Abstract
The pathogenesis of preeclampsia, a hypertensive disorder of pregnancy, involves imbalanced T helper (TH) cell populations and resultant changes in pro- and anti-inflammatory cytokine release. Elevated copeptin (an inert biomarker of arginine vasopressin (AVP)), secretion precedes the development of symptoms in preeclampsia in humans, and infusion of AVP proximal to and throughout gestation is sufficient to initiate cardiovascular and renal phenotypes of preeclampsia in wild-type C57BL/6J mice. We hypothesize that AVP infusion in wild-type mice is sufficient to induce the immune changes observed in human preeclampsia. AVP infusion throughout gestation in mice resulted in increased pro-inflammatory interferon gamma (IFNg) (TH1) in the maternal plasma. The TH17-associated cytokine IL-17 was elevated in the maternal plasma, amniotic fluid, and placenta following AVP infusion. Conversely, the TH2-associated anti-inflammatory cytokine interleukin (IL)-4 was decreased in the maternal and fetal kidneys from AVP-infused dams while IL-10 was decreased in the maternal kidney and all fetal tissues. Collectively, these results demonstrate the sufficiency of AVP to induce the immune changes typical of preeclampsia. We investigated if T cells can respond directly to AVP by evaluating the expression of AVP receptors (AVPRs) on mouse and human CD4+ T cells. Mouse and human T cells expressed AVPR1a, AVPR1b, and AVPR2. The expression of AVPR1a was decreased in CD4+ T cells obtained from preeclampsia-affected women. In total, our data are consistent with a potential initiating role for AVP in the immune dysfunction typical of preeclampsia and identifies putative signaling mechanism(s) for future investigation.
Keywords: APCs= antigen presenting cells, AVP= arginine vasopressin, AVPR= arginine vasopressin receptor, BLD= below the limit of detection, CLII= major histocompatibility complex II, Ct= cycle threshold, DC= dendritic cell, ICS= intracellular staining, IFNg= interferon gamma, IL-= interleukin, LAP= latency associated peptide, MFTB= maternal fetal tissue bank, MNC= mononuclear cell, PD-L1= programmed death ligand 1, pg/g= picogram per gram, PIR B= paired immunoglobulin receptor B, PreE= preeclampsia, TGFb= transforming growth factor beta, TH= T helper cell, Treg= T regulatory cell
Introduction
Preeclampsia, a hypertensive disorder in pregnancy, affects 5–7% of all U.S. pregnancies and yet, disproportionately comprises 15% of all maternal-fetal morbidity and mortality (1). Preeclampsia is known to cause immediate and long-term maternal and fetal morbidities (2–7). Current methods of early diagnosis and prevention of preeclampsia are limited and provide at best a few months of lead time before clinical symptoms appear (8). Aspirin can be used as a preventative agent with varied effectiveness (9, 10). This prevention is partially thought to be due to an early modulation of immune responses. If we can unravel the early immune pathogenic mechanism(s) involved in the development of preeclampsia, there is potential for improved prevention.
Human maternal plasma copeptin, a stable biomarker of arginine vasopressin (AVP) secretion, is elevated mid- to late-pregnancy in women who develop preeclampsia (11–16). Importantly, copeptin is a robust early-pregnancy (as early as the 6th week of gestation) predictor of the development of preeclampsia (17). Further, chronic infusion of AVP in wild-type, pregnant C57BL/6J females is sufficient to model human preeclampsia by inducing pregnancy-specific hypertension, proteinuria, glomerular endotheliosis, and fetal growth restriction (17). These data support a role for AVP in the early pathogenesis of preeclampsia, and provide key insight toward the future development of therapeutic interventions for preeclampsia
While the development of preeclampsia is multifactorial and involves many systems and processes including inadequate trophoblastic invasion and poor spiral artery remodeling, an altered inflammatory response is thought to be involved in the early pathogenesis of preeclampsia (18–23). In healthy pregnancies, pro-inflammatory CD4+ T helper (TH) 1 related activity is dominant early and later shifts to a more anti-inflammatory TH2 type of immune response. An imbalance of these TH1 and TH2 cells occurs during preeclampsia (24–27). This paradigm has expanded to include an increase in pro-inflammatory TH17 cells (24, 28–30). Although the precise mechanisms involved in the development of preeclampsia are complex and poorly understood, an aberrant pro-inflammatory TH cell response is clearly involved (18, 21, 31–34).
Roles for AVP in the regulation of blood pressure and fluid homeostasis are generally accepted, however, a role for AVP in immune system function is less well appreciated. AVP secretion stimulates pro-inflammatory cytokine secretion and activation of lymphocytes (35–38). The immune system is vital to the development of vascular dysfunction in hypertensive diseases. McMaster et al. review the evidence demonstrating T cells and T cell-derived cytokines, such as interferon gamma (IFNg) and interleukin-17 (IL-17), play a role in the renal and vascular dysfunction present in hypertensive diseases (39). As in preeclampsia, hypertension is thought to skew T cells towards pro-inflammatory TH1 (IFNg) and TH17 (IL-17) dominant phenotypes (40). TH IFNg production is a dynamic interaction that requires the presence of IL-2. Suppressor cells absorb IL-2, preventing induction of IFNg secretion and inhibiting TH1-associated responses (36, 37, 41). As reviewed by Chikanza et al., AVP has been shown to boost TH1 responses in vitro and in vivo via enhancement of IFNg (42, 43). Further, AVP has been shown to replace the requirement of IL-2 for the production of IFNg, thus inhibiting appropriate down-regulation of TH1 responses. Further, Johnson et al. show that AVP induces proliferation of C57BL/6 thymocytes in culture (36, 37, 44). Taken together, these data support the potential for AVP to induce increased IFNg (and thus a TH1 response) in an IL-2 independent manner to circumvent appropriate conversion from an inflammatory TH1 response needed for placentation and spiral artery remodeling to a more anti-inflammatory TH2 environment. To our knowledge, the potential role of TH17 and IL-17 in the regulation of AVP production has not been investigated. Further, this immunologic role of AVP has not been assessed in pregnancy. Thus, AVP is uniquely positioned to potentially initiate known mechanisms of preeclampsia, and may therefore represent an early cause of preeclampsia. The objective of this study is to test the hypothesis that AVP infusion during pregnancy in wild-type C57BL/6J mice is sufficient to induce the immunologic alterations observed in human preeclampsia. These data may provide novel insights into immune mechanisms mediated by AVP in preeclampsia.
Materials and Methods
Animal Studies
The animal procedures used were approved by the University of Iowa Institutional Animal Care and Use Committee. Wild-type 12–16 week old C57BL/6J male and female mice were obtained from Jackson Laboratories and maintained on standard chow under standard care conditions. Virgin females were subcutaneously implanted with osmotic mini-pumps (Alzet Model #1004, Cupertine, CA) infusing either saline or AVP (24 ng/hr, Sigma, St. Louis, MO) as previously described (17). Three days after implantation, dams were individually mated for a single overnight period. Pregnancies were timed by post-coital vaginal plugs indicative of gestational day (GD) 0.5. Maternal and fetal tissues were harvested on GD 18. Data were collected from multiple pregnancies from independent experiments. A subset of the cohort presented in the current study was previously published by Santillan et al. (17) and subsequent experiments have confirmed the preeclampsia phenotype (pregnancy-specific hypertension, proteinuria, fetal growth restriction, and kidney glomerular endotheliosis). Each pregnancy was considered N=1 for maternal tissues. Due to fetal tissue mass, 5 pairs of fetal kidneys and 5 fetal livers were pooled for an N=1 from a single pregnancy. Maternal tissues (plasma, kidney, and liver) and fetal tissues (amniotic fluid, kidney, liver, and placenta) had N ≥ 5 per group from at least two independent experiments. All whole tissues (except spleen) and plasma were stored at −80°C until protein extraction and analysis. Maternal spleen was kept on ice in phosphate buffered saline supplemented with 2% fetal bovine serum for immediate dissociation of cells.
Tissue Protein Extraction and Protein Analysis
Total protein lysate was generated by homogenization of tissues in buffer containing 5M NaCl, 1M Tris, 0.5M EDTA, NP-40, protease inhibitor (Roche, Switzerland), and phosphatase inhibitor (Roche, Switzerland). A commercially available bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Waltham, MA) was utilized per protocol to assess total protein concentration. Plasma and tissue protein extracts were diluted where needed and commercially available ELISAs for mouse pro-inflammatory IFNg (TH1) and IL-17 (TH17) and anti-inflammatory IL-4 (TH2), IL-10 (TH2), and TGFb cytokines were performed per protocol in duplicate (eBioscience, San Diego, CA). Cytokine concentrations were normalized to total protein and are presented as picograms/gram of total protein (pg/g).
Cell Preparation and Flow Cytometric Analysis
Single cell suspensions were prepared by mechanical maceration from spleens of saline and AVP-infused dams followed by density gradient separation (FicoLite LM, Atlanta Biologicals, Lawrenceville, GA) to obtain MNCs. Cell suspensions were then stained with fluorochrome-conjugated or biotinylated antibodies (Supplemental Table 1), followed by streptavidin-conjugated fluorochromes. Fluorochrome-conjugated, purified rat immunoglobulins were used as isotype controls for background fluorescence. All cell samples were incubated with anti-CD16/32 (clone 2.4G2) and rat serum during staining to prevent background FcγR binding. Intracellular Fixation and Permeabilization Buffer Set (eBioscience, San Diego, CA) was used per protocol for intracellular staining (ICS) of cytokines. Following staining, cells were fixed with either 0.1% formaldehyde or intracellular fixation buffer (eBioscience, San Diego, CA) where appropriate. Flow cytometric data were obtained within 24 hours using a Becton Dickinson LSR II (San Jose, CA) and analyzed using FlowJo software (Treestar Inc., Ashland, OR). Dead cells were excluded by forward/orthogonal light scatter characteristics. Single cells were identified via forward scatter-area (FSC-A) versus side scatter-width (SSC-W). Gating strategies of CD4+ T cells and dendritic cells (DCs) are shown in Supplemental Figure 2.
