Abstract
Paediatric chronic fatigue syndrome or myalgic encephalomyelitis affects at least 1% of secondary school children in the UK and is very disabling. Treatment is effective but few children get a diagnosis or access treatment. This paper summarises what we currently know about diagnosing and treating this important illness in childhood.
Introduction
Paediatric chronic fatigue syndrome or myalgic encephalomyelitis (CFS/ME)1 is relatively common, affecting between 0.1% and 2% of secondary school children.2–5 It has significant impact on the lives of young people; school absence, periods of being bedbound and long-term consequences for physical health, psychological well-being, social development and overall quality of life.6–10 Young people report a ‘feeling of being forgotten, invisible and left behind’.11 Multiple qualitative studies have found that young people feel a sense of loss in several areas of life, including family, friendships and schooling11–13 and young people report experiencing disbelief and distrust from others.14 This may contribute to why young people with CFS/ME have significantly impaired quality of life, even when compared with children and adolescents with other chronic illnesses.15
Having a child with CFS/ME affects the whole family16 and is costly for the National Health Service (NHS). Specialist treatment is effective, and therefore making a diagnosis and ensuring children can access treatment is important.
Diagnosis and Referral
Young people with CFS/ME have persistent and disabling fatigue. Many teenagers (up to 34% in some studies) have significant fatigue.17–19 The fatigue experienced by children with CFS/ME is qualitatively different and disabling with a significant impact on function. Most (62%) of those attending specialist services attend only 2 days a week of school or less20 and children give up social activities and hobbies.13 Postexertional malaise is a core symptom and is an increase in fatigue and symptoms after doing more than usual. For most children, this means that they miss time off school at the end of the week. In those who are severely affected they may have an increase in symptoms after a small amount of activity such as walking down stairs. Different diagnostic criteria require different numbers of symptoms. Table 1 describes the different diagnostic criteria used. In the UK clinicians use the National Institute for Health and Care Excellence (NICE) guidance1; however, most research studies use the Fukuda21 or Centers for Disease Control and Prevention criteria.22 The Canadian criteria23 and the Institute of Medicine criteria24 are used in some studies. CFS/ME is a diagnosis of exclusion and other explanations for the fatigue must be ruled out via clinical assessment (including blood tests, see box 1).
Table 1.
Comparison of CFS/ME diagnostic criteria
| CDCP criteria | Canadian criteria | NICE guidance | |
|---|---|---|---|
| Principal symptom | Fatigue | Fatigue | Fatigue |
| Other symptoms | At least four of: sore throat, tender lymph nodes, muscle pain, joint pain, headaches, unrefreshing sleep, postexertional malaise, impaired memory or concentration | Postexertional malaise and/or postexertional fatigue, unrefreshing sleep or sleep disturbance, pain cognitive dysfunction | Malaise, headaches, sleep disturbances, difficulties with concentration and muscle pain and/or joint pain, painful lymph nodes, sore throat, dizziness and/or nausea and palpitations with no identifiable heart problem |
| Onset | Of new or definite onset (not lifelong) | Not stated | New, persistent and/or recurrent |
| Duration | ≥6 months Persistent or relapsing |
≥3 months in a child or young person Persistent or reoccurring |
≥3 months in a child or young person |
| Impact on functioning | Results in a substantial reduction in occupational, educational, social or personal functioning | Results in substantial reduction in previous levels of educational, social and personal functioning | Substantial reduction in activity levels |
| Exclusions | Fatigue is not substantially alleviated by rest and is not the result of ongoing exertion. Fatigue is clinically evaluated and unexplained. | Fatigue is clinically evaluated and unexplained. Current psychiatric conditions that may explain the presence of chronic fatigue, including schizophrenia or psychotic disorders, bipolar disorder, alcohol or substance abuse, anorexia nervosa or bulimia nervosa and depressive disorders. | Fatigue not explained by other conditions. The diagnosis of CFS/ME should be reconsidered if none of the following key features are present: postexertional fatigue or malaise, cognitive difficulties, sleep disturbance and chronic pain. |
Adapted from Loades et al.48
CDCP, Centers for Disease Control and Prevention; CFS/ME, paediatric chronic fatigue syndrome or myalgic encephalomyelitis; NICE, National Institute for Health and Care Excellence.
Box 1. Screening tests recommended by National Institute for Health and Care Excellence.
