Abstract
Alzheimer’s disease (AD) and type 2 diabetes mellitus (T2DM) are two progressive and devastating health disorders afflicting millions of people worldwide. The probability and incidence of both have increased considerably in recent years consequent to increased longevity and population growth. Progressively more links are being continuously found between inflammation and central nervous system disorders like AD, Parkinson's disease, Huntington's disease, motor neuron disease, multiple sclerosis, stroke, traumatic brain injury and even cancers of the nervous tissue. The depth of the relationship depends on the timing and extent of anti- or pro-inflammatory gene expression. Inflammation has also been implicated in T2DM. Misfolding and fibrillization (of tissue specific and/or non-specific proteins) are features common to both AD and T2DM and are induced by as well as contribute to inflammation and stress (oxidative/glycation). This review appraises the roles of inflammation and abnormalities in the insulin signaling system as important shared features of T2DM and AD. The capacity of anti-cholinesterases in reducing the level of certain common inflammatory markers in particular if they may provide therapeutic potential to mitigate awry mechanisms leading to AD.
Keywords: Alzheimer’s disease, Anti-cholinesterase, Butyrylcholinesterase, Inflammation, Type 2 diabetes mellitus
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a heterogeneous, multi-factorial and polygenic disorder and one of the most common metabolic diseases. Its incidence is reaching epidemic proportions, and its prevalence increases with age [1]. Impaired insulin action and secretion in T2DM generates a general mayhem specific of this disease [2]. Evidence suggests that T2DM represents a risk factor for developing Alzheimer’s disease (AD) [3]. AD, a progressive neurodegenerative disorder of hitherto unknown aetiology leads progressively to severe cognitive incapacity and ultimately to death, has been described as the pandemic of the 21st century [4]. How T2DM increases the likelihood of AD is currently an area of extensive research.
Stress (inflammatory, oxidative, nitrosative), progressive amyloidosis, atherosclerosis, obesity, metabolic syndrome are pathological mechanisms or risks related to T2DM and AD [5, 6]. In fact metabolic syndrome, T2DM and AD are considered as low-grade systemic inflammatory conditions as recent studies have demonstrated associations between elevated levels of circulating acute phase inflammatory markers, typified by C-reactive protein (CRP), and indices of insulin resistance and the development of T2DM and AD [7–9]. Moreover, elevated butyrylcholinesterase (BuChE) may represent a key trigger factor of the inflammatory processes seen in T2DM and AD consequent to down-regulation of the "cholinergic anti-inflammatory pathway" [10]. In an interesting recent review article [11], the evidence for the association of vascular risk factors such as T2DM, hypertension, obesity and dyslipidemia in relation to dementia was systematically reviewed on the basis of longitudinal population-based studies. These risk factors often occur concomitantly and, although each has been linked to an elevated risk of dementia, its ambiguous as which imposes the greatest risk and when this occurs. During midlife, the population attributable risk of dementia was greatest for hypertension, which accounted for up to 30% of cases that developed thereafter [11]. During later life diabetes emerged as conveying the highest impact on dementia [11]. Clearly, vascular risk factors have long been known to favor the development of dementia, with age and the duration of exposure as important variables. Understanding the mechanisms associated with T2DM, hypertension, obesity and dyslipidemia may aid in offsetting their adverse actions as well as developing potential treatment strategies [11].
Impaired glucose tolerance is likewise associated with impaired cognition, independent of age, and there are reports of increased risk of AD with diabetes [12, 13]. Whereas the localization and pathological features of diabetes and AD differ, there are numerous commonalities. Examples amongst many are that both are chronic progressive conditions that are present before a diagnosis is made or treatment initiated. Insulin resistance (IR), impaired insulin receptor, and insulin growth factor (IGF) signaling, glucose toxicity, advanced glycation end products (AGEs) and the receptor for advanced glycation end products (RAGEs) interactions, cerebrovascular injury, vascular inflammation, are some of the mechanisms lying on the way to T2DM manifestation/complications that influence the pathogenesis of AD [14].
ABNORMAL INSULIN SIGNALING IN AD
T2DM is characterized by IR, hyperinsulinemia and glucose intolerance. The consequent hyperglycemia induces oxidative stress and non enzymatic glycation of key regulatory proteins leading to potential malfunctions in those proteins. Abnormal glucose utilization and insulin resistance or deficiency is also early events in AD pathology that can be present without any correlation to the presence of diabetes. Consequent to this, the term ‘type 3 diabetes’ has been coined by some for AD. In a similar manner, glycation induced conformational changes have been shown to initiate the fibrillization of otherwise non pathogenic proteins that leads to the hypothesis that diabetes is a conformational disease [15].
In accord with evidence suggesting that diabetics have a higher risk for cognitive dysfunction, depression and memory impairment [16], animal models of T2DM have been described to have defective transport, uptake and neuronal concentrations of insulin [17–19]. Indeed, the induction of diabetes accelerates and worsens cognitive dysfunction in transgenic AD animal models, emphasizing the role of altered insulin pathways in AD brain [20].
The brain is clearly an insulin-sensitive organ, where insulin plays a significant role in normal brain physiology [21], and disturbances in its signaling can impact synaptic plasticity as well as cell viability. Insulin receptors are localized throughout the brain, with particularly dense distributions in the hippocampus, entorhinal cortex and hypothalamus [22]. Within brain, the binding of insulin to its receptor brings about rapid autophosphorylation of the tyrosine residues in the β-subunit of the receptor and leads to activation of several second-messenger transduction pathways. One such pathway, the neural Shc/MAP (Src homology collagen mitogen-activated protein) kinase pathway induces gene expression required for neuronal cell and synapse growth, maintenance and repair processes besides modulating the hippocampal synaptic plasticity important for learning and memory [23]. In an alternate pathway, the insulin receptor substrates 1 and 2 (IRS-1 and IRS-2) bind to phosphatidylinositol 3-kinase (PI3K), which stimulates glucose transport and inhibits mitochondrial DNA damage, apoptosis induced death by mechanisms involving the activation of Akt/protein kinase B and the inhibition of glycogen synthase kinase-3β (GSK-3β) [24–26]. These processes are known to influence synaptic plasticity and memory consolidation, retrieval and extinction of contextual memory, and Aβ-induced memory loss [27–29]. This same pathway likewise induces the synthesis of nitric oxide, additionally affecting learning and memory processes [30]. Thus, any defects in insulin signaling and IR may have adverse consequences on synaptic maintenance and remodelling through direct effects on energy production and glucose uptake. Notably, AD patients have reduced insulin levels and a lowered expression of insulin receptors and IRS proteins [31]. Correction of insulin levels in AD subjects has also been correlated with improved cognition [31, 32]. Furthermore, insulin has been also described to regulate tau phosphorylation [33].
Insulin resistance/deficiency results in an inhibition of the PI3K/Akt pathway, a rise in oxidative stress and hence hyperactivation of GSK-3β [5, 34]. This can stimulate tau protein hyperphosphorylation. In this context, it is worth noting that elevated oxidative stress is an established feature of both T2DM and AD. Insulin may have regulatory action on both amyloid-β (Aβ) and amyloid precursor protein [35].
