Abstract
This retrospective cohort study compared real‐world clinical and healthcare‐resource utilization (HCRU) data in patients with type 2 diabetes using basal insulin (BI) who switched to insulin glargine 300 units/mL (Gla‐300) or another BI. Data from the Predictive Health Intelligence Environment database 12 months before (baseline) and 6 months after (follow‐up) the switch date (index date, March 1, 2015 to May 31, 2016) included glycated haemoglobin A1c (HbA1c), hypoglycaemia, HCRU and associated costs. Baseline characteristics were balanced using propensity score matching. Change in HbA1c from baseline was similar in both matched cohorts (n = 1819 in each). Hypoglycaemia incidence and adjusted event rate were significantly lower with Gla‐300. Patients switching to Gla‐300 had a significantly lower incidence of HCRU related to hypoglycaemia. All‐cause and diabetes‐related hospitalization and emergency‐department HCRU were also favourable for Gla‐300. Lower HCRU translated to lower costs in patients using Gla‐300. In this real‐world study, switching to Gla‐300 reduced the risk of hypoglycaemia in patients with type 2 diabetes when compared with those switching to another BI, resulting in less HCRU and potential savings of associated costs.
Keywords: basal insulin, glycaemic control, hypoglycaemia, observational study, type 2 diabetes
1. INTRODUCTION
Patients with type 2 diabetes (T2D) who are unable to reach target glycated haemoglobin A1c (HbA1c) levels with metformin, with or without additional oral antidiabetic drugs (OADs), may be initiated on basal‐insulin (BI) therapy based on recommendations of the American Diabetes Association and the American Association of Clinical Endocrinologists 2017.1, 2
Two new second‐generation BIs, insulin glargine 300 units/mL (Gla‐300) and insulin degludec (IDeg), with smooth action profiles and reduced risk of hypoglycaemia, have been approved recently.3, 4, 5 In the USA, Gla‐300 became available in 2015. The EDITION randomized controlled trial (RCT) programme demonstrated that the use of Gla‐300 leads to glycaemic control comparable to that of insulin glargine 100 units/mL (Gla‐100), with less hypoglycaemia in a wide range of T2D patients.6 However, in RCTs, outcomes do not always mirror those seen in the real world, and healthcare‐resource utilization (HCRU) cannot be evaluated reliably.
The interest in comparative effectiveness research, and a growing demand for real‐world data to support decision‐making, have increased the use of data sources other than RCTs (eg, electronic medical records [EMRs]).7 In view of its relatively recent introduction for clinical use in the USA, the outcomes associated with Gla‐300 therapy compared with other BIs under the conditions of routine real‐world clinical practice have not been characterized. Therefore, this study aimed to evaluate clinical outcomes, HCRU and costs in patients with T2D using BI who switched to either Gla‐300 or other BIs in real‐world clinical settings.
2. METHODS
The Differentiate Gla‐300 clinical and Economic in reaL‐world Via EMR Data study (DELIVER 2) is a retrospective cohort study. Data were collected from the Predictive Health Intelligence Environment (PHIE) database of EMRs, representing 39 integrated healthcare‐delivery networks in the USA, between March 1, 2014 and November 30, 2016 (observation period).
Eligible patients were adults (≥ 18 years of age) with T2D (identified by ICD‐9/ICD‐10 codes)8 who were using BI (Gla‐300, Gla‐100, insulin detemir or IDeg) during the observation period. The index date was the date of first switch to either Gla‐300 or other BIs (Gla‐100, insulin detemir or IDeg) between March 1, 2015 and May 31, 2016 (identification period). Patients were required to have ≥1 prescription for non‐index BI within 12 months prior to the index date (baseline), and were followed for 6 months after the index date (follow‐up). All patients had ≥1 HbA1c values in the 6 months before the index date and between 3 and 6 months follow‐up. Patients using >1 BI on the index date were excluded as it is not possible to use EMR data to identify which BI those patients switched to.