-
►
Full blood count
-
►
Creatinine, urea and electrolytes
-
►
Thyroid function
-
►
Erythrocyte sedimentation rate/plasma viscosity
-
►
C-reactive protein
-
►
Random blood glucose
-
►
Screening blood tests for gluten sensitivity
-
►
Serum calcium
-
►
Creatine kinase
-
►
Ferritin levels
-
►
Urinalysis
Paediatric CFS/ME is heterogeneous with at least three different phenotypes25 described in table 2. This is consistent with adult data where three to five different phenotypes are described. This means that children will present with different clusters of symptoms and may explain why different children respond to different treatments.
Table 2.
Three different phenotypes of CFS/ME in children
| Phenotype | Description and key points |
|---|---|
| Musculoskeletal |
|
| Migraine |
|
| Sore throat |
|
Summarised from May et al.25
CFS/ME, CFS/ME, paediatric chronic fatigue syndrome or myalgic encephalomyelitis.
Diagnosis and referral is valued by families affected by CFS/ME; mothers reported finding specialist CFS/ME services useful in recognising and acknowledging their child’s condition and opening channels of dialogue between healthcare professionals and education providers. Adolescents report that specialist medical care results in better symptom management.26
Treatment and Management
Children with CFS/ME have the right to access specialist treatment.1 Those with mild CFS/ME are typically mobile, able to care for themselves and the majority will by attending school, although they will often be taking days off. They will probably have stopped leisure and social pursuits and will be using the weekend for rest. These young people with mild CFS/ME should be referred to specialist paediatric CFS/ME services within 6 months1. Young people with moderate CFS/ME have reduced mobility and are restricted in all activities of daily living, often having peaks and troughs of ability. They are likely to be missing periods of schooling. These individuals should be referred within 3–4 months1. Young people with severe CFS/ME should be referred immediately.1 A young person is deemed to have severe CFS/ME if he or she is unable to do activities for themselves, can only carry out minimal daily tasks, or if he or she has severe cognitive difficulties and depends on a wheelchair for mobility.1 Few children access specialist services within these time frames.27
NICE recommends that children with CFS/ME are offered either cognitive behavioural therapy (CBT), activity management or graded exercise therapy (GET). These approaches should also include advice on sleep and symptom management. Some young people present with complex presentations, so individualised management plans1 should be developed including treatment for comorbidities such as mood disorders, eating difficulties and obesity. Clinicians may also address more systemic issues such as how the CFS/ME affects family members28 and how families should be involved in treatment.29
The largest evidence-base is for CBT-type interventions; five randomised controlled trials of CBT for CFS/ME in adolescents have shown that it is an effective treatment approach.30–34 There is a limited evidence base for GET. Although there are several trials in adults,35 there are no trials investigating GET in the outpatient setting. A randomised controlled is currently in progress comparing the effectiveness of GET to activity management in children aged 8–1736.
Activity management and GET are behavioural approaches that work on changing the boom–bust pattern of activity (or exercise in GET) to a more stable ‘baseline’ before increasing it. For children and families with more complex needs (such as comorbid mood disorders) and for those who find change too difficult, CBT may be the more appropriate treatment; it includes the behavioural elements but also uses cognitive approaches to support psychological needs and encourage behaviour change.
Activity management
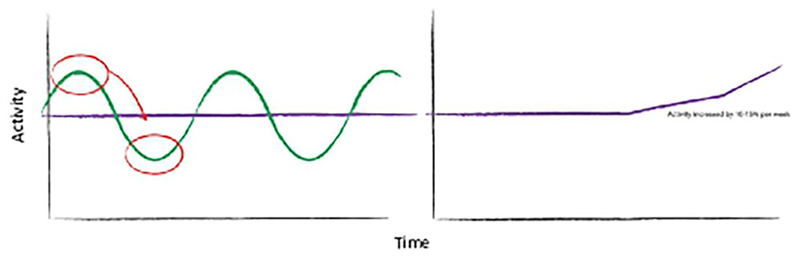
Activity management is delivered by multidisciplinary health professionals (including occupational therapists, physiotherapists, nurses and psychologists). This behavioural intervention targets the ‘boom–bust’ pattern of activity; fluctuations of over activity and underactivity, where young people engage in high levels of activity on ‘good’ days with consequential ‘bad’ days of low activity,37 38 as shown in figure 1.
Figure 1.
The boom–bust pattern of activity and the aims of activity management. The green line represents the boom and bust pattern of activity. Activity management is represented in purple; young people are supported to initially establish a ‘baseline’ of activity, and once they have managed this they are advised to gradually increase activity by 10%–15% per week.
