The molecular structure of (2E)-1,4-bis(4-chlorophenyl)but-2-ene-1,4-dione (1) is composed of two p-chloro phenyl rings, each bonded on opposite ends to a near planar 1,4-trans enedione moiety [–C(= O)—CH=CH—(C=O)–]. (2E)-1,4-Bis(4-bromophenyl)but-2-ene-1,4-dione (2) has a similar structure to (1), but with two p-bromo phenyl rings and a less planar enedione group. In the crystal, molecules of (1) exhibit C—Cl⋯Cl type I interactions, whereas molecules of (2) present C–Br⋯Br type II interactions.
Keywords: crystal structure; synthesis; 1,4-enedione moiety; bis-halo-enedione; NMR
Abstract
The molecular structure of (2E)-1,4-bis(4-chlorophenyl)but-2-ene-1,4-dione [C16H10Cl2O2, (1)] is composed of two p-chlorophenyl rings, each bonded on opposite ends to a near planar 1,4-trans enedione moiety [–C(=O)—CH=CH—(C=O)–] [r.m.s. deviation = 0.003 (1) Å]. (2E)-1,4-Bis(4-bromophenyl)but-2-ene-1,4-dione [C16H10Br2O2, (2)] has a similar structure to (1), but with two p-bromophenyl rings and a less planar enedione group [r.m.s. deviation = 0.011 (1) Å]. Both molecules sit on a center of inversion, thus Z′ = 0.5. The dihedral angles between the ring and the enedione group are 16.61 (8) and 15.58 (11)° for (1) and (2), respectively. In the crystal, molecules of (1) exhibit C—Cl⋯Cl type I interactions, whereas molecules of (2) present C—Br⋯Br type II interactions. van der Waals-type interactions contribute to the packing of both molecules, and the packing reveals face-to-face ring stacking with similar interplanar distances of approximately 3.53 Å.
Chemical context
The 1,4-enedione moiety [–C(=O)—CH=CH—(C=O)–] occurs in many natural and bioactive compounds, including steroids, antibiotics, and antitumor agents (Koft & Smith, 1982 ▸; Ismail et al., 1996 ▸; Connolly & Hill, 2010 ▸; Fouad et al., 2006 ▸; Yang et al., 2013 ▸). Its multifunctionality and versatility make it an excellent building block for novel material syntheses. In certain molecules, the facile and reversible E/Z isomerization of the enedione groups enables them to perform as optical pH and fluorescent sensors (Li et al., 2017 ▸). The title compounds (2E)-1,4-bis(4-chlorophenyl)but-2-ene-1,4-dione (1) and (2E)-1,4-bis(4-bromophenyl)but-2-ene-1,4-dione (2) exhibit two p-halogen phenyl rings, each bonded on opposite ends of the enedione group. We have synthesized these compounds in our laboratory as precursors to 4,4′-(furan-2,5-diyl)dibenzaldehyde cross-linkers. The reduction of the title compounds yields the saturated 1,4-diketones that, under Paal–Knorr reaction conditions, can undergo cyclization to produce the corresponding furans (Sauer et al., 2017 ▸). The aryl halides can be subsequently replaced with formyl groups using the Bouveault aldehyde synthesis to generate the targeted 4,4′-(furan-2,5-diyl)dibenzaldehyde cross-linkers, which can be potentially used for non-toxic, isocyanate-free synthesis of polyurethanes.
Structural commentary
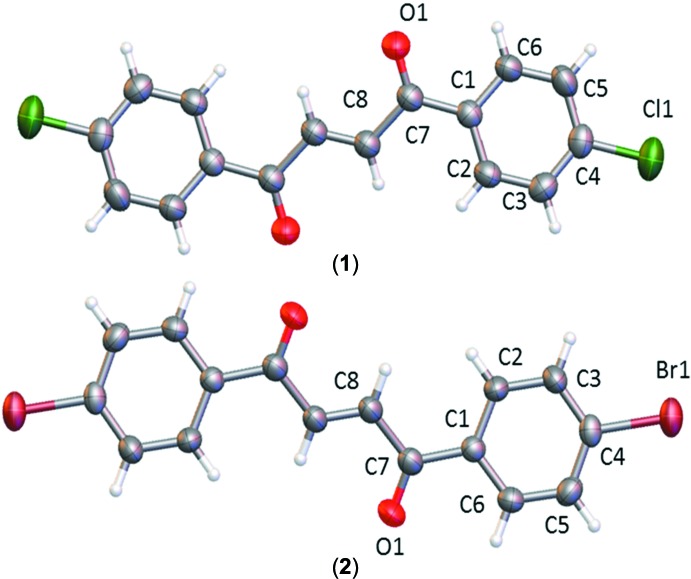
The title compounds exhibit molecular structures typical of biphenyl enedione compounds (Rabinovich et al., 1970 ▸; Xu et al., 2013 ▸; Li et al., 2014 ▸). Bond lengths and angles are in the usual ranges. Fig. 1 ▸ shows that the molecules sit on centers of inversion and that the enedione groups adopt a trans, near planar configuration [r.m.s deviations = 0.003 (1) and 0.011 (1) Å for (1) and (2), respectively]. In molecule (1), the carbonyl group is twisted slightly out of the chlorophenyl plane, as evidenced by the torsion angles C6—C1—C7—O1 [−15.6 (3)°] and C2—C1—C7—O1 [163.9 (2)°]. Molecule (2) shows a similar conformation with torsion angles of 14.5 (4) and −164.7 (3)° for the corresponding atoms of the inverted asymmetric unit (−x + 1, −y, −z + 1). The chlorophenyl ring planes form a dihedral angle of 16.61 (8)° with respect to the enedione plane (O1–C7–C8–C8′–C7′–O1′) in (1), whereas the bromophenyl ring planes form a dihedral angle of 15.58 (11)° relative to the enedione plane in (2). Both molecules exhibit a pair of short intramolecular H⋯H contacts [(1): H2⋯H8 = H2i⋯H8i = 2.127 (2) Å; symmetry code (i): −x, 1 − y, 1 − z; and (2): H2⋯H8 = H2ii⋯H8ii = 2.113 (3) Å; symmetry code: (ii) 1 − x, −y, 1 − z], possibly resulting from steric compression of the large phenyl halogen groups. A best fit of all symmetry independent atoms of both molecules (see Fig. 2 ▸) yields an r.m.s. deviation of 0.05 Å.
