [PubMed] [Google Scholar]
- 6. Yoon SH, Bleiziffer S, De Backer O, Delgado V, Arai T, Ziegelmueller J, Barbanti M, Sharma R, Perlman GY, Khalique OK, et al. Procedural and clinical outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol 2017;69:2579–2589. [DOI] [PubMed] [Google Scholar]
- 7. Jilaihawi H, Kashif M, Fontana G, Furugen A, Shiota T, Friede G, Makhija R, Doctor N, Leon MB, Makkar RR. Cross‐sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol 2012;59:1275–1286. [DOI] [PubMed] [Google Scholar]
- 8. Kasel AM, Cassese S, Bleiziffer S, Amaki M, Hahn RT, Kastrati A, Sengupta PP. Standardized imaging for aortic annular sizing: implications for transcatheter valve selection. JACC Cardiovasc Imag 2013;6:249–262. [DOI] [PubMed] [Google Scholar]
- 9. Piazza N, de Jaegere P, Schultz C, Becker AE, Serruys PW, Anderson RH. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ Cardiovasc Interv 2008;1:74–81. [DOI] [PubMed] [Google Scholar]
- 10. Babaliaros VC, Junagadhwalla Z, Lerakis S, Thourani V, Liff D, Chen E, Vassiliades T, Chappell C, Gross N, Patel A, Howell S, Green JT, Veledar E, Guyton R, Block PC. Use of balloon aortic valvuloplasty to size the aortic annulus before implantation of a balloon‐expandable transcatheter heart valve. JACC Cardiovasc Interv 2010;3:114–118. [DOI] [PubMed] [Google Scholar]
- 11. Alkhouli M, Zack CJ, Sarraf M, Bashir R, Nishimura RA, Eleid MF, Nkomo VT, Sandhu GS, Gulati R, Greason KL, Holmes DR, Rihal CS. Morbidity and mortality associated with balloon aortic valvuloplasty: A national perspective. Circ Cardiovasc Interv 2017;10:e004481. [DOI] [PubMed] [Google Scholar]
- 12. Jilaihawi H, Wu Y, Yang Y, Xu L, Chen M, Wang J, Kong X, Zhang R, Wang M, Lv B, Wang W, Xu B, Makkar RR, Sievert H, Gao R. Morphological characteristics of severe aortic stenosis in China: Imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv 2015;85: 752–761. [DOI] [PubMed] [Google Scholar]
- 13. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: Executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438–2488. [DOI] [PubMed] [Google Scholar]
- 14. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es G‐A, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés‐Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium‐2 consensus document. J Am Coll Cardiol 2012;60:1438–1454. [DOI] [PubMed] [Google Scholar]
- 15. Watanabe Y, Morice M‐C, Bouvier E, Leong T, Hayashida K, Lefèvre T, Hovasse T, Romano M, Chevalier B, Donzeau‐Gouge P, Farge A, Cormier B, Garot P. Automated 3‐dimensional aortic annular assessment by multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Interv 2013;6:955–964. [DOI] [PubMed] [Google Scholar]
- 16. Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007;133:1226–1233. [DOI] [PubMed] [Google Scholar]
- 17. Frick M, Meyer CG, Kirschfink A, Altiok E, Lehrke M, Brehmer K, Lotfi S, Hoffmann R. Evaluation of aortic regurgitation after transcatheter aortic valve implantation: AORTIC root angiography in comparison to cardiac magnetic resonance. EuroIntervention 2016;11:1419–1427. [DOI] [PubMed] [Google Scholar]
- 18. Adele C, Vaitkus PT, Tischler MD. Evaluation of the significance of a transvalvular catheter on aortic valve gradient in aortic stenosis: A direct hemodynamic and Doppler echocardiographic study. Am J Cardiol 1997;79:513–516. [DOI] [PubMed] [Google Scholar]
