Abstract
Small-fiber polyneuropathy SFPN affects unmyelinated and thinly myelinated peripheral axons. Several questionnaires have been developed to assess polyneuropathy from diabeties or chemotherapy, but none for SFPN from other or unknown causes. A comprehensive survey could help clinicians diagnose and assess treatment responses, define prevalence natural history and cures, and identify research subjects. Thus, we developed the one-page Small-fiber Symptom Survey (SSS), using input from patients and 21 medical/scientific experts. Participants comprised consenting consecutive patients evaluated for SFPN at the Massachusetts General Hospital plus normal controls. Participants SFPN status was stratified based on the results of their objective diagnostic tests (distal-leg skin biopsy and autonomic-function testing). We measured internal consistency, test re-test reliability, convergent validity and performed a receiver operating curve analysis.
The 179 participants averaged 46.6±15.6 years old; they were 73.2% female and 92.2% Caucasian. Eighty-five had confirmed SFPN, mostly idiopathic. Principal component analysis revealed 5 symptom clusters. The questionnaire had good internal consistency (Cronbach alpha=0.893), excellent test re-test reliability (r=0.927, p<0.001) and good-to-fair convergent validity. Participants with confirmed SFPN had more severe symptoms than others (p=0.009). The SSS has satisfactory psychometric properties, indicating potential future utility for surveying patient-reported symptoms of SFPN regardless of its cause.
Perspective
This article reports the initial development and early psychometric validation of a new patient-reported outcome measure intended to capture the wide range of multi-system symptoms of small fiber polyneuropathy. Once further developed, it could potentially help clinicians diagnose and monitor patients, and help advance research.
Keywords: peripheral neuropathy, dysautonomia, sensory, neuropathic pain, skin biopsy
Introduction
Distal polyneuropathy is common, with the National Health and Nutrition Examination Survey (NHANES) reporting 14.8% prevalence among people over age 40 [10]. However, these figures do not fully capture patients with small-fiber polyneuropathy (SFPN), although it is the most common presentation of distal polyneuropathy. Multiple labs now report evidence of SFPN in about 40% of patients with fibromyalgia [11, 21, 26]. Since fibromyalgia affects 2-5% of the world’s population [17, 28], SFPN may affect millions. SFPN involves preferential damage to the small diameter, unmyelinated C-fibers and/or thinly myelinated A-delta fibers that signal pain, tissue damage and inflammation, and regulate the body’s tissues and organs [5]. If oxygen, nutrient, or energy supply is compromised, the distal ends of these long axons malfunction and degenerate, which causes diverse symptoms. Sensory symptoms can include spontaneous chronic widespread pain (CWP), stimulus-evoked hyperalgesia/allodynia, reduced nociceptive sensation, and neuropathic itch [20, 25]. The cardiovascular, gastrointestinal, microvascular and/or sweating symptoms can reflect either autonomic or somatic axonopathy since many internal organs and tissues have dual small-fiber innervation [1]. Neurogenic dysregulation of the microvessels alone causes a wide array of symptoms including postural orthostatic tachycardia syndrome [11] fatigue, and even cognitive dysfunction [22].
Current patient reported outcomes (PROs) focus on the polyneuropathies that predominantly affect the large myelinated motor and sensory fibers such as Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy (CIDP), or neuropathies caused by one medical cause [3, 4, 6, 14, 15, 18, 23]. Examples include the Survey of Autonomic Symptoms for diabetic neuropathy [29], the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, the Chemotherapy Induced Peripheral Neuropathy Questionnaire [24] and the Treatment-Induced Neuropathy Assessment Scale [19]. No questionnaire has been designed to capture symptoms from patients with idiopathic SFPN, although they represent the 2nd largest group of SFPN patients (20%-50% in recent series [9, 13]) after those with diabetic SFPN. Thus, we developed and evaluated the Small-Fiber Symptom Survey (SSS), a one-page questionnaire concerning the full spectrum of SFPN symptoms and applicable to patients with undefined causes of their SFPN.
Methods
Subjects and Data Acquisition
Participants provided informed consent to a protocol approved by the hospital’s institutional review board. Most were patients being evaluated for symptoms suggesting SFPN despite no evident medical cause (e.g., diabetes, chemotherapy exposure), thus they had been referred for potentially confirmatory objective testing for SFPN at Massachusetts General Hospital (MGH) in 2014-2015. A small sample of screened healthy volunteers was added to expand to the full range of symptom presence and severity. Eligibility required age ≥18 years, English fluency, and distal-leg, PGP9.5-immunolabeled skin biopsy or diagnostic Autonomic Function Testing (AFT) conducted within 18 months of recruitment for this study. These tests had been performed in JC-accredited clinical diagnostic laboratories using standard clinical diagnostic methods, equipment and interpretations [2, 8, 16]. Patients were stratified into “confirmed SFPN” or “possible SFPN” based on whether the medical report interpreting their skin biopsy and/or AFT diagnosed them with SFPN or not.
