Abstract
Crizotinib, an inhibitor of anaplastic lymphoma kinase (ALK), MET, and ROS1, is approved for treatment of patients with ALK‐positive or ROS1‐positive advanced non‐small‐cell lung cancer (NSCLC). However, ALK rearrangements are also implicated in other malignancies, including anaplastic large‐cell lymphoma and inflammatory myofibroblastic tumors (IMTs). In this ongoing, multicenter, single‐arm, open‐label phase 1b study (PROFILE 1013; NCT01121588), patients with ALK‐positive advanced malignancies other than NSCLC were to receive a starting dose of crizotinib 250 mg twice daily. Primary endpoints were safety and objective responses based on Response Evaluation Criteria in Solid Tumors version 1.1 or National Cancer Institute International Response Criteria. Forty‐four patients were enrolled (lymphoma, n = 18; IMT, n = 9; other tumors, n = 17). The objective response rate was 53% (95% confidence interval [CI], 28–77) for lymphoma, with 8 complete responses (CRs) and 1 partial response (PR); 67% (95% CI, 30–93) for IMTs, with 1 CR and 5 PRs; and 12% (95% CI, 2–36) for other tumors, with 2 PRs in patients affected by colon carcinoma and medullary thyroid cancer, respectively. The median duration of treatment was almost 3 years for patients with lymphoma and IMTs, with 2‐year progression‐free survival of 63% and 67%, respectively. The most common treatment‐related adverse events were diarrhea (45.5%) and vision disorders (45.5%), mostly grade 1. These findings indicate strong and durable activity of crizotinib in ALK‐positive lymphomas and IMTs. The safety profile was consistent with the known safety profile of crizotinib even with long‐term treatment.
1. INTRODUCTION
Activating mutations or translocations of the anaplastic lymphoma kinase (ALK) oncogene have been recurrently identified in several cancer types, including non‐small‐cell lung cancer (NSCLC), anaplastic large‐cell lymphoma (ALCL), inflammatory myofibroblastic tumors (IMTs), and neuroblastoma.1, 2, 3 Activation of the ALK gene usually occurs through chromosomal rearrangement, resulting in the placement of one of several different 5ʹ fusion partners and their associated promoter regions upstream of the ALK kinase domain. ALK point mutations and possibly overexpression of ALK can also lead to activation of ALK signaling in human tumors.4, 5, 6
ALCL is a rare form of non‐Hodgkin lymphoma (NHL), usually consisting of large neoplastic cells with abundant cytoplasm and pleomorphic nuclei, with a translocation involving the ALK gene, and expression of ALK protein and CD30. It accounts for ∼3% of adult and 10%–15% of pediatric NHL cases.7, 8, 9 ALCL may be effectively treated using intensive chemotherapy regimens (e.g. cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP])10, 11 but at the cost of early and long‐term toxicities.8, 12 In addition, if relapse occurs after first‐line treatment, ALCL has a poor prognosis and tends to follow a very aggressive clinical course.13
IMTs are a rare type of mesenchymal neoplasm composed of a variable mixture of myofibroblasts and inflammatory infiltrates that can occur throughout the body, predominantly in the mesentery, retroperitoneum, and pelvis. ALK rearrangements have been implicated in giving rise to oncogenic fusion proteins in ≥50% of IMTs.14, 15, 16, 17, 18 In addition, activating ALK mutations are found in about 7%–9% of cases of neuroblastoma, a childhood cancer of the developing nervous system.19, 20
Single‐agent clinical protocols facilitate rapid enrollment of molecularly profiled patients with genetic alterations linked to targeted therapies, and early signs of clinical activity in such trials provide opportunities for detection of new indications using a patient‐sparing design that could lead to subsequent confirmatory trials.21
Crizotinib is a potent, orally administered, small‐molecule tyrosine kinase inhibitor of ALK, MET, and ROS1 receptor tyrosine kinases.22, 23 Currently, crizotinib has received marketing authorization approvals globally for the treatment of ALK‐positive advanced NSCLC in over 90 countries. In 2016, crizotinib was also approved in the United States and European Union for the treatment of patients with advanced NSCLC whose tumors are ROS1‐positive.24
Here, we report the results of the PROFILE 1013 study that evaluated the safety and antitumor activity of single‐agent, oral crizotinib among patients with advanced ALCL, IMT, or other advanced ALK‐positive malignancies different from NSCLC.
2. METHODS
2.1. Patients
Patients aged ≥15 years with Eastern Cooperative Oncology Group performance status of 0–3 and histologically or cytologically proven diagnosis of ALCL, IMT, or other advanced malignancy (excluding NSCLC) for which no standard therapy is available were eligible if they were positive during local ALK testing with validated methods at study sites for either:
ALK gene translocation or inversion events (e.g., nucleophosmin [NPM]‐ALK fusion) as determined by fluorescence in situ hybridization (FISH), reverse transcriptase (RT)–polymerase chain reaction (PCR) or sequencing, or immunohistochemistry (in the case of ALCL)
ALK‐activating point mutations determined by direct sequencing of the ALK gene locus
ALK amplification events (defined as ALK/CEP2 ratio of ≥5 in ≥15% of evaluated cells by FISH or as >7 copies by quantitative PCR or array comparative genomic hybridization).