Figure 1.
Molecular conformation and atom-numbering scheme of (1) (top) and (2) (bottom). The non-labeled atoms are generated by symmetry operation (−x, 1 − y, 1 − z) for (1) and (1 − x, −y,1 − z) for (2). Non-hydrogen atoms are shown as 50% probability displacement ellipsoids.
Figure 2.
Superimposition of structure (1) (green) onto the inverted structure of (2) (red). Only the asymmetric unit of (1) is presented for clarity.
Supramolecular features
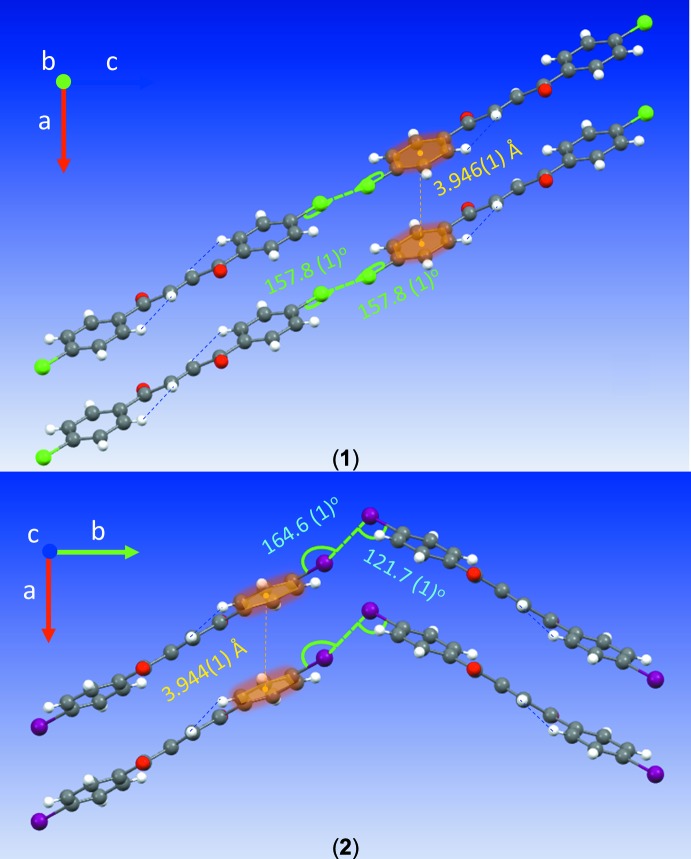
Contacts between the O atoms and H atoms of adjacent molecules [O1⋯H3i = 3.329 (2) Å; symmetry code: (i) −1 + x, 1 + y, z] and between the Cl atoms and Cl atoms of adjacent molecules [Cl1⋯Cl1ii = 3.3841 (1) Å; symmetry code: (ii) 2 − x, −y, −z] contribute to the intermolecular interactions of (1) (see Fig. 3 ▸). The short Cl⋯Cl distances are approximately 0.3 Å shorter than double the Cl van der Waals radius of 3.64 Å (Alvarez, 2013 ▸). The molecules feature type I, Cw—Clx⋯Cly—Cz interactions, where θ1 = angle Cw—Clx⋯Cly, θ2 = angle Clx⋯Cly—Cz, and |θ1 − θ2| = 0 (θ1 = θ2, approximately 157°) (see Fig. 4 ▸), suggesting that the Cl atoms minimize repulsion by interfacing the neutral regions of their electrostatic potential surfaces (Desiraju & Parthasarathy, 1989 ▸; Mukherjee & Desiraju, 2014 ▸). Unlike (1), (2) exhibits trifurcated contacts between the O atoms and H and C atoms of adjacent molecules [O1⋯H2iii = 2.616 (2) Å, O1⋯H3iii = 2.711 (2) Å, and O1⋯C2iii = 3.194 (3) Å; symmetry code: (iii) x,  − y,
− y,  + z]. Furthermore, the Br atoms form bifurcated contacts with the Br atoms of adjacent molecules [Br1⋯Br1v = Br1⋯Br1v = 3.662 (1) Å; symmetry codes: (iv) −x, −
+ z]. Furthermore, the Br atoms form bifurcated contacts with the Br atoms of adjacent molecules [Br1⋯Br1v = Br1⋯Br1v = 3.662 (1) Å; symmetry codes: (iv) −x, − + y,
+ y,  − z; (v) −x,
− z; (v) −x,  + y,
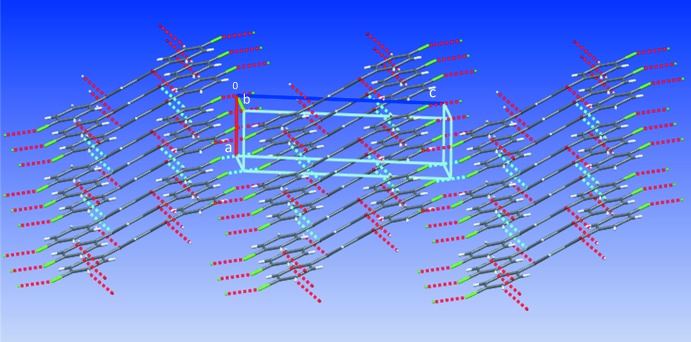
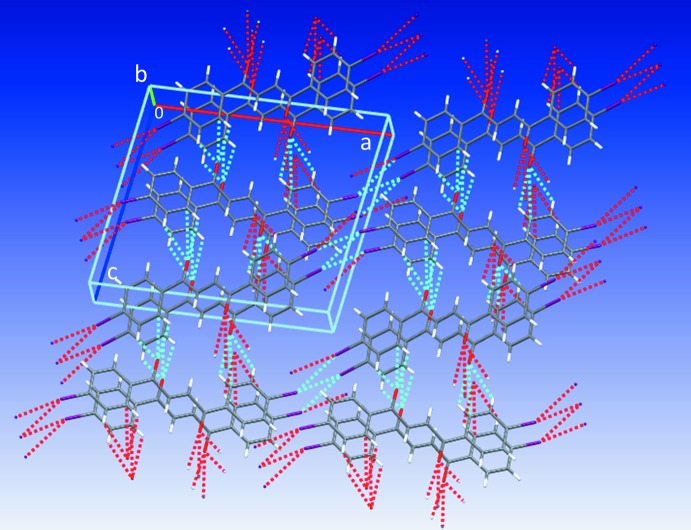