The study endpoints included: change in HbA1c from baseline to follow‐up and proportion of patients reaching the pre‐specified HbA1c target <7.0% (53 mmol/mol) and <8.0% (64 mmol/mol) during the 6‐month follow‐up period (the latest available HbA1c measures were selected during baseline and follow‐up); incidence and event rates of hypoglycaemia identified by ICD‐9/ICD‐10 codes8 and/or plasma glucose level ≤ 70 mg/dL, and hypoglycaemia associated with hospitalization or emergency‐department (ED) visit during the 6‐month follow‐up period; proportion of patients requiring hospitalization, ED and outpatient services (for hypoglycaemia‐related, diabetes‐related and all‐cause events) and HCRU event rates (events/per patient per year [PPPY]). Cost of healthcare and cost difference were derived by multiplying the cost per event (unit cost, data source) (Appendix S1 in File S1) with adjusted mean healthcare events/PPPY and adjusted mean difference in events/PPPY between the 2 cohorts, respectively. All unit‐cost values were adjusted to 2016 US dollars using the medical‐care component of the Consumer Price Index.9
To minimize confounding by indication, patients were matched 1:1 on a propensity score according to baseline demographics (age, sex, race, insurance type, geographic region), clinical features (HbA1c, BMI, Charlson Comorbidity Index score, comorbidities, hypoglycaemia), treatment (baseline BI type and concomitant drug use) and HCRU characteristics (incidence of visits) (Appendix S1 in File S1).10 Adjusted odds ratios (aOR), accounting for baseline hypoglycaemia/HCRU incidence, were calculated for incidence of hypoglycaemia and HCRU to compare risks between the 2 matched cohorts. Adjusted mean and least squares mean (LSM) differences were calculated for event rates of hypoglycaemia and HCRU in the 2 cohorts controlled for baseline hypoglycaemia/HCRU events.
3. RESULTS
The final unmatched cohort included 2196 patients who switched to Gla‐300, and 3837 who switched to other BIs (Appendix S2 in File S1). After propensity score matching, each cohort comprised 1819 patients with substantial aligned baseline values (Appendix S3 in File S1). Both matched cohorts were comparable in terms of sex (approximately 49% male in matched cohorts), and mean age was approximately 59.5 years. At baseline, Gla‐100 was the BI in 73.0% and 71.4% of patients in the Gla‐300 and other‐BI cohorts, respectively. The remainder of patients in each cohort switched from insulin detemir. In the other‐BI cohort, 22.4%, 66.9% and 10.7% of patients switched to Gla‐100, insulin detemir and IDeg, respectively.
At baseline, mean HbA1c was 8.95% in the Gla‐300 cohort and 8.93% in the other‐BI cohort, and significantly decreased to 8.43% and 8.43%, respectively, during follow‐up (P < .001 for both cohorts). The magnitude of HbA1c changes was comparable between cohorts (−0.51% for Gla‐300 vs −0.51% for other BI; P = .928) (Figure 1A). Patients using Gla‐300 and those using other BIs were also equally likely to reach HbA1c <7.0% (16.8% vs 18.4%, respectively; P = .223) and HbA1c <8.0% (44.0% vs 44.2%, respectively; P = .920) during follow‐up (Appendix S4 in File S1).