Human Studies
Human mononuclear cells (MNCs) from control (n=27) and preeclampsia-affected (n=24) pregnancies were obtained from the University of Iowa Maternal Fetal Tissue Bank (MFTB, IRB# 200910784). The MFTB is a pregnancy focused human biorepository with clinically annotated biosamples collected throughout gestation with quality control measures for clinical data and biosample integrity. As a prospective, cross-sectionally collected biorepository and clinical data warehouse, early pregnancy samples are collected before the onset of diagnoses. Coded clinical data were obtained through the MFTB as previously published (45), which derives data from our Clinical Research Data Warehouse. The human control and preeclampsia-affected samples used in this study are a subset of a previously published cohort (17) and no differences in characteristics were observed between groups (Supplemental Table 2). The diagnosis and classification of PreE was based on the standard American College of Obstetrics and Gynecology (ACOG) definitions for analysis (46). PreE cases were identified by cross-referencing the MFTB database with the bioinformatics query of ICD-9 and ICD-10 codes of bank participants at the time of delivery. The electronic medical record of each potential case was evaluated by the MFTB to confirm the diagnosis of PreE by the ACOG definitions (46). Case-control verification training of the MFTB and verification of cases and controls was led by the senior author (MKS) who is a clinical maternal fetal medicine specialist. Maternal age-matched plasma samples and corresponding clinical data for the control population were obtained by querying the MFTB database. Control pregnancies were pregnant women who did not develop PreE. The gestational age at the time of sample collection was recorded. MNCs are all processed and stored for viability in liquid nitrogen as previously described (45).
CD4+ T cell isolation
Human MNCs obtained from the MFTB. Splenic murine mononuclear cells were prepared as described above (cell preparation). Human and mouse CD4+ T cells were negatively selected using species-specific EasySep CD4+ T Cell Enrichment Kits per protocol (Stemcell Technologies Inc., Vancouver, BC). Human and mouse CD4+ T cell purity was determined to be ≥ 90% via flow cytometry.
Quantitative PCR
Total cellular RNA was purified from cells using the mirVana miRNA isolation per protocol (ThermoFisher Scientific, Waltham, MA). RNA concentration and purity were determined using a NanoDrop 1000 spectrophotometer (ThermoFisher Scientific, Waltham, MA). Quantitative PCR (qPCR) gene expression assays of AVP receptors 1a (AVPR1a), 1b (AVPR1b), and 2 (AVPR2) were performed following SuperScript III (ThermoFisher Scientific, Waltham, MA) reverse transcription of fixed total RNA mass (500ng). Resulting cDNAs were amplified using primer sets shown in Table 1. All primer efficiencies were between 93–95%. Amplicons were detected in a PowerSYBR Green qPCR assay carried out on an Applied Biosystems 7900HT Real Time PCR System in the Genomics Division of the Iowa Institute of Human Genetics (IIHG). Raw cycle threshold (Ct) values were normalized (ΔCt) against the 18S rRNA endogenous control. The ΔΔCt and expression fold change were calculated as previously described (47, 48).
Table 1.
qPCR Primer Sequences*
| Target# | Sequence | Tm | Amplicon | |
|---|---|---|---|---|
| hAVPR1a | S | GTGCAGAGCAAGCGGGTGTG | 61.8°C | 438bp |
| AS | CGAGTCCTTCCACATACCCGT | 58.7°C | ||
| mAVPR1a | S | CTCTGCTGGACACCTTTCTTC | 55.5°C | 218bp |
| AS | GTTGGGCTTCGGTTGTTAGA | 55.2°C | ||
| hAVPR1b | S | CCAAGATCCGAACAGTGAAGAT | 54.6°C | 206bp |
| AS | GCTGTTGAAGCCCATGTAGA | 55.0°C | ||
| mAVPR1b | S | AAGATCCGAACCGTGAAGATG | 54.8°C | 320bp |
| AS | TGGGTCAGCAGTGTTGTG | 55.4°C | ||
| hAVPR2 | S | GGCCAAGACTGTGAGGATGA | 56.9°C | 200bp |
| S | ACACGCTGCTGCTGAAAGAT | 57.5°C | ||
| mAVPR2 | S | AGGACACCGGACAGGAA | 55.7°C | 275bp |
| AS | AAAGCAGGCTACGCAACT | 54.8°C | ||
| 18S rRNA | S | AACTTTCGATGGTAGTCGCCG | 57.3°C | 104bp |
| AS | CTTGGATGTGGTAGCCGTTT | 57.6°C |
qPCR primers were designed using the Integrated DNA Technologies PrimerQuest online tool (www.idtdna.com) against GenBank mRNA sequences.
#h = human-specific; m = mouse-specific; S = sense strand; AS = anti-sense strand; All primer efficiencies were between 93–95%.
Statistical Analysis
For continuous variables, a two sided Student’s t test with unequal variance was utilized (GraphPad, Prism 7, La Jolla, CA). In addition, chi square was utilized for categorical variables. Statistical significance was designated at α = 0.05 or as determined by Bonferroni correction for multiple comparisons ANOVA.
Results
AVP infusion throughout gestation heavily skews maternal TH immunity toward a pro-inflammatory TH1 and TH17 phenotype
We previously demonstrated infusion of AVP into pregnant mice results in the hallmark features of human preeclampsia including, pregnancy-specific hypertension, proteinuria, renal glomerular endotheliosis, and fetal growth restriction (17). In the current study, we utilize this established mouse model of preeclampsia to investigate immune alterations caused by elevated AVP in pregnancy. The TH1- and TH17- associated pro-inflammatory cytokines, IFNg and IL-17, were significantly increased in the plasma of AVP-infused dams and unaffected by AVP in the maternal kidney and liver (Figure 1). T cells are activated in secondary lymphoid organs (eg. spleen); and consistent with circulating plasma IFNg and IL-17 elevations, we observed increases in both TH1 and TH17 CD4+ T cells in the spleen of AVP-infused dams (Figure 1). These data suggest elevated AVP during pregnancy induces a pro-inflammatory TH1 and TH17 milieu in pregnant mice similar to that observed in human preeclampsia-affected women.
Figure 1.
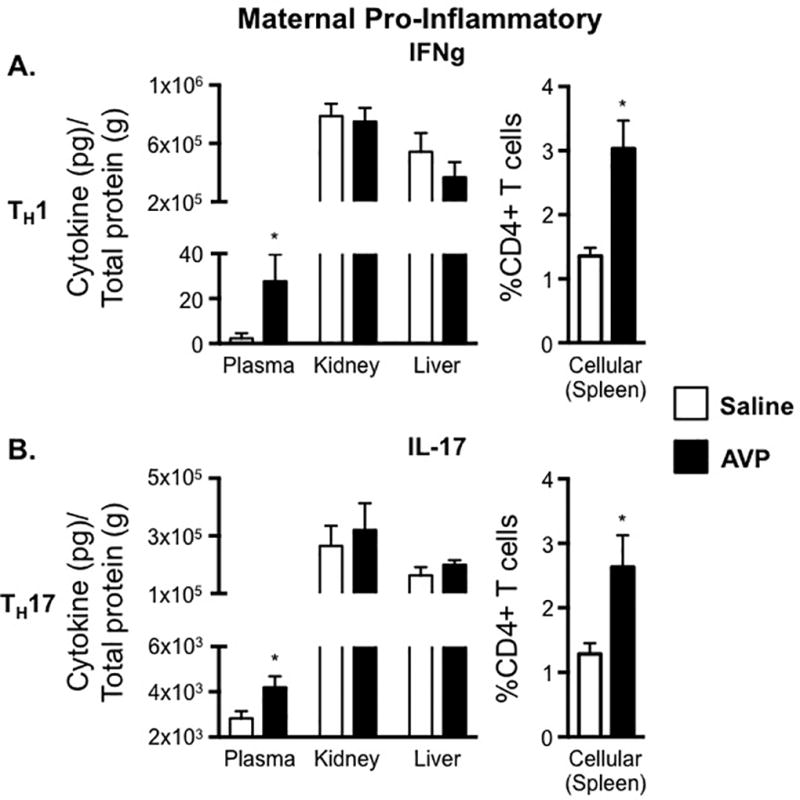
AVP infusion is sufficient to induce TH1 and TH17 maternal immune alterations. IFNg (A) and IL-17 (B) concentrations in maternal plasma, maternal kidney, and maternal liver were normalized to total protein and are represented as pg of cytokine per g of total protein. Lymphocytes were isolated from the spleen of saline and AVP infused dams. Following intracellular cytokine staining, CD3+ CD4+ cells were gated as shown in Supplemental Figure 1A and the frequency of cells producing the pro-inflammatory cytokines IFNg (A) and IL-17 (B) was determined. Open bars= saline-infused; Solid bars= AVP-infused. N ≥ 5 per group from at least two independent experiments. Data are mean ± SEM. Statistical significance was determined using a Student t test and the minimal level of confidence deemed statistically significant was a p value <0.05. *=p<0.05.
