Abstract
Purpose of review
Demonstrate the role of environment as a predictor of child health.
Recent findings
The Children’s Health Exposure Analysis Resource (CHEAR) assists the Environmental influences on Child Health Outcomes (ECHO) program in understanding the time-sensitive and dynamic nature of perinatal and childhood environment on developmental trajectories by providing a central infrastructure for the analysis of biological samples from the ECHO Cohort Awards. CHEAR will assist ECHO cohorts in defining the critical or sensitive period for effects associated with environmental exposures. Effective incorporation of these principles into multiple existing cohorts requires extensive multidisciplinary expertise, creativity, and flexibility. The pursuit of life course–informed research within the CHEAR/ECHO structure represents a shift in focus from single exposure inquiries to one that addresses multiple environmental risk factors linked through shared vulnerabilities. CHEAR provides ECHO both Targeted analyses of inorganic and organic toxicants, nutrients, and social-stress markers and Untargeted analyses to assess the exposome and discovery of exposure-outcome relationships.
Summary
Utilization of CHEAR as a single site for characterization of environmental exposures within the ECHO cohorts will not only support the investigation of the influence of environment on children’s health but also support the harmonization of data across the disparate cohorts that comprise ECHO.
Keywords: biomarkers, children’s health, environment, chemicals
Introduction
It is increasingly recognized that the modern diseases of childhood (e.g., autism, asthma, metabolic syndrome, attention deficit hyperactivity disorder, etc.), are not predictable by genetics, but instead arise due to complex interactions among early life environmental factors, that act independently, via mixtures or via modification by genetic susceptibility [1–3]. The true relational model of disease causation likely reflects interactions between genetic/epigenetic variation, environmental stressors, and the consequent endogenous biological responses that may modify or mediate effects of external exposures (Fig. 1). The NIH Environmental Influences on Child Health Outcomes (ECHO) program emphasizes the need to understand the physical, chemical, biological, genetic, and social/behavioral factors that independently, cumulatively, and interactively influence health and development, starting prenatally [1,2]. The NIEHS Children’s Health Exposure Analysis Resource (CHEAR) addresses such challenges by analyzing biological samples to understand environmental exposures and their timing more accurately and comprehensively [4,5]. CHEAR emphasizes the consideration of mixtures of chemical, as well as non-chemical (social/behavioral/dietary/physical) stressors, to more fully understand health risks [6–8]. Environmental exposures affect multiple physiological processes and key regulatory systems simultaneously, particularly in sensitive periods of development, which shape health and development throughout life [9,10] starting in utero. Coordinated functioning of multiple networks of complex processes are necessary for optimal development and the maintenance of health. If one or more key regulatory systems are stressed by external environmental factors, plasticity—the ability of the organism to respond to an insult and still maintain homeostasis—can be overwhelmed, with consequent health impacts. Programming effects can result from shifts in a number of molecular, cellular, and physiological states and their interacting systems. Environmental exposures affect many overlapping homeostatic processes. As the field advances, our ability to integrate comprehensive assessment of environmental exposures with biological response biomarkers characterized by the evolving high-throughput ‘omics’ approaches will enable systematic analysis of exposure and response and the dynamic influences of the environment on health effects throughout the life course [11].
Figure 1. Organization of Environmental Indicators.