Al eligible patients were sent invitation letters followed by a phone call. Respondents were screened for eligibility, consented, and demographic and medical information including comorbid medical conditions was captured using an approved telephone script. Study aims and instructions were provided. Data were captured using the Research Electronic Data Capture system (REDCap), a secure web-based application for securely capturing medical data [12]. Participants who completed the study were compensated $15.
Questionnaire Development
The initial 23-item first version was based on the most common symptoms reported by patients during medical care, and by review of the literature pertaining to symptoms of SFPN. The questionnaire was then used during patients evaluations for 16 months, during which we added new items that patients reported, and improved comprehensibility. This revised 32-item version was then circulated to 21 experts for input. They consisted of 11 neurologists (including 4 pediatric neurologists and three peripheral-nerve specialists), an internist/pediatrician, a cardiology expert on dysautonomia, 4 gastrointestinal specialists including one pediatrician, 1 urologist, and three experts in design of pain-related PROs. They added one item, rephrased several items, and modified the scale. The third version (33 items) underwent cognitive debriefing interviews with patients to improve comprehensibility.
The fourth version of the survey that was studied here containing 33 items and is one page long. The instructions are “Rate how much you have been affected by each symptom below in the last week”. Participants were asked to rate the first 32 items on a 0-4 scale (0 not at all, 1 a little bit, 2 somewhat, 3 quite a bit, 4 very much), a scale recommended by the NIH Patient Reported Outcomes Measurement Information System (PROMIS) project (http://www.nihpromis.org). The 33rd item is a 0-10 numerical pain rating scale (NPS) asking respondents to rate “Intensity of your chronic widespread pain (on both sides of your body) at its worst during the last week”. A free-text section was available to capture additional symptoms or suggestions for improvement.
Preliminary validation
A link was emailed to each consented subject directing them to the REDCap survey, which included questions on demographics and the SSS. Subjects could mail back paper documents if preferred. To assess convergent validity, subjects also completed the Composite Autonomic Symptom Score (COMPASS-31) [25], the Short-form McGill Pain Questionnaire (SF-MPQ -2) [7] and the Medical Outcomes Study Short-Form Health Survey (SF-36) [27]. Two weeks after completing the first REDCap survey, participants completed the SSS a second time to assess test re-test reliability.
Statistical analyses
Analyses were conducted with SPSS for Windows version 19 (Chicago, IL, USA) and SAS version 9.3 (SAS Institute, Cary NC). Descriptive statistics described prevalence. Symptom scores were reported as means ± standard deviations. Internal validity was assessed by Cronbach’s alpha. Test re-test reliability and convergent validity were assessed by zero order Pearson product-moment correlations. Exploratory factor analysis (EFA) with principal components extraction was conducted on the responses of all 179 participants with varimax rotation, with factor weights sorted and suppressed if less than |4|. This produces an orthogonal (i.e., uncorrelated) factor solution. This process was repeated until the full underlying factor structure emerged. Differences in dependent variables were compared between groups using independent t-test. P-values ≤ 0.05 were considered statistically significant. Receiver operating characteristic (ROC) analysis was conducted with SFPN diagnosis as the state variable and the SSS total score as the test variable, using age and gender as covariates. Due to the exploratory nature of the study, no corrections for multiple comparisons were used.
Results
Cohort characteristics
From 470 eligible potential subjects contacted, 179 (162 patients and 17 healthy volunteers) completed the first REDCap survey (38% response rate). Their mean age was 46.6 ± 15.6 years (range 18-88 years), 73.2% (131/179) identified as female, and 92.2% as Caucasian. Table 1 tallies their most common comorbid medical diagnoses and their most common classes of medications used. Overall, 85 subjects were diagnosed with “confirmed SFPN” by one or both diagnostic tests. Specifically, 67 diagnostic skin biopsies (epidermal nerve fiber density ≤ 5th centile of predicted) and 29 had diagnostic AFTs. The demographic characteristics of participants is presented in Table 2.
Table 1.