Elevated AVP in pregnancy decreases anti-inflammatory TH2 and TGFb cytokines in pregnant dams
Although we did not observe an AVP-induced change in the frequency of splenic CD4+ T cells producing anti-inflammatory cytokines during pregnancy, there were tissue-specific changes in anti-inflammatory cytokines (Figure 2). The TH2-associated cytokines, IL-4 and IL-10, as well as the anti-inflammatory cytokine TGFb, were all decreased in the maternal kidneys of AVP-infused dams. Additionally, there was a significant decrease in plasma TGFb in these animals. The levels of IL-4, IL-10, and TGFb were all comparable between the saline and AVP dams in the maternal liver (Figure 2). These data demonstrate AVP infusion during pregnancy results in an overall reduction in maternal anti-inflammatory cytokines.
Figure 2.
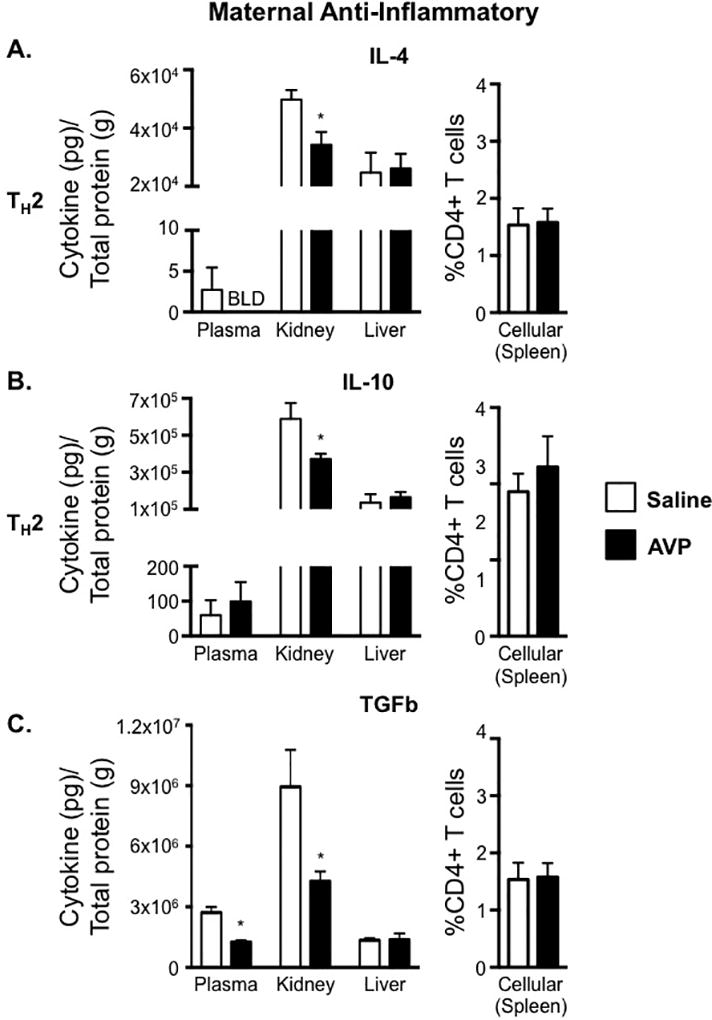
Elevated AVP in pregnancy decreases anti-inflammatory cytokine production in maternal tissues. IL-4 (A), IL-10 (B), and TGFb (C) concentrations in maternal plasma, maternal kidney, and maternal liver were normalized to total protein and are represented as pg of cytokine per g of total protein. Lymphocytes were isolated from the spleen of saline and AVP infused dams. Following intracellular cytokine staining, CD3+ CD4+ cells were gated as shown in Supplemental Figure 1A and the frequency of cells producing the TH2-associated cytokines IL-4 (A) and IL-10 (B) as well as LAP/TGFb (C) was determined. Open bars= saline-infused; Solid bars= AVP-infused. BLD is below the limit of detection. N ≥ 5 per group from at least two independent experiments. Data are mean ± SEM. Statistical significance was determined using a Student t test and the minimal level of confidence deemed statistically significant was a p value <0.05. *=p<0.05.
AVP infusion in pregnancy decreases Treg and alters the ratio of Treg:TH17 cells
It has been observed in both humans and in animal models that Tregs are reduced and the ratio of Treg to TH17 cells is altered in preeclampsia (24, 28, 33). Given the changes observed in the anti-inflammatory cytokines IL-10 and TGFb (Figure 2), we next evaluated Treg. Elevated AVP resulted in a decrease in anti-inflammatory Treg cells (Figure 3A) as well as an altered Treg:TH17 ratio (Figure 3B).
Figure 3.
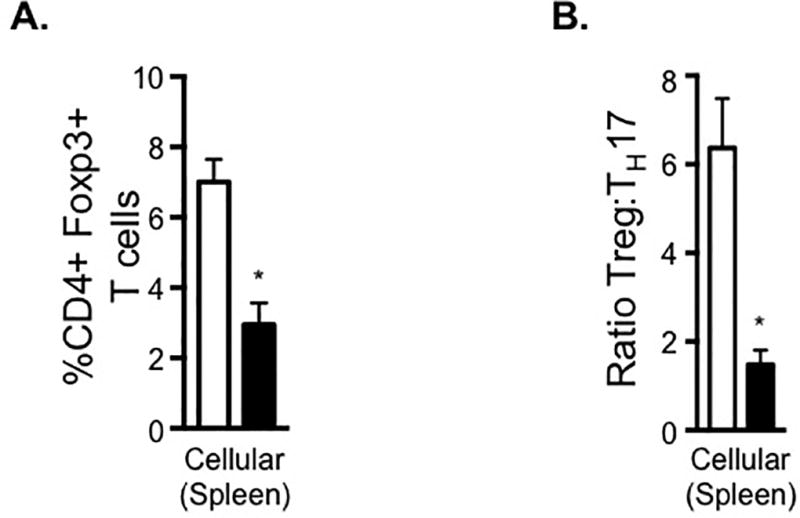
AVP infusion in pregnancy alters the ratio of anti-inflammatory Tregs to pro-inflammatory TH17 cells. Lymphocytes were isolated from the spleen of saline and AVP infused dams. Following intracellular cytokine staining, CD3+ CD4+ cells were gated as shown in Supplemental Figure 1A and the frequency of cells producing the Treg transcription factor Foxp3 (A) or the pro-inflammatory cytokine IL-17 was determined. The ratio of Treg to TH17 cells was calculated (B). Open bars= saline-infused; Solid bars= AVP-infused. BLD is below the limit of detection. N ≥ 5 per group from at least two independent experiments. Data are mean ± SEM. Statistical significance was determined using a Student t test and the minimal level of confidence deemed statistically significant was a p value <0.05. *=p<0.05.
Dendritic cells from AVP-treated dams show enhanced co-stimulatory molecule expression
Dendritic cells (DCs) are potent antigen presenting cells (APCs) that activate and influence TH cell differentiation. This process involves not only cytokine production by both cell types, but also surface molecule interactions. The expression of co-stimulatory and inhibitory molecules by DCs was evaluated to determine if elevations in AVP during pregnancy alters the activation phenotype of DCs. The co-stimulatory surface molecules major histocompatibility complex class II (CLII), CD80, and CD86 are highly expressed on CD11c+ DCs from AVP-treated versus saline-treated dams (Figure 4A). Concomitantly, the expression of the inhibitory surface molecules programmed death ligand 1 (PD-L1) and paired immunoglobulin receptor B (PIR B) were significantly decreased on DCs from AVP-treated dams (Figure 4B). These data show that AVP programs DCs toward an activated, stimulatory phenotype and thus may enhance antigen presentation and pro-inflammatory T cell activation in preeclampsia.
Figure 4.
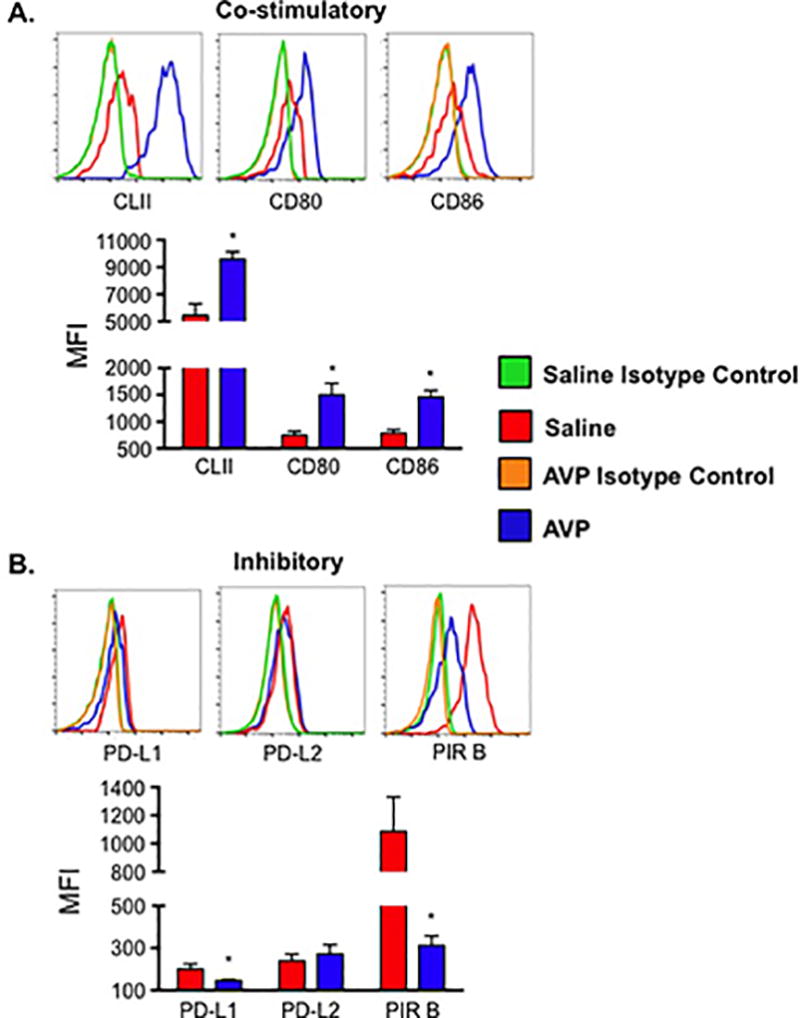
AVP induces altered surface receptor expression on DC. Inhibitory molecules paired immunoglobulin-like receptor B (PIR B) and programmed death ligand 1 (PD-L1) are decreased while co-stimulatory molecules MHC Class II (CLII), CD80, and CD86 were increased on dendritic cells from AVP infused dams. Lymphocytes were isolated from the spleen of saline and AVP infused dams. Following fluorescent antibody staining, CD11c+ DCs were gated as shown in Supplemental Figure 1B. (A) Representative histograms and mean fluorescence intensity (MFI) of co-stimulatory cell surface molecules. (B) Representative histograms and MFI of inhibitory cell surface molecules. Background fluorescence was determined by staining cells with corresponding fluorochrome-conjugated rat immunoglobulin isotype control antibodies. Isotype control subtracted MFIs are shown. N ≥ 5 per group from at least two independent experiments. MFIs are mean ±SEM. Statistical significance was determined using a Student t test and the minimal level of confidence deemed statistically significant was a p value <0.05. *=p<0.05.
