Abstract
Metal dyshomeostasis is involved in the pathogenesis and progression of diseases including cancer and neurodegenerative diseases. Metal chelators and ionophores are well known modulators of transition metal homeostasis, and a number of these molecules are in clinical trials. Metalbinding compounds are not the only drugs capable of targeting transition metal homeostasis. This review presents recent highlights in the development of chelators and ionophores for the treatment of cancer and neurodegenerative disease. Moreover, we discuss the development of small molecules that alter copper and iron homeostasis by inhibiting metal transport proteins. Finally, we consider the emergence of metal regulatory factor 1 as a drug target in diseases where it mediates zinc-induced signalling cascades leading to pathogenesis.
Graphical abstract

Introduction
The proteome is composed of approximately 30% metalloproteins whose functions are dependent on the availability and delivery of transition metals. Disorders of transition metal homeostasis vary from genetic diseases of copper overload (Wilson’s disease) and deficiency (Menkes disease) to iron overload (hereditary haemochromatosis). Aberrant transition metal homeostasis is implicated in many other diseases, with intense interest in its role in cancer and neurodegenerative diseases.
In genetic diseases of metal overload there is an unambiguous link between transition metal status and disease symptoms. For decades, these diseases have been treated with chelators that bind the offending metals, leading to their excretion rather than accumulation in body tissues. Now, chelators and their metal-bound alter egos known as ionophores show promising activity in cancer and neurodegenerative diseases. The relationship between metal status and disease pathology and progression in other diseases is more complex. The inhibition of disease progression via altering metal homeostasis may result from: the elimination of excess metal, the redistribution of metals across cells and tissues or even the accumulation of metals to toxic levels in diseased tissue. To match these diverse objectives, the development of drugs targeting transition metal homeostasis now spans: (1) chelators and ionophores that bind and release metals; (2) inhibitors that target metal uptake and transport proteins; and (3) drugs that impact metal regulatory transcription factors. This review will cover recent developments in the design of drugs targeting iron, copper, zinc and manganese homeostasis in cancer and neurodegenerative diseases, with special emphasis on drugs that interfere with cellular metal trafficking (Figure 1).
Figure 1.
Structures of drugs – referred to in this review – that target transition metal homeostasis.
Metal-binding chelators and metal-releasing ionophores
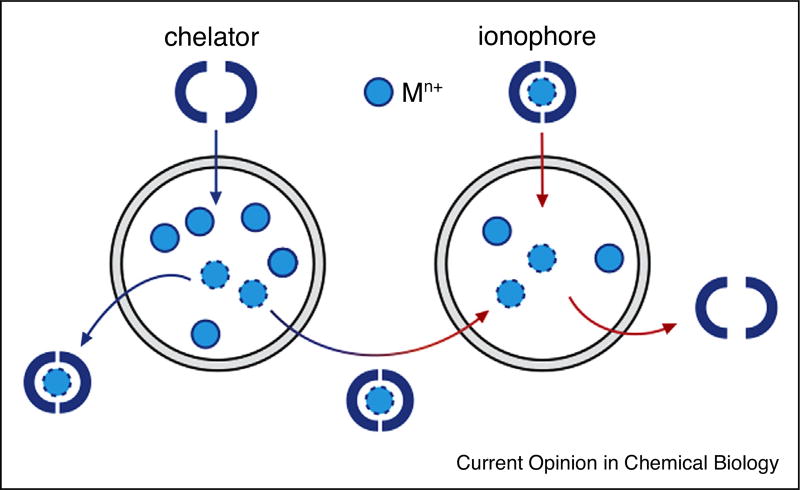
Chelators and ionophores target transition metal homeostasis at the molecular level by binding and releasing metals with the aim of eliminating excess metals, redistributing endogenous metals or depositing exogenous metals (Figure 2). Chelators have traditionally been used to treat heavy metal toxicity and diseases characterised by metal overload due to genetic defects that impair metal uptake or export pathways. While ionophores and chelators may be considered opposite to each other in that the former is responsible for the delivery of metals and the latter for the removal of metals; ultimately they both act as metal-binding compounds. Several recent, comprehensive reviews detail the current state of research into chelators and ionophores [1,2] and the broader application of this class of metal-binding molecules to cancer [3,4] and neurodegenerative diseases [5,6•].
Figure 2.
Metal-binding compounds act as chelators and ionophores. Chelators may eliminate excess metal ions or redistribute endogenous metal ions from regions of excess to regions of deficiency. Ionophores deliver exogenous metal ions.