Top 10 diagnoses and classes of medication (n=179)
| Diagnoses | % (n) | Medication classes | % (n) |
|---|---|---|---|
| Fibromyalgia | 29.1 (52) | Antidepressants | 42.5 (76) |
| Hypertension | 20.1 (36) | Cardiovascular-related | 39.7 (71) |
| Hypothyroidism | 16.2 (29) | Gastrointestinal-related | 38.5 (69) |
| Depression | 15.1 (27) | Antiepileptic | 33.0 (59) |
| Gastroesophageal reflux disease | 12.3 (22) | Corticosteroids | 29.6 (53) |
| Asthma | 11.7 (21) | NSAID/Anti-inflammatory | 29.1 (52) |
| Migraine | 8.4 (15) | Benzodiazepine | 27.4 (49) |
| Anxiety | 7.8 (14) | Opioids | 24.0 (43) |
| Allergic Rhinitis | 7.3 (13) | Antibiotic, antiviral and anti-fungal | 21.8 (39) |
| Headache | 7.3 (13) | Asthma and allergies | 20.7 (37) |
Table 2.
Demographic characteristics of participants stratified by SFPN status
| Characteristic | Confirmed SFPN | Non-SFPN (n=94) n (%) | ||
|---|---|---|---|---|
|
| ||||
| 85 patients with confirmed SFPN n (%) | 77 patients with possible SFPN | 17 healthy controls | ||
| Age | (mean± SD) | 50.18±15.4 | 45.22±14.6 | 34.00±15.2 |
| Gender | ||||
| Female | 64 (75.3%) | 62 (80.5%) | 5 (29.4%) | |
| Male | 21 (24.7%) | 15 (19.5%) | 12 (70.6%) | |
| Race/Ethnicity | ||||
| Hispanic | 2 (2.4%) | 1 (1.3%) | 0 | |
| White | 80 (94.1%) | 71 (92.2%) | 14 (82.4%) | |
| Asian | 1 (1.2%) | 3 (3.9%) | 1 (5.9%) | |
| Other (black and mixed race) | 2 (2.4%) | 2 (2.6%) | 2 (11.8%) | |
| Employment status | ||||
| Employed | 34 (40.0%) | 38 (49.4%) | 12 (70.6) | |
| Disabled | 30 (35.3%) | 17 (22.1%) | 0 | |
| Retired | 17 (20%) | 8 (10.4%) | 0 | |
| Other (including students) | 4 (4.7%) | 14 (18.2%) | 5 (29.4%) | |
| Educational status | ||||
| High school or lower | 33 (38.9%) | 26 (33.8%) | 9 (52.9%) | |
| Bachelor | 21 (24.7%) | 24 (31.2%) | 6 (35.3%) | |
| Graduate or higher | 31 (36.5%) | 27 (35.1%) | 2 (11.8%) | |
| Marital status | ||||
| Single | 21 (24.7%) | 26 (33.8%) | 9 (52.9%) | |
| In relationship | 56 (65.9%) | 49 (63.6%) | 7 (41.1%) | |
| Divorced or widowed | 8 (9.4%) | 2 (2.6%) | 1 (5.9%) | |
Table 3 reveals that participants’ 5 most prevalent symptoms (rated as present in any severity) were “Tiredness (fatigue)”, present in 98.1%, “Reduced endurance or strength for activities” in 96.3%, “Difficulty thinking, concentrating, or remembering” in 90.1%, “Tingling or “pins and needles” in 88.9%, and “Deep pains or aches” in 87.0%. Subjects’ worst pain in the last week averaged 5.3 ± 3.4, with 84.4% reporting having chronic widespread pain (non-zero score) and 67.6% reporting pain scores ≥ 4.
Table 3.
Ranked prevalence of specific symptoms in all 179 participants
| Item | Not at all | A little bit | Somewhat | Quite a bit | Very much |
|---|---|---|---|---|---|
| Tiredness (fatigue) | 1.9% (3) | 10.5% (17) | 17.9% (29) | 37.0% (60) | 32.7% (53) |
| Reduced endurance or strength for activities | 3.7% (6) | 11.1% (18) | 16.7% (27) | 37.7% (61) | 30.9% (50) |
| Difficulty thinking, concentrating, or remembering | 9.9% (16) | 24.1% (39) | 23.5% (38) | 24.7% (40) | 17.9% (29) |
| Tingling or “pins and needles” | 11.1% (18) | 16.0% (26) | 24.7% (40) | 25.9% (42) | 22.2% (36) |
| Deep pains or aches | 13.0% (21) | 7.4% (12) | 19.1% (31) | 27.2% (44) | 33.3% (54) |
| Need to move legs often for comfort | 14.8% (24) | 19.1% (31) | 22.2% (36) | 24.1% (39) | 19.8% (32) |
| Eye difficulties (dry, sensitive to light, hard to focus) | 17.3% (28) | 21.0% (34) | 26.5% (43) | 21.0 (34) | 14.2% (23) |
| Skin that has less sensation (numbness) | 17.3% (28) | 17.9% (29) | 23.5% (38) | 23.5% (38) | 17.9% (29) |
| Feeling dizzy or faint when standing up | 23.5% (38) | 27.8% (45) | 22.2% (36) | 13.0% (21) | 13.6% (22) |