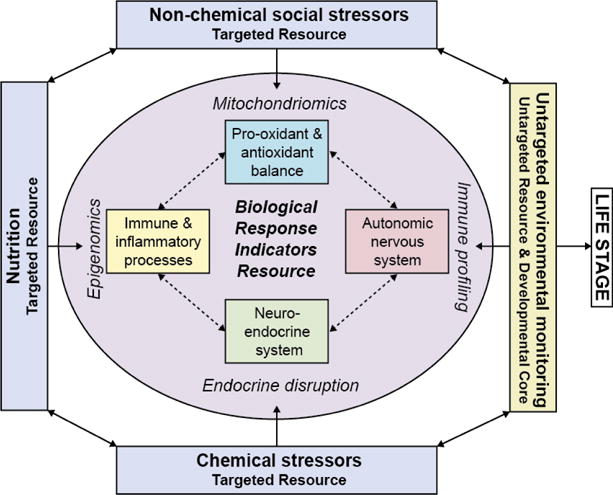
Known and unknown toxicants interact with nutrition and social stressors to elicit a biological response via multiple physiologic pathways. This response depends on the life stage at which exposure occurs. The Targeted Resource will provide validated markers of exposure to known toxicants, nutrients, and stress markers. The Untargeted Resource will undertake exposomic analyses to assess a wide range of unknown chemical signatures that include environmental chemicals, their breakdown products, and biomolecular signatures of disrupted cellular function.
In the last 30 years, significant advances have been made in understanding the influence of environmental factors on children’s health, resulting in policies that have reduced environmental chemical exposure (e.g., removal of lead from gasoline). Nonetheless, there are more than 80,000 chemicals registered for industrial use by the EPA [12], and for most of them, little to no research on their impact on child health has been performed. Just as genomic principles revolutionized the scope and pace of genetics, the CHEAR consortium is designed to quicken the pace of environmental health research and rapidly advance our understanding of the impact of the environmental chemicals, in all their complexity, on child development and physiology. However, many barriers remain to undertaking comprehensive environmental health studies in children. Here, we outline the major challenges facing exposure science and describe how the CHEAR program provides a resource that allows ECHO scientists to overcome these barriers.
First, children are exposed to mixtures of many chemicals, their metabolites, and stress responses in their daily lives; however, most epidemiologic studies examine single toxicants or small groups of compounds seldom taking on complex mixtures. A major barrier to undertaking mixtures research has been a lack of tools to comprehensively assess simultaneous exposure to a variety of environmental chemicals/exposures and the dearth of advanced statistical methods that can unravel the health effects of such chemical mixtures [9]. The CHEAR Targeted Analysis Resources provide a comprehensive suite of analytical capabilities for quantifying metal and organic toxicants, nutritional compounds, and markers of social stressors that can be incorporated into existing child health studies.
The second major barrier to children’s environmental health research is the sheer number of chemicals in the human environment. The concept of the ‘exposome’, i.e., the totality of environmental exposures over the course of a lifetime, captures the scope of what CHEAR is about. Although hundreds of thousands of potential toxicants are present in our environment, only a small portion of them are being studied. It is not possible to keep pace with the rapid rate at which new chemicals are introduced unless we fundamentally change our approach and accept the role of data-driven science as a tool for chemical biomarker discovery [13–16]. Data-driven research has led to an explosion of discoveries in genomics, epigenomics, proteomics, and metabolomics. Exposomics is the new ‘omic’ science to enable the hypothesis-free discovery of exposures associated with health-related endpoints and their subsequent replication in independent populations. The CHEAR Untargeted Analysis Resource will discover and validate these presently unknown toxicants and their metabolic products.
The third major challenge to children’s environmental health research is the lack of methods that can assess exposures which occur during sensitive periods or windows –i.e., developmental stages when individuals have heightened vulnerability to specific environmental exposures [17–19]. In fetal life in particular, profound developmental changes occur on a time scale of days to weeks; consequently, single measures of environmental exposure in blood or urine designed to characterize exposure over a trimester or entire pregnancy will undoubtedly miss critical windows during which a sensitive biological process occurs. Methods that can capture repeated and/or integrated exposure over these time points would rapidly advance our understanding of how environment and developmental biology interact. Thus, the CHEAR Lab Network includes Developmental Cores to overcome these limitations by developing and validating methods for exposure analysis whether that is for emerging exposures (replacements for pesticides, flame, retardants, etc.), novel biological matrices (placenta, cerebral spinal fluid, etc.) or to enable retrospective exposure analysis (teeth, hair, nails). These approaches will provide more precise, unbiased, retrospective biomarkers of multi-chemical exposures at specific life stages, enabling a more comprehensive record of environmental exposures than is currently possible on a study of the magnitude of ECHO. These developmental cores, as well as cores in biological response assays (epigenetics, inflammation biomarkers etc.) are unique to CHEAR and outside the scope of the ECHO-CHEAR collaboration but can nonetheless be accessed by ECHO cohorts through partnerships with CHEAR labs. Fig. 2 illustrates CHEAR’s overall integration into ECHO with its primary role being integration of biomarkers into the larger ECHO program.