+ y,  − z] (see Fig. 5 ▸). Inspection of the C—Br⋯Br—C angles, reveals that the molecules exhibit type II interactions (|θ1 − θ2| ≥ 30°, where θ1 (164.58°) − θ2 (121.71°) = 42.87°, suggesting the electrophilic region of one Br atom approaches the nucleophilic region of the companion Br atom, unlike the Cl⋯Cl interactions (Mukherjee & Desiraju, 2014 ▸; Tothadi et al., 2013 ▸; Nuzzo et al., 2017 ▸). The chlorophenyl rings (1) are stacked in close proximity along the vicinity of the a axis with an interplanar separation of 3.528 Å [centroid-to-centroid distance = 3.946 (1) Å] (see Figs. 4 ▸ and 5 ▸). Similarly, the bromo phenyl rings of (2) stack along the vicinity of the a axis with an interplanar separation of 3.525 Å [centroid-to-centroid distance = 3.994 (1) Å], but in a crisscross-like pattern when viewed along the c axis (see Figs. 3 ▸ and 5 ▸). The intersecting ring planes subtend dihedral angles of 48.09 (6)°.
− z] (see Fig. 5 ▸). Inspection of the C—Br⋯Br—C angles, reveals that the molecules exhibit type II interactions (|θ1 − θ2| ≥ 30°, where θ1 (164.58°) − θ2 (121.71°) = 42.87°, suggesting the electrophilic region of one Br atom approaches the nucleophilic region of the companion Br atom, unlike the Cl⋯Cl interactions (Mukherjee & Desiraju, 2014 ▸; Tothadi et al., 2013 ▸; Nuzzo et al., 2017 ▸). The chlorophenyl rings (1) are stacked in close proximity along the vicinity of the a axis with an interplanar separation of 3.528 Å [centroid-to-centroid distance = 3.946 (1) Å] (see Figs. 4 ▸ and 5 ▸). Similarly, the bromo phenyl rings of (2) stack along the vicinity of the a axis with an interplanar separation of 3.525 Å [centroid-to-centroid distance = 3.994 (1) Å], but in a crisscross-like pattern when viewed along the c axis (see Figs. 3 ▸ and 5 ▸). The intersecting ring planes subtend dihedral angles of 48.09 (6)°.
Figure 3.
Crystal packing of (1) along the vicinity of the a axis. Dashed lines depict Cl1⋯Cl1i and O1⋯H3ii interactions [symmetry codes: (i) 2 − x, −y, −z; (ii) −1 + x, 1 + y, z].
Figure 4.
Molecular conformations of (1) and (2) viewed along the b and c axes, respectively, showing type I and II halogen interactions, centroid-to-centroid distances, and short intramolecular H⋯H interactions.
Figure 5.
Crystal packing of (2) along the b axis. Dashed blue lines represent bifurcated Br1⋯Br1iv,v interactions [symmetry codes: (iv) −x, − + y,
+ y,  − z; (v) −x,
− z; (v) −x,  + y,
+ y,  − z] and trifurcated interactions involving the O1 atoms.
− z] and trifurcated interactions involving the O1 atoms.