As IDE (insulin degrading enzyme) is known to degrade insulin along with Aβ, at high insulin concentrations that may be prevalent due to IR (e.g., hyperinsulinemia), insulin may compete with Aβ for IDE in brain, including within the hippocampus, to cause a decline in the normal clearance of Aβ, leading to Aβ brain accumulation and a higher risk of AD [36]. Insulin may also promote Aβ secretion resulting in excessive Aβ deposition in senile plaques [37]. Zhao et al. [38] have reported that Aβ soluble oligomers (AβOs) may initiate the loss of insulin receptors from the neuronal membranes. This may again, potentiate IR, impacting Aβ clearance and its deposition. Hence, IR and Aβ may act in cyclical manner exacerbating the process. Taken together, research suggests that insulin signaling plays a central role in learning and memory, and, in particular, in deficits when signaling is aberrant.
Bomfim et al. [39] experimentally demonstrated that insulin signaling is substantially disrupted in AD brain, rodents and non-human primate models of the disease. In accord with earlier reports, they proposed that in AD brain insulin signaling becomes stalled by processes leading to IR, as it does in diabetes. Amongst key mechanisms, c-Jun NH2-terminal kinase (JNK) activity is induced in conditions of chronic hyperglycaemia and IR, which ultimately leads to oxidative stress and apoptosis of pancreatic β-cells [40]. It has been suggested that AβOs activate JNK/tumor necrosis factor-α (TNF-α) pathways leading to the abnormal serine phosphorylation of IRS-1, which thereby blocks the downstream insulin signaling [41]. A high immunoreactivity for IRS-1pSer636/639 was detected in the hippocampal CA1 region in human AD brains compared to normal subjects, suggesting that serine phosphorylation of IRS-1 triggers central nervous system IR [39]. In hippocampal neuronal cell cultures AβOs induced abnormal elevations in IRS-1pSer636/639 levels and blocked IRS-1 tyrosine phosphorylation. Similar findings were additionally obtained in non-human primates. Using JNK inhibitors or transfecting the neuronal cells with GFP-fused dominant negative JNK, it was further demonstrated that JNK plays a pivotal role in mediating the neuronal IR in response to AβOs. In peripheral IR, JNK activity appears to be mediated by TNF-α. In AD also, TNF-α has been found to mediate the JNK signaling pathway [39]. Two crucial regulators of peripheral IR and stress sensitive kinases, are the double-stranded RNA dependent protein kinase and IκB kinase (IKK) that are likewise activated by AβOs. This provides additional support for a striking resemblance between inflammation-associated brain IR in AD and chronic inflammation-induced IR in peripheral tissues in T2DM (that is discussed later) [39]. Extending this work, Talbot et al., [42] elegantly probed insulin and IGF-1 responsiveness in 2 brain regions - the hippocampus and cerebellar cortex that are differentially impacted by AD - retrieved from early post mortem AD cases and age-matched controls. By the use of an ex vivo stimulation protocol and near-physiological doses of insulin or IGF-1, the AD brain was determined to be insulin and IGF-1 resistant in the absence of T2DM. Two candidate biomarkers of brain insulin resistance were identified that are common to systemic IR and T2DM; specifically, elevated levels of IRS-1pS616 and IRS-1pS636/639 [42] in concordance with Bomfim et al. [39]. The bottom line from both studies is that insulin orchestrates numerous neuronal processes that impact synaptic plasticity, involving both the expression as well as trafficking of key receptors (e.g., N-methyl-D-aspartate, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and gamma-aminobutyric acid), the translation of critical scaffolding proteins, such as PSD-95, which are essential in regulating postsynaptic density. The development of insulin resistance at the level of the insulin receptor in AD, as a consequence of AβOs, neuroinflammation and other mechanisms, may down regulate insulin mediated signaling pathways at postsynaptic sites within the hippocampus and other key brain regions engaged in memory consolidation and retrieval.
Extending their results, Bomfim et al. [39] reported that insulin and the clinically approved T2DM drug exendin-4 (Byetta/Bydurion), (a long-acting glucagon-like peptide-1 (GLP-1) agonist that stimulates the insulin signaling pathway via the GLP-1 receptor that is present both on pancreatic-β cells as well as on neurons within the brain [43]), prevented the AβO-dependent IRS-1pSer636/639 increase and IRS-1pTyr465 decrease. Prior studies with exendin-4 have demonstrated that it provides neurotrophic and neuroprotective actions in cellular and animal models of a host of neurological disorders, including AD, Parkinson’s disease, Huntington's disease, motor neuron disease, peripheral neuropathy, stroke and traumatic brain injury [43–51]. Confirming prior studies [49–51], exendin-4 treatment in AD transgenic mice in the Bomfim et al., study [39] induced a reduction in brain amyloid plaque load and improved memory and cognition [39]. These prior studies, moreover, demonstrated that the initiation of diabetes in AD transgenic mice caused an elevated induction of Aβ and tau, that was mitigated by exendin-4 [49].
In addition, it has been suggested that the JNK pathway induction, likewise, leads to phosphorylation of c-Jun and tau in brains of AD patients [52–54]. The protein JIP-1 [JNK-interacting protein 1, also known as ‘islet brain 1’ (IB1) protein] has been found co-localized with JNK, hyperphosphorylated tau and amyloid deposits in neurofibrillary tangles in the brain and pancreatic islets, pointing to a further potential link between AD and T2DM [55].
Obesity is a major determinant of IR and hence T2DM. Studies have associated dementia/AD with obesity [56]. However, the exact molecular mechanisms linking the two remain underinvestigated. It is suggested that disturbances in insulin cause hyperactivation of adipocyte hormone sensitive lipase, which eventually leads to high levels of free fatty acids (FFA). These FFAs are pro-inflammatory, proamyloidogenic, and reduce the Aβ clearance [as reviewed in 2]. Hence, new comprehensive research approaches towards dissecting mechanisms that underpin the coexistence of T2DM and AD may be helpful in not only understanding the pathophysiologic similarities between these two disorders but also ways to potentially offset or ameliorate these two progressive disorders.
LOW-GRADE SYSTEMIC INFLAMMATION IN AD AND T2DM
Evidence supports the concept that AD and T2DM can be considered related systemic inflammatory conditions that can potentially be mitigated, at least in part, by normalizing common pathways associated with this systemic inflammation. As an example, alterations in the levels of key inflammatory markers, such as CRP, TNF-α, interleukin-6 (IL-6) and lipid peroxides, are common to T2DM and AD.