Figure 2.
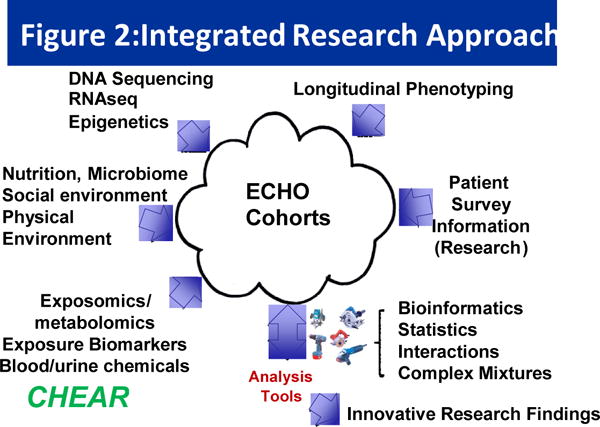
Schematic of various inputs into the ECHO cohorts. CHEAR’s role is to provide biomarker measures of environmental exposure through both targeted and untargeted.
The Mission of CHEAR
CHEAR is based on the concept of “transdisciplinary exposure science.” The consortium integrates expertise from a number of disciplines (e.g., exposure science, analytical chemistry, pediatrics, toxicology, molecular biology and ‘omics’, epidemiology, biostatistics, bioinformatics, data science). While CHEAR provides state-of-the-art analyses of targeted and untargeted biomarkers of exposure in many different matrices (Table 1), it also develops and implements new exposure biomarkers that can measure thousands of chemical signatures simultaneously, or objectively determine the dose and timing of past exposures. Our broad expertise and support for collaborative consultation distinguishes CHEAR from other service oriented or commercial labs and illustrates the value of transdisciplinary approaches that bring together chemistry, pediatric development, and data analytics.
Table 1.
Preliminary list of endogenous and exogenous chemicals CHEAR consortium can measure. The list of chemicals offered will be updated as more assays are developed by the consortium’s developmental cores and the needs of ECHO become more clear.
| Matrices | Elements/Metals | Organics |
|---|---|---|
| Whole Blood Serum Plasma Dried blood Urine Toenails Teeth Hair Bone Milk Semen Nasal Lavage Lung Lavage Placenta Umbilical cord Cord blood Kidney Amniotic fluid Saliva |
Aluminum (Al) Antimony (Sb) Arsenic (As) With speciation Barium (Ba) Cadmium (Cd) Cesium (Ce) Chromium (Cr) Cobalt (Co) Copper (Cu) Iron(Fe), Lead(Pb) Manganese (Mn) Mercury(Hg) With speciation Nickel (Ni) Selenium (Se) Strontium (Sr) Tin (Sn) Vanadium (V) Uranium (U) Zinc (Zn) |
Organochlorine Pesticides:hexachlorocyclohexane, hexachlorocyclohexane (Lindane) residues, dieldrin, hexachlorobezene, heptachlor & chlordane residues, mirex, DDT residues Organophosphate pesticides and metabolites: diazinon, ethylparathion, chlorpyrifos, malathion Environmental tobacco smoke markers: nicotine, cotinine, hydroxycotinine, nitrosamines Brominated flame retardants: polybrominated diphenyl ethers; halogenated organophosphates Polychlorinated biphenyls (PCBs) Environmental phenols and phthalates: bisphenol-A, triclosan, parabens, phthalate monoesters (ethyl – decyl) and their validated oxidative metabolites Polycyclic aromatic hydrocarbons (PAHs) Polyfluoroalkyl Chemicals; PFOS PFOA, PFGxS Water and fat soluble vitamins Cortisol, sex hormones, thyroid hormones |
Engagement with ECHO Cohorts
A major strength of CHEAR is the combination of interdisciplinary expertise and years of children’s environmental health research experience that can be offered to ECHO [20–25]. The integration of CHEAR into ECHO will facilitate well-powered analyses of the impact of exposures to mixtures of environmental chemicals/agents/? in children. It would be naïve to think that fundamental principles of child development, exposure biology, and study design that will apply can be addressed post hoc. Thus, all cohorts serviced by our Hubs undergo a rigorous pre-sample analysis consultation process, in order to maximize the potential benefits of the exposure biomarkers to be assayed. This discussion is critical, as it combines the in-depth knowledge of the ECHO