Database survey
A search of the Cambridge Structural Database (CSD web interface; Groom et al., 2016 ▸) and the Crystallography Open Database (Gražulis et al., 2009 ▸) yields the crystal structures of a number of compounds containing the 1,4-enedione moiety. For examples, see Rabinovich et al. (1970 ▸), Xu et al. (2013 ▸), Li et al. (2014 ▸), Deng et al. (2012 ▸); Gao et al. (2010 ▸), and Wu et al. (2011 ▸). The compounds trans-1,2-diphenylethylene (3) (Xu et al., 2013 ▸; CCDC 918566, BZOYEY01) and cis-1,2-dichlorobenzoylethylene (4) (Rabinovich et al., 1970 ▸; CCDC 112151, CBOZET) merit discussion because the former has a similar structure to the title compounds, whereas the latter is a stereoisomer of (1). The title compounds adopt an E configuration, similar to (3). They contain halogen atoms in the para position of the phenyl groups, unlike (3), but the rings are nearly planar as are those of (3), whose r.m.s value = 0.008 Å. The r.m.s. value, reflecting the planarity of the enedione moiety, in (1) is different to that of (3) (0.003 vs 0.0035 Å), and the value determined for (2) (0.011 Å). The dihedral angles between the ring planes of (1) and (2) are nearly identical to those of (3) [16° (average) vs 15.7 (1)°]. Unlike (1), its diastereomer (4) does not exhibit a planar enedione moiety and its near planar chlorophenyl rings (r.m.s deviation = 0.018 Å) form a dihedral angle of 77.4 (3)° with respect to each other. Superimposition of atom C1 of the E/Z diastereomers through the C7, Cl1, and O1 atoms yields an r.m.s. deviation of 0.033 Å. The remaining parts of the molecules are twisted from each other, with the planes containing the chlorophenyl group and adjoining carbonyl group of each stereoisomer forming a dihedral angle of approximately 79°.
Synthesis and crystallization
The title compounds were synthesized following a modified literature procedure (Sauer et al., 2017 ▸). The reactions were run ‘neat’ with chloro- or bromobenzene used in excess and serving also as the reaction solvent. Under a stream of nitrogen, aluminum chloride (3.6 g, 27 mmol, 2.9 equiv.) was dissolved in chloro- or bromobenzene (9.0 and 9.3 ml, respectively, 89 mmol, 9.6 equiv.) at room temperature. The reaction mixture was subsequently cooled to 273 K and fumaryl chloride (1.0 ml, 9.3 mmol, 1.0 equiv.) was added dropwise under constant stirring, at which point an instantaneous color change from clear to deep red was observed. The reaction mixture was then heated to 333 K for 2–4 days until fumaryl chloride was no longer detected on a TLC plate (SiO2, DCM). At the conclusion of the reaction, the mixture was cooled to room temperature, poured into ice-cold aqueous 1 M HCl, and extracted several times with DCM. The combined organic layers were washed with 0.5 M NaOH and dried over Na2SO4, and the volatiles were removed under reduced pressure. The resulting red–brown solid was recrystallized in DCM, further purified with a series of cold DCM washes, and dried under reduced pressure, affording either compound (1) (burnt orange solid, 1.5 g, 4.9 mmol, 53% yield) or (2) (yellow solid, 1.9 g, 4.8 mmol, 50% yield). Slow evaporation of DCM solutions saturated with either (1) or (2) yielded single crystals suitable for X-ray diffraction.
NMR spectra were recorded on a Bruker 400 MHz spectrometer. Chemical shifts (δ) are given in ppm and are referenced to tetramethylsilane (TMS) using the residual solvent (1H: CDCl3, 7.26 ppm; 13C: CDCl3, 77.16 ppm). (1): 1H NMR (CDCl3, 400.13 MHz): δ 7.51 (d, J = 8.6 Hz, 4H), 7.97 (s, 2H), 8.00 (d, J = 8.6 Hz, 4H) ppm. 13C NMR (CDCl3, 100.62 MHz): δ 129.48, 130.40, 135.06, 135.31, 140.77, 188.51 ppm. (2): 1H NMR (CDCl3, 400.13 MHz): δ 7.67 (d, J = 8.6 Hz, 4H), 7.92 (d, J = 8.6 Hz, 4H), 7.96 (s, 2H) ppm. 13C NMR (CDCl3, 100.62 MHz): δ 129.53, 130.44, 132.45, 135.03, 135.69, 188.69 ppm.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 1 ▸. The hydrogen atoms of both compounds were refined using a riding model with C—H = 0.93 Å and U iso(H) = 1.2U eq(C).
Table 1. Experimental details.