Chelators and ionophores are of acute interest in Alzheimer’s disease (AD) where the metal hypothesis holds that it is a disease of metal dyshomeostasis with elevated metal levels associated with amyloid plaques and neurofibrillary tangles and, importantly, reduced metal levels elsewhere in the brain tissue. Derivatives of the 8-hydroxyquinoline clioquinol chelate Cu2+ and Zn2+ in the extracellular matrix and transfer them into cells, restoring crucial metalloprotease activity and leading to improved outcomes in mouse models of AD. With some drugs already in clinical trials, efforts continue to develop derivatives of 8-hydroxyquinolines with improved ionophoric activity and selectivity for copper and zinc over iron [7]. Derivatives of bis(8-aminoquinolines) are a novel class of chelators that can, at least in vitro, chelate metals associated with amyloid plaques and then release them in the presence of glutathione [8,9].
Metal dyshomeostasis, especially of iron and manganese, is implicated in the pathology of Parkinson’s disease. To this end, a hydroxyquinoline-based chelator was designed to decrease the mitochondrial iron pool, which led to reduced oxidative damage in cell and mouse models of Parkinson’s disease [10]. Strengthening the case for manganese homeostasis as a target in Parkinson’s disease, mutations in the SLC39A14 gene encoding for the divalent metal transporter ZIP14 have been shown to cause childhood-onset parkinsonism-dystonia, the symptoms of which are alleviated by the use of EDTA to reduce blood manganese levels [11]. Along with iron(II), biologically abundant calcium(II) also competes with manganese binding, posing challenges to the design of specific manganese binding compounds.
Cancer may be amenable to treatment with metal chelators and ionophores and a number of these drugs, especially from the thiosemicarbazone class of compounds, have entered or are in the clinical trial phase [3,4,12]. The development of these drugs is complicated by the disease’s heterogeneity and uncertainty around the relationship between metal status and tumour development, growth and metastasis. This is exemplified by recent results of in vitro experiments into the use of the iron chelator deferoxamine in breast cancer cells: in MCF-7 cells treatment led to decreased iron levels, but in more aggressive MDA-MB-231 cells, iron levels were increased and cell migration was enhanced [13]. Nonetheless, preliminary reports from a Phase II clinical trial indicate that treatment with tetrathiomolybdate – to lower copper levels to within normal limits – extended progression-free survival in patients with breast cancer [14]. (The activity of tetrathiomolybdate is not limited to chelation: it is known to bind the copper chaperone protein ATOX1 [15] and inhibits copper-dependent superoxide dismutase 1 (SOD1) activity [16].) The zinc chelator PAC-1 and its derivatives also show promise for cancer treatment. PAC-1 competes with procaspase-3 for loosely-bound zinc, activating its cleavage to caspase-3 and leading to apoptosis. The weak affinity of PAC-1 for zinc limits the potential for off-target effects that would result from competition for tightly-bound zinc in proteins like the matrix metalloproteinases [17].
In the dithiocarbamate class of chelators, disulfiram had proved disappointing in clinical trials in patients with prostate cancer, but evidence of its copper-dependent activity has led to the design of new clinical trials based on its use as an ionophore [18]. Also headed to clinical trials is a drug from a class of zinc thiosemicarbazones that exert their cytotoxicity through transmetalation with copper in lysosomes [19•]. The complexity of the relationship between ionophores, tumour metal levels and anticancer activity inspired research using a model of prostate cancer, which found that treatment with copper ionophores generated toxic levels of reactive oxygen species in the tumour cells, but not normal epithelial cells. Furthermore, mutation of the copper transport protein ATP7B reduced the tumour burden in this mouse model, suggesting a dependence of prostate tumour growth on copper supply from the liver [20••]. These findings lead us to the question: can we develop drugs to target this copper supply and other metal transport pathways?
Small molecule inhibitors of metal transport proteins
The development of drugs targeting metal transport proteins is dependent on an understanding of cellular metal transport pathways and their roles in disease pathogenesis. Our knowledge of the proteins involved in metal uptake, transport and export in humans is most detailed for copper [21], zinc [22] and iron [23], but even for these metals, much remains to be discovered.
In keeping with our limited understanding of metal transport in humans there are few drugs that directly target proteins involved in these pathways. Some drugs are known to interact with the metal-binding sites of transition metal transport proteins. Medicinal gold compounds [24] and cisplatin [25,26] form stable adducts with ATOX1 due to the affinity of gold and platinum for thiols in the copper-binding site. However, drugs targeted to the CXXC copper-binding motif lack specificity given the appearance of that motif in proteins that do not bind metals. Small molecule inhibition of metalloenzymes can be effective, and selective inhibitors exist for a range of metalloenzymes [27]. Small molecular inhibitors of histone deacetylases (HDACs) are in clinical trials for the anticancer activity. These inhibitors target HDACs by chelating zinc at the active site, and research continues into altering the affinity of the zinc-binding moiety, to tune the selectivity of the inhibitors [28,29].