Fetal exposure to elevated AVP in utero results in enhanced TH17 and impaired TH2 cytokine profiles
Unlike maternal tissues, IFNg was not elevated in the fetal tissues from GD 18 AVP-infused dams (Figure 5A). Congruent with increased maternal IL-17, the amniotic fluid, placenta, and fetal kidney all showed increased IL-17 levels compared to tissues from saline infused dams, suggesting increased TH17 cells (Figure 5B). In addition to increases in pro-inflammatory IL-17, fetal tissues had marked decreases in the anti-inflammatory cytokines IL-4 (TH2), IL-10 (TH2), and TGFb. IL-4 was significantly decreased in the amniotic fluid, placenta, and fetal kidney, while IL-10 was decreased in all fetal tissues, including fetal liver (Figure 6A and 6B). These data suggest a global alteration in TH2 cells in fetal tissues when exposed to elevated AVP during gestation. Further contributing to an elevated pro-inflammatory over an anti-inflammatory cytokine balance, the anti-inflammatory cytokine TGFb was also decreased in the placenta and fetal kidney from AVP-infused pregnancies (Figure 6C). In humans, it is unknown if maternal AVP crosses the placenta. Radiolabeled I125-AVP experiments in pregnant ewes suggest that AVP does not cross the placenta. Further, rat studies (49) suggest that the source of placental AVP is from the fetus. In total, our findings and others suggest that AVP is sufficient directly to cause preeclampsia related physiologic and immunologic phenotypes in the mother. Yet, the fetal physiologic and immunologic phenotypes may be due to a combination of direct stimulation at the maternal-fetal interface and/or a fetal response to the AVP induced maternal phenotypes.
Figure 5.
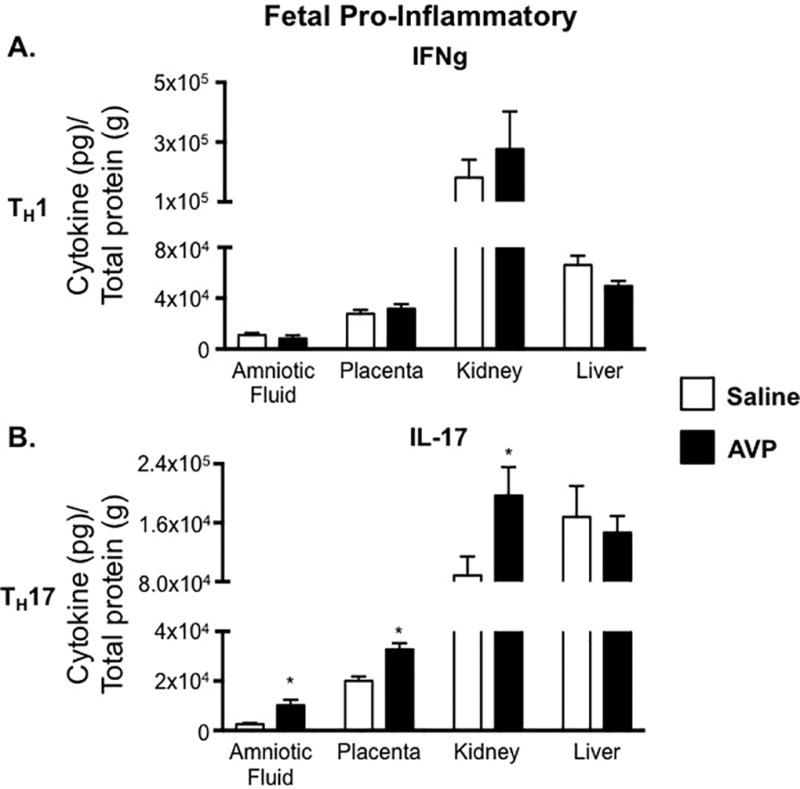
Fetal tissue IL-17 cytokine production is altered by AVP. The TH17-associated cytokine IL-17 is increased in fetal tissues obtained from AVP-infused dams. IFNg (A) and IL-17 (B) concentrations in amniotic fluid, placenta, fetal kidney, and fetal liver were normalized to total protein and are represented as pg of cytokine per g of total protein. Open bars= saline-infused; Solid bars= AVP-infused. Due to fetal tissue mass, 5 pairs of fetal kidneys and 5 fetal livers were pooled for an N=1 from a single pregnancy. N ≥ 5 per group from at least two independent experiments. Data are mean ±SEM. Statistical significance was determined using a Student t test and the minimal level of confidence deemed statistically significant was a p value <0.05. *=p<0.05.
Figure 6.
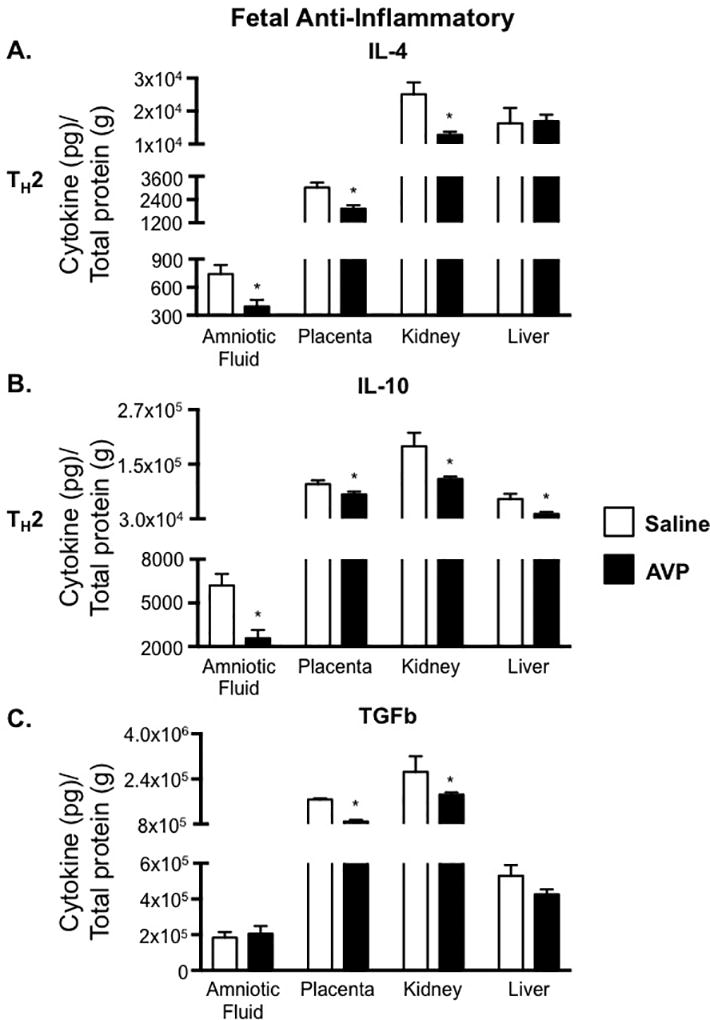
Fetal concentrations of anti-inflammatory cytokines are decreased in the presence of elevated AVP during gestation. IL-4 (A), IL-10 (B), and TGFb (C) concentrations in amniotic fluid, placenta, fetal kidney, and fetal liver were normalized to total protein and are represented as pg of cytokine per g of total protein. Open bars= saline-infused; Solid bars= AVP-infused. Due to fetal tissue mass, 5 pairs of fetal kidneys and 5 fetal livers were pooled for an N=1 from a single pregnancy. N ≥ 5 per group from at least two independent experiments. Data are mean ± SEM. Statistical significance was determined using a Student t test and the minimal level of confidence deemed statistically significant was a p value <0.05. *=p<0.05.