Changes in human behaviour and lifestyle over the last century have resulted in burgeoning rates of obesity and metabolic syndrome, leading to a dramatically increasing prevalence of T2DM. The mechanisms that tie obesity to various metabolic abnormities in T2DM, such as insulin resistance, dyslipidemia and hyperglycemia, remain to be fully elucidated. However, research has linked obesity to the manifestations of diabetes through various fat-derived proteins, termed “adipocytokines”, including TNF-α, interleukin 1 (IL-1), IL-6, plasminogen activator inhibitor-1 (PAI-1), monocyte chemoattractant protein-1 (MCP-1) and others [8]. The deleterious effects of these include down-regulation of insulin-action at the level of target tissues (muscle, liver and adipose), acceleration of inflammatory processes as well as induction of apoptosis of pancreatic β-cells [57]. Moreover, several transcription factors and kinases, such as JNK, IKK also participate in processes [58]. Hence, these inflammatory cytokines, adipocytokines and transcription factors result in hyperglycemia, which is the biochemical hallmark of T2DM. Notably, an inflammatory response is likewise involved in atherosclerosis, which can lead to diabetic cardiovascular complications, stroke and AD [59, 60]. It has been shown that pancreatic β-cells from T2DM subjects have elevated IL-1β expression and reduced levels of the natural inhibitor of the proinflammatory effect of IL1β, the endogenous IL-1β receptor antagonist, IL-1RA [61]. Such high levels of the proinflammatory cytokine IL-1β are associated with islet cell damage, impaired insulin secretion and apoptosis [62, 63]. Conversely, IL-1RA treatment at high doses improves glucose sensitivity, insulin processing, and suppresses inflammation and infiltration of immune cells in a diabetic rat model [64]. Diabetes is associated with the production of AGE, the derivatives of lipids, proteins and nucleic acids. AGE interacts with their receptors RAGE and this interaction induces reactive oxygen species mediated inflammatory responses that are widely considered as the major culprits behind diabetic complications [65–67]. Different molecular forms of RAGE are present, which include the alternatively spliced variant, esRAGE (endogenous secretory RAGE) and the enzymatic cleavage generated sRAGE (soluble RAGE). esRAGE competes for the full length membrane bound form for AGEs and prevents the initiation of downstream inflammatory responses [68]. sRAGE has been found to be negatively correlated with the full length form in monocytes from T2DM patients, suggesting its protective role during inflammation [69, 70]. Toll like receptor (TLR) signal pathways have also been implicated in mediating inflammation associated with diabetes. Particularly notable, in this regard, are TLR2 and TLR4 whose expression increases in the presence of high glucose and FFAs [71, 72] and whose effects are mediated by NFκB activation [73]. CD36 (oxidized low-density lipoprotein receptor, oxLDL receptor), a co-receptor for TLR2 and TLR6 heterodimers in addition to TLR4 and TLR6 heterodimers [74], is likewise induced by high glucose, oxLDL, FFAs and low high density lipoprotein, cholesterol concentrations in monocytes/macrophages. This promotes vascular oxidative injury, leukocyte adhesion, and atherogenesis [75]. To the contrary, CD36 knockout transgenic mice exhibit improved insulin signaling and reduced inflammation [76, 77].
It has been widely shown that inflammatory mechanisms are classically associated with AD [78]. In support of this, elevated plasma and cerebrospinal fluid levels of inflammatory cytokines (IL-1, IL-6 as well as TNF-α) have been reported in patients with AD [79–82]. Studies have revealed that systemic administration of IL-1 lowered extracellular acetylcholine (ACh) levels within the hippocampus and suggest that raised levels of IL-1 could be involved in AD consequent to ACh reductions [83]. In support of this, raised IL-1 levels in brain, induced by intracerebroventricular IL-1β administration, induced a significant reduction in hippocampal ACh release (that could be blocked by IL-1 RA) and this decline correlated both with memory deficits and a lowered mRNA expression of hippocampal nerve growth factor (NGF) [84].
The neurotrophin NGF is indispensable for the maintenance and differentiation of basal forebrain cholinergic neurons [85], which are one of the major neuronal populations affected that progressive degeneration in AD. It has become increasingly clear that alterations in NGF transport and signaling may play a key role both in development and progression of sporadic AD, leading to the proposition that "neurotrophic imbalance" (i.e., too little mature NGF and too much unprocessed pro-NGF, as found in AD11 mice) is an upstream driver for AD. Lowered levels of NGF as well as other neurotrophic factors can impact APP expression as well as its processing to Aβ, in addition to the induction of neuroinflammation that, in turn, impacts NGF expression and ACh release; thereby providing a self-propagating destructive cycle of events [85]. An alike NGF transport failure leading to degeneration of basal forebrain cholinergic neurons, and elevations in brain APP levels has been described in the Ts65Dn mouse model of Down’s syndrome [86].
The involvement of a heightened neuroinflammatory process in AD is further supported by the observation that inhibiting or neutralizing the actions of TNF-α and other inflammatory cytokines has been beneficial to AD patients [87, 88]. In this regard, studies in subjects with mild cognitive impairment (MCI) that progress to develop AD suggest that raised CSF TNF-α levels represent an early event, and their increase correlates with disease progression [89]. In accord with this, Janelsins and colleagues [90] noted an increased expression of TNF-α transcripts within the entorhinal cortex of transgenic AD mice at 2 months, occurring before the appearance of classical amyloid and tau pathology, and this increase correlated with the onset of cognitive deficits in these mice [91]. These studies, together with others reporting that (i) TNF-α polymorphisms that increase TNF-α production may elevate AD risk, particularly in patients carrying one or more apolipoprotein E ε4 alleles [92–94] and that (ii) genetic ablation of TNF-α receptor 1 (TNFR1) in APP23 AD mice [95] or a selective lowering of secreted TNF-α brain levels in AD transgenic mice [96–98] reduces AD progression, reinforce the view that TNF-α inhibition/reduction may be beneficial treatment strategy for AD [99,100]. From a mechanistic perspective, a pre-pathological upregulation of TNF-α and associated enhancement of activated microglia have been consistently described in AD transgenic mouse models [90, 99, 100], and it has been proposed that such activated immune cells are essential in the clearance of extracellular Aβ. A likely result of increased Aβ exposure, however, is microglia TLR4 stimulation and a resulting elevated cytokine production and release [101]. TNF-α as well as IL-1β can correspondingly elevate Aβ generation by stimulating γ-secretase activity [102], thereby initiating a self-propagating positive feedback loop of Aβ induction of inflammation and TNF-α signaling that, in turn, may provoke further Aβ generation.
The formation and accumulation of AGEs has also been identified immunohistochemically within senile plaques and neurofibrillary tangles, the pathological hallmarks of AD [103]. Elevated AGE levels in AD brain upregulate CD36, augmenting proinflammatory responses, oxidative stress, and altering the microglial uptake of Aβ [104]. RAGE is also expressed in neurons, microglia and astrocytes [105–107].
An increased expression of RAGE has been observed in AD pathology afflicted regions of brain, including the hippocampus [108]. Notably, Aβ has been reported to be a specific RAGE ligand, interacting with its N-terminal domain and inducing an oxidative stress mediated activation of NF-κB, expression of inflammatory genes and proteins [105]. RAGE mediated microglia activation and neuro-inflammation has been experimentally demonstrated using double transgenic mice that over-express both the human RAGE gene and human amyloid precursor protein [109]. Such mice exhibited elevated levels of IL-1β and TNF-α, increased amyloid plaque infiltration by microglia and astrocytes, increased levels of Aβ40 and Aβ42, reduced AChE activity, and a rapid loss of memory. These effects were mitigated by blocking the RAGE mediated signal transduction. In addition to this, the level of sRAGE and hence its anti-inflammatory effects are reported reduced in AD, a scenario quite similar to T2DM [110]. Brought together, these findings support the concept that inflammation is an underlying thread in the coexistence of T2DM and AD.