| Skin that hurts for no reason | 27.2% (44) | 13.6% (22) | 21.0% (34) | 16.0% (26) | 22.2% (36) |
| Headaches | 29.6% (48) | 25.9% (42) | 16.7% (27) | 19.1% (31) | 8.6% (14) |
| Stomach quickly full or bloated after meals | 32.1% (52) | 16.0% (26) | 16.0% (26) | 18.5% (30) | 17.3% (28) |
| Skin that itches for no reason | 34.0% (55) | 25.3% (41) | 16.7% (27) | 11.7% (19) | 12.3% (20) |
| Swelling in hands or feet | 37.0% (60) | 24.7% (40) | 17.9% (29) | 9.9% (16) | 10.5% (17) |
| Constipation | 38.3% (62) | 15.4% (25) | 15.4% (25) | 17.3% (28) | 13.6% (22) |
| Rapid heartbeat | 41.4% (67) | 24.1% (39) | 8.0 (13) | 16.0% (26) | 10.5% (17) |
| Abdominal pain | 41.4% (67) | 24.1% (39) | 13.6% (22) | 9.3% (15) | 11.7% (19) |
| Urinary frequency, urgency, or accidents | 41.4% (67) | 15.4% (25) | 22.2% (36) | 14.2% (23) | 6.8% (11) |
| Skin that hurts after gentle contact (touch, breeze) | 42.6% (69) | 12.3% (20) | 17.9% (29) | 15.4% (25) | 11.7% (19) |
| Skin that burns or requires cooling for comfort | 42.6% (69) | 13.6% (22) | 16.0% (26) | 12.3% (20) | 15.4% (25) |
| Changed pattern of sweating on body | 43.2% (70) | 15.4% (25) | 16.0% (26) | 17.3% (28) | 8.0% (13) |
| Skin with unusual color or changes in color | 44.4% (72) | 16.0% (26) | 19.8% (32) | 8.0% (13) | 11.7% (19) |
| Difficulty with sexual function | 47.5% (77) | 17.3% (28) | 14.2% (23) | 9.9% (16) | 11.1% (18) |
| Diarrhea | 50.0% (81) | 27.2% (44) | 13.6% (22) | 5.6% (9) | 3.7% (6) |
| Deep vibration or fluttering | 50.6% (82) | 18.5% (30) | 13.0% (21) | 10.5% (17) | 7.4% (12) |
| Nausea or vomiting | 51.9% (84) | 21.0% (34) | 9.3% (15) | 8.6% (14) | 9.3% (15) |
| Difficulty completely emptying bladder | 52.5% (85) | 16.7% (27) | 13.6% (22) | 10.5% (17) | 6.8% (11) |
| Less hair growth on lower legs or feet | 53.1% (86) | 14.2% (23) | 13.6% (22) | 12.3% (20) | 6.8% (11) |
| Less appetite or unintended weight loss | 58.0% (94) | 21.6% (35) | 9.3% (15) | 5.6% (9) | 5.6% (9) |
| Blisters or sores inside mouth | 60.5% (98) | 22.8% (37) | 12.3% (20) | 3.1% (5) | 1.2% (2) |
| Difficulty starting to urinate | 66.0% (107) | 13.0% (21) | 9.3% (15) | 8.0% (13) | 3.7% (6) |
| Blister, sores or ulcers on feet or hands | 84.6% (137) | 10.5% (17) | 3.1% (5) | 1.2% (2) | 0.6% (1) |
Principal components analysis
Extraction of the 33 items produced a 7-component solution that explained 61.2% of the variance. The 7 items that correlated with more than one component were: “Intensity of chronic widespread pain”, “Tingling or “pins and needles”, “Need to move legs often for comfort”, Abdominal pain”, “Feeling dizzy or faint when standing up”, “Deep pains or aches”, and “Skin that has less sensation (numbness)”. The second iteration restricted to the remaining 26 items yielded a 6-component solution that explained 58.7% of the variance. After “Difficulty thinking, concentrating or remembering” was excluded, a third analysis of 25 items yielded 6 components that explained 58.8% of the variance. After “Deep vibration or fluttering” and “Blisters, sores or ulcers on feet and hands” were excluded, a forth analysis yielded a 5 component 23-item version that explained 58.7% of the variance. After “Blisters or sores inside mouth” was excluded, a fifth iteration yielded 5 components (each item falling in a single component), that explained 57.8% of the variance (Table 4). Since some of the items detected are medically important, we decided to re-evaluate the overlapping items to see which made medical sense to delete, and which should be revised to improve their clinical and statistical utility in a subsequent study.
Table 4.