Mouse and human CD4+ T cells express AVP receptors
As TH cells are altered in response to AVP during pregnancy in mice, CD4+ T cells were isolated and analyzed for expression of AVP receptors to determine if CD4+ T cells may be able to respond directly to elevated AVP. Interestingly, mouse and human CD4+ T cells expressed AVP receptors 1a, 1b, and 2 (Table 2 and 3). Similar to mouse CD4+ T cells, human CD4+ T cells highly expressed AVPR2, but AVPR1a was also highly expressed (Table 3). To focus on potential early and late pregnancy changes in circulating CD4+ T cells, AVPR expression was determined in the first and third trimester of human pregnancies. CD4+ T cells isolated from human preeclampsia-affected pregnancies expressed significantly lower AVPR1a in the first trimester, with a 6.3 fold decrease in expression (Table 3). Additionally, although AVPR1b was not as highly expressed AVPR1a and 2, there was a 3.7 fold decrease in expression by CD4+ T cells isolated from preeclampsia-affected pregnancies during the first trimester. Interestingly, the changes in AVPR1a and AVPR1b expression observed normalized by the third trimester. Although AVPR2 expression was not altered in the preeclampsia-affected group in the first trimester, it was 1.7 fold increased the third trimester. These data not only demonstrate that CD4+ T cells express AVP receptors, but also that expression of AVPRs is differentially regulated throughout pregnancy in human preeclampsia-affected CD4+ T cells.
Table 2.
Murine CD4+ T cell expression of AVPRs
| Saline | AVP | Expression Fold Change |
||
|---|---|---|---|---|
| Average ΔCT | p-value | |||
| AVPR1a | 20.7 ±0.9 | 21.0 ±0.5 | −1.3 | 0.72 |
| AVPR1b | 20.6 ±0.7 | 21.6 ±0.3 | −2.0 | 0.19 |
| AVPR2 | 9.7 ±0.6 | 10.5 ±0.4 | −1.7 | 0.26 |
Mouse splenic CD4+ T cells were negatively purified from saline and AVP-infused dams. Expression of AVP receptors was determined via qPCR. Raw cycle threshold (Ct) values were normalized (ΔCt) against the18S rRNA endogenous control. The lower the ΔCt value of a specific target, the higher the expression. The ΔΔCt and expression fold change of AVP versus saline and preeclampsia-affected versus control pregnancy were calculated as previously described (47, 48). Data are mean ±SEM. Statistical significance was determined using a Student’s t test and the minimal level of confidence deemed statistically significant was a p value <0.05.
Table 3.
CD4+ T cells isolated from human preeclampsia-affected women have trimester-specific alterations in AVPR expression
| 1st Trimester | 3rd Trimester | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | PreE | Control | PreE | |||||
| Average ΔCT | Fold Change |
p-value | Average ΔCT | Fold Change |
p-value | |||
| AVPR1a | 9.4±0.6 | 12.1±0.3 | −6.3 | 0.0009 | 10.2±0.4 | 10.5±0.3 | −1.1 | 0.56 |
| AVPR1b | 20.4±0.7 | 22.3±0.5 | −3.7 | 0.04 | 21.2±0.6 | 21.6±0.4 | −1.3 | 0.62 |
| AVPR2 | 8.0±0.3 | 8.7±0.3 | −1.7 | 0.14 | 8.8±0.2 | 8.0±0.1 | 1.7 | 0.01 |
Human CD4+ T cells were negatively selected from peripheral blood mononuclear cells of control (1st trimester N=6; 3rd trimester N=15) and preeclampsia-affected (PreE; 1st trimester N=9; 3rd trimester N=12–14) pregnancies. Expression of AVP receptors was determined via qPCR. Raw cycle threshold (Ct) values were normalized (ΔCt) against the18S rRNA endogenous control. The lower the ΔCt value of a specific target, the higher the expression. The ΔΔCt and expression fold change of AVP versus saline and preeclampsia-affected versus control pregnancy were calculated as previously described (47, 48). Data are mean ±SEM. Statistical significance was determined using a Student’s t test and the minimal level of confidence deemed statistically significant was a p value <0.05.
Discussion
The early pathogenesis of preeclampsia involves the failure of the maternal immune system to normally tolerate the pregnancy (21). Previous studies have indicated that during a healthy human pregnancy, a shift occurs from a pro-inflammatory TH1 (IFNg dominant) CD4+ T cell response required early for appropriate placentation to an anti-inflammatory TH2 (IL-4 and IL-10 dominant) response required for fetal tolerance. The concept that persistent TH1 related feto-placental intolerance is important in the pathogenesis of preeclampsia has been demonstrated by our lab and others (50–53). TH1 responses and related cytokines (IFNg) directly inhibit the development of TH2 responses and vice versa (31, 54, 55). IFNg is elevated in human preeclampsia with a concurrent decrease in IL-10 and IL-4 production, driving a TH1 response (55–58). These TH1 cells further increase IFNg production, resulting in increased recruitment and activation of antigen presenting cells and cytotoxic CD8+ T cells. Recently, the production of IL-17 and the percent of TH17 cells have been shown to be increased in preeclampsia and potentially contribute to poor fetal tolerance (28, 33). Taken together, our data extend previous studies indicating there is both an increase in TH1 and TH17 cells, by identifying AVP as a potential, novel contributor to poor fetal tolerance and the development of preeclampsia.
For the first time, our data show that AVP-infusion during mouse pregnancy induces the TH subset changes and pro-inflammatory milieu similar to that observed in human preeclampsia. In mouse maternal circulation, there is a pro-inflammatory TH1 and TH17 milieu with elevated IFNg and IL-17 and a concomitant decrease in anti-inflammatory TGFb. Correlating with previously observed AVP-induced renal changes (17), there were maternal kidney specific decreases in the TH2-associated anti-inflammatory cytokines IL-4 and IL-10, as well as anti-inflammatory TGFb. Our observed differences in cytokines between the mouse maternal circulation and target tissues, underscores the concept that circulating immune responses may not completely represent tissue-specific immune responses. In addition to producing soluble proteins that are detectable in the circulation, T cells travel to the site of inflammation to exert their function. Although IFNg is elevated in the maternal plasma of our AVP-infused dams, but not in the tissues evaluated, it is likely IFNg is elevated in the spleen (given the observed increase in splenic CD4+ IFNg+ T cells). As T cells are activated in the secondary lymphoid organs and also influence other immune responses, the observed changes in maternal plasma IFNg may be acting at the level of the spleen. In regards to IL-17, the elevation observed in the circulation does correlate with a known target organ in preeclampsia, the placenta. Lastly, immune cells in the target organs may also locally produce cytokines and further contribute to tissue inflammation and disease progression. Collectively, our data expands what other groups have observed in mouse models and in human preeclampsia (18, 25, 27, 28, 33, 56, 59, 60), in that we observed changes in TH1/TH2/TH17 associated cytokines in our AVP-induced mouse model of preeclampsia, highlighting AVP as a novel, potential immunologic agent in the development of preeclampsia.
We contend that AVP is an important initiating pathway in the early immunologic phenotype of preeclampsia. TH1 cells, seen in abundance in preeclampsia, are potent sources of IFNg which can induce AVP production (61). AVP secretion (via copeptin detection) is elevated very early in human preeclampsia (17) and is capable of inducing the early immunologic changes observed in the first trimester preeclampsia. Additionally, it has been demonstrated that the immune system, more specifically TH1 and TH17 cells, play a pivotal role in the development of vascular dysfunction (39, 40). We observed AVP-induced changes in both the maternal and fetal compartments. Although toward the end of mouse gestation, GD 18 mouse pups developmentally correspond to GD 58–60 in humans (62–64), which falls well within the first trimester of human gestation. Our mouse fetal immune findings may translate into early first trimester immunologic changes in the human fetus. This is consistent with the current understanding of a very early initiating cause of preeclampsia, as first trimester immunologic dysregulation lies upstream of the placental/vascular phenotype (21, 65). These observations also further the concept that AVP has immune effects beyond the canonical blood pressure/volume control. Together, these data suggest that AVP, whether via direct interaction with immune cells or indirectly via alterations in cardiovascular and renal function, may be an early cause of immune changes in preeclampsia.
Although the exact role of AVP in immune responses still requires further investigation, AVP is thought to stimulate immune cell activity in non-pregnant environments (35–38, 44, 66). As reviewed by Chikanza et al., rats with chronic inflammatory disease also had elevated levels of plasma AVP. A reduction in circulating AVP decreased the pro-inflammatory response in these animals (67). In vitro experiments demonstrated a dose-dependent augmentation of autologous mixed lymphocyte reactions, including the requirement of IL-2 for IFNg production (36, 44). Further, addition of IL-1 beta (IL-1b) or IL-6 to in vitro cultures resulted in a dose-dependent increase in AVP production (68). Lastly, human cancer patients administered IL-6 had significantly higher circulating AVP levels within two hours of administration (69).
To our knowledge, the role of AVP in immune responses has not been investigated in pregnancy, and more specifically, not in preeclampsia-affected pregnancies. Here, we demonstrate that in pregnancy, mouse and human CD4+ T cells express AVP receptors, and that AVPR1a and AVPR2 are highest expressed. Interestingly, AVPR1a and 1b expression by human CD4+ T cells is significantly down-regulated in preeclampsia-affected pregnancies in the first trimester and this normalizes by the third trimester. AVPR1a is expressed in blood vessels and is known to play a role in baroreceptor reflexes and blood volume homeostasis (70–72). Although high placental AVPR1a expression has also been purposed as a potential mediator early in pregnancy for increased blood flow and appropriate placental growth (73), these data suggest that AVPR1A may also mediate immune responses during preeclampsia and provide insight into potential therapeutic targets. In the third trimester, CD4+ T cells from preeclampsia-affected pregnancies increased expression of AVPR2 compared to cells from normotensive control pregnancies. AVPR2 is highly expressed in the kidney and is pivotal in renal water reabsorption and thus urine concentration (74, 75) and under conditions of elevated AVP, it effects renal vasoconstriction (76). T cells have been shown to play a role in hypertension and renal dysfunction. More specifically, Increased CD4+ IL-17 producing T cells are seen in hypertension and these cells traffic to the kidney and vasculature to cause dysfunction that leads to hypertension (77, 78). Renal dysfunction and hypertension are often observed in PreE in the third trimester. Our data showing an increase in inflammatory TH17 cells with altered AVPR2 expression suggests this receptor may play a currently unknown role in immune responses and PreE toward the end of pregnancy. Although the function of the expression of AVPRs on CD4+ T cells in PreE is currently unknown, our data suggests these receptors may be a previously uncharacterized link between renal, cardiovascular, and immune dysfunction in PreE.