COMMON THERAPEUTIC TARGETS OF AD AND T2DM
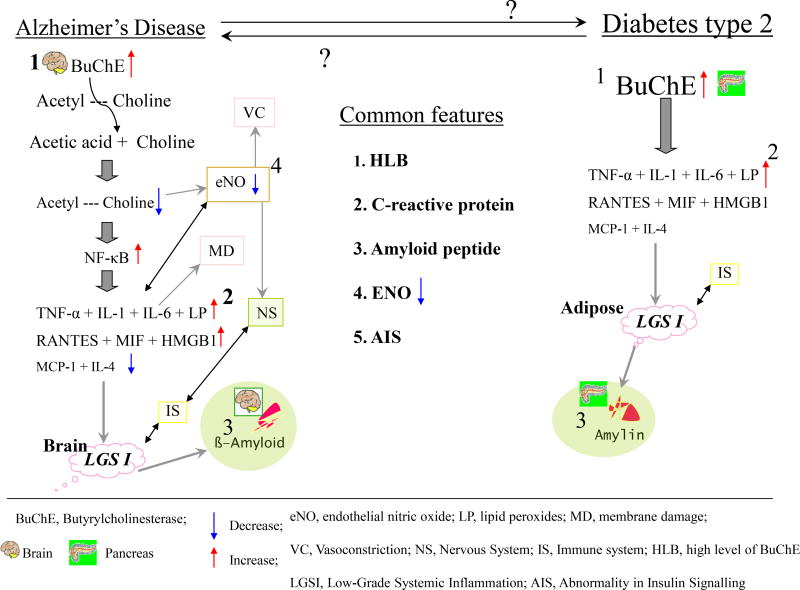
In general, drug developments, particularly for neurological disorders, are a field of high risk and attrition, but also of huge gain - if successful [111–113]. As alterations in the levels of key inflammatory markers (CRP, TNF-α, IL-6) and lipid peroxides are common to T2DM and AD and a close association exists between these two frequent progressive disorders, selectively blocking inflammatory signal cascades and restoring appropriate insulin signaling appears to be a justifiable approach towards treatment of both these disorders. For example, the inhibition of BuChE and TNF-α by selective and well-tolerated inhibitors may have potential for the treatment of both. In addition to the examples provided, BuChE and TNF-α, there are likely further endogenous proteins that are gatekeepers of awry biological cascades that impact the pancreas and brain that may warrant investigation and thereby provide additional apparent links between T2DM and AD, as new avenues for potential treatment [114]. BuChE, in addition to its role in co-regulating ACh levels and cholinergic neurotransmission, has non-cholinergic functions related to differentiation, proliferation and apoptosis [115]. Likewise, ACh modulates interactions between the nervous system and the immune system. Indeed, the ACh mediated cholinergic anti-inflammatory pathway acts by inhibiting the production of TNF-α, IL-1, macrophage migration inhibitory factor (MIF), and high-mobility group B1 protein (HMGB1) and suppresses the activation of NF-κB expression [116]. Progress in the characterization of both disease mechanisms and drug targets has provided the insight that a number of common players are involved across a broad variety of biological cascades, and thus relatively focused approaches such as (i) restoring ACh levels by selectively inhibiting the catalytic activity of BuChE, and (ii) selectively inhibiting pro-inflammatory cytokines (e.g., TNF-α) can potentially inhibit a number of destructive cycles of events. ACh, to extend this example, has a regulatory role on dopamine, serotonin as well as several neuropeptides, providing a close interaction between immune responses and neurotransmission [117]. Elevated levels of BuChE, are reported in diabetes and AD and have been hypothesized to result in a low-grade systemic inflammation (often seem in the elderly, and associated with geriatric depression [118] as well as sarcopenia [119, 120] and other disorders [121]), consequent to dysregulation of the described pathway (Fig. 1). Elevated systemic BuChE activity is a marker of low-grade inflammation, is found in Alzheimer brain, and may have a role in the altered lipoprotein metabolism in hyper triglyceridemia associated with insulin insensitivity or insulin deficiency in T2DM, and thus may be a target worth pursuing [122, 123]. Interestingly, the BuChE K variant allele is more common among subjects with T2DM versus non-diabetics, suggesting a close association of the BuChE gene (3q26) with T2DM that could be related to an identified susceptibility locus on chromosome 3q27, but independent of islet function [123, 124]. The normalization of elevated BuChE activity found in AD [125–127] may hence be of value for T2DM, which, as indicated, is associated with incidence of AD and drives its progression. The design and development of cymserine analogues for brain BuChE inhibition [128–131], and application of innovative and quantitative enzyme kinetic analyses [132–135] provides an immediately clinically translatable approach [136]. Such studies aid in moving agents from the laboratory to clinical assessment to both test new hypotheses and define the role of key proteins in health, aging and disease, and the compound bisnorcymserine has just entered the clinic [http://clinicaltrials.gov/ct2/show/NCT01747213?term=bisnorcymserine&rank=1]. Other approaches too have moved forward, as exemplified by Nizri et al. [137] investigating both the use of bi-functional compounds designed for multiple targeting and enhanced CNS permeability, and of recombinant alpha-fetoprotein (AFP), for the treatment of CNS inflammation. Their bi-functional compounds showed a novel pharmacokinetic profile providing an alternative potential strategy for future drug modalities.
Fig. (1).
Similarities between Alzheimer’s disease and type 2 diabetes mellitus reflecting the key role of BuChE in regulating acetylcholine (the critical neurotransmitter linking the nervous and immune systems).
CONCLUSIONS
A growing body of research continues to associate AD to T2DM, as well as with obesity and cardiovascular disease. Certainly, people with T2DM have an increased incidence of sporadic AD. Neuropatholological studies suggest that vascular disease, in particular, is the key pathology that drives the elevated risk of dementia in T2DM. Other features underpinning how diabetes potentially exacerbates AD pathology include the common denominators - amyloidogenesis, impaired brain insulin signaling, abnormal brain glucose metabolism, mitochondrial dysfunction, inflammation and oxidative stress. These factors also underlie brain dysfunction and/or cognitive decline in a number of neurological disorders [138–140]. The interrelations between these factors are multiple, complex and require extensive research to pinpoint intersecting points of regulation amenable to pharmacological manipulation. A better understanding of risk factors across a lifetime is additionally needed for AD, and other neurological disorders ans T2DM, to identify potentially modifiable ones to support promising lifestyle changes for those most vulnerable and to define the optimal window(s) of opportunity to initiate remedy.
Acknowledgments
N.G. is supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Baltimore, MD, USA. SP and NAA are grateful for support by the Section of Digestive Diseases and Nutrition, Department of Medicine, University of Illinois, Chicago, USA. MAK, JNR and ST also extend their appreciation to the deanship of scientific research at KSU for funding the work through the research group project No- RGP-VPP-215.