cohort investigators on their populations and the outcome data available with CHEAR’s expertise in child development, exposure science, analytical chemistry, and pediatric phenotyping. Simply adding an exposure biomarker to an existing cohort without considering these factors would reduce the effectiveness of the program and limit its ability to meet its goals. Cohort clients are active participants in this process. Although the client/PI often have more expertise than CHEAR personnel in the scientific fields relevant to their Cohort’s aims (psychology, asthma, obesity, etc.) s/he is likely to unfamiliar with all relevant issues in exposure analysis, analytical chemistry, toxicology, or environmental health. Collaboration is necessary to merge exposure science into ECHO and avoid pitfalls of a scientific, analytic, biologic nature as neither field can do this work alone. To provide expert counsel, it is incumbent upon CHEAR to comprehensively understand the client’s study, its design, the types of biological specimens collected, their storage conditions, the participants’ age at time of collection, and unusual characteristics.
Targeted Analysis
The hallmark of targeted analysis is the hypothesis-driven application of state-of-the-art biomonitoring for specific chemical analytes both endogenous and exogenous, known or suspected to affect or modify risk for health outcomes in children. CHEAR run analyses provide quantitative accuracy, sensitivity, and specificity due to rigorous quality control and the incorporation of standard benchmark materials which facilitate the direct comparison of analyses across studies. The CHEAR laboratory hubs focus is on three key categories of exposures: 1) inorganic biomarkers, including metals (e.g., lead) metalloids (e.g. arsenic) and minerals (e.g. selenium); 2) organic compounds, including plastic stabilizers, halogenated hydrocarbons, organic pesticides, tobacco smoke, and polycyclic aromatic hydrocarbons; and 3) nutritional and social environment and stress biomarkers, including cortisol and lipid profiles in various media. We encourage a broad range of assays be run whenever feasible (barring sample type, condition, study design, or toxicologic concerns), to determine the potential interactions implicit in simultaneous exposure to multiple chemicals or the interactions among chemicals and social stressors or nutrition (or its deficit). Although studies of environmental mixtures are complex, they represent the real-life scenario that children, pregnant women, and their families face daily.
Untargeted Analysis
Most studies of toxic chemicals have used a reductionist, “single chemical at a time” approach, but humans are not exposed to a single chemical at a time. Given the multifaceted nature of biologic interactions, it is difficult to conceive that one environmental exposure can ever primarily account for the complexity of the current “morbidities of childhood.” In addition, there are certainly chemicals/metabolites important to disease pathogenesis or child development whose existence is not yet known, and the “single chemical at a time” approach might never comprehensively identify such chemicals. Because population studies take years to conduct and are expensive, methods that have the capacity to measure the exposome have the potential to allow the research community to more fully understand how chemicals and environment affect children’s health.