| (1) | (2) | |
|---|---|---|
| Crystal data | ||
| Chemical formula | C16H10Cl2O2 | C16H10Br2O2 |
| M r | 305.14 | 394.06 |
| Crystal system, space group | Triclinic, P

|
Monoclinic, P21/c |
| Temperature (K) | 298 | 298 |
| a, b, c (Å) | 3.9455 (3), 6.0809 (5), 14.6836 (11) | 14.4391 (7), 3.9937 (2), 12.7244 (7) |
| α, β, γ (°) | 82.653 (6), 88.638 (6), 84.601 (7) | 90, 97.827 (5), 90 |
| V (Å3) | 347.82 (5) | 726.92 (7) |
| Z | 1 | 2 |
| Radiation type | Mo Kα | Mo Kα |
| μ (mm−1) | 0.46 | 5.57 |
| Crystal size (mm) | 0.34 × 0.22 × 0.15 | 0.35 × 0.14 × 0.12 |
| Data collection | ||
| Diffractometer | Agilent SuperNova, Dualflex, EosS2 | Agilent SuperNova, Dualflex, EosS2 |
| Absorption correction | Multi-scan (CrysAlis PRO; Bourhis et al., 2015 ▸) | Multi-scan (CrysAlis PRO; Bourhis et al., 2015 ▸) |
| T min, T max | 0.928, 1.000 | 0.370, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 5641, 1416, 1256 | 6231, 1470, 1228 |
| R int | 0.023 | 0.031 |
| (sin θ/λ)max (Å−1) | 0.625 | 0.625 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.048, 0.104, 1.16 | 0.029, 0.065, 1.08 |
| No. of reflections | 1416 | 1470 |
| No. of parameters | 91 | 92 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.18, −0.20 | 0.36, −0.43 |
Supplementary Material
Crystal structure: contains datablock(s) 1, 2. DOI: 10.1107/S205698901800230X/zl2724sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S205698901800230X/zl27241sup4.hkl
Structure factors: contains datablock(s) 2. DOI: 10.1107/S205698901800230X/zl27242sup5.hkl
Supporting information file. DOI: 10.1107/S205698901800230X/zl27241sup4.cml
Supporting information file. DOI: 10.1107/S205698901800230X/zl27242sup5.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
(2E)-1,4-Bis(4-chlorophenyl)but-2-ene-1,4-dione (1) . Crystal data
| C16H10Cl2O2 | Z = 1 |
| Mr = 305.14 | F(000) = 156 |
| Triclinic, P1 | Dx = 1.457 Mg m−3 |
| a = 3.9455 (3) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 6.0809 (5) Å | Cell parameters from 2009 reflections |
| c = 14.6836 (11) Å | θ = 2.8–26.3° |
| α = 82.653 (6)° | µ = 0.46 mm−1 |
| β = 88.638 (6)° | T = 298 K |
| γ = 84.601 (7)° | Irregular, orange |
| V = 347.82 (5) Å3 | 0.34 × 0.22 × 0.15 mm |
(2E)-1,4-Bis(4-chlorophenyl)but-2-ene-1,4-dione (1) . Data collection
| Agilent SuperNova, Dualflex, EosS2 diffractometer | 1256 reflections with I > 2σ(I) |
| Detector resolution: 8.0945 pixels mm-1 | Rint = 0.023 |
| ω scans | θmax = 26.4°, θmin = 2.8° |
| Absorption correction: multi-scan (CrysAlisPro; Bourhis et al., 2015) | h = −4→4 |
| Tmin = 0.928, Tmax = 1.000 | k = −7→7 |
| 5641 measured reflections | l = −18→18 |
| 1416 independent reflections |
(2E)-1,4-Bis(4-chlorophenyl)but-2-ene-1,4-dione (1) . Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.048 | H-atom parameters constrained |
| wR(F2) = 0.104 | w = 1/[σ2(Fo2) + (0.0335P)2 + 0.118P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.16 | (Δ/σ)max < 0.001 |
| 1416 reflections | Δρmax = 0.18 e Å−3 |
| 91 parameters | Δρmin = −0.19 e Å−3 |
(2E)-1,4-Bis(4-chlorophenyl)but-2-ene-1,4-dione (1) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
(2E)-1,4-Bis(4-chlorophenyl)but-2-ene-1,4-dione (1) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.3800 (5) | 0.4949 (3) | 0.30832 (13) | 0.0411 (4) | |