APCs are critical to the activation and programming of TH cells. AVP in pregnancy is sufficient to induce the up-regulation of co-stimulatory (CD80, CD86, and CLII) and down-regulation of inhibitory (PD-L1 and PIR B) molecule expression on DC, resulting in the generation of more TH1 and TH17 cells, which are necessary in the early pathogenesis of preeclampsia. Increased expression of co-stimulatory molecule CD86 has been shown to correlate highly with poor maternal-fetal immune balance (79). Blockade of CD86 in pregnancy allows for expansion of protective Treg cells and promotes the differentiation of a more tolerant TH2 environment (80). The expression of PD-L1 during pregnancy has been shown to be T cell dependent and required to confer fetal tolerance (81). Both, co-stimulatory and inhibitory molecules, play a key role in maternal-fetal tolerance. Our data show that elevated AVP during pregnancy results in the up-regulation of co-stimulatory and down-regulation of inhibitory molecules by DCs. These alterations in signals received by the DCs are a likely mechanism contributing to the altered TH phenotype induced by elevated AVP during pregnancy and the development of preeclampsia. These alterations in the co-stimulatory phenotype of DCs in the spleen leads to a differentiation of CD4+ T cells toward a TH1 and TH17 phenotype. Peripheral TH1 and TH17 cells, as well as, decidual cell populations skew the placenta toward a milieu rich in pro-inflammatory cytokines that furthers contributes to the development and pathogenesis of preeclampsia (Figure 7).
Figure 7.
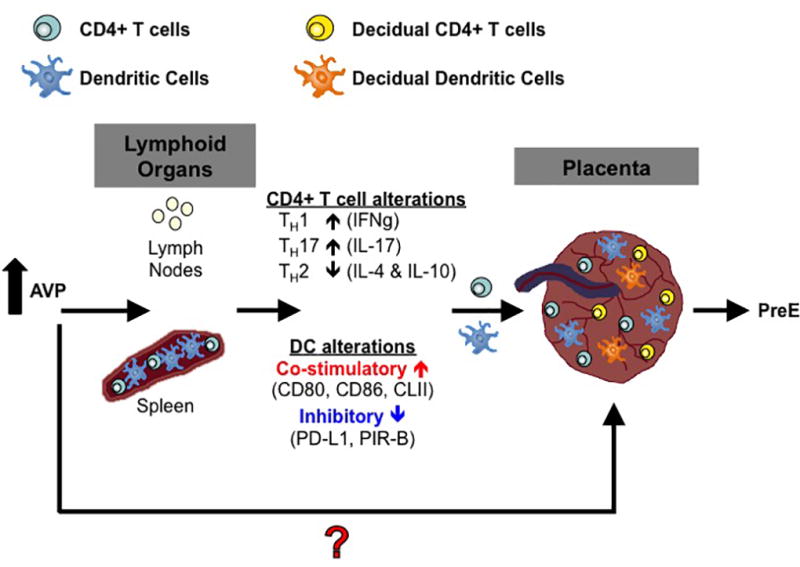
Proposed model of AVP action in pregnancy. Elevated AVP during pregnancy induces DCs in secondary lymphoid organs to upregulate co-stimulatory molecules and down-regulate inhibitory molecules. These DCs activate T cells and the resultant helper T cells secrete more pro-inflammatory TH1 and TH17 and less anti-inflammatory TH2 cytokines. These cells traffic to the placenta and along with resident decidual cell populations alter cytokine profiles in the placenta and fetal tissues. The red question mark indicates possibility of AVP acting directly on the placenta and tissue resident cell populations. PreE=preeclampsia.
Our data demonstrate that elevated AVP in mice during pregnancy induces maternal and fetal TH related changes; these alterations mimic those previously observed in human preeclampsia-affected pregnancies. Additionally, the expression of AVP receptors by CD4+ T cells identifies a putative signaling mechanism and new therapeutic targets for future investigation. From our studies, we propose that manipulation of the AVP pathway may be a novel preventative and therapeutic target to address the vascular, renal, and immune causes of preeclampsia.
Supplementary Material
Supplemental Figure 1. Flow cytometric cell gating. Lymphocytes were isolated from the spleen of saline and AVP infused dams. Dead cells were excluded by forward/orthogonal light scatter characteristics. Single cells were identified via forward scatter-area (FSC-A) versus side scatter-width (SSC-W). CD3+ CD4+ T cells (A) and CD11c+ DCs (B) were gated as shown.
Supplemental Table 1. Human MNC group characteristics. The samples used in this study are a subset of a previously published study (17). Statistical significance was determined using a Student’s t test or chi squared and the minimal level of confidence deemed statistically significant was a p value <0.05.
Clinical Perspectives.
AVP secretion during pregnancy, via copeptin detection, has been shown to be elevated very early in women who later develop preeclampsia. AVP infusion into pregnant mice recapitulates the physiological aspects of human preeclampsia. It is unknown if elevated AVP during pregnancy is sufficient to cause the maternal and fetal immune phenotypes consistent with human preeclampsia
Elevated AVP in pregnant mice results in increased TH1 and TH17 associated cytokines and T cells with a concomitant decrease in the TH2 associated cytokines. Additionally, CD4+ T cells express AVP receptors and expression of AVPR1a by human CD4+ T cells from preeclampsia-affected women is decreased.
The data presented demonstrate a novel role for AVP in altering immune phenotypes in pregnancy. Additionally, the expression of AVP receptor expression by CD4+ T cells lends to the possibility of therapies utilizing AVP modulation to mitigate preeclampsia.
Acknowledgments
The authors thank the staff of the following facilities and departments: the University of Iowa Department of Obstetrics and Gynecology, the University of Iowa Office of Animal Resources, the University of Iowa Women’s Health Tissue Repository, the University of Iowa Institute for Clinical and Translational Science, and the Carver College of Medicine Flow Cytometry Facility.
Financial Support:
American Heart Association Innovative Research Grant (14IRG18710013)
American Heart Association Postdoctoral Fellowship (16POST30960016)
American Heart Association Strategically Focused Research Network (15 SFRN 23730000, 18679000, 18679001,18679002, 18679003)
Burroughs Wellcome Fund (1015358)
Clinical and Translational Science Award (NIH U54TR001356)
John Warner Maternal Health Grant, Shelly Bridgewater Dreams Foundation K99/R00 (NIH HL098276)
March of Dimes Foundation (4-FY15-415)
NIH R01 (NIH HL134850)
Program Project Grant (NIH HL084207)
Reproductive Scientist Development Program (NIH K12 HD000849) (NIH K12 HD000849-28)
University of Iowa Carver College of Medicine Collaborative Grant
University of Iowa Center for Hypertension Research
University of Iowa Immunology Postdoctoral Fellowship (NIH 5 T32 AI 7260-29)
University of Iowa Institute for Clinical and Translational Science (NIH KL2 RR024980-2)
Footnotes
Disclosure Statement: The authors report no conflict of interest.
Author Contribution
S.M.S, D.A.S., W.S.H., E.J.D., H.A.D., C.D.S., J.L.G., and M.K.S. designed the experiments, experiments and data collection were performed by S.M.S., D.A.S., J.M.L., J.A.S., L.K.K., W.S.H., E.J.D., H.A.D., K.N.G-C., J.L.G., and M.K.S., data analysis was performed by S.M.S., D.A.S., E.J.D., and M.K.S., data interpretation was conducted by S.M.S., D.A.S., E.J.D., G.L.P., C.D.S., J.L.G., and M.K.S., and all authors contributed to and approved the final manuscript.