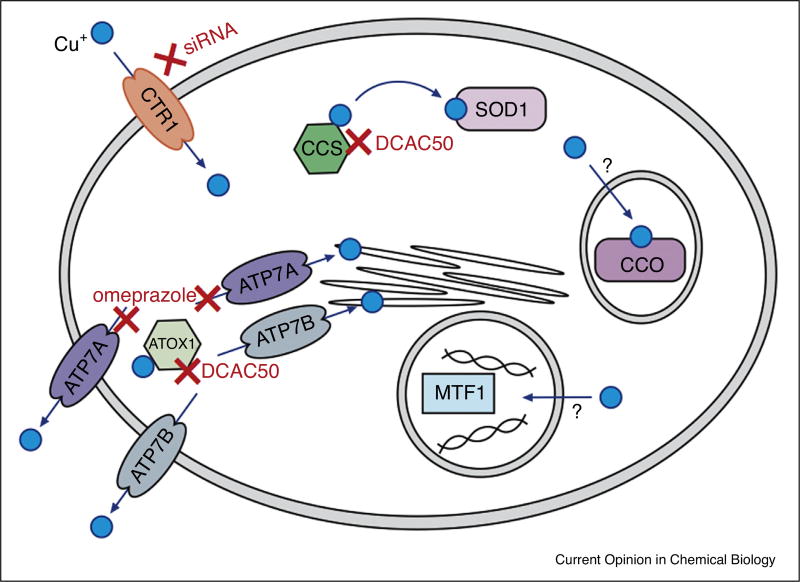
An alternative approach to altering metal homeostasis is the inhibition of metal transfer between proteins. There are several copper transport proteins ripe for targeting by small molecule inhibitors. DCAC50 is the most thoroughly-characterised drug developed specifically to target metal transport proteins [30••]. The compound was identified by virtual screening of known structures evaluated against the established copper transfer mechanisms of ATOX1 to ATP7A and ATP7B and the copper chaperone for superoxide dismutase (CCS) to SOD1 (Figure 3). Micromolar binding affinity to ATOX1 and CCS was confirmed in vitro. The drug was shown to impact the copper transport pathways with increased copper levels, reduced SOD1 and cytochrome c oxidase (CCO) activity all observed in lung cancer cells, as expected from the inhibition of the intracellular copper transport pathway. The resulting ROS generation and reduced levels of lipid synthesis contributed to reduced cell proliferation. Furthermore, tumorigenesis was inhibited in a mouse lung cancer xenograft.
Figure 3.
A simplified scheme of cellular copper transport pathways relevant to this review. Copper uptake by CTR1 can be inhibited by siRNAs that knock down CTR1 expression. DCAC50 inhibits copper transfer between the CCS and SOD1. DCAC50 also inhibits copper transfer between ATOX1 and the membrane transport proteins ATP7A and ATP7B. Omeprazole inhibits tyrosinase activity, possibly by inhibiting copper transport to the trans-Golgi network via ATP7A and therefore preventing the metallation of tyrosinase. The specifics of copper transport to the mitochondria, where copper is required for CCO activity, and the nucleus, where MTF1 senses and regulates copper levels, are not yet known.
A drug recently identified as a likely inhibitor of the transmembrane copper transporter ATP7A is omeprazole, a P-type ATPase inhibitor already used in the treatment of peptic ulcer disease. It was identified in a high-throughput screen for the inhibition of melanin production in melanocytes. Investigation of the mechanism of action revealed that omeprazole reduced tyrosinase activity, presumably through the inhibition of copper trafficking by ATP7A, also a P-type ATPase. However, the authors note that omeprazole likely inhibits tyrosinase activity through multiple mechanisms, and direct binding of omeprazole to ATP7A is yet to be proven [31••]. Finally, the high affinity copper importer CTR1 has been verified as a potential pharmacological target in tumour angiogenesis: reducing the mRNA and protein expression levels of CTR1 using an siRNA resulted in reduced copper uptake and the inhibition of angiogenesis in endothelial cells [32].