Local ALK testing results were reviewed by the Sponsor to confirm eligibility. Solid tumors had to be measurable per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and lymphomas had to be measurable per the National Cancer Institute (NCI) International Response Criteria for NHL. Patients with mutations or amplifications involving c‐MET, who were receiving concurrent treatment on another clinical trial, or who had received prior therapy specifically directed against ALK, were excluded.
2.2. Trial design and treatment
This ongoing, multicenter, open‐label, single‐arm exploratory phase 1b study (PROFILE 1013; http://ClinicalTrials.gov identifier: NCT01121588)25 enrolled patients with advanced malignancies (NHL, IMT, and solid tumors) harboring a translocation, inversion, mutation, or amplification event involving the ALK gene locus. Crizotinib, at a starting dose of 250 mg twice daily, was to be orally administered on a continuous dosing schedule in 21‐day cycles to facilitate scheduling of visits and assessments. Patients were permitted to continue treatment with crizotinib if there was evidence of clinical benefit in the opinion of the investigators.
2.3. Trial assessments
Patients were to be screened before receiving crizotinib. Subsequent visits were to be scheduled for days 1 and 15 of cycle 1 and then day 1 of all following treatment cycles, with a final visit at the end of treatment. After a protocol amendment (August 2015), a reduced visit frequency schedule (visits on day 1 of every 6 cycles) was to be followed for ongoing patients. Assessments of adverse events (AEs), laboratory safety (hematology, chemistry, and coagulation [cycle 1 only]), physical examinations, and electrocardiograms (cycles 1 and 2 only) were to be performed at each visit. Safety assessments included collection of AEs, serious AEs (SAEs), vital signs, physical examinations, 12‐lead electrocardiograms, laboratory assessments (including pregnancy tests), ophthalmologic examinations, and documentation of concomitant treatments. On‐study tumor assessments were to be performed at the beginning of cycle 3 and every other cycle up to and including cycle 10. After cycle 10, tumor assessments were to be performed every 12 weeks, reduced to every 24 weeks from cycle 26 and to every 52 weeks from cycle 52. Tumor assessments were reviewed by the investigators at study sites.
2.4. Endpoints
The primary endpoints were objective response rate (ORR) based on RECIST v1.1 or the NCI International Response Criteria, the type, incidence, severity, seriousness, and relationship to study medications of AEs, and laboratory abnormalities. Secondary endpoints included progression‐free survival (PFS), overall survival (OS) at 6 months and 1 and 2 years, OS, and duration of response (DOR).
2.5. Trial oversight
PROFILE 1013 was designed by Pfizer and conducted in collaboration with the principal investigators (see Supporting Information). All patients provided written informed consent before enrollment. The institutional review board or independent ethics committee at each participating center approved the protocol and amendments, which complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws.
2.6. Statistical analysis
The sample size for this study was determined empirically based on expected small numbers of patients in the population of interest, and it was anticipated that approximately 40 patients would be enrolled. The study population for all analyses (except tumor response‐related endpoints) included patients enrolled in the study who received at least one dose of crizotinib. For response‐related endpoints, enrolled patients who received at least one dose of crizotinib also had to have an adequate baseline tumor assessment.
Descriptive statistics were used to summarize patient characteristics, treatment administration, antitumor activity, and safety. The ORR was summarized with the corresponding exact 2‐sided 95% confidence interval (CI) calculated using a method based on the F distribution. Secondary time‐to‐event endpoints were summarized using Kaplan‐Meier methods. The data cutoff date was March 4, 2016. Data were summarized for the overall patient population, as well as separately for antitumor activity in the ALCL, IMT, and “other tumors” patient groups.
3. RESULTS
3.1. Patients
From March 2011 through October 2013, a total of 44 patients were enrolled in the study (lymphoma, n = 18; IMT, n = 9; and other tumors, n = 17). Demographic characteristics are shown in Table 1, and patients' diagnoses in the other tumors group are shown in Supporting Information Table S1. All enrolled patients were either white or Asian in nearly equal proportions (Japanese, Korean, or Chinese).
Table 1.