The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
References
- 1.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstetrics and gynecology. 2009;113(6):1299–306. doi: 10.1097/AOG.0b013e3181a45b25. [DOI] [PubMed] [Google Scholar]
- 2.Garovic VD, Hayman SR. Hypertension in pregnancy: an emerging risk factor for cardiovascular disease. Nature clinical practice Nephrology. 2007;3(11):613–22. doi: 10.1038/ncpneph0623. [DOI] [PubMed] [Google Scholar]
- 3.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53(6):944–51. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 4.Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstetrics and gynecology. 2009;114(5):961–70. doi: 10.1097/AOG.0b013e3181bb0dfc. [DOI] [PubMed] [Google Scholar]
- 5.Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke; a journal of cerebral circulation. 2009;40(4):1176–80. doi: 10.1161/STROKEAHA.108.538025. [DOI] [PubMed] [Google Scholar]
- 6.Wu CS, Sun Y, Vestergaard M, Christensen J, Ness RB, Haggerty CL, et al. Preeclampsia and risk for epilepsy in offspring. Pediatrics. 2008;122(5):1072–8. doi: 10.1542/peds.2007-3666. [DOI] [PubMed] [Google Scholar]
- 7.Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J. Health of children born to mothers who had preeclampsia: a population-based cohort study. American journal of obstetrics and gynecology. 2009;201(3):269. doi: 10.1016/j.ajog.2009.06.060. e1 - e10. [DOI] [PubMed] [Google Scholar]
- 8.Rana S, Karumanchi SA, Lindheimer MD. Angiogenic factors in diagnosis, management, and research in preeclampsia. Hypertension. 2014;63(2):198–202. doi: 10.1161/HYPERTENSIONAHA.113.02293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695–703. doi: 10.7326/M13-2844. [DOI] [PubMed] [Google Scholar]
- 10.Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. The New England journal of medicine. 2017;377(7):613–22. doi: 10.1056/NEJMoa1704559. [DOI] [PubMed] [Google Scholar]
- 11.Akinlade KS, Adediji IO, Rahamon SK, Fawole AO, Tongo OO. Serum copeptin and pregnancy outcome in preeclampsia. Niger Med J. 2015;56(5):362–8. doi: 10.4103/0300-1652.170385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foda AA, Abdel Aal IA. Maternal and Neonatal Copeptin Levels at Cesarean Section and Vaginal Delivery. European Journal of Obsterics & Gynecology and Reproductive Biology. 2012;165:215–8. doi: 10.1016/j.ejogrb.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Tuten A, Oncul M, Kucur M, Imamoglu M, Ekmekci OB, Acikgoz AS, et al. Maternal serum copeptin concentrations in early- and late-onset pre-eclampsia. Taiwan J Obstet Gynecol. 2015;54(4):350–4. doi: 10.1016/j.tjog.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 14.Yesil A, Kanawati A, Helvacioglu C, Kaya C, Ozgun CG, Cengiz H. Identification of patients at risk for preeclampsia with the use of uterine artery Doppler velocimetry and copeptin. J Matern Fetal Neonatal Med. 2016:1–6. doi: 10.1080/14767058.2016.1262841. [DOI] [PubMed] [Google Scholar]
- 15.Yeung EH, Liu A, Mills JL, Zhang C, Männistö T, Lu Z, et al. Increased Levels of Copeptin Before Clinical Diagnosis of Preelcampsia. Hypertension. 2014;64(6):1362–7. doi: 10.1161/HYPERTENSIONAHA.114.03762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zulfikaroglu E, Islimye M, Tonguc EA, Payasli A, Isman F, Var T, et al. Circulating levels of copeptin, a novel biomarker in pre-eclampsia. The journal of obstetrics and gynaecology research. 2011;37(9):1198–202. doi: 10.1111/j.1447-0756.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- 17.Santillan MK, Santillan DA, Scroggins SM, Min JY, Sandgren JA, Pearson NA, et al. Vasopressin in preeclampsia: a novel very early human pregnancy biomarker and clinically relevant mouse model. Hypertension. 2014;64(4):852–9. doi: 10.1161/HYPERTENSIONAHA.114.03848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laresgoiti-Servitje E, Gómez-López N, Olson DM. An immunological insight into the origins of pre-eclampsia. Human Reproduction Update. 2010;16(5):510–24. doi: 10.1093/humupd/dmq007. [DOI] [PubMed] [Google Scholar]
- 19.Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CW, Sargent IL, et al. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27(1):56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Formigli L, Papucci L, Tani A, Schiavone N, Tempestini A, Orlandini GE, et al. Aponecrosis: morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. Journal of cellular physiology. 2000;182(1):41–9. doi: 10.1002/(SICI)1097-4652(200001)182:1<41::AID-JCP5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63(6):534–43. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 22.DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999;155(1):293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadyrov M, Kingdom JC, Huppertz B. Divergent trophoblast invasion and apoptosis in placental bed spiral arteries from pregnancies complicated by maternal anemia and early-onset preeclampsia/intrauterine growth restriction. American journal of obstetrics and gynecology. 2006;194(2):557–63. doi: 10.1016/j.ajog.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 24.Mjösberg J, Berg G, Jenmalm MC, Ernerudh J. FOXP3+ Regulatory T Cells and T Helper 1, T Helper 2, and T Helper 17 Cells in Human Early Pregnancy Decidua. Biology of Reproduction. 2010;82(4):698–705. doi: 10.1095/biolreprod.109.081208. [DOI] [PubMed] [Google Scholar]
- 25.Darmochwal-Kolarz D, Leszczynska-Gorzelak B, Rolinski J, Oleszczuk J. T helper 1- and T helper 2-type cytokine imbalance in pregnant women with pre-eclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1999;86(2):165–70. doi: 10.1016/s0301-2115(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 26.Wilczyński JR. Th1/Th2 cytokines balance—yin and yang of reproductive immunology. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2005;122(2):136–43. doi: 10.1016/j.ejogrb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clinical and Experimental Immunology. 1999;117(3):550–5. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darmochwal-Kolarz D, Kludka-Sternik M, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, et al. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. Journal of Reproductive Immunology. 2012;93(2):75–81. doi: 10.1016/j.jri.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima A, Ito M, Yoneda S, Shiozaki A, Hidaka T, Saito S. SHORT COMMUNICATION: Circulating and Decidual Th17 Cell Levels in Healthy Pregnancy. American Journal of Reproductive Immunology. 2010;63(2):104–9. doi: 10.1111/j.1600-0897.2009.00771.x. [DOI] [PubMed] [Google Scholar]
- 30.Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, et al. Systemic Increase in the Ratio between Foxp3+ and IL-17-Producing CD4+ T Cells in Healthy Pregnancy but Not in Preeclampsia. The Journal of Immunology. 2009;183(11):7023–30. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 31.Wilczynski JR. Immunological analogy between allograft rejection, recurrent abortion and pre-eclampsia - the same basic mechanism? Human Immunology. 2006;67(7):492–511. doi: 10.1016/j.humimm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 33.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, et al. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension. 2011;57(5):949–55. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallace K, Morris R, Kyle PB, Cornelius D, Darby M, Scott J, et al. Hypertension, inflammation and T lymphocytes are increased in a rat model of HELLP syndrome. Hypertension in pregnancy : official journal of the International Society for the Study of Hypertension in Pregnancy. 2014;33(1):41–54. doi: 10.3109/10641955.2013.835820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrianov IG, Dobkin AN, Kiselev OI, Okulov VB, Semiglazov VF. [Immunomodulating activity of natural killers in patients with breast tumors using vasopressin and interleukin 2 in vitro] Voprosy onkologii. 1989;35(10):1186–91. [PubMed] [Google Scholar]
- 36.Johnson HM, Farrar WL, Torres BA. Vasopressin replacement of interleukin 2 requirement in gamma interferon production: lymphokine activity of a neuroendocrine hormone. The Journal of Immunology. 1982;129(3):983–6. [PubMed] [Google Scholar]
- 37.Johnson HM, Torres BA. Regulation of lymphokine production by arginine vasopressin and oxytocin: modulation of lymphocyte function by neurohypophyseal hormones. Journal of Immunology. 1985;135(2 Suppl):773s–5s. [PubMed] [Google Scholar]
- 38.Kruimel JW, Pesman GJ, Sweep CG, van der Vliet JA, Liem T, Jansen JB, et al. Depression of plasma levels of cytokines and ex-vivo cytokine production in relation to the activity of the pituitary-adrenal axis, in patients undergoing major vascular surgery. Cytokine. 1999;11(5):382–8. doi: 10.1006/cyto.1999.0440. [DOI] [PubMed] [Google Scholar]
- 39.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, Immunity, and Hypertensive End-Organ Damage. Circulation research. 2015;116(6):1022–33. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wenzel U, Turner JE, Krebs C, Kurts C, Harrison DG, Ehmke H. Immune Mechanisms in Arterial Hypertension. Journal of the American Society of Nephrology. 2015 doi: 10.1681/ASN.2015050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chikanza IC, Grossman AS. Hypothalamic-pituitary-mediated immunomodulation: arginine vasopressin is a neuroendocrine immune mediator. Rheumatology. 1998;37(2):131–6. doi: 10.1093/rheumatology/37.2.131. [DOI] [PubMed] [Google Scholar]
- 42.Chikanza CI, Grossman BA. Neuroendocrine Immune Respinses to Inflammation: The Concept of the Neuroendocrine Immune Loop. Neuroendocrine Immune Loop. 1996;10(2):199–225. doi: 10.1016/s0950-3579(96)80015-x. [DOI] [PubMed] [Google Scholar]
- 43.Chikanza IC, Grossman AB. Reciprocal Interactions Between the Neuroendocrine and Immune System During Inflammation. Rheumatic Disease Clinics of North America. 2000;26(4):693–711. doi: 10.1016/s0889-857x(05)70165-4. [DOI] [PubMed] [Google Scholar]