| C2 | 0.5146 (5) | 0.2732 (3) | 0.31865 (14) | 0.0482 (5) | |
| H2 | 0.493021 | 0.185559 | 0.374837 | 0.058* | |
| C3 | 0.6788 (6) | 0.1826 (4) | 0.24675 (15) | 0.0529 (5) | |
| H3 | 0.771626 | 0.035173 | 0.254438 | 0.063* | |
| C4 | 0.7048 (5) | 0.3111 (4) | 0.16369 (14) | 0.0500 (5) | |
| C5 | 0.5749 (6) | 0.5314 (4) | 0.15096 (15) | 0.0559 (6) | |
| H5 | 0.595485 | 0.617285 | 0.094315 | 0.067* | |
| C6 | 0.4149 (5) | 0.6214 (3) | 0.22341 (14) | 0.0495 (5) | |
| H6 | 0.327970 | 0.770037 | 0.215556 | 0.059* | |
| C7 | 0.2047 (5) | 0.6004 (3) | 0.38415 (14) | 0.0459 (5) | |
| C8 | 0.0820 (5) | 0.4582 (3) | 0.46605 (13) | 0.0452 (5) | |
| H8 | 0.123290 | 0.304322 | 0.469025 | 0.054* | |
| Cl1 | 0.90353 (18) | 0.19244 (12) | 0.07252 (4) | 0.0768 (3) | |
| O1 | 0.1558 (5) | 0.8021 (2) | 0.38003 (11) | 0.0699 (5) |
(2E)-1,4-Bis(4-chlorophenyl)but-2-ene-1,4-dione (1) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0389 (10) | 0.0429 (10) | 0.0419 (10) | −0.0081 (8) | −0.0005 (8) | −0.0038 (8) |
| C2 | 0.0565 (13) | 0.0421 (11) | 0.0450 (11) | −0.0066 (9) | 0.0017 (9) | −0.0003 (9) |
| C3 | 0.0558 (13) | 0.0447 (11) | 0.0587 (13) | −0.0013 (10) | −0.0009 (10) | −0.0115 (10) |
| C4 | 0.0463 (12) | 0.0595 (13) | 0.0471 (12) | −0.0076 (10) | 0.0021 (9) | −0.0164 (10) |
| C5 | 0.0616 (14) | 0.0622 (13) | 0.0413 (11) | −0.0054 (11) | 0.0049 (10) | 0.0017 (10) |
| C6 | 0.0539 (12) | 0.0437 (11) | 0.0481 (12) | −0.0008 (9) | 0.0031 (9) | 0.0017 (9) |
| C7 | 0.0480 (11) | 0.0440 (11) | 0.0450 (11) | −0.0066 (9) | 0.0022 (9) | −0.0017 (8) |
| C8 | 0.0477 (12) | 0.0431 (10) | 0.0440 (11) | −0.0046 (9) | 0.0015 (8) | −0.0023 (8) |
| Cl1 | 0.0799 (5) | 0.0926 (5) | 0.0621 (4) | −0.0021 (4) | 0.0148 (3) | −0.0326 (3) |
| O1 | 0.1034 (14) | 0.0410 (8) | 0.0617 (10) | −0.0006 (8) | 0.0245 (9) | −0.0015 (7) |
(2E)-1,4-Bis(4-chlorophenyl)but-2-ene-1,4-dione (1) . Geometric parameters (Å, º)
| C1—C2 | 1.392 (3) | C4—Cl1 | 1.737 (2) |
| C1—C6 | 1.390 (3) | C5—H5 | 0.9300 |
| C1—C7 | 1.481 (3) | C5—C6 | 1.372 (3) |
| C2—H2 | 0.9300 | C6—H6 | 0.9300 |
| C2—C3 | 1.374 (3) | C7—C8 | 1.488 (3) |
| C3—H3 | 0.9300 | C7—O1 | 1.218 (2) |
| C3—C4 | 1.369 (3) | C8—C8i | 1.307 (4) |
| C4—C5 | 1.379 (3) | C8—H8 | 0.9300 |
| C2—C1—C7 | 122.61 (18) | C4—C5—H5 | 120.6 |
| C6—C1—C2 | 118.30 (19) | C6—C5—C4 | 118.8 (2) |
| C6—C1—C7 | 119.08 (18) | C6—C5—H5 | 120.6 |
| C1—C2—H2 | 119.6 | C1—C6—H6 | 119.3 |
| C3—C2—C1 | 120.72 (19) | C5—C6—C1 | 121.3 (2) |
| C3—C2—H2 | 119.6 | C5—C6—H6 | 119.3 |
| C2—C3—H3 | 120.3 | C1—C7—C8 | 119.63 (17) |
| C4—C3—C2 | 119.4 (2) | O1—C7—C1 | 120.95 (18) |
| C4—C3—H3 | 120.3 | O1—C7—C8 | 119.41 (19) |
| C3—C4—C5 | 121.4 (2) | C7—C8—H8 | 118.8 |
| C3—C4—Cl1 | 118.98 (17) | C8i—C8—C7 | 122.3 (2) |
| C5—C4—Cl1 | 119.60 (17) | C8i—C8—H8 | 118.8 |
| C1—C2—C3—C4 | −1.1 (3) | C4—C5—C6—C1 | −0.4 (3) |
| C1—C7—C8—C8i | −178.2 (2) | C6—C1—C2—C3 | 0.3 (3) |
| C2—C1—C6—C5 | 0.5 (3) | C6—C1—C7—C8 | 163.48 (18) |
| C2—C1—C7—C8 | −17.0 (3) | C6—C1—C7—O1 | −15.6 (3) |
| C2—C1—C7—O1 | 163.9 (2) | C7—C1—C2—C3 | −179.24 (19) |
| C2—C3—C4—C5 | 1.2 (3) | C7—C1—C6—C5 | −179.94 (19) |
| C2—C3—C4—Cl1 | −178.37 (16) | Cl1—C4—C5—C6 | 179.15 (16) |
| C3—C4—C5—C6 | −0.4 (3) | O1—C7—C8—C8i | 0.9 (4) |
Symmetry code: (i) −x, −y+1, −z+1.