Patient baseline characteristics and demographics
| Lymphomas (n = 18) | IMT (n = 9) | Other tumors (n = 17) | Total (N = 44) | |
|---|---|---|---|---|
| Sex | ||||
| Male | 12 (66.7) | 5 (55.6) | 8 (47.1) | 25 (56.8) |
| Age, years | ||||
| Median | 25.0 | 32.0 | 53.0 | 32.0 |
| Range | 15–37 | 16–73 | 26–73 | 15–73 |
| ECOG performance status | ||||
| 0 | 4 (22.2) | 4 (44.4) | 5 (29.4) | 13 (29.5) |
| 1 | 7 (38.9) | 4 (44.4) | 9 (52.9) | 20 (45.5) |
| 2 | 5 (27.8) | 1 (11.1) | 2 (11.8) | 8 (18.2) |
| 3 | 2 (11.1) | 0 (0.0) | 1 (5.9) | 3 (6.8) |
| Race | ||||
| White | 12 (66.7) | 4 (44.4) | 7 (41.2) | 23 (52.3) |
| Asian | 6 (33.3) | 5 (55.6) | 10 (58.8) | 21 (47.7) |
| Japanese | 2 (11.1) | 1 (11.1) | 2 (11.8) | 5 (11.4) |
| Korean | 2 (11.1) | 1 (11.1) | 4 (23.5) | 7 (15.9) |
| Chinese | 2 (11.1) | 3 (33.3) | 4 (23.5) | 9 (20.5) |
| Smoking classification | ||||
| Never | 10 (55.6) | 6 (66.7) | 14 (82.4) | 30 (68.2) |
| Former | 5 (27.8) | 3 (33.3) | 2 (11.8) | 10 (22.7) |
| Current | 3 (16.7) | 0 (0.0) | 1 (5.9) | 4 (9.1) |
| Prior systemic therapy | ||||
| No | 0 (0.0) | 6 (66.7) | 1 (5.9) | 7 (15.9) |
| Yes | 18 (100) | 3 (33.3) | 16 (94.1) | 37 (84.1) |
| No. of regimens | ||||
| 1 | 2 (11.1) | 2 (22.2) | 3 (17.6) | 7 (15.9) |
| 2 | 6 (33.3) | 1 (11.1) | 4 (23.5) | 11 (25.0) |
| 3 | 5 (27.8) | 0 (0.0) | 3 (17.6) | 8 (18.2) |
| 4 | 3 (16.7) | 0 (0.0) | 3 (17.6) | 6 (13.6) |
| 5 | 1 (5.6) | 0 (0.0) | 0 (0.0) | 1 (2.3) |
| 6 | 1 (5.6) | 0 (0.0) | 2 (11.8) | 3 (6.8) |
| 7 | 0 (0.0) | 0 (0.0) | 1 (5.9) | 1 (2.3) |
Data are presented as n (%) unless otherwise noted.
ECOG, Eastern Cooperative Oncology Group; IMT, inflammatory myofibroblastic tumor.
3.2. Antitumor activity
3.2.1. Lymphoma group
In the lymphoma group, 17 of 18 patients were evaluable for response (Table 2), as 1 patient was retrospectively found not to have measurable disease. The patient was followed for antitumor activity based on positive RT‐PCR status for NPM‐ALK, which predicts a poor prognosis,26 and became RT‐PCR negative within 4 months of treatment; this patient was still on treatment and relapse‐free at the data cutoff (at cycle 74), after more than 4 years on crizotinib. Patients in the lymphoma group had an ORR of 52.9% (95% CI, 27.8–77.0). This comprised 8 patients (47.1%) who experienced a complete response (CR) and 1 patient (5.9%) with a partial response (PR). Median DOR was 2.6 years with all but 1 patient still in response or ongoing at the time of data cutoff (Supporting Information Figure S1). In addition, 3 patients (17.6%) had stable disease (SD), including one lasting over 3.5 years and ongoing at data cutoff in a patient with diffuse large B‐cell lymphoma (DLBCL). Median PFS had not been reached (Figure 1) because of the small number of events (33%) and the high percentage of patients with lymphoma still in follow‐up for progression (44.4%). PFS at 2 years was 63.0% (95% CI, 35.3–81.4). Of note, all responding patients who progressed/relapsed did so within the initial 90 days of treatment and no further progressions/relapses were noted thereafter. Median OS was also not reached as OS data were immature, with 72.2% of patients with lymphoma still alive at the cutoff (Figure 2). One patient underwent a cord stem cell transplant approximately 4 months after starting crizotinib treatment and was subsequently censored for evaluation of tumor response‐related endpoints. The patient resumed crizotinib after the transplant and was still ongoing and disease‐free at the data cutoff, after more than 4 years on crizotinib.
Table 2.
Patient response to crizotinib treatment
| Lymphoma (n = 17) | IMT (n = 9) | Other (n = 17) | |
|---|---|---|---|
| Best overall response, n (%) | |||
| Complete response | 8 (47.1) | 1 (11.1) | 0 (0.0) |
| Partial response | 1 (5.9) | 5 (55.6) | 2 (11.8) |
| Stable disease | 3 (17.6) | 3 (33.3) | 4 (23.5) |
| Objective progression | 3 (17.6) | 0 (0.0) | 7 (41.2) |
| Early death | 1 (5.9) | 0 (0.0) | 3 (17.6) |
| Indeterminate | 1 (5.9) | 0 (0.0) | 1 (5.9) |
| Objective response rate, % | 52.9 | 66.7 | 11.8 |
| 95% CI | 27.8–77.0 | 29.9–92.5 | 1.5–36.4 |
| Duration of response, weeks | |||
| Median | 135.9 | 74.1 | 59.7 |
| Range | 6.0–205.9 | 29.6–138.3 | 16.1–103.3 |
| Stable disease duration, n (%)a | |||
| <3 months | 2 (66.7) | 0 (0.0) | 0 (0.0) |
| 3–<6 months | 0 (0.0) | 1 (33.3) | 2 (50.0) |
| 6–<9 months | 0 (0.0) | 0 (0.0) | 1 (25.0) |
| 9–<12 months | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ≥12 months | 1 (33.3) | 2 (66.7) | 1 (25.0) |
Percentages based on the number of patients with stable disease.