Figure 1.
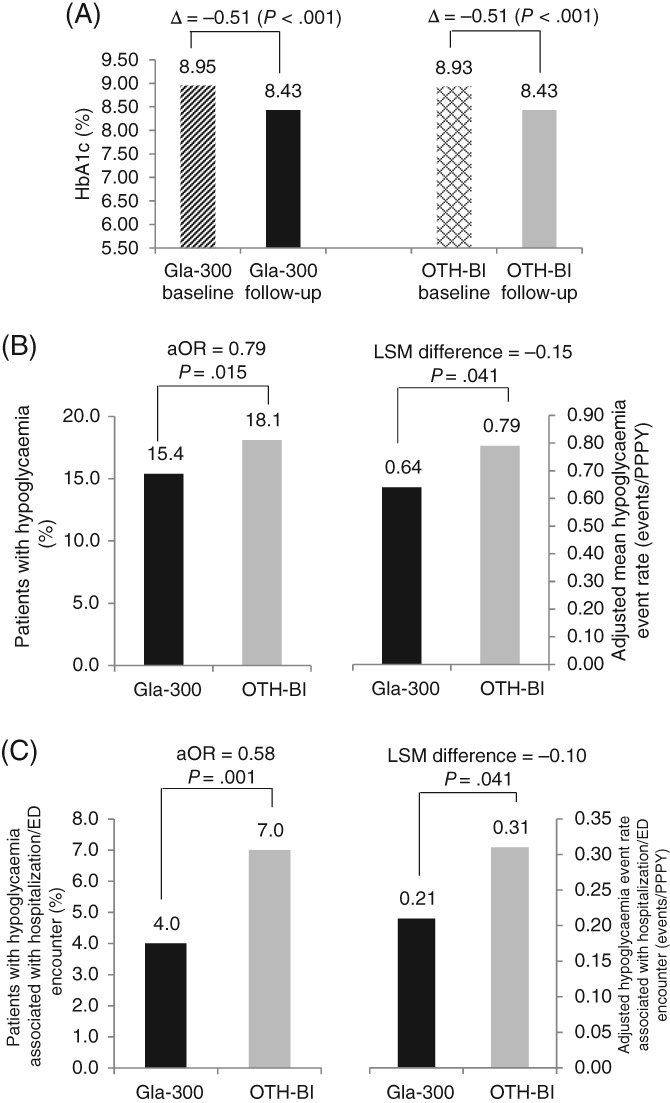
HbA1c change and hypoglycaemia during the 6‐month follow‐up period. A, Change in HbA1ca. B, Hypoglycaemia incidenceb and event rate (events/PPPY)c. C, Hypoglycaemia incidenceb and event rate (events/PPPY)c associated with hospitalization/ED encounter. aComparison of mean reduction in Gla‐300 vs other basal insulin (P = .928). baOR: Adjusted for baseline hypoglycaemia incidence. cAdjusted for baseline hypoglycaemia event rate. OTH‐BI, other basal insulin
During follow‐up, significantly fewer patients using Gla‐300 experienced hypoglycaemia compared to those using other BIs (incidence: 15.4% vs 18.1%, respectively; P = .015). Patients who switched to Gla‐300 were 21% less likely to have hypoglycaemia compared to other BIs adjusted for baseline hypoglycaemia incidence (aOR = 0.79; P = .015). Adjusting for baseline hypoglycaemia rate, switching to Gla‐300 was associated with a 19% lower hypoglycaemia event rate at 6 months' follow‐up (LSM difference: −0.15 events/PPPY; P = .041) (Figure 1B). A similar trend was observed for hypoglycaemia associated with hospitalization or ED visits, with patients switching to Gla‐300 having 32% fewer hypoglycaemia events related to hospitalization/ED during follow‐up (Figure 1C).
Patients switching to Gla‐300 had a lower risk of hypoglycaemia‐related hospitalization (33% less likely), ED services (38% less likely) and outpatient visits (23% less likely) at follow‐up compared to those switching to other BIs (aOR: hospitalization, 0.67 [P = .037]; ED services, 0.62 [P = .007]; outpatient visits, 0.77 [P = .011]) (Figure 2A). The outcomes of overall diabetes‐related and all‐cause HCRU incidences were favourable for Gla‐300, showing a significant reduction in the risk of requiring ED services (Appendix S5 in File S1).
Figure 2.
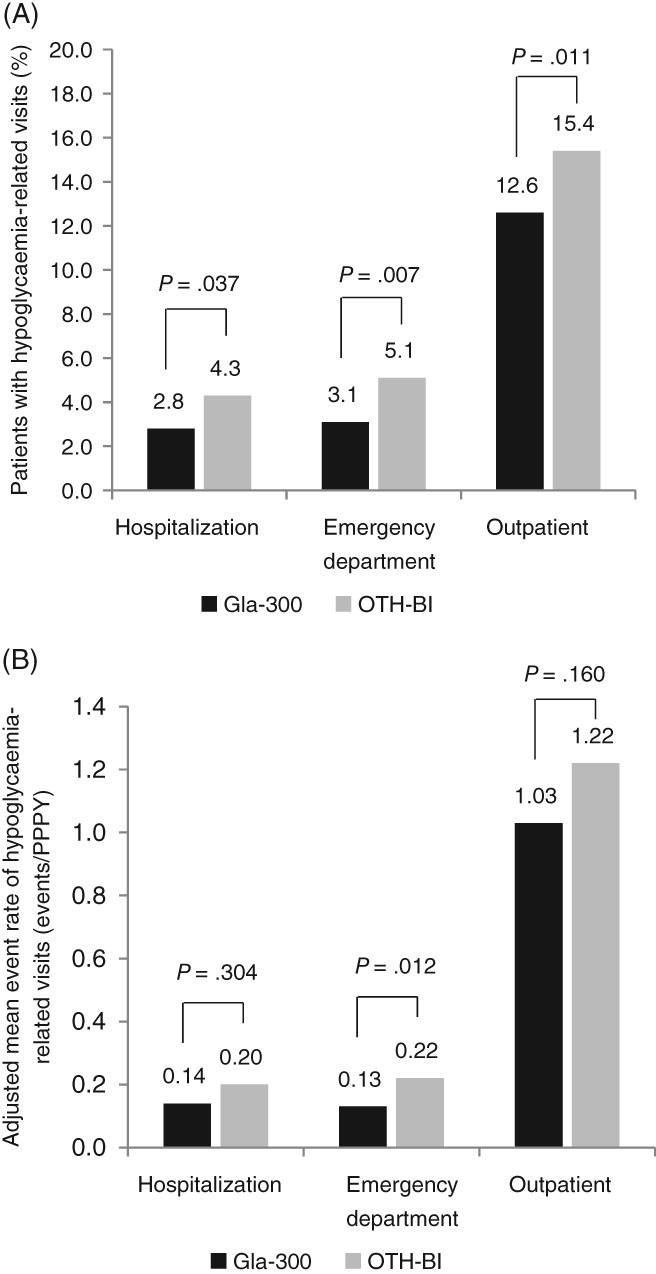
Hypoglycaemia‐related healthcare‐resource utilization during the 6‐month follow‐up period. A, Patients requiringa hypoglycaemia‐related healthcare‐resource utilization. B, Event rateb of hypoglycaemia‐related healthcare‐resource utilization. aaOR: adjusted for baseline healthcare‐resource utilization incidence. bAdjusted mean event rate: adjusted for baseline healthcare‐resource utilization event rate
There were significantly fewer adjusted hypoglycaemia‐related ED events (42% fewer) during follow‐up in patients treated with Gla‐300 (LSM difference: −0.09 events/PPPY; P = .012) (Figure 2B), which resulted in savings of US $161 PPPY. Considering all hypoglycaemia‐related HCRU including hospitalizations, ED and outpatient visits, switching to Gla‐300 resulted in overall savings of US $1439 PPPY. Patients switching to Gla‐300 also experienced significantly fewer diabetes‐related ED events (28% fewer) during follow‐up compared to patients switching to other BIs, which translated to savings of US $138 PPPY. Fewer all‐cause ED and outpatient events during follow‐up in patients treated with Gla‐300 resulted in savings of US $185 and US $69 PPPY for the 2 outcomes, respectively (Appendix S6 in File S1).