The clinician assesses the patient’s current levels of activity, recording detailed information about cognitive activities (school work, reading, socialising and screen time (phone, laptop, TV, games)), emotional activities and physical activities. The clinician agrees a ‘baseline’ of activity with the young person; a daily sustainable level of activity, which is typically the average amount of activity that the young person reports at assessment.28
Young people are taught how to record the total number of minutes spent each day doing different levels of activity (high energy and low energy). Recording activity is used to help participants understand whether they are doing the same each day or varying their activity and whether the baseline has been set at the correct level. Young people are able to use either paper diaries or digital applications to record and monitor activity.
When participants have achieved their baseline, they are supported to increase this. NICE guidance recommends a gradual increase.1 Studies have used different approaches, most studies increase activity by 10%–20% each week36 with a gradual return to school if this is the child’s goal, and to normal social activity.39 Activity management should be structured, tailored to the child’s goals, active and an incremental approach to rehabilitation.1 40 Therapists discuss problems encountered by participants and provide possible solutions. Setbacks are discussed and managed by appropriately adapting behavioural plans.
Graded exercise therapy
Graded exercise therapy (GET) uses a similar approach but focuses only on exercise. This means that there needs to be an appropriate physical assessment, with goal setting and a rehabilitation plan created based on physical goals.1 It should be prescribed by a suitably qualified/trained therapist and should be offered to young people with mild or moderate CFS/ME.1 GET probably works in several ways. For some, GET stops children and young people doing too much exercise on good days and helps to establish a consistent amount of physical activity every day. For other children, it helps them to gain stamina and increase their cardiovascular tolerance to exercising.
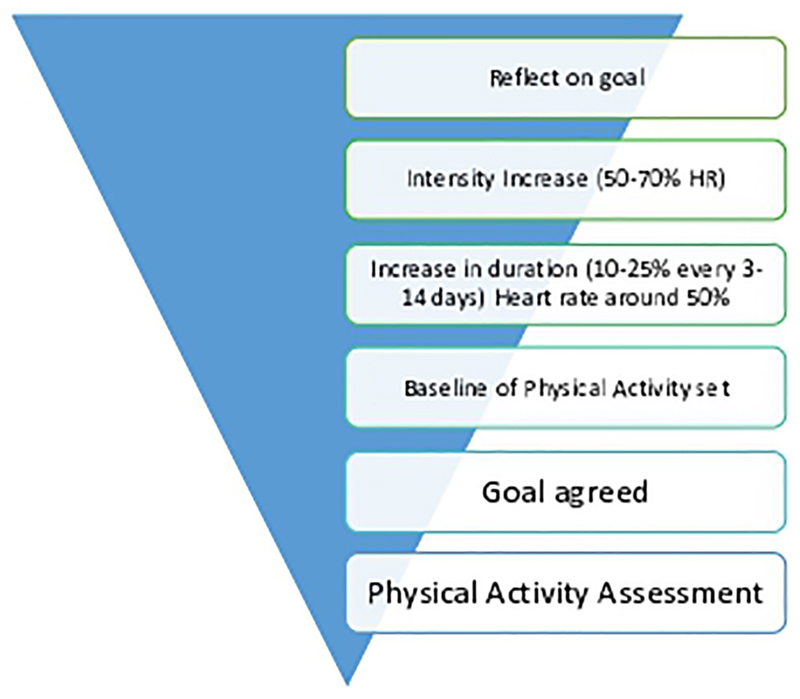
The young person’s physical activity is assessed and a baseline is set by the clinician. The total amount of physical activity the young person does that is, walking around school, playing and participating in active hobbies are all taken into consideration when devising the tailored treatment plan of a physical activity baseline level and a GET training programme. A musculoskeletal assessment may also be conducted to check for muscle tightness, weakness or joint hypermobility. Short-term and long-term goals are set. The GET programme starts at a low intensity. Walking is the easiest exercise to access as it is functional and cheap. The walk duration is initially set by the therapist following an assessment of the young person’s current ability. The walk should be carried out at their own steady pace with a heart rate between 40% and 50% of their maximum heart rate and be continuous. This should be carried out at least five times a week. Once this has been achieved and maintained, without a daily fluctuation in overall activity levels, the young person can increase the duration of the walk to reach 30 minutes. This is done carefully with a graduated approach, increasing 10%–25% every 3–14 days. Once the young person is able to carry out 30 minutes of low intensity exercise 5–7 days a week, they can begin to increase the intensity of the walk, using interval training. The training heart rate will aim to rise between 50% and 70%1 of their maximum heart rate. Using a heart rate monitor can avoid over exercising which can lead to an exacerbation in symptoms. The graded exercise model is displayed in figure 2.