The divalent metal transporter DMT1 has been the target of drug development research in recent years. Highly expressed in proximal duodenum enterocytes, it facilitates the uptake of dietary non-haem iron as well as a variety of other metals. Its inhibition is therefore potentially useful in diseases of iron overload in which DMT1 is upregulated. Efforts to identify DMT1 inhibitors have existed for at least a decade, all using a cell-based calcein assay adapted to monitor iron uptake in human cancer cells overexpressing human DMT1. In recent years, screening and lead optimisation has generated three series of substituted pyrazole compounds as well as diaryl isothiourea and benzylisothiourea compounds that all significantly reduced serum iron levels in an acute rat model of iron hyperabsorption [33,34]. More recently, pyrimidinone has been shown to inhibit iron uptake in the same screen [35], however there was no indication that its iron-chelating properties had been tested, which is crucial given the related compound pyrazolopyrimidinone is known to chelate iron and does not directly inhibit the mycobacterial iron uptake system [36]. With several different DMT1 inhibitors now known, the mechanistic, biochemical and pre-clinical studies should follow the chemical discovery.
Ferristatin II is an iron transport inhibitor that appears to act via DMT1 inhibition, amongst other mechanisms. Initially identified in a screen for the inhibition of transferrin-mediated iron delivery, ferristatin II was shown to induce the degradation of transferrin receptor 1, thus reducing the cellular import of transferrin-bound iron. More recently, it has been shown to induce DMT1 internalisation and thereby reduce the uptake of non-haem iron [37]. A third mode of action is its ability to upregulate the synthesis of the peptide hormone hepcidin, which acts as negative regulator of iron uptake through the initiation of the degradation of the iron exporter ferroportin [38,39]. A number of hepcidin agonists and antagonists have been developed in recent years to target iron overload and deficiency, as reviewed in Refs. [40,41].
Targeting metal homeostasis through metal regulatory proteins
The ability of ferristatin II and other compounds to induce or inhibit the synthesis of hepcidin represents a third means of targeting metal homeostasis: through metal sensing and the regulation of metal homeostasis. Metal regulatory factors sense fluctuations in metal ion levels and, in response, alter gene expression via transcriptional, posttranscriptional and posttranslational mechanisms to maintain metal ion homeostasis [42]. Targeting these factors will enable the manipulation of transition metal homeostasis independent of metal ion sensing. Part of the difficulty lies in the fact that metal regulatory transcription factor 1 (MTF1) is the only regulatory factor to have been identified in mammals. Several transcription factors have been identified in yeast that sense zinc, copper and iron [43].
MTF1 is a zinc-sensing transcription factor that responds to elevated zinc levels by repressing the transcription of zinc uptake genes and upregulating the expression of genes that mitigate the toxic effects of high zinc levels. It is a prospective therapeutic target in epileptogenesis where an increase in zinc concentration activates MTF1 which then binds to metalloregulatory elements in a voltage-dependent calcium channel implicated in the emergence of epileptic seizures [44•]. MTF1 is also implicated in osteoarthritis pathogenesis where upon the upregulation of zinc levels and ZIP8 transporters it mediates the expression of catabolic factors that contribute to cartilage degeneration [45•]. In both cases, MTF1 is not the only possible drug target: excess zinc and, in the case of osteoarthritis, ZIP8 transporters, are also possible targets for pharmaceutical intervention.
The identification of roles for MTF1 in disease pathogenesis and the effect of the manipulation of hepcidin synthesis on iron levels combine to make a compelling argument for continued efforts to discover and characterise mammalian metal sensing and regulatory elements.
Conclusions and outlook
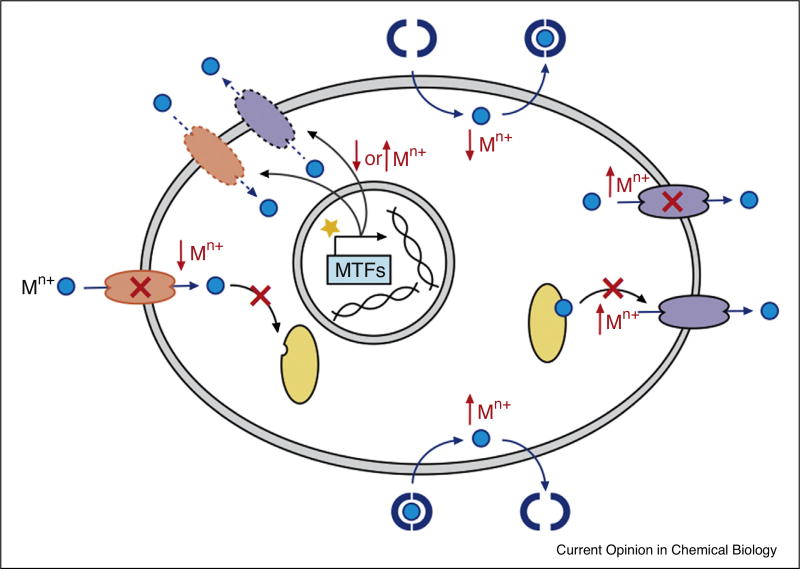
We have described three broad classes of drugs that target transition metal homeostasis: chelators and ionophores that remove, redistribute and deposit metals; small molecule inhibitors of proteins involved in metal transport and a nascent class of drugs that target metal regulatory proteins. A summary of their potential impacts on cellular metal homeostasis is shown in Figure 4.