The 5 component solution of the Principal Component Analysis
| Rotated Component Matrix | |||||
|---|---|---|---|---|---|
|
| |||||
| Component
|
|||||
| 1 | 2 | 3 | 4 | 5 | |
| Nausea or vomiting | .815 | ||||
| Stomach quickly full or bloated after meals | .744 | ||||
| Less appetite or unintended weight loss | .686 | ||||
| Headaches | .564 | ||||
| Constipation | .476 | ||||
|
| |||||
| Skin that hurts for no reason | .762 | ||||
| Skin that hurts after gentle contact (touch, breeze) | .754 | ||||
| Skin that burns or requires cooling for comfort | .744 | ||||
| Difficulty with sexual function | .577 | ||||
|
| |||||
| Reduced endurance or strength for activities | .784 | ||||
| Tiredness (fatigue) | .759 | ||||
| Eye difficulties (dry, sensitive to light, hard to focus) | .521 | ||||
| Diarrhea | .471 | ||||
| Rapid heartbeat | .450 | ||||
|
| |||||
| Skin with unusual color or changes in color | .722 | ||||
| Less hair growth on lower legs or feet | .679 | ||||
| Changed pattern of sweating on body | .619 | ||||
| Swelling in hands or feet | .587 | ||||
| Skin that itches for no reason | .467 | ||||
|
| |||||
| Difficulty completely emptying bladder | .851 | ||||
| Difficulty starting to urinate | .786 | ||||
| Urinary frequency, urgency, or accidents | .587 | ||||
Extraction Method: Principal Component Analysis. Rotation Method: Varimax with Kaiser Normalization. Rotation converged in 5 iterations.
Preliminary validation of the SSS
Cronbach’s alpha analysis on all 179 subjects revealed good internal consistency for the entire survey (0.893) and for each of the 5 components (0.785, 0.799, 0.759, 0.708 and 0.715 respectively; Table 5). One hundred sixty-four subjects (147 patients and 17 healthy controls) completed the surveys twice. Stability over time was excellent for the entire questionnaire (Pearson r=0.927, p<0.001) and for each component (0.884, 0.867, 0.887, 0.883, 0.837, respectively; P<0.001 for all tests). There was good convergent validity between total scores of the SSS and the McGill (r=0.795, p<0.001), the COMPASS-31 (r=0.769, p<0.001) and fair convergence with the SF-36 (r=-0.644, p<0.001). Diagnostic potential of SSS for SFPN was evaluated by ROC analysis. The SSS had poor accuracy in predicting SFPN, with area under the curve of 0.599.
Table 5.
Means in cohort (±SD), Cronbach’s alpha values of the 5 components
| Component | Number of items | Cronbach’s alpha | Cohort Mean (±SD) | SFPN Mean (±SD) | Non-SFPN Mean (±SD) | p-value |
|---|---|---|---|---|---|---|
| The entire survey | 22 | 0.893 | 29.06 (16.35) | 32.38 (14.76) | 26.05 (17.20) | 0.009 |
| Component 1 | 5 | 0.785 | 6.02 (4.99) | 6.24 (4.97) | 5.82 (5.01) | 0.578 |
| Component 2 | 4 | 0.799 | 5.41 (4.64) | 6.27 (4.63) | 4.64 (4.54) | 0.018 |
| Component 3 | 5 | 0.759 | 9.04 (4.48) | 9.82 (3.99) | 8.33 (4.80) | 0.026 |
| Component 4 | 5 | 0.708 | 5.83 (4.59) | 6.72 (4.50) | 5.03 (4.54) | 0.014 |
| Component 5 | 3 | 0.715 | 2.75 (2.96) | 3.33 (3.08) | 2.23 (2.75) | 0.013 |
Symptom profile in patients with objectively confirmed SFPN
The results from the 85 “gold-standard” patients with objective confirmation of SFPN are presented in Figure 1. Their 5 most severe symptoms were “Tiredness (fatigue)”, “Reduced endurance or strength for activities”, “Deep pains or aches”, “Tingling or “Pins and needles”, and “Difficulty thinking, concentrating, or remembering”. Table 5 summarizes the analysis of the questionnaire’s discriminative ability. This showed higher overall symptom severity among the patients with confirmed SFPN (32.4 ± 14.8) than in the others (26.1 ± 17.2; p=0.009). Those with confirmed SFPN also had higher scores in 4/5 components. The range of symptoms reported by the 17 healthy volunteers was far lower, with total scores ranging from 0 – 15 and a mean of 5.06 ± 5.3
Figure 1.