Figure 2.
Graded exercise model.
It is important to warn the young person and their parents that there can be a slight increase in symptoms when physical activity levels are increased which is normal. Frequent assessments are used to check that the young person is not over doing it. Children are warned that a reduction in function may mean the baseline needs to be reassessed.
The use of technology (activity monitors/pedometers/heart rate monitors) can help the young person to understand the principles of GET and aid them to monitor and adjust their individual programme.
Cognitive behavioural therapy
The CB model of CFS/ME suggests that although fatigue is triggered by (for example) an infection, it is maintained by behavioural responses to fatigue such as overexertion or excessive rest and emotional factors such as depression, frustration and anxiety.41 In some cases, children (and parents) are understandably anxious about reproducing symptoms, and this makes engagement with rehabilitation much harder.
CBT aims to counter the cognitive and behavioural factors that maintain fatigue. When a child experiences chronic fatigue, they change what they do (eg, by reducing activity levels), driven by thoughts such as ‘I need to rest to recover’. A graded approach to stabilising and then increasing activity (physical and mental) and sleep management strategies are used to tackle the behavioural cycles.1 Identifying and challenging unhelpful cognitions and developing more helpful cognitions is done with the behavioural programme.1 Cognitive processes are tackled by shifting attention away from the symptom of fatigue using attentional retraining principles.42 Children and families are reminded that it is normal to feel tired at times, just not consistently so and not to the extent of significantly limiting functioning. Therefore, the aim of treatment is not for an individual never to be tired again; instead, the aim is for the child to be capable of doing everything that they want to do, which typically includes a return to fulltime education.30 Parents are commonly involved in treatment sessions.
Engaging in CBT requires a degree of concentration and attention, and its success is premised on an individual doing work in between sessions to notice patterns and make changes in their daily lives, at both a cognitive and behavioural level. Therefore, for some children, even if the CBT is adapted by the therapist to accommodate for their symptoms of fatigue, the demands of the therapy may be too much.
More recently, studies have looked beyond face-face delivery, exploring telephone33 and online delivery of CBT.31 Despite NICE guideline that young people should be referred to specialist CFS/ME services, most young people in the UK are unable to access such services locally. Many children experience significant barriers to accessing care with delayed access and prolonged periods of ill health before treatment.27 Once solution may be to provide online CBT, which has been shown to be effective in the Netherlands.31 A large randomised controlled trial, Fatigue In Teenagers on the inter-NET-NHS ‘FITNET-NHS’, is currently in progress to investigate the clinical effectiveness and cost-effectiveness of internet-delivered CBT in UK NHS services.
Managing sleep
Young people with CFS/ME characteristically present with sleep difficulties, unusual sleep patterns (such as day–night reversal) and unrefreshing sleep.2 43 Clinicians should take a good sleep history, identify the individual’s problems and then work with the family to improve sleep quality. The most important strategies are to reduce excessive sleeping as this will improve sleep architecture. Clinicians should agree the maximum number of hours the young person will sleep and then set a wake-up time. The going to sleep time can be calculated by subtracting the maximum time from this.
Adrenal function is disturbed in adolescents with CFS/ME with low salivary cortisol in the morning.28 This gets better with treatment.44 Setting a regular wake-up time for weekends as well as weekdays is important to restore normal diurnal cortisol patterns.
Melatonin can also be used to help young people get off to sleep if they still take more than 20 minutes to get off to sleep after sleep restriction and a regular waking up time. Suggested regimens include starting with 2 mg doses 30 minutes before sleep is desired, if the child is in bed and ready for sleep. The dose can be increased incrementally but if it is ineffective at 6 mg, it is not likely to work at higher doses. It does not usually help to maintain sleep through the night, even if the slow release preparation is used. No preparations are licensed for use in children, but Circadin (slow release) has recently been added to the British National Formulary for Children (BNFC, UK guidance on prescribing and pharmacology) for shared care prescribing. It should be noted that there is a limited evidence base for melatonin.45
Managing pain
Pain is a common symptom; around 20% of patients have a pain phenotype, where musculoskeletal pain is the predominant symptoms alongside fatigue.46 Adult patients who experience pain have a less favourable outcome, and this may also be true for younger patients.