To contextualize the scope of this issue, as mentioned earlier, more than 80,000 chemicals are registered with the EPA for commercial use in the U.S [12]. If we are to tackle the challenge of understanding how our environment impacts health/disease, scholars are asserting that we must rethink how we conduct environmental health research [26,27]. Just as genetics capitalized on the genome-wide scan to advance gene “discoveries,” environmental health studies utilizing untargeted scans allow for screening of the exposome/ metabolome (or subsets of it) to “discover” novel environmental risk factors [15] and their effects [13]. Although we cannot measure the entirety of the exposome, we have demonstrated the potential of applying untargeted metabolomics technologies to discover possible unknown exposures associating with outcome signatures. Given access to the multiple populations within ECHO, CHEAR will have the opportunity to mirror genomic studies in which hypotheses are generated in one population and then test those hypotheses in separate populations, as a validation/replication method [28,29].
Thus, CHEAR’s Untargeted Resources provide a comprehensive unbiased approach to biomarker discovery by using exposomic technologies such as: metabolomics, which profiles the unique breakdown products of both exogenous and endogenous molecules; lipidomics, to profile the effects on the molecular building blocks of cells; and adductomics, to quantify modification of, or adducts to, biomolecules.
Benefits of Dedicated Exposure Analysis Resources
Epidemioology studies commonly use Core labs that use medium to high throughput validated assays, often through contracts with commercial vendors. Such labs differ in key ways from research laboratories that service only a single researcher, or handful of lab faculty. Perhaps more importantly, a consortium, such as ECHO will have special needs that even a core lab or commercial vendor cannot typically provide. For example, the sheer volume of samples is likely to overwhelm all but a handful of individual labs. Even if capacity exists, careful attention to batch effects, inter-instrument/inter-lab variability is needed to reduce bias introduced by nonrandom measurement error. The STROBE-ME statement [29] outlines many of the critical special requirements for laboratories used for epidemiologic studies, all of which CHEAR follows. Of greatest importance is the need for careful consideration of biological sample processing and storage, and the validity/reliability of biomarker assays. The complexity of the CHEAR network mandates thoughtful consideration of laboratory information management and tracking such as the OpenSpecimen software system, and OpenLAB software. These integrated programs support cross-laboratory data and information management. Laboratory information management systems (LIMS) and sample tracking systems tailor the electronic management system specifically to the needs of both internal users and external stakeholders. CHEAR runs extensive QA/QC procedures, including Round Robin studies in collaboration with all six lab hubs to determine the practical limits for detecting and quantifying each of the proposed analytes. These efforts address accuracy, precision and reproducibility, between labs as well as the within lab variability. CHEAR has a collection of pooled quality assurance specimens that will be included in all analyses supported by the consortium. These “technical replicates” offer the ability to adjust results for both batch effects and inter-laboratory variability. This is an important and unique feature that an ad hoc collection of labs could not offer. As additional Standard Reference Materials (SRMs) become available from the National Institute of Standards and Technology (NIST), CHEAR will validate its methods using NIST-recommended concentrations for these analytes in the respective biological matrices. Finally, because we are a lab consortium, conducting QA/QC in tandem, this allows for unprecedented coordination of methods, development of common standards and the ability to understand and adjust for too often unseen sources of bias.
Conclusion
ECHO represents a shift in focus from single exposure–disease inquiries to one that addresses multiple risk factors linked through shared vulnerabilities and shared biological networks. The life-course approach emphasizes the need to understand the chemical, biological, genetic, and social/behavioral factors that independently, cumulatively, and interactively influence health and development, starting prenatally and continuing through each life stage [8,30]. CHEAR also emphasizes the analysis of mixtures of chemical as well as nonchemical (social, nutritional) stressors [6–8]. We developed statistical approaches to assess the combinability of existing studies with a variety of exposure-sampling protocols, covariate data, child health phenotypes, and archived biological samples. Given CHEAR’s expertise and analytic capabilities, we can provide ECHO with intellectual and analytical resources needed to address the challenges of cohort harmonization for chemical exposure. Such work can inform the upstream early life environmental cause of complex chronic diseases of childhood (e.g., perinatal health, obesity, respiratory health, neurodevelopment) while setting the stage for large scale, big data genome by exposome interaction research.