(2E)-1,4-Bis(4-bromophenyl)but-2-ene-1,4-dione (2) . Crystal data
| C16H10Br2O2 | F(000) = 384 |
| Mr = 394.06 | Dx = 1.800 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 14.4391 (7) Å | Cell parameters from 2527 reflections |
| b = 3.9937 (2) Å | θ = 2.9–26.2° |
| c = 12.7244 (7) Å | µ = 5.57 mm−1 |
| β = 97.827 (5)° | T = 298 K |
| V = 726.92 (7) Å3 | Irregular, yellow |
| Z = 2 | 0.35 × 0.14 × 0.12 mm |
(2E)-1,4-Bis(4-bromophenyl)but-2-ene-1,4-dione (2) . Data collection
| Agilent SuperNova, Dualflex, EosS2 diffractometer | 1228 reflections with I > 2σ(I) |
| Detector resolution: 8.0945 pixels mm-1 | Rint = 0.031 |
| ω scans | θmax = 26.4°, θmin = 2.9° |
| Absorption correction: multi-scan (CrysAlisPro; Bourhis et al., 2015) | h = −18→18 |
| Tmin = 0.370, Tmax = 1.000 | k = −4→4 |
| 6231 measured reflections | l = −15→15 |
| 1470 independent reflections |
(2E)-1,4-Bis(4-bromophenyl)but-2-ene-1,4-dione (2) . Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.029 | w = 1/[σ2(Fo2) + (0.0234P)2 + 0.4382P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.065 | (Δ/σ)max < 0.001 |
| S = 1.08 | Δρmax = 0.36 e Å−3 |
| 1470 reflections | Δρmin = −0.43 e Å−3 |
| 92 parameters | Extinction correction: SHELXL-2016/4 (Sheldrick, 2015b), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.0047 (8) |
(2E)-1,4-Bis(4-bromophenyl)but-2-ene-1,4-dione (2) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
(2E)-1,4-Bis(4-bromophenyl)but-2-ene-1,4-dione (2) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.06905 (2) | 0.86344 (8) | 0.66837 (3) | 0.05337 (16) | |
| C1 | 0.31059 (19) | 0.3876 (7) | 0.5110 (2) | 0.0355 (6) | |
| C2 | 0.3212 (2) | 0.4543 (8) | 0.6188 (2) | 0.0444 (7) | |
| H2 | 0.377230 | 0.400535 | 0.660470 | 0.053* | |
| C3 | 0.2503 (2) | 0.5990 (8) | 0.6653 (2) | 0.0471 (8) | |
| H3 | 0.258517 | 0.646056 | 0.737543 | 0.057* | |
| C4 | 0.1673 (2) | 0.6726 (6) | 0.6034 (2) | 0.0391 (7) | |
| C5 | 0.1538 (2) | 0.6073 (7) | 0.4962 (2) | 0.0463 (7) | |
| H5 | 0.097052 | 0.657253 | 0.455312 | 0.056* | |
| C6 | 0.2258 (2) | 0.4666 (8) | 0.4507 (2) | 0.0422 (7) | |
| H6 | 0.217497 | 0.423632 | 0.378154 | 0.051* | |
| C7 | 0.3859 (2) | 0.2399 (8) | 0.4570 (2) | 0.0418 (7) | |
| C8 | 0.4672 (2) | 0.0760 (7) | 0.5218 (2) | 0.0410 (7) | |
| H8 | 0.471561 | 0.081856 | 0.595399 | 0.049* | |
| O1 | 0.38162 (17) | 0.2478 (7) | 0.36132 (17) | 0.0665 (7) |
(2E)-1,4-Bis(4-bromophenyl)but-2-ene-1,4-dione (2) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.0433 (2) | 0.0504 (2) | 0.0706 (3) | 0.00725 (15) | 0.02309 (16) | 0.00106 (17) |
| C1 | 0.0354 (15) | 0.0380 (14) | 0.0334 (15) | −0.0010 (12) | 0.0057 (12) | 0.0011 (12) |
| C2 | 0.0380 (17) | 0.0601 (19) | 0.0340 (16) | 0.0088 (14) | 0.0012 (13) | −0.0001 (14) |
| C3 | 0.0465 (18) | 0.0598 (19) | 0.0359 (16) | 0.0119 (15) | 0.0082 (13) | −0.0026 (15) |
| C4 | 0.0371 (16) | 0.0344 (15) | 0.0487 (18) | 0.0009 (12) | 0.0158 (13) | 0.0028 (13) |
| C5 | 0.0371 (16) | 0.0514 (18) | 0.0487 (18) | 0.0054 (14) | 0.0002 (13) | 0.0034 (15) |
| C6 | 0.0439 (17) | 0.0491 (17) | 0.0328 (15) | 0.0031 (14) | 0.0030 (13) | −0.0006 (13) |
| C7 | 0.0395 (16) | 0.0480 (16) | 0.0382 (17) | −0.0016 (13) | 0.0060 (13) | −0.0057 (13) |
| C8 | 0.0364 (16) | 0.0514 (18) | 0.0357 (15) | −0.0005 (13) | 0.0060 (12) | −0.0064 (14) |
| O1 | 0.0591 (15) | 0.109 (2) | 0.0322 (12) | 0.0232 (14) | 0.0078 (11) | −0.0071 (12) |
(2E)-1,4-Bis(4-bromophenyl)but-2-ene-1,4-dione (2) . Geometric parameters (Å, º)
| Br1—C4 | 1.896 (3) | C4—C5 | 1.376 (4) |
| C1—C2 | 1.385 (4) | C5—H5 | 0.9300 |
| C1—C6 | 1.390 (4) | C5—C6 | 1.377 (4) |
| C1—C7 | 1.485 (4) | C6—H6 | 0.9300 |
| C2—H2 | 0.9300 | C7—C8 | 1.492 (4) |
| C2—C3 | 1.378 (4) | C7—O1 | 1.211 (3) |
| C3—H3 | 0.9300 | C8—C8i | 1.310 (5) |
| C3—C4 | 1.374 (4) | C8—H8 | 0.9300 |
| C2—C1—C6 | 118.3 (3) | C4—C5—H5 | 120.6 |
| C2—C1—C7 | 123.1 (3) | C4—C5—C6 | 118.8 (3) |
| C6—C1—C7 | 118.7 (3) | C6—C5—H5 | 120.6 |
| C1—C2—H2 | 119.4 | C1—C6—H6 | 119.4 |
| C3—C2—C1 | 121.2 (3) | C5—C6—C1 | 121.3 (3) |
| C3—C2—H2 | 119.4 | C5—C6—H6 | 119.4 |
| C2—C3—H3 | 120.5 | C1—C7—C8 | 119.3 (2) |
| C4—C3—C2 | 119.0 (3) | O1—C7—C1 | 121.0 (3) |
| C4—C3—H3 | 120.5 | O1—C7—C8 | 119.7 (3) |
| C3—C4—Br1 | 118.8 (2) | C7—C8—H8 | 119.0 |
| C3—C4—C5 | 121.5 (3) | C8i—C8—C7 | 121.9 (4) |
| C5—C4—Br1 | 119.7 (2) | C8i—C8—H8 | 119.0 |
| Br1—C4—C5—C6 | −179.6 (2) | C3—C4—C5—C6 | −0.4 (5) |
| C1—C2—C3—C4 | 1.0 (5) | C4—C5—C6—C1 | 0.6 (5) |
| C1—C7—C8—C8i | 175.8 (3) | C6—C1—C2—C3 | −0.8 (5) |
| C2—C1—C6—C5 | −0.1 (4) | C6—C1—C7—C8 | −164.8 (3) |
| C2—C1—C7—C8 | 16.0 (4) | C6—C1—C7—O1 | 14.5 (4) |
| C2—C1—C7—O1 | −164.7 (3) | C7—C1—C2—C3 | 178.4 (3) |
| C2—C3—C4—Br1 | 178.8 (2) | C7—C1—C6—C5 | −179.3 (3) |
| C2—C3—C4—C5 | −0.5 (5) | O1—C7—C8—C8i | −3.5 (6) |
Symmetry code: (i) −x+1, −y, −z+1.