LIST OF ABBREVIATIONS
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- AD
Alzheimer’s disease
- AGEs
Advanced glycation endproducts
- Aβ
Amyloid-β peptide
- AβOs
Amyloid-β oligomers
- BuChE
Butyrylcholinesterase
- BuChE-Is
Butyrylcholinesterase inhibitors
- CNS
Central nervous system
- IL-6
Interleukin 6
- IRS
Insulin receptor substrate
- JNK
c-Jun N-terminal kinase
- RAGE
Receptor for advanced glycation endproducts
- T2DM
Type 2 diabetes mellitus
- TNF-α
Tumor necrosis factor α
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Kumar S, Singh R, Vasudeva N, Sharma S. Acute and chronic animal models for the evaluation of anti-diabetic agents. Cardiovasc Diabetol. 2012;11(1):9. doi: 10.1186/1475-2840-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Priyadarshini M, Kamal MA, Greig NH, et al. Alzheimer’s Disease And Type 2 Diabetes: Exploring The Association To Obesity And Tyrosine Hydroxylase. CNS & Neurol Disord - Drug Targets. 2012;11(4):482–9. doi: 10.2174/187152712800792767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leibson CL. Risk of dementia among persons with diabetes mell-itus: a population-based cohort study. Am J Epidemiol. 1997;145:301–8. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 4.Jellinger KA. Alzheimer 100 - highlights in the history of Alzheimer research. J Neural Transm. 2006;113:1603–23. doi: 10.1007/s00702-006-0578-3. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Holscher C. Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev. 2007;56:384–402. doi: 10.1016/j.brainresrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed I, Goldstein BJ. Cardiovascular risk in the spectrum of type 2 diabetes mellitus. Mt Sinai J Med. 2006;73:759–68. [PubMed] [Google Scholar]
- 7.Das UN. Is metabolic syndrome X a disorder of the brain with the initiation of low-grade systemic inflammatory events during the perinatal period? J Nutr Biochem. 2007;18:701–13. doi: 10.1016/j.jnutbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo JT, Yu J, Grass D, et al. Inflammation-dependent cerebral deposition of serum amyloid a protein in a mouse model of amyloidosis. J Neurosci. 2002;15:5900–9. doi: 10.1523/JNEUROSCI.22-14-05900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allam AR, Sridhar GR, Thota H, et al. Alzheimer's disease and Type 2 diabetes mellitus: the cholinesterase connection? Lipids Health Dis. 2006;5:28. doi: 10.1186/1476-511X-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kloppenborg RP, van den Berg E, Kappelle LJ, Biessels GJ. Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur J Pharmacol. 2008;585(1):97–108. doi: 10.1016/j.ejphar.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 12.Ott A, Stolk RP, van Harskamp F, et al. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–42. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 13.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–6. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 14.Lue LF, Andrade C, Sabbagh M, Walker D. Is There Inflammatory Synergy in Type II Diabetes Mellitus and Alzheimer’s Disease? Intl J Alzheimer’s Dis. 2012;2012:918680. doi: 10.1155/2012/918680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vetter SW, Indurthi VSK. Moderate glycation of serum albumin affects folding, stability, and ligand binding. Clin Chim Acta. 2011;412:2105–16. doi: 10.1016/j.cca.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Akter K, Lanza EA, Martin SA, et al. Diabetes mellitus and Alzheimer’s disease: shared pathology and treatment? Br J Clin Pharmacol. 2012;71(3):365–76. doi: 10.1111/j.1365-2125.2010.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baskin DG, Stein LJ, Ikeda H, et al. Genetically obese Zucker rats have abnormally low brain insulin content. Life Sci. 1985;36:627–33. doi: 10.1016/0024-3205(85)90166-3. [DOI] [PubMed] [Google Scholar]
- 18.Banks WA, Jaspan JB, Kastin AJ. Effect of diabetes mellitus on the permeability of the blood-brain barrier to insulin. Peptides. 1997;18:1577–84. doi: 10.1016/s0196-9781(97)00238-6. [DOI] [PubMed] [Google Scholar]
- 19.Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–33. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- 20.Takeda S, Sato N, Uchio-Yamada K, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Ab deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci USA. 2010;107:7036–41. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biessels GJ, Bravenboer B, Gispen WH. Glucose, insulin and the brain: modulation of cognition and synaptic plasticity in health and disease: a preface. Eur J Pharmacol. 2004;490:1–4. doi: 10.1016/j.ejphar.2004.02.057. [DOI] [PubMed] [Google Scholar]
- 22.Marks JL, King MG, Baskin DG. Localization of insulin and type 1 IGF receptors in rat brain by in vitro autoradiography and in situ hybridization. In: Raizada MK, LeRoith D, editors. Molecular Biology and Physiology of Insulin and Insulin-Like Growth Factors. Plenum Press; New York: 1991. pp. 459–70. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto Y, Chen L, Sato M, et al. Hippocampal synaptic modulation by the phosphotyrosine adapter protein AhcC/N-Shc via interaction with the NMDA receptor. J Neurosci. 2005;16:1826–35. doi: 10.1523/JNEUROSCI.3030-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam K, Carpenter CL, Ruderman NB, Friel JC, Kelly KL. The phosphatidylinositol 3-kinase serine kinase phosphorylates IRS-1 Stimulation by insulin and inhibition by Wortmannin. J Biol Chem. 1994;269:20648–52. [PubMed] [Google Scholar]
- 25.Halestrap AP, Doran E, Gillespie JP, O'Toole A. Mitochondria and cell death. Biochem Soc Trans. 2000;28:170–7. doi: 10.1042/bst0280170. [DOI] [PubMed] [Google Scholar]
- 26.Dudek H, Datta SR, Franke TF, et al. Regulation of neuronal survival by the serine- threonine protein kinase Akt. Science. 1997;275:661–5. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 27.Horwood JM, Dufour F, Laroche S, Davis S. Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci. 2006;23:3375–84. doi: 10.1111/j.1460-9568.2006.04859.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Garelick MG, Wang H, et al. PI3 kinase signalling is required for retrieval and extinction of contextual memory. Nat Neurosci. 2005;8:925–31. doi: 10.1038/nn1482. [DOI] [PubMed] [Google Scholar]
- 29.Chiang HC, Wang L, Xie Z, Yau A, Zhong Y. PI3 kinase signalling is involved in Ab-induced memory loss in Drosophila. Proc Natl Acad Sci USA. 2010;107:7060–5. doi: 10.1073/pnas.0909314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wortwein G, Gustafson B, Hansen KL, Mogensen J. Behavioral symptoms in adult rats after postnatal L-nitro-arginine. Int J Dev Neurosci. 1997;15:147–54. doi: 10.1016/s0736-5748(97)00002-6. [DOI] [PubMed] [Google Scholar]
- 31.de la Monte SM. Insulin resistance and Alzheimer’s disease. BMB Rep. 2009;42:475–81. doi: 10.5483/bmbrep.2009.42.8.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivera EJ, Goldin A, Fulmer N, et al. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–68. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 33.Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem. 1997;272:19547–53. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- 34.Chen GJ, Xu J, Lahousse SA, Caggiano NL, de la Monte SM. Transient hypoxia causes Alzheimer-type molecular and biochemical abnormalities in cortical neurons: potential strategies for neuroprotection. J Alzheimers Dis. 2003;5:209–28. doi: 10.3233/jad-2003-5305. [DOI] [PubMed] [Google Scholar]