4. DISCUSSION
Real‐world studies provide information that is complementary to RCTs and may be more generalizable and pertinent to clinicians and healthcare‐delivery systems.11 In this real‐world study with well‐balanced matched cohorts, patients switching to Gla‐300 reached similar HbA1c control with a lower risk of hypoglycaemia events compared with those switching to other BIs. In addition, patients using Gla‐300 had a lower risk of requiring hypoglycaemia‐related, diabetes‐related and all‐cause ED services. This lower HCRU translated to lower costs in patients using Gla‐300.
This is the first real‐world study examining the comparative effects of switching to Gla‐300 from another BI in patients with T2D. The results showing comparable HbA1c reduction and reduced hypoglycaemia with Gla‐300 are consistent with results from the phase III EDITION RCT programme. Real‐world evidence concerning switching to Gla‐300 is very limited. A recent medical chart study showed that switching to Gla‐300 from another BI resulted in significantly lower HbA1c, hypoglycaemia events and frequency of dosing.12 However, that study compared only data before and after switching, without a comparator cohort. In our study, HbA1c was significantly improved in both cohorts, although the residual mean HbA1c level was still relatively poor (>8.0%), reflecting that treatment may not be sufficiently intensified in the real‐world clinical practice. The results of HbA1c control are similar to those of other studies, which can be explained by the fact that a suboptimal HbA1c level is one of the potential reasons for insulin switching. Nevertheless, our data provide real‐world evidence of effectiveness, that is, switching to Gla‐300 from other BIs (mainly Gla‐100) is associated with benefits in terms of reduced hypoglycaemia. Insulins with a lower risk of hypoglycaemia may lead to better adherence to and persistence with therapy, and potentially, to longer‐term glycaemic control.13, 14
This study demonstrated that switching to Gla‐300 is associated with reduction in HCRU, which could result in significant cost savings in patients with T2D. The different prescribing patterns based on insurance coverage in our study, which remained even after propensity score matching, suggest that up‐front medication costs may be driving some prescription decisions. The better health‐economic outcomes with Gla‐300 shown here may provide important information to healthcare professionals, patients and payers when considering the overall cost consequences of prescribing decisions.