CI, confidence interval; IMT, inflammatory myofibroblastic tumor.
Figure 1.
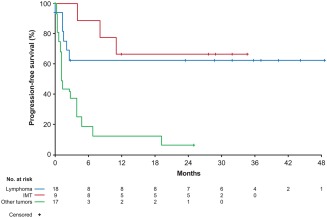
Progression‐free survival. IMT, inflammatory myofibroblastic tumor
Figure 2.
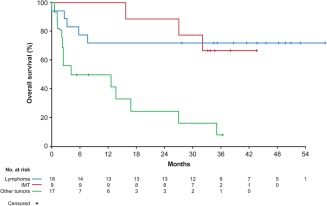
Overall survival. IMT, inflammatory myofibroblastic tumor
3.2.2. IMT group
All patients with IMT were evaluable for response (Table 2). Patients in the IMT group had an ORR of 66.7% (95% CI, 29.9–92.5), which comprised 1 CR and 5 PRs. Three patients are still in response, with responses lasting over 2 years, and these patients remain in follow‐up (Supporting Information Figure S1). In addition, 3 patients (33.3%) had SD. Median PFS was not reached in the IMT group (Figure 1) because of the small number of events (33%) and 55.6% of patients with IMT who remain in follow‐up for progression. PFS at 2 years was 66.7% (95% CI, 28.2–87.8). In addition, median OS was not reached as 66.7% of patients with IMT were alive at the data cutoff (Figure 2).
3.2.3. “Other tumors” group
All patients in the “other tumors” group were evaluable for response (Table 2), and the ORR was 11.8% (95% CI, 1.5–36.4). This comprised 2 of 17 patients (11.8%) ALK‐positive for rearrangement on FISH who achieved a PR (one with mucinous carcinoma of the colon [response duration, 103.3 weeks] and one with medullary thyroid cancer [response duration, 16.1 weeks]). In addition, 4 patients (23.5%) had SD, of which 1 patient with advanced neuroblastoma harboring ALK F1174L mutation had SD lasting 19 months. Among patients with other tumors, median PFS was 1.3 months (95% CI, 1.1–3.9) (Figure 1), PFS at 2 years was 6.3% (95% CI, 0.4–24.7), and median OS was 8.3 months (95% CI, 2.2–16.9) (Figure 2).
3.3. Safety
At the time of data cutoff, the duration of crizotinib treatment ranged from 0.1 to 249.3 weeks (57 months) across all patients, with median treatment durations of 35 months, 33 months, and 1 month for the lymphoma, IMT, and “other tumors groups”, respectively; a total of 16 of 44 patients (36.4%) were still receiving crizotinib treatment. At least one AE of any cause was reported in 43 of 44 patients (97.7%) (Supporting Information Table S2), and treatment‐related AEs (TRAEs) occurred in a total of 39 patients (88.6%) (Table 3). Mild‐to‐moderate diarrhea and vision disorder were the most common TRAEs reported (both 45.5%), most of which were grade 1.
Table 3.
Treatment‐related AEs and SAEs in patients treated with crizotinib (N = 44)
| Maximum CTCAE | ||||
|---|---|---|---|---|
| Event, n (%) | Any grade | Grade 3 | Grade 4 | Grade 5 |
| Any AEa | 39 (88.6) | 16 (36.4) | 6 (13.6) | 2 (4.5) |
| AEs in ≥10% of patients | ||||
| Diarrhea | 20 (45.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vision disorderb | 20 (45.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nausea | 17 (38.6) | 2 (4.5) | 0 (0.0) | 0 (0.0) |
| Elevated transaminasesb | 15 (34.1) | 3 (6.8) | 0 (0.0) | 0 (0.0) |
| Vomiting | 15 (34.1) | 3 (6.8) | 0 (0.0) | 0 (0.0) |
| Neutropeniab | 14 (31.8) | 8 (18.2) | 2 (4.5) | 0 (0.0) |
| Edemab | 11 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Abdominal painb | 10 (22.7) | 1 (2.3) | 0 (0.0) | 0 (0.0) |
| Leukopeniab | 9 (20.5) | 1 (2.3) | 0 (0.0) | 0 (0.0) |
| Fatigue | 7 (15.9) | 2 (4.5) | 0 (0.0) | 0 (0.0) |
| Headache | 7 (15.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Constipation | 6 (13.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| CPK increased | 5 (11.4) | 1 (2.3) | 3 (6.8) | 0 (0.0) |
| Maximum CTCAE | ||||
|---|---|---|---|---|
| Any grade | Grade 3 | Grade 4 | Grade 5 | |
| Any SAEa | 9 (20.5) | 1 (2.3) | 4 (9.1) | 2 (4.5) |
| CPK increased | 3 (6.8) | 0 (0.0) | 3 (6.8) | 0 (0.0) |
| Cardiac failureb | 1 (2.3) | 0 (0.0) | 1 (2.3)c | 0 (0.0) |
| Cerebral infarction | 1 (2.3) | 0 (0.0) | 0 (0.0) | 1 (2.3) |
| Deep vein thrombosis | 1 (2.3) | 1 (2.3)c | 0 (0.0) | 0 (0.0) |
| Interstitial lung diseaseb | 1 (2.3) | 0 (0.0) | 0 (0.0) | 1 (2.3) |
| Myocardial ischemia | 1 (2.3) | 1 (2.3)c | 0 (0.0) | 0 (0.0) |
Patients are counted once under the highest CTCAE grade.