If chronic pain is a predominant feature, clinicians should consider referral to a specialist pain management clinic. Psychological treatments for pain are principally, but not exclusively, cognitive and behavioural treatments. These treatments encourage active engagement and self-management by the young person with behavioural strategies to support normal daily activities, an increased awareness of the role of cognition in suffering, a focus on the self-regulation of emotion and the use of techniques for reducing aversive arousal (eg, relaxation).47 In terms of medical management of pain, simple analgesics such as ibuprofen are first-line treatments. Amitriptyline may be used an initial 10 mg dose gradually increased up to 1 mg/kg (maximum 50 mg daily). Amitriptyline side effects can include drowsiness, so it can also be useful given in the evening, for those who have difficulty getting off to sleep. It may take up to 3 weeks for the effects to be seen. Opiates should not be prescribed to children and adolescents with CFS/ME and pain.
Managing Comorbid Conditions
Managing mood
Depression and anxiety are more common in children with CFS/ME than in the general population, and are associated with markers of disease severity.9 In qualitative studies, the majority (but not all) of the young people interviewed, described their CFS/ME as preceding either their depression13 or anxiety.14 However, the direction of causality is not known.9 While the treatment approaches for CFS/ME may help children with either depression or anxiety, it is not clear which treatment approaches should be used in those with comorbid mood disorders.48 49 For some children, their negative thinking, hopelessness and helplessness or anxiety is so severe that it may impede treatment for CFS/ME. In these children, it may be helpful to refer children for specialist assessment and treatment for their mood. NICE recommends treating any comorbid mood disorders as per standard clinical management, including selective serotonin reuptake inhibitor medications and talking therapies.
Managing eating difficulties
An estimated 10% of young people with CFS/ME who access specialist services experience eating difficulties, which can have a significant impact on their quality of life, illness and on their families.50 Not eating increases fatigue, low mood and anxiety which further exacerbates the eating difficulties. Clinicians should screen for eating difficulties in those with symptoms of nausea and abdominal pain, warn adolescents and their families of the risk of developing eating difficulties and provide interventions and support as early as possible.50 NICE recommends offering advice such as eating little and often, snacking on dry starchy foods and sipping fluids. The use of antiemetic drugs should be considered only if nausea is severe.1 Exclusion diets are not generally recommended by NICE, and dieticians should be consulted in this instance due to risk of malnutrition. Individuals may find exclusion diets helpful in managing symptoms, including bowel symptoms.1
Managing weight
Young people with CFS/ME are at increased risk of obesity.51 This may be due to decreased levels of physical activity and/or increased time spent doing sedentary activities, although the temporal relationship between CFS/ME and weight has not been established. Obesity is associated with a range of diseases, and clinicians should consider screening and offering advice for managing weight as per NICE guidance when they see patients with CFS/ME. For most children, this is likely to involve dietary changes as increasing exercise to manage weight is not always possible.
Do Children Get Better?
Reported outcomes vary, but the prognosis in children and young people is more optimistic that in adults.1 Four small studies (n=15–31) from the 1990s report that between 50% and 94% of children make a good or complete recovery at 13–72 months52. The largest trial to date demonstrated that most children with CFS/ME will recover within 6 to 12 months if they receive internet-delivered CBT as treatment.31 For those who do not receive specialist care, recovery is much slower with less than 10% recovering at 6 months.31
Future Directions
We need to develop better treatments for children with CFS/ME because even with the best treatments available, approximately 35% will still be ill after 6 months31. We also need to develop new approaches for those with mood disorders and younger children because outcomes for children in these groups are worse. Most children in the UK are still unable to access treatment and therefore we need to investigate different ways to deliver treatment (eg, video conferencing or internet-delivered CBT).
Useful Further Reading
Action for ME’s webpage on children and young people https://www.actionforme.org.uk/children-and-young-people/introduction.
Burgess M, Chalder T. Overcoming Chronic Fatigue: A self-help guide using cognitive behavioural techniques. London: Robinson; 2009.
NICE. Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy): Diagnosis and management of CFS/ME in adults and children (NICE guidelines CG53). London 2007 2007. CG53.
Report to the Chief Medical Officer of an Independent Working Group http://www.erythos.com/gibsonenquiry/docs/cmoreport.pdf.
Acknowledgements
EC was funded by the NIHR (Senior Research Fellowship, SRF-2013-06-013). Dr Loades is funded by the NIHR (Doctoral Research Fellowship, DRF-2016-09-021). This report is independent research. The views expressed in this publication are those of the authors(s) and not necessarily those of the NHS, The National Institute for Health Research or the Department of Health. Thank you to Dr Melanie Parker for providing an overview of the medical management of pain and sleep.