These results should be interpreted in light of certain limitations resulting from the retrospective nature of the real‐world design. Patients from the PHIE EMR database were, for the most part, from the Midwest and Southern regions of the USA, which could limit extrapolating the results to other regional populations. Patients included in the study were early users of second‐generation basal insulins; therefore, the sample size was limited and the patients' demographics and clinical characteristics might differ from basal insulin‐experienced patients in general. The follow‐up period in this study is relatively short, although it is on a par with or longer than that of most RCTs. The study is likely to underreport overall hypoglycaemia, as only clinically significant events were captured and there were no self‐monitored blood‐glucose or continuous blood‐glucose monitoring data in the database. Thus, the treatment effect on hypoglycaemia could be underestimated. The reason for switching was not available from the EMRs, so selection bias may not be completely excluded after propensity score matching. The diabetes‐related HCRU was identified based on ICD‐9/ICD‐10 codes,8 but EMR data may not link the actual diagnosis name, which could result in misclassification. The unit cost of HCRU may differ based on integrated‐delivery‐network size, features, geographic location, etc. However, the current analysis and data should provide a useful perspective on potential cost savings related to the lower HCRU resulting from a reduction in hypoglycaemia events.
In conclusion, the current study provides the first comparative real‐world evidence concerning the benefits of switching to the new‐generation BI Gla‐300. From the perspective of healthcare practitioners and healthcare systems, the clinical benefits of lower hypoglycaemia associated with Gla‐300 translated to less HCRU. With the known increased economic burden of T2D care on the healthcare system, less HCRU and associated cost‐savings can be an important consideration for payers. Considering the limited sample size because of the early users in this study, further research with a larger sample size will be needed.
Supporting information
File S1. Supporting information files.
Appendix S1. Supplementary Methods.
Appendix S2. Patient selection in Gla‐300 and other basal‐insulin switcher cohorts.
Appendix S3. Patient baseline characteristics before and after propensity score matching.
Appendix S4. Patients reaching pre‐specified HbA1c targets during follow‐up.
Appendix S5. Healthcare‐resource utilization incidence during the 6‐month follow‐up. A, percent patients requiring all‐cause healthcare‐resource utilization, a and B, percent patients requiring diabetes‐related healthcare‐resource utilizationa.
Appendix S6. Healthcare resource utilization events and costs.
ACKNOWLEDGEMENTS
The authors received writing/editorial support in the preparation of this manuscript which was provided by Yunyu Huang, PhD, of Excerpta Medica, and was funded by Sanofi.
Early analyses from this study were presented in poster format at the 99th Annual Meeting of the Endocrine Society, Orlando, Florida, April 1–4, 2017 and at the 26th Annual Meeting & Clinical Congress of the American Association of Clinical Endocrinologists, Austin, Texas, May 3–7, 2017.
Conflict of interest
F. L. Z., P. B. and J. W. are employees of and stockholders in Sanofi. F. Y. is an employee of Sanofi. V. G. and R. G. are employees of Accenture, under contract with Sanofi. J. S. is an employee of Novartis Pharmaceuticals Corporation and was an employee of Sanofi at the time the study was conducted. T. B. provides research support for Abbott, Ambra, Ascensia, BD, Boehringer Ingelheim, Calibra, Companion Medical, Dexcom, Elcelyx, Glysens, Janssen, Lexicon, Lilly, Medtronic, Novo Nordisk, Sanofi, Senseonics, Versartis and Xeris; is a consultant for Astra Zeneca, Bayer, BD, Calibra, Lilly, Medtronic, Novo Nordisk and Sanofi; and is a speaker for Abbott, Insulet, Lilly, Medtronic, Novo Nordisk and Sanofi. L. B. has received grants from and provided research support to AstraZeneca, Janssen Pharmaceuticals, Inc., Lexicon Pharmaceuticals, Inc., Merck & Co., Novo Nordisk and Sanofi; is a speaker for AstraZeneca, Janssen Pharmaceuticals, Inc., Merck & Co., Novo Nordisk and Sanofi; and is a consultant for AstraZeneca, GlaxoSmithKline, Intarcia Therapeutics, Inc., Janssen Pharmaceuticals, Inc., Merck & Co., Inc., Novo Nordisk and Sanofi.