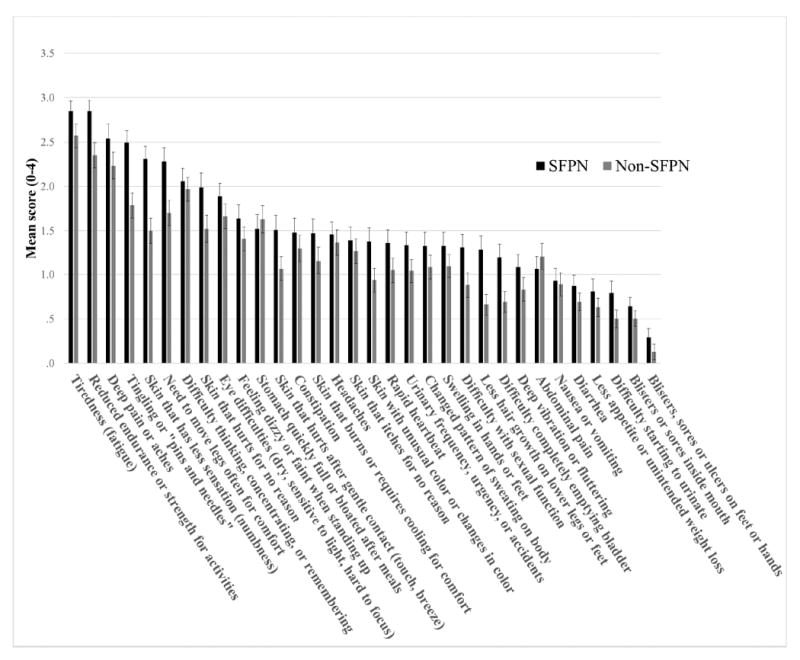
Severity of symptoms in patients with objectively confirmed SFPN (n = 85) and in Non-SFPN (n=94).
Discussion
The SSS version 4 questionnaire had good psychometric properties including excellent test re-test reliability, good internal consistency and convergent validity. Participants with confirmed SFPN had higher scores than non-SFPN healthy controls, providing evidence of potential diagnostic utility.
The Principal Component Analysis yielded a 5-component solution that mostly divided into medically appropriate categories, containing symptoms likely to share underlying mechanisms. Component 1 was mainly gastrointestinal symptoms and Component 2 mainly somatosensory symptoms. Component 2 had the largest severity differences between the two groups, suggesting that sensory symptoms might be a potentially useful discriminator for screening. Component 3 was a cluster of miscellaneous symptoms with no evident link at present. Component 4 comprised largely vascular symptoms and Component 5 contained only 3 questions about urological symptoms. It had the lowest severity score overall and it differed only modestly between groups, perhaps because urological symptoms are common and often multifactorial. However, some of the items that “fell out” were medically important to retain, so they were flagged for further revision to improve their psychometric performance. This illustrates a limitation of principal component analyses, and the need to balance them with real-world considerations.
This study’s strengths included rigorous development over 3 years using input from hundreds of patients and a broad spectrum of experts in relevant medical specialties and in questionnaire design (see Acknowledgements). Another strength is the large sample of 85 patients with objective confirmation of SFPN. These gold-standard subjects provide some assurance of the specificity of symptoms, although having SFPN does not preclude having other unrelated causes of symptoms, e.g., urological complaints from prostate hypertrophy.
One limitation is that the SSS cannot distinguish between primary versus secondary symptoms, for instance caused by medications, co-morbidities, or inactivity. For instance, 24% of the entire cohort used opioids (which cause constipation), and 15% were also diagnosed as suffering from depression. These are among the factors that might explain why cognitive concerns, not traditional symptoms of polyneuropathy, were prevalent, although there is increasing evidence that SFPN causes trans-synaptic network effects on brain neurons [22]. However, whether primary or secondary, all SFPN-associated symptoms are important to capture. Another limitation is that the SSS was administered up to 18 months after participants had their objective tests, whereas ideally, they should have been administered concomitantly.
Another consideration is that both of the recommended objective diagnostic tests for SFPN were used to identify the gold-standard patients, skin biopsy and AFT. Using both permitted us to capture patients with the full spectrum of somatic and autonomic symptoms, but each test can capture different patients. The cohort had too few patients with confirmation by only one of these tests but not the other, to perform subgroup analyses in this initial study. As additional data are collected, clusters of questions might be identified that predict skin biopsy or AFT results sufficiently well to serve as non-invasive surrogates for these expensive tests, or to identify which patients should or should not undergo these tests.
The ROC analysis of the SSS for identifying SFPN had an AUC of 0.599 using autonomic function testing and skin biopsy to define SFPN. It is likely adding other potential risk factors and easily measured clinical characteristics (e.g., heart rate) along with a more advanced version of the SSS would lead to a higher AUC that could identify at-risk patients who should have more in-depth clinical testing. Further, such a model could also be used to improve population-based research to identify novel risk factors for SFPN that could further enhance prediction. More research is needed to expand the predictive capacity of the model.