This item comprised a cluster of AEs that may represent similar clinical symptoms or syndromes, listed in Supporting Information Table S3.
Deep vein thrombosis (grade 3; n = 1) was concurrent with a course of corticosteroids; myocardial ischemia (grade 3) and cardiac failure (grade 4) occurred in the same patient, who had preexisting hypertension and coronary artery disease. These grade ≥3 SAEs were considered possibly treatment‐related by the investigator; however, their relationship to concomitant medications, coexisting medical conditions, or tumor progression could not be clearly established.
AE, adverse event; CPK, creatine phosphokinase; CTCAE, Common Terminology Criteria for Adverse Events; SAE, serious adverse event.
Overall, 9 patients (20.5%) experienced an SAE considered to be related to crizotinib treatment (Table 3). The most common SAE considered possibly related to treatment was grade 4 blood creatine phosphokinase increase reported in 3 patients (6.8%), all in clinical response and concurrent with strenuous physical activity that is known to cause brisk elevations of creatine phosphokinase levels.27
Grade 5 AEs considered by the investigator as related to treatment occurred in 2 patients: cerebral infarction in a patient with unknown primary tumor and multiple brain metastases, concurrent with disease progression, and interstitial lung disease in a patient with nasopharyngeal carcinoma. Subsequent review by an independent radiology committee, consisting of a pulmonologist, radiologist, and oncologist, indicated that this event was aspiration pneumonia and not related to the study drug.
Permanent discontinuation of crizotinib associated with TRAEs occurred in the 2 patients with cerebral infarction (2.3%) and interstitial lung disease (2.3%). Disease progression led to permanent discontinuation from treatment in 50% of patients.
Overall, 21 patients died during the study. Seventeen patients died because of disease progression, 1 patient died from interstitial lung disease/aspiration pneumonia, and 1 patient died because of cerebral infarction (the latter 2 as previously described). During long‐term follow‐up, 1 patient died because of small bowel obstruction and hernia complication (13 months after treatment discontinuation) and 1 patient's cause of death (10 months after treatment discontinuation) remained unknown.
4. DISCUSSION
Crizotinib has been shown to be superior to standard chemotherapy in previously treated and untreated patients with ALK‐positive advanced NSCLC28, 29 and has previously been shown to have therapeutic activity in relapsed and resistant ALK‐positive lymphomas.30, 31, 32, 33, 34 These findings, together with previous studies, encourage the use of crizotinib as a potential treatment option in ALK‐positive lymphoma, although some lack long‐term follow‐up data.
Patients with relapsed ALK‐positive lymphoma generally have a poor prognosis and, presently, there is no consensus on the optimal chemotherapy combination for relapsed ALCL.7, 8 Brentuximab vedotin induced objective responses in a majority (86%) of 58 patients (57% CR, 29% PR) with recurrent systemic ALCL (median DOR and PFS of 12.6 and 13.3 months, respectively)35 and has been approved for the treatment of systemic ALCL after failure of ≥1 prior multiagent chemotherapy regimen. However, brentuximab vedotin is unlikely to represent a long‐term treatment for advanced ALCL because of its side effects (incremental neurotoxicity), parenteral route of administration, and general use as a bridge therapy.