Funding Statement
This work was funded by Army Research Laboratory grant . Oak Ridge Institute for Science and Education grant . U.S. Department of Energy grant .
References
- Alvarez, S. (2013). Dalton Trans. 42, 8617–8636. [DOI] [PubMed]
- Bourhis, L. J., Dolomanov, O. V., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2015). Acta Cryst. A71, 59–75. [DOI] [PMC free article] [PubMed]
- Connolly, J. D. & Hill, R. A. (2010). Nat. Prod. Rep. 27, 79–132. [DOI] [PubMed]
- Deng, C., Yang, Y., Gao, M., Zhu, Y.-P., Wu, A.-X., Ma, J.-R. & Yin, G.-D. (2012). Tetrahedron, 68, 3828–3834.
- Desiraju, G. R. & Parthasarathy, R. (1989). J. Am. Chem. Soc. 111, 8725–8726.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Fouad, M., Edrada, R. A., Ebel, R., Wray, V., Müller, W. E. G., Lin, W. H. & Proksch, P. J. (2006). J. Nat. Prod. 69, 211–218. [DOI] [PubMed]
- Gao, M., Yang, Y., Wu, Y.-D., Deng, C., Cao, L.-P., Meng, X. G. & Wu, A.-X. (2010). J. Org. Chem. 12(8), 1856–1859. [DOI] [PubMed]
- Gražulis, S., Chateigner, D., Downs, R. T., Yokochi, A. F. T., Quirós, M., Lutterotti, L., Manakova, E., Butkus, J., Moeck, P. & Le Bail, A. (2009). J. Appl. Cryst. 42, 726–729. [DOI] [PMC free article] [PubMed]
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Ismail, K. A., El-Tombary, A. A., Aboulwafa, O. M., Omar, A. M. E. & El-Rewini, S. H. (1996). Arch. Pharm. Pharm. Med. Chem. 329, 433–437. [DOI] [PubMed]
- Koft, E. R. & Smith, A. B. (1982). J. Am. Chem. Soc. 104, 2659–2661.
- Li, S.-Y., Wang, X.-B., Jiang, N. & Kong, L.-Y. (2014). Eur. J. Org. Chem. pp. 8035–8039.
- Li, M., Wang, Y. X., Wang, J. & Chen, Y. (2017). J. Mater. Chem. C5, 3408–3414.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Mukherjee, A. & Desiraju, G. R. (2014). IUCrJ, 1, 49–60. [DOI] [PMC free article] [PubMed]
- Nuzzo, S., Twamley, B. & Baker, R. J. (2017). J. Chem. Crystallogr. 47, 182–186.
- Rabinovich, D., Schmidt, G. M. J. & Shaked, D. (1970). J. Chem. Soc. B, pp. 17–24.
- Rigaku OD (2015). CrysAlis PRO. Rigaku Oxford Diffraction Ltd, Yarnton, England.
- Sauer, B., Skinner-Adams, T. S., Bouchut, A., Chua, M. J., Pierrot, C., Erdmann, F., Robaa, D., Schmidt, M., Khalife, J., Andrews, K. T. & Sippl, W. (2017). Eur. J. Med. Chem. 127, 22–40. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Tothadi, S., Joseph, S. & Desiraju, G. R. (2013). Cryst. Growth Des. 13, 3242–3254.
- Wu, L., Deng, C. & Yang, Y. (2011). Acta Cryst. E67, o1499. [DOI] [PMC free article] [PubMed]
- Xu, K., Fang, Y., Yan, Z., Zha, Z. & Wang, Z. (2013). Org. Lett. 15, 2148–2151. [DOI] [PubMed]
- Yang, Y., Ni, F., Shu, W.-M., Yu, S.-B., Gao, M. & Wu, A.-X. (2013). J. Org. Chem. 78, 5418–5426. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) 1, 2. DOI: 10.1107/S205698901800230X/zl2724sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S205698901800230X/zl27241sup4.hkl
Structure factors: contains datablock(s) 2. DOI: 10.1107/S205698901800230X/zl27242sup5.hkl
Supporting information file. DOI: 10.1107/S205698901800230X/zl27241sup4.cml
Supporting information file. DOI: 10.1107/S205698901800230X/zl27242sup5.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report