To conclude, we report preliminary evidence of good psychometric properties and clinical relevance of the SSS. Responses from of the 85 gold standard patients with confirmed SFPN suggest that patients with SFPN have more symptoms than classically reported, including fatigue, chronic headache, deep aches, and reduced endurance. Future tasks include clarifying which questionnaire items should be removed and which should be rewritten to improve their psychometric performance, defining scoring algorithms for clinical and research use, and validating performance in subgroups with common causes of SFPN such as diabetes or chemotherapy exposure. Correlation with outcomes of skin biopsy and AFT can be explored to assess the potential utility of the SSS as an inexpensive patient-administered screening tool for SFPN.
Highlights.
Current neuropathy-related PRO’s do not capture the full range of SFPN symptoms
We report the development and initial validation of a new survey for SFPN symptoms
This survey is suitable for patients with ill-defined causes of SFPN
It can support diagnosis and monitoring symptoms, for clinically and research wise
Acknowledgments
We thank participating patients, their families and their referring physicians for their help, and we gratefully acknowledge the help of Heather Downs and Kate O’Neill with subject recruitment and data entry and Gary R. Zirpoli for assisting with data analyses. We thank the medical and scientific experts who reviewed this questionnaire and helped contribute to its development: Jaime Belkind-Gerson MD, Aimee Boegle MD PhD, Daniel Carr MD, Verne S. Caviness Jr. MD DPhil, H. Thomas Cheng MD PhD, Robert H. Dworkin PhD, Florian Eichler MD, Khosro Farhad MD, John Farrar MD PhD, Cosmas Giallourakis MD, Steven Horowitz MD, Pablo Gomery MD, Nancy Gracin MD, Amel Karaa MD, Braden Kuo MD, Stephen Massaquoi MD PhD, Allan Ropper MD, Katherine Sims MD, Shivraj Sohur MD PhD, Kathryn Swoboda MD, Andrea Thurler NP.
This work was supported by the National Institutes of Health [R01-NS093653 and UL1 TR001102]; Lundbeck Foundation Scholarship in Neurology; and the U.S. Department of Defense [GW140169].
Footnotes
Disclosures
Conflicts of interest: The authors declare no financial or other conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albrecht PJ, Hou Q, Argoff CE, Storey JR, Wymer JP, Rice FL. Excessive peptidergic sensory innervation of cutaneous arteriole-venule shunts (AVS) in the palmar glabrous skin of fibromyalgia patients: Implications for widespread deep tissue pain and fatigue. Pain Medicine. 2013;14(6):895–915. doi: 10.1111/pme.12139. [DOI] [PubMed] [Google Scholar]
- 2.Amato AA, Oaklander AL. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 16-2004. A 76-year-old woman with numbness and pain in the feet and legs. New England Journal of Medicine. 2004;350(21):2181–9. doi: 10.1056/NEJMcpc049005. [DOI] [PubMed] [Google Scholar]
- 3.Bastyr EJ, 3rd, Price KL, Bril V MBBQ Study Group. Development and validity testing of the neuropathy total symptom score-6: questionnaire for the study of sensory symptoms of diabetic peripheral neuropathy. Clinical Therapeutics. 2005;27(8):1278–94. doi: 10.1016/j.clinthera.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Binda D, Cavaletti G, Cornblath DR, Merkies IS CI-PeriNomS study group. Rasch-Transformed Total Neuropathy Score clinical version (RT-TNSc (©)) in patients with chemotherapy-induced peripheral neuropathy. Journal of Peripheral Nervous System. 2015;20(3):328–32. doi: 10.1111/jns.12140. [DOI] [PubMed] [Google Scholar]
- 5.Chan AC, Wilder-Smith EP. Small fiber neuropathy: Getting bigger! Muscle Nerve. 2016;53(5):671–82. doi: 10.1002/mus.25082. [DOI] [PubMed] [Google Scholar]
- 6.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Annals of the Academy of Medicine Singapore. 1994;23(2):129–38. [PubMed] [Google Scholar]
- 7.Dworkin RH, Turk DC, Revicki DA. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2) Pain. 2009 Jul;144(1-2):35–42. doi: 10.1016/j.pain.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 8.England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, Asbury AK, Szigeti K, Lupski JR, Latov N, Lewis RA, Low PA, Fisher MA, Herrmann D, Howard JF, Lauria G, Miller RG, Polydefkis M, Sumner AJ American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Practice parameter: the evaluation of distal symmetric polyneuropathy: the role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM&R. 2009;1:14–22. doi: 10.1016/j.pmrj.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Farhad K, Traub R, Ruzhansky KM, Brannagan TH., 3rd Causes of neuropathy in patients referred as “idiopathic neuropathy”. Muscle Nerve. 2015;53(6):856–61. doi: 10.1002/mus.24969. [DOI] [PubMed] [Google Scholar]