Figure 4.
General drug targets for altering transition metal homeostasis and their probable impact on metal (Mn+) levels. Chelators and ionophores remove or introduce metal ions. Import, export and other metal transport proteins can be inhibited by drugs that occupy the metal-binding site or prevent metal transfer (as indicated by crosses). Metal regulatory transcription factors (MTFs) can be targeted by agonists or antagonists (represented by a star) to alter expression levels of proteins involved in metal homeostasis. Not depicted here is the potential impact of these drugs on the redistribution of metals across cellular compartments and between cells.
As the best-developed class of drugs targeting metal homeostasis, chelators and ionophores are making their way into clinical trials for the treatment of neurodegenerative diseases and cancer. Research continues into the development of better-targeted chelators and ionophores [46–48]. Speciation calculations have highlighted the potential for iron chelators, provided in excess, to bind zinc and copper [49•]. Caution must be exercised to minimise the impact of chelators on the homeostasis of other metals and to avoid deleterious impacts on metal levels in non-target tissues.
Drugs targeting metal transport, signalling and regulatory pathways may offer a more finely-tuned approach to targeting transition metal homeostasis. However, targeting these proteins does not guarantee specificity to a single metal as the signalling, regulation and transport pathways of metals are frequently intertwined. As its full name implies, DMT1 is involved in the trafficking of metals other than iron. MTF1 is best understood as a regulator of zinc homeostasis, but also responds to fluctuations in the levels of other metals. ZIP8 and ZIP14, in addition to their established zinc uptake activity, are divalent metal transporters that have been linked to the uptake of iron and manganese [50]. These intricacies must be kept in mind when developing therapies for complex diseases of metal dyshomeostasis.
By developing diverse approaches to targeting transition metal homeostasis we will have a robust arsenal of drug candidates for the treatment of diseases of metal dyshomeostasis. From these candidates we may select the point at which we alter transition metal homeostasis for greatest efficacy with the fewest side effects. Bringing this vision to fruition depends on broader and deeper knowledge of metal regulation, transport pathways and the role of metals in disease pathogenesis.
Acknowledgments
The authors acknowledge colleagues whose work was not cited due to space limitations. CMW is supported by a National Health and Medical Research Council (NHMRC) CJ Martin Fellowship. CH is an investigator of the Howard Hughes Medical Institute (HHMI) and is supported by National Institutes of Health GM071440.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Nurchi VM, Crisponi G, Lachowicz JI, Medici S, Peana M, Zoroddu MA. Chemical features of in use and in progress chelators for iron overload. J Trace Elem Med Biol. 2016 doi: 10.1016/j.jtemb.2016.05.010. http://dx.doi.org/10.1016/j.jtemb.2016.05.010. [DOI] [PubMed]
- 2.Franz KJ. Clawing back: broadening the notion of metal chelators in medicine. Curr Opin Chem Biol. 2013;17:143–149. doi: 10.1016/j.cbpa.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santini C, Pellei M, Gandin V, Porchia M, Tisato F, Marzano C. Advances in copper complexes as anticancer agents. Chem Rev. 2014;114:815–862. doi: 10.1021/cr400135x. [DOI] [PubMed] [Google Scholar]
- 4.Helsel ME, Franz KJ. Pharmacological activity of metal binding agents that alter copper bioavailability. Dalton Trans. 2015;44:8760–8770. doi: 10.1039/c5dt00634a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert A, Liu Y, Nguyen M, Meunier B. Regulation of copper and iron homeostasis by metal chelators: a possible chemotherapy for Alzheimer’s disease. Acc Chem Res. 2015;48:1332–1339. doi: 10.1021/acs.accounts.5b00119. [DOI] [PubMed] [Google Scholar]
- 6•.Barnham KJ, Bush AI. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem Soc Rev. 2014;43:6727–6749. doi: 10.1039/c4cs00138a. A comprehensive review of the use and role of metal chelators and ionophores in neurodegenerative diseases, particularly Alzheimer’s disease. [DOI] [PubMed] [Google Scholar]