Our study cohorts show notable and durable antitumor activity of crizotinib as monotherapy for patients with advanced, relapsed or refractory ALK‐positive ALCL and ALK‐positive IMT, together with a favorable safety profile that was consistent over long‐term treatment. This study suggests that crizotinib may also offer a potential long‐term treatment option, with an OS at 2 years similar to that reported with first‐line CHOP chemotherapy in patients with ALK‐positive ALCL36 and a PFS at 2 years that exceeds 60% in ALK‐positive ALCL. Abrupt relapses of ALK‐positive lymphoma following crizotinib discontinuation have been reported, and it has been suggested that caution must be exercised when interrupting treatment in patients with ALK‐positive lymphomas, since ALCL may recur rapidly.32
One patient in this study had ALK‐positive DLBCL and experienced SD lasting almost 4 years, a clinically meaningful response for this rare form of lymphoma that exhibits a more aggressive clinical course and worse prognosis than typical DLBCL.37
Sustained and durable responses to crizotinib in lymphoma were observed in another study performed in 26 pediatric patients with relapsed or refractory ALK‐positive ALCL, which represent an update of a previous publication.33 Although a high response rate of 90% was observed in the Children's Oncology Group study, one‐half of patients ultimately received stem cell transplantation,34 thus rendering the evaluation of the long‐term effects of crizotinib in this population problematic. In PROFILE 1013, this was not a confounding factor as only 1 responder underwent bone marrow transplantation and was censored at that time.
Previous reports have shown that crizotinib has antitumor activity in patients with unresectable ALK‐positive IMTs34 as well as in those with refractory ALK‐positive IMT.33, 38 A case report of a patient with ALK‐positive IMT documented complete radiographic remission 2 years after initiating crizotinib treatment.39 The results of our study indicate a marked antitumor activity, with nearly two‐thirds of objective responses lasting >2 years and a PFS at 2 years of 67%. The durable responses observed in this small set of patients further support the National Comprehensive Cancer Network's recommendation of crizotinib as a treatment for IMTs with an ALK rearrangement40 and suggest that ALK testing should be routinely performed in all patients with IMT.
In contrast, a low level of response was observed in the “other tumors” group, which included a variety of ALK‐positive tumors. Potential reasons for lack of clinical activity include differences in ALK signaling events in different tumor types (i.e., “early” vs “late” events during tumor progression), differing tumor types, and genetics of the tumor cell, which may affect the response to ALK tyrosine kinase inhibitors.41 In addition, point mutations permitted in this study, for example F1174L and F1245C (common ALK‐activating mutations seen in neuroblastoma), have been shown to confer resistance to crizotinib in neuroblastoma and other cancers.33, 42, 43 MYCN overexpression has also been associated with resistance to ALK inhibitors.44 However, in this subgroup, 1 patient with mucinous carcinoma of the colon achieved a long‐lasting response of almost 2 years, 1 patient with medullary thyroid carcinoma also responded to crizotinib with a response duration of 4 months, and 1 patient with neuroblastoma had SD for 19 months, suggesting that ALK alterations may act as an oncogenic driver and can potentially be targeted in other malignancies.
Previously, safety data were reported from studies of crizotinib with median treatment durations of only 10.9 months,28 which is substantially less than the median treatment duration in the ALCL and IMT groups in this study (35 and 33 months, respectively). The treatment durations (up to 57 months) in this study are currently the longest reported in the literature in adult patients and therefore provide new information about the long‐term safety of crizotinib.
Crizotinib was generally well tolerated; the safety profile observed was similar to that reported previously with crizotinib, and no new safety signals were identified.28, 29, 45 Limitations of this study include its single‐arm, open‐label design, small patient population, and the heterogeneity of the patients' tumors.
In conclusion, this study indicated strong and durable activity of crizotinib in ALK‐positive lymphomas and IMTs. Treatment duration was much longer than usual treatment in patients with NSCLC, and the safety profile was consistent with the known safety profile of crizotinib, even with long‐term treatment.
CONFLICT OF INTERESTS
CG‐P received a research grant from Pfizer. FB received honorarium for advisory and speaking from Pfizer. TE received honoraria from Chugai, Eisai, Eli Lilly, Merck Serono, Nihon Kayaku, Ono and Taiho and research funding from AstraZeneca, Boehringer Ingelheim, Daiichi‐Sankyo, DS Pharma, Eli Lilly, Merck, Merck Serono, Novartis, Ono and Taiho. J‐SA received honoraria from Boehringer Ingelheim, Bristol‐Myers Squibb, Eisai, Janssen, Menarini and Roche. JTB received a research grant from Pfizer. MT received honoraria and served as a consultant or advisor for Blue Print Medicines, Bristol‐Myers Squibb, Eisai and Trilium Pharma and as a speaker for Bristol‐Myers Squibb and Eisai and received travel, accommodations or expenses from Blue Print Medicines, Bristol‐Myers Squibb and Eisai. BAVT received a research grant from Pfizer. JP is a Pfizer employee and owns stock in Pfizer. PS is a Pfizer employee and owns stock in 3M, Colgate Palmolive, EnteroMedics, GE, Nxstage Medical and Zoetis. SO, LZ, HH, KH, WJE, YS, KT, S‐JW and TMK declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Carlo Gambacorti‐Passerini, Sergey Orlov, Li Zhang, Fadi Braiteh, Huiqiang Huang, Taito Esaki, Keizo Horibe, Jin‐Seok Ahn, Joseph T. Beck, William Jeffrey Edenfield, Yuankai Shi, Matthew Taylor, Kenji Tamura, Brian A. Van Tine, Shang‐Ju Wu, Jolanda Paolini, Tae Min Kim
Collection and assembly of data: Carlo Gambacorti‐Passerini, Sergey Orlov, Li Zhang, Fadi Braiteh, Huiqiang Huang, Taito Esaki, Keizo Horibe, Jin‐Seok Ahn, Joseph T. Beck, William Jeffrey Edenfield, Yuankai Shi, Matthew Taylor, Kenji Tamura, Brian A. Van Tine, Shang‐Ju Wu, Tae Min Kim.