Funding EC is funded by the NIHR Senior Research Fellowship (SRF-2013-06-013) and ML is funded by the NIHR Doctoral Research Fellowship (DRF-2016-09-021).
Footnotes
Contributors All authors contributed to the writing of the manuscript.
Disclaimer This report is independent research. The views expressed in this publication are those of the authors(s) and not necessarily those of the NHS, The National Institute for Health Research or the Department of Health.
Competing interests EC leads the Bath Specialist CFS/ME service. She is the principal investigator for FITNET-NHS, a trial investigating internet-delivered CBT and MAGENTA which is investigating the effectiveness and cost-effectiveness of Graded Exercise Therapy.
Provenance and peer review Commissioned; externally peer reviewed.
References
- 1.NICE. Vol. 2007. London: 2007. Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy): diagnosis and management of CFS/ME in adults and children (NICE guidelines CG53) CG53. [Google Scholar]
- 2.Nijhof SL, Maijer K, Bleijenberg G, et al. Adolescent chronic fatigue syndrome: prevalence, incidence, and morbidity. Pediatrics. 2011;127:e1169–75. doi: 10.1542/peds.2010-1147. [DOI] [PubMed] [Google Scholar]
- 3.Crawley E, Hughes R, Northstone K, et al. Chronic disabling fatigue at age 13 and association with family adversity. Pediatrics. 2012;130:e71–9. doi: 10.1542/peds.2011-2587. [DOI] [PubMed] [Google Scholar]
- 4.Crawley EM, Emond AM, Sterne JA. Unidentified chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is a Major cause of school absence: surveillance outcomes from school-based clinics. BMJ Open. 2011;1:e000252. doi: 10.1136/bmjopen-2011-000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalder T, Goodman R, Wessely S, et al. Epidemiology of chronic fatigue syndrome and self reported myalgic encephalomyelitis in 5– 15 year olds: cross sectional study. BMJ. 2003;327:654–5. doi: 10.1136/bmj.327.7416.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garralda ME, Rangel L. Impairment and coping in children and adolescents with chronic fatigue syndrome: a comparative study with other paediatric disorders. J Child Psychol Psychiatry. 2004;45:543–52. doi: 10.1111/j.1469-7610.2004.00244.x. [DOI] [PubMed] [Google Scholar]
- 7.Patel MX, Smith DG, Chalder T, et al. Chronic fatigue syndrome in children: a cross sectional survey. Arch Dis Child. 2003;88:894–8. doi: 10.1136/adc.88.10.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter BD, Edwards JF, Kronenberger WG, et al. Case control study of chronic fatigue in pediatric patients. Pediatrics. 1995;95:179–86. [PubMed] [Google Scholar]
- 9.Bould H, Lewis G, Emond A, et al. Depression and anxiety in children with CFS/ME: cause or effect? Arch Dis Child. 2011;96:211–4. doi: 10.1136/adc.2009.173161. [DOI] [PubMed] [Google Scholar]
- 10.Dowsett EG, Colby J. Long-term sickness absence due to ME/CFS in UK Schools: an epidemiological study with medical and educational implications. J Chronic Fatigue Syndr. 1997;3:29–42. [Google Scholar]
- 11.Winger A, Ekstedt M, Wyller VB, et al. 'Sometimes it feels as if the world goes on without me': adolescents' experiences of living with chronic fatigue syndrome. J Clin Nurs. 2014;23:2649–57. doi: 10.1111/jocn.12522. [DOI] [PubMed] [Google Scholar]
- 12.Jelbert R, Stedmon J, Stephens A. A qualitative exploration of adolescents' experiences of chronic fatigue syndrome. Clin Child Psychol Psychiatry. 2010;15:267–83. doi: 10.1177/1359104509340940. [DOI] [PubMed] [Google Scholar]
- 13.Taylor AK, Loades M, Brigden AL, et al. 'It's personal to me': a qualitative study of depression in young people with CFS/ME. Clin Child Psychol Psychiatry. 2017;22 doi: 10.1177/1359104516672507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher H, Crawley E. Why do young people with CFS/ME feel anxious? A qualitative study. Clin Child Psychol Psychiatry. 2013;18:556–73. doi: 10.1177/1359104512460862. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy G, Underwood C, Belch JJ. Physical and functional impact of chronic fatigue syndrome/myalgic encephalomyelitis in childhood. Pediatrics. 2010;125:e1324–30. doi: 10.1542/peds.2009-2644. [DOI] [PubMed] [Google Scholar]