- 7.Liang SH, Southon AG, Fraser BH, Krause-Heuer AM, Zhang B, Shoup TM, Lewis R, Volitakis I, Han Y, Greguric I, et al. Novel fluorinated 8-hydroxyquinoline based metal ionophores for exploring the metal hypothesis of Alzheimer’s disease. ACS Med Chem Lett. 2015;6:1025–1029. doi: 10.1021/acsmedchemlett.5b00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen M, Robert A, Sournia-Saquet A, Vendier L, Meunier B. Characterization of new specific copper chelators as potential drugs for the treatment of Alzheimer’s disease. Chem Eur J. 2014;20:6771–6785. doi: 10.1002/chem.201402143. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen M, Bijani C, Martins N, Meunier B, Robert A. Transfer of copper from an amyloid to a natural copper-carrier peptide with a specific mediating ligand. Chem Eur J. 2015;21:17085–17090. doi: 10.1002/chem.201502824. [DOI] [PubMed] [Google Scholar]
- 10.Mena NP, García-Beltrán O, Lourido F, Urrutia PJ, Mena R, Castro-Castillo V, Cassels BK, Núñez MT. The novel mitochondrial iron chelator 5-((methylamino)methyl)-8-hydroxyquinoline protects against mitochondrial-induced oxidative damage and neuronal death. Biochem Biophys Res Commun. 2015;463:787–792. doi: 10.1016/j.bbrc.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Meyer E, Valdivia LE, Zhao N, Dadswell C, Abdul-Sada A, Hung CY, Simpson MA, Chong WK, Jacques TS, Woltjer RL, et al. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism-dystonia. Nat Commun. 2016;7:1–16. doi: 10.1038/ncomms11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park KC, Fouani L, Jansson PJ, Wooi D, Sahni S, Lane DJR, Palanimuthu D, Lok HC, Kovačević Z, Huang MLH, et al. Copper and conquer: copper complexes of di-2-pyridylketone thiosemicarbazones as novel anti-cancer therapeutics. Metallomics. 2016;8:874–886. doi: 10.1039/c6mt00105j. [DOI] [PubMed] [Google Scholar]
- 13.Liu P, He K, Song H, Ma Z, Yin W, Xu LX. Deferoxamine-induced increase in the intracellular iron levels in highly aggressive breast cancer cells leads to increased cell migration by enhancing TNF-α-dependent NF-κB signaling and TGF-β signaling. J Inorg Biochem. 2016;160:40–48. doi: 10.1016/j.jinorgbio.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Garber K. Targeting copper to treat breast cancer. Science. 2015;349:128–129. doi: 10.1126/science.349.6244.128. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez HM, Xue Y, Robinson CD, Canalizo-Hernandez MA, Marvin RG, Kelly RA, Mondragon A, Penner-Hahn JE, O’Halloran TV. Tetrathiomolybdate inhibits copper trafficking proteins through metal cluster formation. Science. 2010;327:331–334. doi: 10.1126/science.1179907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juarez JC, Betancourt O, Pirie-Shepherd SR, Guan X, Price ML, Shaw DE, Mazar AP, Doñate F. Copper binding by tetrathiomolybdate attenuates angiogenesis and tumor cell proliferation through the inhibition of superoxide dismutase 1. Clin Cancer Res. 2006;12:4974–4982. doi: 10.1158/1078-0432.CCR-06-0171. [DOI] [PubMed] [Google Scholar]
- 17.Roth HS, Hergenrother PJ. Derivatives of procaspase-activating compound 1 (PAC-1) and their anticancer activities. Curr Med Chem. 2016;23:201–241. doi: 10.2174/0929867323666151127201829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safi R, Nelson ER, Chitneni SK, Franz KJ, George DJ, Zalutsky MR, McDonnell DP. Copper signaling axis as a target for prostate cancer therapeutics. Cancer Res. 2014;74:5819–5831. doi: 10.1158/0008-5472.CAN-13-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Stacy AE, Palanimuthu D, Bernhardt PV, Kalinowski DS, Jansson PJ, Richardson DR. Zinc(II)–thiosemicarbazone complexes are localized to the lysosomal compartment where they transmetallate with copper ions to induce cytotoxicity. J Med Chem. 2016;59:4965–4984. doi: 10.1021/acs.jmedchem.6b00238. Demonstration of the transmetallation, with copper, of zinc ionophores in lysosomes, leading to ROS generation and lysosomal membrane permeabilisation. [DOI] [PubMed] [Google Scholar]