Data analysis and interpretation: All authors.
Manuscript writing: All authors.
Final approval of manuscript: All authors.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
We would like to thank all of the participating patients and their families, as well as the investigators, research nurses, study coordinators, and operations staff. This study was sponsored by Pfizer Inc. Medical writing support was provided by Jade Drummond and Simon Lancaster of inScience Communications, Springer Healthcare (Chester, UK), and funded by Pfizer Inc.
PRIOR PRESENTATION
Presented at the 59th American Society of Hematology (ASH) Annual Meeting & Exposition, December 9–12, 2017, Atlanta, GA.
Gambacorti‐Passerini C, Orlov S, Zhang L, et al. Long‐term effects of crizotinib in ALK‐positive tumors (excluding NSCLC): A phase 1b open‐label study. Am J Hematol. 2018;93:607–614. https://doi.org/10.1002/ajh.25043 xs
Funding information Pfizer Inc
REFERENCES
- 1. Mano H. Non‐solid oncogenes in solid tumors: EML4‐ALK fusion genes in lung cancer. Cancer Sci. 2008;99(12):2349–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009;420(3):345–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pulford K, Morris SW, Turturro F. Anaplastic lymphoma kinase proteins in growth control and cancer. J Cell Physiol. 2004;199(3):330–358. [DOI] [PubMed] [Google Scholar]
- 4. George RE, Sanda T, Hanna M, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455(7215):975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013;13(10):685–700. [DOI] [PubMed] [Google Scholar]
- 6. Murugan AK, Xing M. Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK gene. Cancer Res. 2011;71(13):4403–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferreri AJ, Govi S, Pileri SA, Savage KJ. Anaplastic large cell lymphoma, ALK‐positive. Crit Rev Oncol Hematol. 2012;83(2):293–302. [DOI] [PubMed] [Google Scholar]
- 8. Turner SD, Lamant L, Kenner L, Brugières L. Anaplastic large cell lymphoma in paediatric and young adult patients. Br J Haematol. 2016;173(4):560–572. [DOI] [PubMed] [Google Scholar]
- 9. National Cancer Institute . Surveillance, Epidemiology, and End Results Program; 2017.
- 10. d'Amore F, Gaulard P, Trumper L, et al. Peripheral T‐cell lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2015;26(suppl 5):v108–v115. [DOI] [PubMed] [Google Scholar]
- 11. Schmitz N, Trumper L, Ziepert M, et al. Treatment and prognosis of mature T‐cell and NK‐cell lymphoma: an analysis of patients with T‐cell lymphoma treated in studies of the German High‐Grade Non‐Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–3425. [DOI] [PubMed] [Google Scholar]
- 12. Lowe EJ, Sposto R, Perkins SL, et al. Intensive chemotherapy for systemic anaplastic large cell lymphoma in children and adolescents: final results of Children's Cancer Group Study 5941. Pediatr Blood Cancer. 2009;52(3):335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T‐cell lymphoma after first relapse or progression: spectrum of disease and rare long‐term survivors. J Clin Oncol. 2013;31(16):1970–1976. [DOI] [PubMed] [Google Scholar]
- 14. Antonescu CR, Suurmeijer AJ, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol. 2015;39(7):957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cessna MH, Zhou H, Sanger WG, et al. Expression of ALK1 and p80 in inflammatory myofibroblastic tumor and its mesenchymal mimics: a study of 135 cases. Mod Pathol. 2002;15(9):931–938. [DOI] [PubMed] [Google Scholar]
- 16. Coffin CM, Patel A, Perkins S, et al. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14(6):569–576. [DOI] [PubMed] [Google Scholar]
- 17. Tsuzuki T, Magi‐Galluzzi C, Epstein JI. ALK‐1 expression in inflammatory myofibroblastic tumor of the urinary bladder. Am J Surg Pathol. 2004;28(12):1609–1614. [DOI] [PubMed] [Google Scholar]
- 18. Coffin CM, Fletcher JA. Inflammatory myofibroblastic tumour In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed Lyon: IARC, 2013:2. [Google Scholar]
- 19. De Brouwer S, De Preter K, Kumps C, et al. Meta‐analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin Cancer Res. 2010;16(17):4353–4362. [DOI] [PubMed] [Google Scholar]
- 20. Pugh TJ, Morozova O, Attiyeh EF, et al. The genetic landscape of high‐risk neuroblastoma. Nat Genet. 2013;45(3):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peguero JA, Knost JA, Bauer MT, et al. Successful implementation of a novel trial model: the Signature program. J Clin Oncol. 2015;33(Suppl):Abstract 106. [Google Scholar]
- 22. Christensen JG, Zou HY, Arango ME, et al. Cytoreductive antitumor activity of PF‐2341066, a novel inhibitor of anaplastic lymphoma kinase and c‐Met, in experimental models of anaplastic large‐cell lymphoma. Mol Cancer Ther. 2007;6(12):3314–3322. [DOI] [PubMed] [Google Scholar]
- 23. Yasuda H, de Figueiredo‐Pontes LL, Kobayashi S, Costa DB. Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1‐translocated lung cancer. J Thorac Oncol. 2012;7(7):1086–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med. 2014;371(21):1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. http://ClinicalTrials.gov. An investigational drug, crizotinib (PF‐02341066), is being studied in tumors, except non‐small cell lung cancer, that are positive for anaplastic lymphoma kinase (ALK). http://ClinicalTrials.gov Identifier. NCT01121588; 2012.