- 16.Velleman S, Collin SM, Beasant L, et al. Psychological wellbeing and quality-of-life among siblings of paediatric CFS/ME patients: a mixed-methods study. Clin Child Psychol Psychiatry. 2016;21:618–33. doi: 10.1177/1359104515602373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ter Wolbeek M, van Doornen LJ, Kavelaars A, et al. Severe fatigue in adolescents: a common phenomenon? Pediatrics. 2006;117:e1078–86. doi: 10.1542/peds.2005-2575. [DOI] [PubMed] [Google Scholar]
- 18.ter Wolbeek M, van Doornen LJ, Kavelaars A, et al. Fatigue, depressive symptoms, and anxiety from adolescence up to young adulthood: a longitudinal study. Brain Behav Immun. 2011;25:1249–55. doi: 10.1016/j.bbi.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Luntamo T, Sourander A, Santalahti P, et al. Prevalence changes of pain, sleep problems and fatigue among 8-year-old children: years 1989, 1999, and 2005. J Pediatr Psychol. 2012;37:307–18. doi: 10.1093/jpepsy/jsr091. [DOI] [PubMed] [Google Scholar]
- 20.Crawley E, Sterne JA. Association between school absence and physical function in paediatric chronic fatigue syndrome/myalgic encephalopathy. Arch Dis Child. 2009;94:752–6. doi: 10.1136/adc.2008.143537. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International chronic Fatigue syndrome Study Group. Ann Intern Med. 1994;121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Chronic fatigue syndrome: 1994 case definition. 2012 [Google Scholar]
- 23.Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. 2011;270:327–38. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clayton EW. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: an IOM report on redefining an illness. JAMA. 2015;313:1101–2. doi: 10.1001/jama.2015.1346. [DOI] [PubMed] [Google Scholar]
- 25.May M, Emond A, Crawley E. Phenotypes of chronic fatigue syndrome in children and young people. Arch Dis Child. 2010;95:245–9. doi: 10.1136/adc.2009.158162. [DOI] [PubMed] [Google Scholar]
- 26.Beasant L, Mills N, Crawley E. Adolescents and mothers value referral to a specialist service for chronic fatigue syndrome or myalgic encephalopathy (CFS/ME) Prim Health Care Res Dev. 2014;15:134–42. doi: 10.1017/S1463423613000121. [DOI] [PubMed] [Google Scholar]
- 27.Webb CM, Collin SM, Deave T, et al. What stops children with a chronic illness accessing health care: a mixed methods study in children with chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) BMC Health Serv Res. 2011;11:308. doi: 10.1186/1472-6963-11-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viner R, Gregorowski A, Wine C, et al. Outpatient rehabilitative treatment of chronic fatigue syndrome (CFS/ME) Arch Dis Child. 2004;89:615–9. doi: 10.1136/adc.2003.035154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalder T, Tong J, Deary V. Family cognitive behaviour therapy for chronic fatigue syndrome: an uncontrolled study. Arch Dis Child. 2002;86:95–7. doi: 10.1136/adc.86.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stulemeijer M, de Jong LW, Fiselier TJ, et al. Cognitive behaviour therapy for adolescents with chronic fatigue syndrome: randomised controlled trial. BMJ. 2005;330:14. doi: 10.1136/bmj.38301.587106.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nijhof SL, Bleijenberg G, Uiterwaal CS, et al. Effectiveness of internet-based cognitive behavioural treatment for adolescents with chronic fatigue syndrome (FITNET): a randomised controlled trial. Lancet. 2012;379:1412–8. doi: 10.1016/S0140-6736(12)60025-7. [DOI] [PubMed] [Google Scholar]
- 32.Al-Haggar MS, Al-Naggar ZA, Abdel-Salam MA. Biofeedback and cognitive behavioural therapy for Egyptian adolescents suffering from chronic fatigue syndrome. J Child Neurol. 2006;4:161–9. [Google Scholar]
- 33.Chalder T, Deary V, Husain K, et al. Family-focused cognitive behaviour therapy versus psycho-education for chronic fatigue syndrome in 11- to 18-year-olds: a randomized controlled treatment trial. Psychol Med. 2010;40:1269–79. doi: 10.1017/S003329170999153X. [DOI] [PubMed] [Google Scholar]