- 20••.Denoyer D, Pearson HB, Clatworthy SAS, Smith ZM, Francis PS, Llanos RM, Volitakis I, Phillips WA, Meggyesy PM, Masaldan S, et al. Copper as a target for prostate cancer therapeutics: copper-ionophore pharmacology and altering systemic copper distribution. Oncotarget. 2016;7:37064–37080. doi: 10.18632/oncotarget.9245. A detailed study into the role of endogenous and exogenous copper in the activity of ionophores in prostate cancer. It extends to the role and source of copper in the progression of prostate tumour growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nevitt T, Öhrvik H, Thiele DJ. Charting the travels of copper in eukaryotes from yeast to mammals. BBA Mol Cell Res. 2012;1823:1580–1593. doi: 10.1016/j.bbamcr.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kambe T, Tsuji T, Hashimoto A, Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev. 2015;95:749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- 23.Montalbetti N, Simonin A, Kovacs G, Hediger MA. Mammalian iron transporters: families SLC11 and SLC40. Mol Asp Med. 2013;34:270–287. doi: 10.1016/j.mam.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Gabbiani C, Scaletti F, Massai L, Michelucci E, Cinellu MA, Messori L. Medicinal gold compounds form tight adducts with the copper chaperone Atox-1: biological and pharmacological implications. Chem Commun. 2012;48:11623–11625. doi: 10.1039/c2cc36610j. [DOI] [PubMed] [Google Scholar]
- 25.Boal AK, Rosenzweig AC. Crystal structures of cisplatin bound to a human copper chaperone. J Am Chem Soc. 2009;131:14196–14197. doi: 10.1021/ja906363t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnesano F, Banci L, Bertini I, Felli IC, Losacco M, Natile G. Probing the interaction of cisplatin with the human copper chaperone Atox1 by solution and in-cell NMR spectroscopy. J Am Chem Soc. 2011;133:18361–18369. doi: 10.1021/ja207346p. [DOI] [PubMed] [Google Scholar]
- 27.Day JA, Cohen SM. Investigating the selectivity of metalloenzyme inhibitors. J Med Chem. 2013;56:7997–8007. doi: 10.1021/jm401053m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobera M, Madauss KP, Pohlhaus DT, Wright QG, Trocha M, Schmidt DR, Baloglu E, Trump RP, Head MS, Hofmann GA, et al. Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group. Nat Chem Biol. 2013;9:319–325. doi: 10.1038/nchembio.1223. [DOI] [PubMed] [Google Scholar]
- 29.Madsen AS, Kristensen HME, Lanz G, Olsen CA. The effect of various zinc binding groups on inhibition of histone deacetylases 1–11. Chem Med Chem. 2013;9:614–626. doi: 10.1002/cmdc.201300433. [DOI] [PubMed] [Google Scholar]
- 30••.Wang J, Luo C, Shan C, You Q, Lu J, Elf S, Zhou Y, Wen Y, Vinkenborg JL, Fan J, et al. Inhibition of human copper trafficking by a small molecule significantly attenuates cancer cell proliferation. Nat Chem. 2015;7:968–979. doi: 10.1038/nchem.2381. A prime example of the development of a small molecule inhibitor of copper transport proteins and the elucidation of its mechanism of action cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Matsui MS, Petris MJ, Niki Y, Karaman-Jurukovska N, Muizzuddin N, Ichihashi M, Yarosh DB. Omeprazole, a gastric proton pump inhibitor, inhibits melanogenesis by blocking ATP7A trafficking. J Investig Dermatol. 2015;135:834–841. doi: 10.1038/jid.2014.461. The identification of omeprazole as a potential inhibitor of copper trafficking by ATP7A as evidenced by its reduction of tyrosinase activity. [DOI] [PubMed] [Google Scholar]
- 32.Narayanan G, Bharathidevi SR, Vuyyuru H, Muthuvel B, Konerirajapuram Natrajan S. CTR1 silencing inhibits angiogenesis by limiting copper entry into endothelial cells. PLoS One. 2013;8:e71982–e71989. doi: 10.1371/journal.pone.0071982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cadieux JA, Zhang Z, Mattice M, Brownlie-Cutts A, Fu J, Ratkay LG, Kwan R, Thompson J, Sanghara J, Zhong J, et al. Synthesis and biological evaluation of substituted pyrazoles as blockers of divalent metal transporter 1 (DMT1) Bioorg Med Chem Lett. 2012;22:90–95. doi: 10.1016/j.bmcl.2011.11.069. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Kodumuru V, Sviridov S, Liu S, Chafeev M, Chowdhury S, Chakka N, Sun J, Gauthier SJ, Mattice M, et al. Discovery of benzylisothioureas as potent divalent metal transporter 1 (DMT1) inhibitors. Bioorg Med Chem Lett. 2012;22:5108–5113. doi: 10.1016/j.bmcl.2012.05.129. [DOI] [PubMed] [Google Scholar]