- 26. Mussolin L, Damm‐Welk C, Pillon M, et al. Use of minimal disseminated disease and immunity to NPM‐ALK antigen to stratify ALK‐positive ALCL patients with different prognosis. Leukemia. 2013;27(2):416–422. [DOI] [PubMed] [Google Scholar]
- 27. Latham J, Campbell D, Nichols W. How much can exercise raise creatine kinase level—and does it matter? J Fam Pract. 2008;57(8):3. [PubMed] [Google Scholar]
- 28. Solomon BJ, Mok T, Kim DW, et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. [DOI] [PubMed] [Google Scholar]
- 29. Shaw AT, Kim D‐W, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK‐positive lung cancer. N Engl J Med. 2013;368(25):2385–2394. [DOI] [PubMed] [Google Scholar]
- 30. Gambacorti Passerini C, Farina F, Stasia A, et al. Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase–positive lymphoma patients. J Natl Cancer Inst. 2014;106(2):djt378. [DOI] [PubMed] [Google Scholar]
- 31. Gambacorti‐Passerini C, Messa C, Pogliani EM. Crizotinib in anaplastic large‐cell lymphoma. N Engl J Med. 2011;364(8):775–776. [DOI] [PubMed] [Google Scholar]
- 32. Gambacorti‐Passerini C, Mussolin L, Brugieres L. Abrupt relapse of ALK‐positive lymphoma after discontinuation of crizotinib. N Engl J Med. 2016;374(1):95–96. [DOI] [PubMed] [Google Scholar]
- 33. Mossé YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large‐cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14(6):472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mossé YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a Children's Oncology Group Study. J Clin Oncol. 2017;35(28):3215–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN‐35) in patients with relapsed or refractory systemic anaplastic large‐cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190–2196. [DOI] [PubMed] [Google Scholar]
- 36. Savage KJ, Harris NL, Vose JM, et al. ALK‐ anaplastic large‐cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T‐cell lymphoma, not otherwise specified: report from the International Peripheral T‐Cell Lymphoma Project. Blood. 2008;111(12):5496–5504. [DOI] [PubMed] [Google Scholar]
- 37. Laurent C, Do C, Gascoyne RD, et al. Anaplastic lymphoma kinase‐positive diffuse large B‐cell lymphoma: a rare clinicopathologic entity with poor prognosis. J Clin Oncol. 2009;27(25):4211–4216. [DOI] [PubMed] [Google Scholar]
- 38. Kimbara S, Takeda K, Fukushima H, et al. A case report of epithelioid inflammatory myofibroblastic sarcoma with RANBP2‐ALK fusion gene treated with the ALK inhibitor, crizotinib. Jpn J Clin Oncol. 2014;44(9):868–871. [DOI] [PubMed] [Google Scholar]
- 39. Butrynski JE, D'Adamo DR, Hornick JL, et al. Crizotinib in ALK‐rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363(18):1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Soft Tissue Sarcoma v3; 2012.
- 41. Hallberg B, Palmer RH. The role of the ALK receptor in cancer biology. Ann Oncol. 2016;27(suppl_3):iii4–iii15. [DOI] [PubMed] [Google Scholar]
- 42. Bresler SC, Wood AC, Haglund EA, et al. Differential inhibitor sensitivity of anaplastic lymphoma kinase variants found in neuroblastoma. Sci Transl Med. 2011;3(108):108ra114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kodityal S, Elvin JA, Squillace R, et al. A novel acquired ALK F1245C mutation confers resistance to crizotinib in ALK‐positive NSCLC but is sensitive to ceritinib. Lung Cancer. 2016;92:19–21. [DOI] [PubMed] [Google Scholar]
- 44. Berry T, Luther W, Bhatnagar N, et al. The ALK(F1174L) mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer Cell. 2012;22(1):117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Camidge DR, Bang Y‐J, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK‐positive non‐small‐cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