- 34.Lloyd S, Chalder T, Sallis HM, et al. Telephone-based guided self-help for adolescents with chronic fatigue syndrome: a non-randomised cohort study. Behav Res Ther. 2012;50:304–12. doi: 10.1016/j.brat.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 35.White PD, Goldsmith KA, Johnson AL, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet. 2011;377:823–36. doi: 10.1016/S0140-6736(11)60096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brigden A, Beasant L, Hollingworth W, et al. Managed Activity Graded Exercise iN Teenagers and pre-Adolescents (MAGENTA) feasibility randomised controlled trial: study protocol. BMJ Open. 2016;6:e011255. doi: 10.1136/bmjopen-2016-011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nijs J, Paul L, Wallman K. Chronic fatigue syndrome: an approach combining self-management with graded exercise to avoid exacerbations. J Rehabil Med. 2008;40:241–7. doi: 10.2340/16501977-0185. [DOI] [PubMed] [Google Scholar]
- 38.Friedberg F, Jason LA. Chronic fatigue syndrome and fibromyalgia: clinical assessment and treatment. J Clin Psychol. 2001;57:433–55. doi: 10.1002/jclp.1040. [DOI] [PubMed] [Google Scholar]
- 39.Wright B, Ashby B, Beverley D, et al. A feasibility study comparing two treatment approaches for chronic fatigue syndrome in adolescents. Arch Dis Child. 2005;90:369–72. doi: 10.1136/adc.2003.046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashby B, Wright B, Jordan J. Chronic Fatigue syndrome: an evaluation of a community based Management Programme for Adolescents and their families. Child Adolesc Ment Health. 2006;11:13–18. doi: 10.1111/j.1475-3588.2005.00383.x. [DOI] [PubMed] [Google Scholar]
- 41.Browne T, Chalder T. Chronic fatigue syndrome. Psychiatry. 2006;5:48–51. [Google Scholar]
- 42.Heins MJ, Knoop H, Bleijenberg G. The role of the therapeutic relationship in cognitive behaviour therapy for chronic fatigue syndrome. Behav Res Ther. 2013;51:368–76. doi: 10.1016/j.brat.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Jason LA, Barker K, Brown A. Pediatric myalgic encephalomyelitis/chronic fatigue syndrome. Rev Health Care. 2012;3:257–70. [PMC free article] [PubMed] [Google Scholar]
- 44.Nijhof SL, Rutten JM, Uiterwaal CS, et al. The role of hypocortisolism in chronic fatigue syndrome. Psychoneuroendocrinology. 2014;42:199–206. doi: 10.1016/j.psyneuen.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 45.(RCPCH) RcoPacH. Evidence based guidelines for the management of CFS/ME in children and young people. London: 2004. [Google Scholar]
- 46.Collin SM, Nikolaus S, Heron J, et al. Chronic fatigue syndrome (CFS) symptom-based phenotypes in two clinical cohorts of adult patients in the UK and the Netherlands. J Psychosom Res. 2016;81:14–23. doi: 10.1016/j.jpsychores.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Fisher E, Heathcote L, Palermo TM, et al. Systematic review and meta-analysis of psychological therapies for children with chronic pain. J Pediatr Psychol. 2014;39:763–82. doi: 10.1093/jpepsy/jsu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loades ME, Sheils EA, Crawley E. Treatment for paediatric chronic fatigue syndrome or myalgic encephalomyelitis (CFS/ME) and comorbid depression: a systematic review. BMJ Open. 2016;6:e012271. doi: 10.1136/bmjopen-2016-012271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoll S, Crawley E, Loades M. Anxiety in paediatric chronic fatigue syndrome (CFS/ME): a systematic review. BMJ Open. 2017 doi: 10.1136/bmjopen-2016-015481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris S, Gilbert M, Beasant L, et al. A qualitative investigation of eating difficulties in adolescents with chronic fatigue syndrome/myalgic encephalomyelitis. Clin Child Psychol Psychiatry. 2017;22:128–39. doi: 10.1177/1359104516646813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norris T, Hawton K, Hamilton-Shield J, et al. Obesity in adolescents with chronic fatigue syndrome: an observational study. Arch Dis Child. 2017;102:35–9. doi: 10.1136/archdischild-2016-311293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joyce J, Hotopf M, Wessely S. The prognosis of chronic fatigue and chronic fatigue syndrome: a systematic review. QJM. 1997;90:223–33. doi: 10.1093/qjmed/90.3.223. [DOI] [PubMed] [Google Scholar]