Author contributions
F. L. Z. contributed to designing the study, acquiring the data and writing the first draft of the manuscript. F. Y., J. S. and J. W. contributed to designing the study. P. B. contributed to designing the study and writing the first draft of the manuscript. V. G. and R. G. contributed to acquiring the data. All authors contributed to the data analysis and interpretation, and critically reviewed the manuscript.
Zhou FL, Ye F, Berhanu P, et al. Real‐world evidence concerning clinical and economic outcomes of switching to insulin glargine 300 units/mL vs other basal insulins in patients with type 2 diabetes using basal insulin. Diabetes Obes Metab. 2018;20:1293–1297. https://doi.org/10.1111/dom.13199
Funding information This study was funded by Sanofi.
REFERENCES
- 1. American Diabetes Association . Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(suppl 1):S1‐S135.27979885 [Google Scholar]
- 2. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm ‐ 2017 Executive Summary. Endocr Pract. 2017;23:207‐238. [DOI] [PubMed] [Google Scholar]
- 3. Maiorino MI, Petrizzo M, Capuano A, Giugliano D, Esposito K. The development of new basal insulins: is there any clinical advantage with their use in type 2 diabetes? Expert Opin Biol Ther. 2014;14:799‐808. [DOI] [PubMed] [Google Scholar]
- 4. Russell‐Jones D, Gall MA, Niemeyer M, Diamant M, Del Prato S. Insulin degludec results in lower rates of nocturnal hypoglycaemia and fasting plasma glucose vs. insulin glargine: a meta‐analysis of seven clinical trials. Nutr Metab Cardiovasc Dis. 2015;25:898‐905. [DOI] [PubMed] [Google Scholar]
- 5. Clements JN, Bello L. Insulin glargine 300 units/mL: a new basal insulin product for diabetes mellitus. Am J Health Syst Pharm. 2016;73:359‐366. [DOI] [PubMed] [Google Scholar]
- 6. White JR Jr. Advances in insulin therapy: a review of new insulin glargine 300 units/mL in the management of diabetes. Clin Diabetes. 2016;34:86‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanenbaum SJ. Comparative effectiveness research: evidence‐based medicine meets health care reform in the USA. J Eval Clin Pract. 2009;15:976‐984. [DOI] [PubMed] [Google Scholar]
- 8. American Association of Clinical Endocrinologists . Diabetes ICD9‐CM Crosswalk to ICD10‐CM Web site. 2015. https://www.aace.com/files/socioeconomics/crosswalk/icd9-icd10-crosswalk-for-dm-2015.pdf. Accessed February 12, 2017.
- 9. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41‐55. [Google Scholar]
- 10. Bureau of Labor Statistics . Consumer Price Index. https://www.bls.gov/cpi. Accessed August 2017.
- 11. Sherman RE, Anderson SA, Dal Pan GJ, et al. Real‐world evidence – what is it and what can it tell us? N Engl J Med. 2016;375:2293‐2297. [DOI] [PubMed] [Google Scholar]
- 12. Tong L, Wang H, Gupta S, et al. Effect of switching to insulin glargine 300 u/ml on clinical outcomes in patients with type 2 diabetes mellitus: a medical chart review study in the US. J Manag Care Spec Pharm. 2016;22(suppl 10):S35‐S36. [Google Scholar]
- 13. Ross SA, Tildesley HD, Ashkenas J. Barriers to effective insulin treatment: the persistence of poor glycemic control in type 2 diabetes. Curr Med Res Opin. 2011;27(suppl 3):13‐20. [DOI] [PubMed] [Google Scholar]
- 14. García‐Pérez LE, Alvarez M, Dilla T, Gil‐Guillén V, Orozco‐Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4:175‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Supporting information files.
Appendix S1. Supplementary Methods.
Appendix S2. Patient selection in Gla‐300 and other basal‐insulin switcher cohorts.
Appendix S3. Patient baseline characteristics before and after propensity score matching.
Appendix S4. Patients reaching pre‐specified HbA1c targets during follow‐up.
Appendix S5. Healthcare‐resource utilization incidence during the 6‐month follow‐up. A, percent patients requiring all‐cause healthcare‐resource utilization, a and B, percent patients requiring diabetes‐related healthcare‐resource utilizationa.
Appendix S6. Healthcare resource utilization events and costs.