- 35.Montalbetti N, Simonin A, Simonin C, Awale M, Reymond J-L, Hediger MA. Discovery and characterization of a novel non-competitive inhibitor of the divalent metal transporter DMT1/SLC11A2. Biochem Pharmacol. 2015;96:216–224. doi: 10.1016/j.bcp.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Dragset MS, Poce G, Alfonso S, Padilla-Benavides T, Ioerger TR, Kaneko T, Sacchettini JC, Biava M, Parish T, Argüello JM, et al. A novel antimycobacterial compound acts as an intracellular iron chelator. Antimicrob Agents Chemother. 2015;59:2256–2264. doi: 10.1128/AAC.05114-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanatori I, Yasui Y, Noguchi Y, Kishi F. Inhibition of iron uptake by ferristatin II is exerted through internalization of DMT1 at the plasma membrane. Cell Biol Int. 2015;39:427–434. doi: 10.1002/cbin.10403. [DOI] [PubMed] [Google Scholar]
- 38.Byrne SL, Buckett PD, Kim J, Luo F, Sanford J, Chen J, Enns C, Wessling-Resnick M. Ferristatin II promotes degradation of transferrin receptor-1 in vitro and in vivo. PLoS One. 2013;8:e70199. doi: 10.1371/journal.pone.0070199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alkhateeb AA, Buckett PD, Gardeck AM, Kim J, Byrne SL, Fraenkel PG, Wessling-Resnick M. The small molecule ferristatin II induces hepatic hepcidin expression in vivo and in vitro. Am J Physiol Gastrointest Liver Physiol. 2015;308:G1019–G1026. doi: 10.1152/ajpgi.00324.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Sun B, Yin H, Liu S. Hepcidin: a promising therapeutic target for iron disorders: a systematic review. Medicine (Baltimore) 2016;95:e3150. doi: 10.1097/MD.0000000000003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sebastiani G, Wilkinson N, Pantopoulos K. Pharmacological targeting of the hepcidin/ferroportin axis. Front Pharmacol. 2016;7:160. doi: 10.3389/fphar.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bird AJ. Cellular sensing and transport of metal ions: implications in micronutrient homeostasis. J Nutr Biochem. 2015;26:1103–1115. doi: 10.1016/j.jnutbio.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waldron KJ, Rutherford JC, Ford D, Robinson NJ. Metalloproteins and metal sensing. Nature. 2009;460:823–830. doi: 10.1038/nature08300. [DOI] [PubMed] [Google Scholar]
- 44•.van Loo KMJ, Schaub C, Pitsch J, Kulbida R, Opitz T, Ekstein D, Dalal A, Urbach H, Beck H, Yaari Y, et al. Zinc regulates a key transcriptional pathway for epileptogenesis via metal-regulatory transcription factor 1. Nat Commun. 2015;6:1–12. doi: 10.1038/ncomms9688. This work explores the roles of zinc and MTF1 in a signaling cascade that is linked to epileptogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Kim J-H, Jeon J, Shin M, Won Y, Lee M, Kwak J-S, Lee G, Rhee J, Ryu J-H, Chun C-H, et al. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014;156:730–743. doi: 10.1016/j.cell.2014.01.007. This paper identifies zinc, ZIP8 and MTF1 as targets for therapeutic approaches in the treatment of osteoarthritis. [DOI] [PubMed] [Google Scholar]
- 46.Perez LR, Franz KJ. Minding metals: tailoring multifunctional chelating agents for neurodegenerative disease. Dalton Trans. 2010;39:2177–2187. doi: 10.1039/b919237a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akam EA, Chang TM, Astashkin AV, Tomat E. Intracellular reduction/activation of a disulfide switch in thiosemicarbazone iron chelators. Metallomics. 2014;6:1905–1912. doi: 10.1039/c4mt00153b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akam EA, Tomat E. Targeting iron in colon cancer via glycoconjugation of thiosemicarbazone prochelators. Bioconjug Chem. 2016;27:1807–1812. doi: 10.1021/acs.bioconjchem.6b00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Crisponi G, Nurchi VM, Crespo-Alonso M, Sanna G, Zoroddu MA, Alberti G, Biesuz R. A speciation study on the perturbing effects of iron chelators on the homeostasis of essential metal ions. PLoS One. 2015;10:14-e0133050. doi: 10.1371/journal.pone.0133050. A calculation of the speciation of metal ions under physiologically-relevant conditions of iron chelation therapy. The predicted chelation of copper and zinc when chelators are in excess of iron is supported by clinical reports of zinc deficiency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jenkitkasemwong S, Wang C-Y, Mackenzie B, Knutson MD. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. BioMetals. 2012;25:643–655. doi: 10.1007/s10534-012-9526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]