- 44.Torres BA, Johnson HM. Arginine vasopressin (AVP) replacement of helper cell requirement in IFN-gamma production. Evidence for a novel AVP receptor on mouse lymphocytes. The Journal of Immunology. 1988;140(7):2179–83. [PubMed] [Google Scholar]
- 45.Santillan MK, Leslie KK, Hamilton WS, Boese BJ, Ahuja M, Hunter SK, et al. Collection of a lifetime: a practical approach to developing a longitudinal collection of women’s healthcare biological samples. European journal of obstetrics, gynecology, and reproductive Biology. 2014;179:94–9. doi: 10.1016/j.ejogrb.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American College of O, Gynecologists, Task Force on Hypertension in P. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstetrics and gynecology. 2013;122(5):1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 47.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 48.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 49.Oosterbaan HP, Swaab DF, Boer GJ. Oxytocin and vasopressin in the rat do not readily pass from the mother to the amniotic fluid in late pregnancy. Journal of developmental physiology. 1985;7(1):55–62. [PubMed] [Google Scholar]
- 50.Santillan MK, Pelham CJ, Ketsawatsomkron P, Santillan DA, Davis DR, Devor EJ, et al. Pregnant mice lacking indoleamine 2,3-dioxygenase exhibit preeclampsia phenotypes. Physiol Rep. 2015;3(1) doi: 10.14814/phy2.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 52.Mellor AL, Munn DH. Tryptophan catabolism prevents maternal T cells from activating lethal anti-fetal immune responses. J Reprod Immunol. 2001;52(1–2):5–13. doi: 10.1016/s0165-0378(01)00118-8. [DOI] [PubMed] [Google Scholar]
- 53.Nishizawa H, Hasegawa K, Suzuki M, Achiwa Y, Kato T, Saito K, et al. Mouse model for allogeneic immune reaction against fetus recapitulates human pre-eclampsia. The journal of obstetrics and gynaecology research. 2008;34(1):1–6. doi: 10.1111/j.1447-0756.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 54.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2(9):656–63. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 55.Wilczyński JR, Tchórzewski H, Banasik M, Głowacka E, Wieczorek A, Lewkowicz P, et al. Lymphocyte subset distribution and cytokine secretion in third trimester decidua in normal pregnancy and preeclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2003;109(1):8–15. doi: 10.1016/s0301-2115(02)00350-0. [DOI] [PubMed] [Google Scholar]
- 56.Hsu P, Nanan RKH. Innate and adaptive immune interactions at the fetal-maternal interface in healthy human pregnancy and preeclampsia. Frontiers in Immunology. 2014:5. doi: 10.3389/fimmu.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chatterjee P, Kopriva SE, Chiasson VL, Young KJ, Tobin RP, Newell-Rogers K, et al. Interleukin-4 deficiency induces mild preeclampsia in mice. Journal of Hypertension. 2013;31(7):1414–23. doi: 10.1097/HJH.0b013e328360ae6c. discussion 23. [DOI] [PubMed] [Google Scholar]
- 58.Saito S, Sakai M. Th1/Th2 balance in preeclampsia. J Reprod Immunol. 2003;59(2):161–73. doi: 10.1016/s0165-0378(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 59.LaMarca B, Cornelius D, Wallace K. Elucidating Immune Mechanisms Causing Hypertension During Pregnancy. physiology. 2013;28(4):225–33. doi: 10.1152/physiol.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. American Journal of Physiology - Heart and Circulatory physiology. 2012;303(1):H1–H8. doi: 10.1152/ajpheart.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dinan TG, Cryan J, Shanahan F, Keeling PW, Quigley EM. IBS: An epigenetic perspective. Nat Rev Gastroenterol Hepatol. 2010;7(8):465–71. doi: 10.1038/nrgastro.2010.99. [DOI] [PubMed] [Google Scholar]
- 62.O’Rahilly R. Early human development and the chief sources of information on staged human embryos. European journal of obstetrics, gynecology, and reproductive Biology. 1979;9(4):273–80. doi: 10.1016/0028-2243(79)90068-6. [DOI] [PubMed] [Google Scholar]
- 63.O’Rahilly R, Muller F. Developmental stages in human embryos: revised and new measurements. Cells Tissues Organs. 2010;192(2):73–84. doi: 10.1159/000289817. [DOI] [PubMed] [Google Scholar]
- 64.Otis EM, Brent R. Equivalent ages in mouse and human embryos. Anat Rec. 1954;120(1):33–63. doi: 10.1002/ar.1091200104. [DOI] [PubMed] [Google Scholar]
- 65.Redman CWG, Staff AC. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. American Journal of Obstetrics & gynecology. 2015;213(4) doi: 10.1016/j.ajog.2015.08.003. S9.e1-S9.e4. [DOI] [PubMed] [Google Scholar]
- 66.Carr DJ, DeCosta BR, Jacobson AE, Rice KC, Blalock JE. Corticotropin-releasing hormone augments natural killer cell activity through a naloxone-sensitive pathway. J Neuroimmunol. 1990;28(1):53–61. doi: 10.1016/0165-5728(90)90040-t. [DOI] [PubMed] [Google Scholar]
- 67.Chikanza IC, Petrou P, Chrousos G. Perturbations of arginine vasopressin secretion during inflammatory stress. Pathophysiologic implications. Annals of the New York Academy of Sciences. 2000;917:825–34. doi: 10.1111/j.1749-6632.2000.tb05448.x. [DOI] [PubMed] [Google Scholar]
- 68.Yasin SA, Costa A, Forsling ML, Grossman A. Interleukin-1 beta and interleukin-6 stimulate neurohypophysial hormone release in vitro. J Neuroendocrinol. 1994;6(2):179–84. doi: 10.1111/j.1365-2826.1994.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 69.Mastorakos G, Weber JS, Magiakou MA, Gunn H, Chrousos GP. Hypothalamic-pituitary-adrenal axis activation and stimulation of systemic vasopressin secretion by recombinant interleukin-6 in humans: potential implications for the syndrome of inappropriate vasopressin secretion. The Journal of clinical endocrinology and metabolism. 1994;79(4):934–9. doi: 10.1210/jcem.79.4.7962300. [DOI] [PubMed] [Google Scholar]
- 70.Littlejohn NK, Siel RB, Jr, Ketsawatsomkron P, Pelham CJ, Pearson NA, Hilzendeger AM, et al. Hypertension in mice with transgenic activation of the brain renin-angiotensin system is vasopressin dependent. American journal of physiology Regulatory, integrative and comparative physiology. 2013;304(10):R818–28. doi: 10.1152/ajpregu.00082.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aoyagi T, Koshimizu TA, Tanoue A. Vasopressin regulation of blood pressure and volume: findings from V1a receptor-deficient mice. Kidney international. 2009;76(10):1035–9. doi: 10.1038/ki.2009.319. [DOI] [PubMed] [Google Scholar]
- 72.Koshimizu TA, Nasa Y, Tanoue A, Oikawa R, Kawahara Y, Kiyono Y, et al. V1a vasopressin receptors maintain normal blood pressure by regulating circulating blood volume and baroreflex sensitivity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(20):7807–12. doi: 10.1073/pnas.0600875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koukoulas I, Risvanis J, Douglas-Denton R, Burrell LM, Moritz KM, Wintour EM. Vasopressin receptor expression in the placenta. Biol Reprod. 2003;69(2):679–86. doi: 10.1095/biolreprod.102.013458. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura T, Sakamaki T, Kurashina T, Hoshino J, Sato K, Ono Z, et al. Effect of vasopressin V (OPC-21268) and V2 (OPC-31260) antagonists on renal hemodynamics and excretory function. Life sciences. 1994;55(4):PL67–72. doi: 10.1016/0024-3205(94)00736-5. [DOI] [PubMed] [Google Scholar]
- 75.Schliebe N, Strotmann R, Busse K, Mitschke D, Biebermann H, Schomburg L, et al. V2 vasopressin receptor deficiency causes changes in expression and function of renal and hypothalamic components involved in electrolyte and water homeostasis. Am J Physiol Renal Physiol. 2008;295(4):F1177–90. doi: 10.1152/ajprenal.00465.2007. [DOI] [PubMed] [Google Scholar]
- 76.Rose CE, Jr, Rose KY, Kinter LB. Effect of V1/V2-receptor antagonism on renal function and response to vasopressin in conscious dogs. The American journal of physiology. 1991;260(2 Pt 2):F273–82. doi: 10.1152/ajprenal.1991.260.2.F273. [DOI] [PubMed] [Google Scholar]
- 77.Harrison DG, Marvar PJ, Titze JM. Vascular inflammatory cells in hypertension. Front Physiol. 2012;3:128. doi: 10.3389/fphys.2012.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Itani HA, McMaster WG, Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, et al. Activation of Human T Cells in Hypertension: Studies of Humanized Mice and Hypertensive Humans. Hypertension. 2016;68(1):123–32. doi: 10.1161/HYPERTENSIONAHA.116.07237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jin LP, Fan DX, Zhang T, Guo PF, Li DJ. The costimulatory signal upregulation is associated with Th1 bias at the maternal-fetal interface in human miscarriage. Am J Reprod Immunol. 2011;66(4):270–8. doi: 10.1111/j.1600-0897.2011.00997.x. [DOI] [PubMed] [Google Scholar]
- 80.Zhu X-Y, Zhou Y-H, Wang M-Y, Jin L-P, Yuan M-M, Li D-J. Blockade of CD86 Signaling Facilitates a Th2 Bias at the Maternal-Fetal Interface and Expands Peripheral CD4+CD25+ Regulatory T Cells to Rescue Abortion-Prone Fetuses. Biology of Reproduction. 2005;72(2):338–45. doi: 10.1095/biolreprod.104.034108. [DOI] [PubMed] [Google Scholar]
- 81.Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. The Journal of experimental medicine. 2005;202(2):231–7. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Flow cytometric cell gating. Lymphocytes were isolated from the spleen of saline and AVP infused dams. Dead cells were excluded by forward/orthogonal light scatter characteristics. Single cells were identified via forward scatter-area (FSC-A) versus side scatter-width (SSC-W). CD3+ CD4+ T cells (A) and CD11c+ DCs (B) were gated as shown.
Supplemental Table 1. Human MNC group characteristics. The samples used in this study are a subset of a previously published study (17). Statistical significance was determined using a Student’s t test or chi squared and the minimal level of confidence deemed statistically significant was a p value <0.05.