Key Points.
Biomarkers can both inform environmental exposure and their biological effects
Early life environmental exposures can predict health and development decades later
Lab consortiums can reduce error and bias in biomarker research compared to ad hoc laboratory use
Acknowledgments
Timothy Fennell, Research Triangle Institute, RTP, North Carolina, Kenneth Aldous Wadsworth Center, Albany New York, John Meeker University of Michigan, Ann Arbor MI, Lisa Peterson, University of Minnesota, Minneapolis MN, Gary Miller, Emory University, Atlanta GA, Susan Teitelbaum, Icahn School of Medicine at Mount Sinai, New York NY, Barbara O’Brien, Westat Inc. Rockville MD, Robert Wright, Icahn School of Medicine at Mount Sinai, New York NY. Claudia Thompson NIEHS, and David Balshaw NIEHS, Research Triangle Park, NC.
Funding
Funded in part by NIH Grants U2CES026561; UG3OD023337; UG3OD023320; U2CES026555; U2CES026560; U2CES26553; U2CES026533;U2CES026542; U2CES026544; U24ES026539.
Footnotes
COI
No relevant conflicts to be reported.
References and recommended reading
Papers of particular interest, published within the annual period of review are highlighted as:
*Of special interest
**Of outstanding interest
- 1.Robinson O, Vrijheid M. The Pregnancy Exposome. Curr Environ Health Rep. 2015;2:204–213. doi: 10.1007/s40572-015-0043-2. [DOI] [PubMed] [Google Scholar]
- 2.Wright RO. Environment, susceptibility windows, development, and child health. Curr Opin Pediatr. 2017;29:211–217. doi: 10.1097/MOP.0000000000000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller GW, Jones DP. The nature of nurture: refining the definition of the exposome. Toxicol Sci. 2014;137:1–2. doi: 10.1093/toxsci/kft251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balshaw DM, Collman GW, Gray KA, et al. The Children’s Health Exposure Analysis Resource: enabling research into the environmental influences on children’s health outcomes. Curr Opin Pediatr. 2017;29:385–389. doi: 10.1097/MOP.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stingone JA, Buck Louis GM, Nakayama SF, et al. Toward Greater Implementation of the Exposome Research Paradigm within Environmental Epidemiology. Annu Rev Public Health. 2017;38:315–327. doi: 10.1146/annurev-publhealth-082516-012750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gennings C, Sabo R, Carney E. Identifying subsets of complex mixtures most associated with complex diseases: polychlorinated biphenyls and endometriosis as a case study. Epidemiology. 2010;21:S77–S84. doi: 10.1097/EDE.0b013e3181ce946c. [DOI] [PubMed] [Google Scholar]
- 7.Teuschler L, Klaunig J, Carney E, et al. Support of science-based decisions concerning the evaluation of the toxicology of mixtures: a new beginning. Regulatory Toxicology Pharmacology. 2002;36:34–39. doi: 10.1006/rtph.2002.1570. [DOI] [PubMed] [Google Scholar]
- 8.Block ML, Elder A, Auten RL, et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33:972–984. doi: 10.1016/j.neuro.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlin DJ, Rider CV, Woychik R, et al. Unraveling the health effects of environmental mixtures: an NIEHS priority. Environ Health Perspect. 2013;121:A6–8. doi: 10.1289/ehp.1206182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eriksson JG. Developmental Origins of Health and Disease - from a small body size at birth to epigenetics. Ann Med. 2016;48:456–467. doi: 10.1080/07853890.2016.1193786. [DOI] [PubMed] [Google Scholar]
- 11.Vineis P, van Veldhoven K, Chadeau-Hyam M, et al. Advancing the application of omics-based biomarkers in environmental epidemiology. Environ Mol Mutagen. 2013;54:461–467. doi: 10.1002/em.21764. [DOI] [PubMed] [Google Scholar]