- 35.Watson GS, Peskind ER, Asthana S, et al. Insulin increases CSF Abeta42 levels in normal older adults. Neurology. 2003;60:1899–903. doi: 10.1212/01.wnl.0000065916.25128.25. [DOI] [PubMed] [Google Scholar]
- 36.Roriz-Filho JS, Sá-Roriz TM, Rosset I, et al. (Pre) diabetes, brain aging, and cognition. Biochim Biophys Acta. 2009;1792(5):432–43. doi: 10.1016/j.bbadis.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Milionis HJ, Florentin M, Giannopoulos S. Metabolic Syndrome and Alzheimer’s disease: A link to a vascular hypothesis? CNS Spectr. 2008;13(7):606–13. doi: 10.1017/s1092852900016886. [DOI] [PubMed] [Google Scholar]
- 38.Zhao WQ, De Felice FG, Fernandez S, et al. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22(1):246–60. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
- 39.Bomfim TR, Forny-Germano L, Sathler LB, et al. An anti-diabetes agent protects the mouse brain from defective insulin signalling caused by Alzheimer’s disease- associated Aβ oligomers. J Clin Invest. 2012;122(4):1339–53. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaneto H, Matsuoka TA, Katakami N, et al. Oxidative stress and the JNK pathway are involved in the development of type 1 and type 2 diabetes. Curr Mol Med. 2007;7:674–86. doi: 10.2174/156652407782564408. [DOI] [PubMed] [Google Scholar]
- 41.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 42.Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–38. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salcedo I, Tweedie D, Li Y, Greig NH. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol. 2012;166:1586–99. doi: 10.1111/j.1476-5381.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perry T, Greig NH. Enhancing central nervous system endogenous GLP-1 receptor pathways for intervention in Alzheimer's disease. Curr Alzheimer Res. 2005;2:377–85. doi: 10.2174/1567205054367892. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Chigurupati S, Holloway HW, et al. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS One. 2012;7(2):e32008. doi: 10.1371/journal.pone.0032008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Perry T, Kindy MS, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci USA. 2009;106:1285–90. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rachmany L, Tweedie D, Li Y, et al. Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. Age. 2013;35(5):1621–36. doi: 10.1007/s11357-012-9464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tweedie D, Rachmany L, Rubovitch V, et al. Exendin-4: a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp Neurol. 2013;239:170–82. doi: 10.1016/j.expneurol.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Duffy KB, Ottinger MA, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J Alzheimers Dis. 2010;19:1205–19. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perry T, Lahiri DK, Sambamurti K, et al. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res. 2003;72:603–12. doi: 10.1002/jnr.10611. [DOI] [PubMed] [Google Scholar]
- 51.Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther. 2002;302:881–8. doi: 10.1124/jpet.102.037481. [DOI] [PubMed] [Google Scholar]
- 52.Ando K, Oishi M, Takeda S, et al. Role of phosphorylation of Alzheimer’s amyloid precursor protein during neuronal differentiation. J Neurosci. 1999;19:4421–7. doi: 10.1523/JNEUROSCI.19-11-04421.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dias-Santagata DD, Fulga TA, Duttaroy A, Feany MB. Oxidative stress medicates tau-induced neurodegeneration in Drosophila. J Clin Invest. 2007;117:236–45. doi: 10.1172/JCI28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thakur A, Wang X, Siedlak SL, et al. c-Jun phosphorylation in Alzheimer disease. J Neurosci Res. 2007;85:1668–73. doi: 10.1002/jnr.21298. [DOI] [PubMed] [Google Scholar]
- 55.Beeler N, Riederer BM, Waeber G, Abderrahmani A. Role of the JNK-interacting protein 1/islet brain 1 in cell degeneration in Alzheimer disease and diabetes. Brain Res Bull. 2009;80:274–81. doi: 10.1016/j.brainresbull.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev. 2008;9:204–18. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roy E, Raz I. Lipotoxicity versus adipotoxicity - The deleterious effects of adipose tissue on beta cells in the pathogenesis of type 2 diabetes. Diabetes Res Clin Pract. 2006;7:S3–8. [Google Scholar]
- 58.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14:222–31. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith U. Introduction: Symposium on diabetes, inflammation and cardiovascular disease. J Intern Med. 2007;262:142–4. doi: 10.1111/j.1365-2796.2007.01827.x. [DOI] [PubMed] [Google Scholar]
- 60.Watson GS, Craft S. The role of insulin resistance in the pathogenesis of Alzheimer’s disease: implications for treatment. CNS Drugs. 2003;17:27–45. doi: 10.2165/00023210-200317010-00003. [DOI] [PubMed] [Google Scholar]
- 61.Böni-Schnetzler M, Thorne J, Parnaud G, et al. Increased interleukin (IL)-1β messenger ribonucleic acid expression in β-cells of individuals with type 2 diabetes and regulation of IL-1β in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93(10):4065–74. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palmer JP, Helqvist S, Spinas GA, et al. Interaction of β-cell activity and IL-1 concentration and exposure time in isolated rat islets of langerhans. Diabetes. 1989;38(10):1211–6. doi: 10.2337/diab.38.10.1211. [DOI] [PubMed] [Google Scholar]
- 63.Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41(5):1203–17. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 64.Ehses JA, Lacraz G, Giroix MH, et al. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci USA. 2009;106(33):13998–4003. doi: 10.1073/pnas.0810087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamagishi S. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp Gerontol. 2011;46(4):217–24. doi: 10.1016/j.exger.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 66.Win MT, Yamamoto Y, Munesue S, et al. Regulation of RAGE for attenuating progression of diabetic vascular complications. Exp Dia Res. 2012;2012:894605. doi: 10.1155/2012/894605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamagishi S, Takeuchi M, Inagaki Y, Nakamura K, Imaizumi T. Role of advanced glycation end products (AGEs) and their receptor (RAGE) in the pathogenesis of diabetic microangiopathy. Int J Clin Pharmcol Res. 2003;23(4):129–34. [PubMed] [Google Scholar]
- 68.Yonekura H, Yamamoto Y, Sakurai S, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370(3):1097–109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raucci A, Cugusi S, Antonelli A, et al. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) FASEB J. 2008;22(10):3716–27. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- 70.Tam XH, Shiu SW, Leng L, et al. Enhanced expression of receptor for advanced glycation end-products is associated with low circulating soluble isoforms of the receptor in Type 2 diabetes. Clin Sci. 2011;120(2):81–9. doi: 10.1042/CS20100256. [DOI] [PubMed] [Google Scholar]