- 19. Takagi K, Latib A, Al‐Lamee R, Mussardo M, Montorfano M, Maisano F, Godino C, Chieffo A, Alfieri O, Colombo A. Predictors of moderate‐to‐severe paravalvular aortic regurgitation immediately after CoreValve implantation and the impact of postdilatation. Catheter Cardiovasc Interv 2011;78:432–443. [DOI] [PubMed] [Google Scholar]
- 20. Ali OF, Schultz C, Jabbour A, Rubens M, Mittal T, Mohiaddin R, Davies S, Di Mario C, Van der Boon R, Ahmad AS, Amrani M, Moat N, De Jaegere PPT, Dalby M. Predictors of paravalvular aortic regurgitation following self‐expanding Medtronic CoreValve implantation: the role of annulus size, degree of calcification, and balloon size during pre‐implantation valvuloplasty and implant depth. Int J Cardiol 2015;179:539–545. [DOI] [PubMed] [Google Scholar]
- 21. Zorn GL III, Little SH, Tadros P, Deeb GM, Gleason TG, Heiser J, Kleiman NS, Oh JK, Popma JJ, Adams D, et al. Prosthesis‐patient mismatch in high‐risk patients with severe aortic stenosis: A randomized trial of a self‐expanding prosthesis. J Thorac Cardiovasc Surg 2016;151:1014–1022. 1023.e1–3. [DOI] [PubMed] [Google Scholar]
- 22. Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010;55:2789–2800. [DOI] [PubMed] [Google Scholar]
- 23. Zhao Z‐G, Jilaihawi H, Feng Y, Chen M. Transcatheter aortic valve implantation in bicuspid anatomy. Nat Rev Cardiol 2015;12:123–128. [DOI] [PubMed] [Google Scholar]
- 24. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron‐Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1–S44. [DOI] [PubMed] [Google Scholar]
- 25. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 26. Mylotte D, Lefevre T, Søndergaard L, Watanabe Y, Modine T, Dvir D, Bosmans J, Tchetche D, Kornowski R, Sinning J‐M, Thériault‐Lauzier P, O'Sullivan CJ, Barbanti M, Debry N, Buithieu J, Codner P, Dorfmeister M, Martucci G, Nickenig G, Wenaweser P, Tamburino C, Grube E, Webb JG, Windecker S, Lange R, Piazza N. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014;64:2330–2339. [DOI] [PubMed] [Google Scholar]
- 27. Liu X‐b, Jiang J‐b, Zhou Q‐j, Pu Z‐x, He W, Dong A‐q, Feng Y, Jiang J, Sun Y, Xiang M‐x, He Y‐x, Fan Y‐q, Dong L, Wang J‐a. Evaluation of the safety and efficacy of transcatheter aortic valve implantation in patients with a severe stenotic bicuspid aortic valve in a Chinese population. J Zhejiang Univ Sci B 2015;16:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shivaraju A, Thilo C, Ott I, Mayr PN, Schunkert H, von Scheidt W, Thoenes M, Byrne R, Kastrati A, Kasel AM. Tools and techniques—Clinical: Fluoroscopic balloon sizing of the aortic annulus before transcatheter aortic valve replacement (TAVR) ‐ follow the “right cusp rule”. EuroIntervention 2015;11:840–842. [DOI] [PubMed] [Google Scholar]
- 29. Cerillo AG, Mariani M, Glauber M, Berti S. Sizing the annulus for transcatheter aortic valve implantation: More than a simple measure?. Eur J Cardiothorac Surg 2012;41:717–718. author reply 718‐9. [DOI] [PubMed] [Google Scholar]
- 30. Auffret V, Regueiro A, Campelo‐Parada F, Del Trigo M, Chiche O, Chamandi C, Puri R, Rodes‐Cabau J. Feasibility, safety, and efficacy of transcatheter aortic valve replacement without balloon predilation: A systematic review and meta‐analysis. Catheter Cardiovasc Interv 2017;90:839–850. [DOI] [PubMed] [Google Scholar]