- 12.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 13.Kortenkamp A, Faust M, Scholze M, et al. Low-level exposure to multiple chemicals: reason for human health concerns? Environmental health perspectives. 2007;115(Suppl 1):106–114. doi: 10.1289/ehp.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wild CP. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiology Biomarkers and Prevention. 2005;14:1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 15.Rappaport SM, Barupal DK, Wishart D, et al. The blood exposome and its role in discovering causes of disease. Environ Health Perspect. 2014;122:769–774. doi: 10.1289/ehp.1308015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Bello GA, Arora M, Austin C, et al. Extending the Distributed Lag Model framework to handle chemical mixtures. Environ Res. 2017;156:253–264. doi: 10.1016/j.envres.2017.03.031. Combines time dependent analysis of mixtures with an index of mixture effects allowing researchers to understand the role of susceptiblity windows in predicting mixture effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren JL, Son JY, Pereira G, et al. Investigating the Impact of Maternal Residential Mobility on Identifying Critical Windows of Susceptibility to Ambient Air Pollution During Pregnancy. Am J Epidemiol. 2017 doi: 10.1093/aje/kwx335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B, Du Y. Cadmium and its neurotoxic effects. Oxid Med Cell Longev. 2013;2013:898034. doi: 10.1155/2013/898034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu SH, Bobb JF, Lee KH, et al. Lagged kernel machine regression for identifying time windows of susceptibility to exposures of complex mixtures. Biostatistics. 2017 doi: 10.1093/biostatistics/kxx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin C, Smith TM, Bradman A, et al. Barium distributions in teeth reveal early-life dietary transitions in primates. Nature. 2013;498:216–219. doi: 10.1038/nature12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Liu SH, Bobb JF, Lee KH, et al. Lagged kernel machine regression for identifying time windows of susceptibility to exposures of complex mixtures. Biostatistics. 2017 doi: 10.1093/biostatistics/kxx036. One of the first studies to demonstrate machine learning can be implemented to assess the role of chemical mixtures predicting child health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claus Henn B, Ettinger AS, Schwartz J, et al. Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology. 2010;21:433–439. doi: 10.1097/ede.0b013e3181df8e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright RJ, Fisher K, Chiu YH, et al. Disrupted prenatal maternal cortisol, maternal obesity, and childhood wheeze. Insights into prenatal programming. Am J Respir Crit Care Med. 2013;187:1186–1193. doi: 10.1164/rccm.201208-1530OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zota AR, Ettinger AS, Bouchard M, et al. Maternal blood manganese levels and infant birth weight. Epidemiology. 2009;20:367–373. doi: 10.1097/EDE.0b013e31819b93c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niedzwiecki MM, Miller GW. The Exposome Paradigm in Human Health: Lessons from the Emory Exposome Summer Course. Environ Health Perspect. 2017;125:064502. doi: 10.1289/EHP1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarigiannis DA. Assessing the impact of hazardous waste on children’s health: The exposome paradigm. Environ Res. 2017;158:531–541. doi: 10.1016/j.envres.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 28*.Andra SS, Austin C, Wright RO, et al. Reconstructing pre-natal and early childhood exposure to multi-class organic chemicals using teeth: Towards a retrospective temporal exposome. Environ Int. 2016;83:137–145. doi: 10.1016/j.envint.2015.05.010. Illustrates the potential of novel biomarkers to retrospectively measure the chemical exposome. This may prove critical in assessing how environment drives health as it allows for case control studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallo V, Egger M, McCormack V, et al. STrengthening the Reporting of OBservational studies in Epidemiology–Molecular Epidemiology (STROBE-ME): an extension of the STROBE Statement. PLoS Med. 2011;8:e1001117. doi: 10.1371/journal.pmed.1001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright RJ. Moving towards making social toxins mainstream in children’s environmental health. Curr Opin Pediatr. 2009;21:222–229. doi: 10.1097/MOP.0b013e3283292629. [DOI] [PMC free article] [PubMed] [Google Scholar]