- 71.Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. High glucose induces toll-like receptor expression in human monocytes mechanism of activation. Diabetes. 2008;57(11):3090–8. doi: 10.2337/db08-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via toll-like receptors. Am J Physiol. 2011;300(1):E145–54. doi: 10.1152/ajpendo.00490.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barton GM, Medzhitov R. Toll-like receptor signalling pathways. Science. 2003;300(5625):1524–5. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 74.Stewart CR, Stuart LM, Wilkinson K, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11(2):155–61. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gautam S, Banerjee M. The macrophage Ox-LDL receptor, CD36 and its association with type II diabetes mellitus. Mol Genet Metab. 2011;102(4):389–98. doi: 10.1016/j.ymgme.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 76.Kennedy DJ, Kashyap SR. Pathogenic role of scavenger receptor CD36: metabolic syndrome and diabetes. Metab Syn Related Disorders. 2011;9(4):239–45. doi: 10.1089/met.2011.0003. [DOI] [PubMed] [Google Scholar]
- 77.Kennedy DJ, Kuchibhotla S, Westfall KM, et al. CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc Res. 2011;89(3):604–13. doi: 10.1093/cvr/cvq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gupta A, Pansari K. Inflammation and Alzheimer's disease. Int J Clin Pract. 2003;57:36–9. [PubMed] [Google Scholar]
- 79.Cacabelos R, Barquero M, García P, et al. Cerebrospinal fluid interleukin-1 beta (IL-1 beta) in Alzheimer’s disease and neurological disorders. Methods Find Exp Clin Pharmacol. 1991;13:455–8. [PubMed] [Google Scholar]
- 80.Fillit H, Ding WH, Buee L, et al. Elevated circulating tumor necrosis factor levels in Alzheimer’s disease. Neurosci Lett. 1991;129:318–20. doi: 10.1016/0304-3940(91)90490-k. [DOI] [PubMed] [Google Scholar]
- 81.Koivisto AM, Helisalmi S, Pihlajamäki J, et al. Interleukin-6 promoter polymorphism and late-onset Alzheimer's disease in the Finnish population. J Neurogenet. 2005;19:155–61. doi: 10.1080/01677060600569721. [DOI] [PubMed] [Google Scholar]
- 82.Reale M, Kamal MA, Velluto L, et al. Relationship between inflammatory mediators, Aβ levels and ApoE genotype in Alzheimer disease. Curr Alzheimer Res. 2012;9:447–57. doi: 10.2174/156720512800492549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rada P, Mark GP, Vitek MP, et al. Interleukin-1 beta decreases acetylcholine measured by microdialysis in the hippocampus of freely moving rats. Brain Res. 1991;550:287–90. doi: 10.1016/0006-8993(91)91330-4. [DOI] [PubMed] [Google Scholar]
- 84.Taepavarapruk P, Song C. Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1beta administrations: effects of omega-3 fatty acid EPA treatment. J Neurochem. 2010;112:1054–64. doi: 10.1111/j.1471-4159.2009.06524.x. [DOI] [PubMed] [Google Scholar]
- 85.Capsoni S, Brandi R, Arisi I, D'Onofrio M, Cattaneo A. A dual mechanism linking NGF/proNGF imbalance and early inflammation to Alzheimer's disease neurodegeneration in the AD11 anti-NGF mouse model. CNS Neurol Disord Drug Targets. 2011;10:635–47. doi: 10.2174/187152711796235032. [DOI] [PubMed] [Google Scholar]
- 86.Salehi A, Delcroix JD, Belichenko PV, et al. Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 87.Tobinick E. Deciphering the physiology underlying the rapid clinical effects of perispinal etanercept in Alzheimer’s disease. Curr Alzheimer Res. 2012;9:99–109. doi: 10.2174/156720512799015073. [DOI] [PubMed] [Google Scholar]
- 88.Rosenberg PB. Cytokine inhibition for treatment of Alzheimer’s disease. Med Gen Med. 2006;8:24–32. [PMC free article] [PubMed] [Google Scholar]
- 89.Tarkowski E, Andreasen N, Tarkowski A, Blennow K. Intrathecal inflammation precedes development of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:1200–5. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Janelsins MC, Mastrangelo MA, Oddo S, et al. Early correlation of microglial activation with enhanced tumor necrosis factor-alpha and monocyte chemoattractant protein-1 expression specifically within the entorhinal cortex of triple transgenic Alzheimer’s disease mice. J Neuroinflammation. 2005;2:23. doi: 10.1186/1742-2094-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–88. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 92.Perry RT, Collins JS, Wiener H, Acton R, Go RC. The role of TNF and its receptors in Alzheimer’s disease. Neurobiol Aging. 2001;22:873–83. doi: 10.1016/s0197-4580(01)00291-3. [DOI] [PubMed] [Google Scholar]
- 93.Laws SM, Perneczky R, Wagenpfeil S, et al. TNF polymorphisms in Alzheimer disease and functional implications on CSF beta-amyloid levels. Hum Mutat. 2005;26:29–35. doi: 10.1002/humu.20180. [DOI] [PubMed] [Google Scholar]
- 94.Aleong R, Blain JF, Poirier J. Pro-inflammatory cytokines modulate glial apolipoprotein E secretion. Curr Alzheimer Res. 2008;5:33–7. doi: 10.2174/156720508783884666. [DOI] [PubMed] [Google Scholar]
- 95.He P, Zhong Z, Lindholm K, et al. Deletion of tumor necrosis factor death receptor inhibits amyloid-β generation and prevents learning and memory deficits in Alzheimer’s mice. J Cell Biol. 2007;178:829–41. doi: 10.1083/jcb.200705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tweedie D, Ferguson RA, Fishman K, et al. Tumor necrosis factor-α synthesis inhibitor 3:6'-dithiothalidomide attenuates markers of inflammation, Alzheimer pathology and behavioral deficits in animal models of neuroinflammation and Alzheimer's disease. J Neuroinflammation. 2012;9:106. doi: 10.1186/1742-2094-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Belarbi K, Jopson T, Tweedie D, et al. TNF-alpha protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J Neuroinflammation. 2012;9:23. doi: 10.1186/1742-2094-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tweedie D, Sambamurti K, Greig NH. TNF-α inhibition as a treatment strategy for neurodegenerative disorders: New drug candidates and targets. Curr Alzheimer Res. 2007;4:378–85. doi: 10.2174/156720507781788873. [DOI] [PubMed] [Google Scholar]
- 100.Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2011;10:391–403. doi: 10.2174/187152711794653751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jin JJ, Kim HD, Maxwell JA, Li L, Fukuchi K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer’s disease. J Neuroinflammation. 2008;5:23. doi: 10.1186/1742-2094-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liao YF, Wang BJ, Cheng HT, Kuo LH, Wolfe MS. Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J Biol Chem. 2004;279:49523–32. doi: 10.1074/jbc.M402034200. [DOI] [PubMed] [Google Scholar]
- 103.Yamagishi S, Nakamura K, Inoue H, Kikuchi S, Takeuchi M. Serum or cerebrospinal fluid levels of glyceraldehyde-derived advanced glycation end products (AGEs) may be a promising biomarker for early detection of Alzheimer's disease. Med Hypotheses. 2005;64:1205–7. doi: 10.1016/j.mehy.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 104.Coraci IS, Husemann J, Berman JW, et al. CD36: a class B scavenger receptor, is expressed onmicroglia in Alzheimer’s disease brains and can mediate production of reactive oxygen species in response to β-amyloid fibrils. Am J Pathol. 2002;160(1):101–12. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lue LF, Walker DG, Brachova L, et al. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: identification of a cellular activation mechanism. Exp Neurol. 2001;171(1):29–45. doi: 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- 106.Sasaki N, Toki S, Chowei H, et al. Immunohistochemical distribution of the receptor for advanced glycation end products in neurons and astrocytes in Alzheimer’s disease. Brain Res. 2001;888(2):256–62. doi: 10.1016/s0006-8993(00)03075-4. [DOI] [PubMed] [Google Scholar]