- 10.Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, Burt V, Curtin L, Engelgau M, Geiss L. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care. 2004;27(7):1591–7. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 11.Haensch CA, Tosch M, Katona I, Weis J, Isenmann S. Small-fiber neuropathy with cardiac denervation in postural tachycardia syndrome. Muscle Nerve. 2014;50(6):956–61. doi: 10.1002/mus.24245. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman EM, Staff NP, Robb JM, St Sauver JL, Dyck PJ, Klein CJ. Impairments and comorbidities of polyneuropathy revealed by population-based analyses. Neurology. 2015;84:1644–1651. doi: 10.1212/WNL.0000000000001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoitsma E, De Vries J, Drent M. The small fiber neuropathy screening list: Construction and cross-validation in sarcoidosis. Respiratory Medicine. 2011;105(1):95–100. doi: 10.1016/j.rmed.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Huang HQ, Brady MF, Cella D, Fleming G. Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a gynecologic oncology group study. International Journal of Gynecolological Cancer. 2007;17(2):387–93. doi: 10.1111/j.1525-1438.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 16.Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies IS, Polydefkis M, Smith AG, Sommer C, Valls-Solé J European Federation of Neurological Societies; Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. European Journal of Neurology. 2010;17:903–909. doi: 10.1111/j.1468-1331.2010.03023.x. [DOI] [PubMed] [Google Scholar]
- 17.Lindell L, Bergman S, Petersson IF, Jacobsson LT, Herrstrom P. Prevalence of fibromyalgia and chronic widespread pain. ScandJ PrimHealth Care. 2000;18(3):149–53. doi: 10.1080/028134300453340. [DOI] [PubMed] [Google Scholar]
- 18.Meijer JW, Smit AJ, Sonderen EV, Groothoff JW, Eisma WH, Links TP. Symptom scoring systems to diagnose distal polyneuropathy in diabetes: the Diabetic Neuropathy Symptom score. Diabetetic Medicine. 2002;19(11):962–5. doi: 10.1046/j.1464-5491.2002.00819.x. [DOI] [PubMed] [Google Scholar]
- 19.Mendoza TR, Wang XS, Williams LA. Measuring Therapy-Induced Peripheral Neuropathy: Preliminary Development and Validation of the Treatment-Induced Neuropathy Assessment Scale. Journal of Pain. 2015;16(10):1032–43. doi: 10.1016/j.jpain.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oaklander AL. Common neuropathic itch syndromes. Acta Dermato-Venereologica. 2012;92(2):118–25. doi: 10.2340/00015555-1318. [DOI] [PubMed] [Google Scholar]
- 21.Oaklander AL1, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain. 2013;154(11):2310–6. doi: 10.1016/j.pain.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ocon AJ, Messer ZR, Medow MS, Stewart JM. Increasing orthostatic stress impairs neurocognitive functioning in chronic fatigue syndrome with postural tachycardia syndrome. Clinical Science (Lond) 2012;122(5):227–38. doi: 10.1042/CS20110241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldenburg J, Fosså SD, Dahl AA. Scale for chemotherapy-induced long-term neurotoxicity (SCIN): psychometrics, validation, and findings in a large sample of testicular cancer survivors. Quality of Life Research. 2006;15(5):791–800. doi: 10.1007/s11136-005-5370-6. [DOI] [PubMed] [Google Scholar]
- 24.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, Hoang-Xuan K, Lantéri-Minet M, Grant R, Huddart R, Moynihan C, Maher J, Lucey R EORTC Quality of Life Group. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. European Journal of Cancer. 2005;41(8):1135–9. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Treister R, O’Neil K, Downs HM, Oaklander AL. Validation of the composite autonomic symptom scale 31 (COMPASS-31) in patients with and without small fiber polyneuropathy. European Journal of Neurology. 2015;22:1124–30. doi: 10.1111/ene.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Üçeyler N, Sommer C. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain. 2013;154(11):2569. doi: 10.1016/j.pain.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 28.White KP, Speechley M, Harth M, Ostbye T. The London Fibromyalgia Epidemiology Study: the prevalence of fibromyalgia syndrome in London, Ontario. J Rheumatol. 1999;26(7):1570–6. [PubMed] [Google Scholar]
- 29.Zilliox L, Peltier AC, Wren PA. Assessing autonomic dysfunction in early diabetic neuropathy: the Survey of Autonomic Symptoms. Neurology. 2011;76(12):1099–105. doi: 10.1212/WNL.0b013e3182120147. [DOI] [PMC free article] [PubMed] [Google Scholar]