- 107.Yan SD, Chen X, Fu J, et al. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nat. 1996;382(6593):685–91. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 108.Chaney MO, Stine WB, Kokjohn TA, et al. RAGE and amyloid β interactions: atomic force microscopy and molecular modelling. Biochim Biophys Acta. 2005;1741(1–2):199–205. doi: 10.1016/j.bbadis.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 109.Fang F, Lue LF, Yan S, et al. RAGE-dependent signalling in microglia contributes to neuroinflammation, Aβ accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. FASEB J. 2010;24(4):1043–55. doi: 10.1096/fj.09-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lue LF, Kuo YM, Beach T, Walker DG. Microglia activation and anti-inflammatory regulation in alzheimer’s disease. Mol Neurobiol. 2010;41(2–3):115–28. doi: 10.1007/s12035-010-8106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pangalos MN, Schechter LE, Hurko O. Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nat Rev Drug Discov. 2007;6:521–32. doi: 10.1038/nrd2094. [DOI] [PubMed] [Google Scholar]
- 112.Becker RE, Greig NH. Lost in translation: neuropsychiatric drug development. Sci Transl Med. 2010;2(61):61rv6. doi: 10.1126/scitranslmed.3000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Becker RE, Greig NH. Why so few drugs for Alzheimer's disease? Are methods failing drugs? Curr Alzheimer Res. 2010;7:642–51. doi: 10.2174/156720510793499075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rao AA, Sridhar GR, Das UN. Elevated butyrylcholinesterase and acetylcholinesterase may predict the development of type 2 diabetes mellitus and Alzheimer's disease. Med Hypotheses. 2007;69:1272–6. doi: 10.1016/j.mehy.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 115.Vidal CJ. Expression of cholinesterases in brain and non-brain tumours. Chem Biol Interact. 2005;157–158:227–32. doi: 10.1016/j.cbi.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 116.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 117.Das UN. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med Sci Monit. 2007;13:214–21. [PubMed] [Google Scholar]
- 118.Kelley KW, O'Connor JC, Lawson MA, et al. Aging leads to prolonged duration of inflammation-induced depression-like behavior caused by Bacillus Calmette-Guérin. Brain Behav Immun. 2013:S0889–1591. doi: 10.1016/j.bbi.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–54. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 120.Hamer M, Molloy GJ. Association of C-reactive protein and muscle strength in the English Longitudinal Study of Ageing. Age. 2009;31:171–7. doi: 10.1007/s11357-009-9097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vasto S, Carruba G, Lio D, et al. Inflammation, ageing and cancer. Mech Ageing Dev. 2009;130:40–5. doi: 10.1016/j.mad.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 122.Abbott CA, Mackness MI, Kumar S, et al. Relationship between serum butyrylcholinesterase activity, hypertriglyceridaemia and insulin sensitivity in diabetes mellitus. Clin Sci. 1993;85:77–81. doi: 10.1042/cs0850077. [DOI] [PubMed] [Google Scholar]
- 123.Sridhar GR, Thota H, Allam AR, et al. Alzheimer's disease and type 2 diabetes mellitus: the cholinesterase connection? Lipids Health Dis. 2006;11:28. doi: 10.1186/1476-511X-5-28. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hashim Y, Shepherd D, Wiltshire S, et al. Butyrylcholinesterase K variant on chromosome 3q is associated with Type II diabetes in white Caucasian subjects. Diabetologia. 2001;44:2227–30. doi: 10.1007/s001250100033. [DOI] [PubMed] [Google Scholar]
- 125.Perry EK. The cholinergic hypothesis - ten years on. Br Med Bull. 1986;42:63–9. doi: 10.1093/oxfordjournals.bmb.a072100. [DOI] [PubMed] [Google Scholar]
- 126.Arendt T, Bigl V, Walther F, Sonntag M. Decreased ratio of CSF acetylcholinesterase to butyrylcholinesterase activity in Alzheimer's disease. Lancet. 1984;1:173. doi: 10.1016/s0140-6736(84)90116-8. [DOI] [PubMed] [Google Scholar]
- 127.Arendt T, Brückner MK, Lange M, Bigl V. Changes in acetyl-cholinesterase and butyrylcholinesterase in Alzheimer's disease resemble embryonic development--a study of molecular forms. Neurochem Int. 1992;21(3):381–96. doi: 10.1016/0197-0186(92)90189-x. [DOI] [PubMed] [Google Scholar]
- 128.Yu QS, Holloway HW, Utsuki T, Brossi A, Greig NH. Phenserine-based synthesis of novel selective inhibitors of butyrylcholinesterase for Alzheimer’s disease. J Med Chem. 1999;42:1855–61. doi: 10.1021/jm980459s. [DOI] [PubMed] [Google Scholar]
- 129.Luo W, Yu QS, Holloway HW, Greig NH, Brossi A. Syntheses of tetrahydrofurobenzofurans and dihydro-methanobenzodioxepines from 5-hydroxy-3-methyl-3H-benzofuran-2-one. Re-arrangement and ring expansion under reductive conditions on treatment with hydrides. J Org Chem. 2005;70:6171–6. doi: 10.1021/jo0503052. [DOI] [PubMed] [Google Scholar]
- 130.Luo X, Yu QS, Zhan M, et al. Novel anticholinesterases based on the molecular skeletons of furobenzofuran and benzodioxepine. J Med Chem. 2005;48:986–94. doi: 10.1021/jm049309+. [DOI] [PubMed] [Google Scholar]
- 131.Luo W, Yu QS, Kulkarni SS, et al. (−) And (+)-o-carbamoyl phenols of pyrroloindole, furoindole, furobenzofuran and benzodioxepine: enantiomeric syntheses and structure/activity relationship for human acetyl- and butyrylcholinesterase inhibitory action. J Med Chem. 2006;49:2174–85. doi: 10.1021/jm050578p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kamal MA, Yu QS, Holloway HW, et al. Kinetics of human serum butyrylcholinesterase and its inhibition by a novel experimental Alzheimer therapeutic, bisnorcymserine. J Alzheimers Dis. 2006;10(1):43–51. doi: 10.3233/jad-2006-10108. [DOI] [PubMed] [Google Scholar]
- 133.Kamal MA, Al-Jafari AA, Yu QS, Greig NH. Kinetic analysis of the inhibition of human butyrylcholinesterase with cymserine. Biochem Biophys Acta. 2006;1760:200–6. doi: 10.1016/j.bbagen.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 134.Kamal MA, Qu X, Yu QS, et al. Tetrahydrofurobenzofuran cymserine, a potent butyrylcholinesterase inhibitor and experimental Alzheimer drug candidate, enzyme kinetic analysis. J Neural Trans. 2008;115(6):889–98. doi: 10.1007/s00702-008-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kamal MA, Klein P, Luo W, et al. Kinetics of human serum butyrylcholinesterase inhibition by a novel experimental Alzheimer therapeutic, dihydrobenzodioxepine cymserine. Neurochem Res. 2008;33(5):745–53. doi: 10.1007/s11064-007-9490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Greig NH, Ingram DK, Wang Y, et al. Selective butyrylcholinsterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer’s β-amyloid peptide in rodent. Proc Natl Acad Sci USA. 2005;102:17213–8. doi: 10.1073/pnas.0508575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nizri E, Irony-Tur-Sinai M, Grigoriadis N, et al. Novel approaches to treatment of autoimmune neuroinflammation and lessons for drug development. Pharmacol. 2007;79(1):42–9. doi: 10.1159/000097628. [DOI] [PubMed] [Google Scholar]
- 138.Cholerton B, Baker LD, Craft S. Insulin resistance and pathological brain ageing. Diabet Med. 2011;28:1463–75. doi: 10.1111/j.1464-5491.2011.03464.x. [DOI] [PubMed] [Google Scholar]
- 139.Moreno-Gonzalez I, Soto C. Misfolded protein aggregates: mechanisms, structures and potential for disease transmission. Semin Cell Dev Biol. 2011;22:482–7. doi: 10.1016/j.semcdb.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nunomura A, Moreira PI, Castellani RJ, et al. Oxidative damage to RNA in aging and neurodegenerative disorders. Neurotox Res. 2012;22:231–48. doi: 10.1007/s12640-012-9331-x. [DOI] [PubMed] [Google Scholar]