Abstract
Objective
Several cross-sectional studies have reported an association between visual contrast sensitivity (a functional measure of low contrast vision) and poor cognitive performance or dementia, but no studies have investigated this association prospectively in a population based cohort with final adjudication of mild cognitive impairment (MCI)/dementia.
Methods
In a prospective, community-based study of aging women (Study of Osteoporotic Fractures), we analyzed whether visual contrast sensitivity was associated with increased risk of MCI or dementia and/or worse performance on various cognitive tests assessed 10 years later. Contrast sensitivity was assessed at baseline in each eye using a VISTECH VCTS 6500 wall chart. MCI/dementia was adjudicated by an expert panel. Multivariable logistic and linear regression models were analyzed.
Results
Of 1,352 White (88.2%) and African-American (11.8%) women with a mean age of 77.7 years (SD 3.3), 536 (39.6%) went on to develop MCI/dementia over 10 years. MCI/Dementia risk was more than doubled (OR 2.16, 95% CI 1.58 to 2.96) in women with the lowest quartile of contrast sensitivity compared to highest (p<0.0001 for the linear trend). Reduced baseline contrast sensitivity was also associated with lower performance on several cognitive measures assessed 10 years later.
Interpretation
Among older women, reduced contrast sensitivity is associated with a greater risk of MCI/dementia. These findings suggest that visual system neurodegeneration or dysfunction may parallel or precede dementia-related cortical or subcortical degeneration, and that contrast sensitivity testing may be useful in identifying aging adults at high risk for dementia.
INTRODUCTION
Age-related neurodegenerative diseases, such as Alzheimer’s disease (AD), are leading causes of death and disability and are expected to affect >100 million people worldwide by 2050.1 Abnormal brain pathology can precede cognitive impairment by decades. Accumulation of amyloid-beta plaques may develop years before clinical symptoms in AD and functional impairment may only become manifest in day-to-day life once substantial neuronal injury has occurred. It is therefore likely that future dementia therapies will be more effective if initiated in pre-symptomatic individuals. Current methods to identify at-risk individuals for dementia are either very expensive (e.g. amyloid imaging) or invasive (e.g. lumbar puncture) and present challenges for population-based screening. Development of inexpensive and non-invasive tests to identify those at higher risk for dementia are needed to help stratify those who may benefit most from additional screening.
Several cross-sectional studies have shown an association between visual contrast sensitivity, a measurement of visual function, and dementia.2-5 Reduced visual contrast sensitivity has also been observed in patients with Mild Cognitive Impairment (MCI), a cognitive decline syndrome that often precedes AD, suggesting that impairment in contrast sensitivity may begin to occur early in the neurodegenerative disease process.4 Measurement of contrast sensitivity is inexpensive, non-invasive, and rapid, and may be particularly well suited to identify individuals at-risk for AD or related neurodegenerative disorders who may benefit from earlier or more invasive evaluations.
In this study, we sought to evaluate whether visual contrast sensitivity, as a simple office-based neuro-visual metric, was associated with risk of developing dementia and poor cognitive performance in a well-phenotyped, longitudinal, community-based cohort of aging women. We hypothesized that low visual contrast sensitivity would be associated with development of MCI/dementia and with poor cognitive function 10 years later.
METHODS
Participants and Cohort
The Study of Osteoporotic Fractures (SOF) is a longitudinal, multi-site cohort study that was originally designed to examine predictors of falls and fractures.6 The cohort has also been utilized to study additional age-related conditions, including dementia.7, 8 In the total SOF cohort, 10,366 women were originally enrolled, including 9,704 white women enrolled between September 1986 and October 1988, and 662 African-American women enrolled between February 1997 to December 1998. Women were excluded if they were unable to walk or had bilateral hip replacements at the time of enrollment. A detailed ophthalmological assessment that included testing of contrast sensitivity in each eye was subsequently added to the protocol and performed at Year 10 (which was the study baseline for the African-American women). We therefore designated Year 10 of the SOF cohort as the baseline for our study. A detailed cognitive assessment and dementia adjudication was performed 10 years later (Year 20 of the original SOF cohort).
For this analysis, we only included women from the three sites that participated in the ancillary cognitive study – Monongahela Valley near Pittsburgh in PA, Minneapolis, MN, and Portland, OR. Of these 7,760 women, we excluded the 4,516 who died prior to the Year 20 study visit and the 876 who terminated participation, were lost to follow-up, or had minimal information status/missing data. Of the remaining 2,368 women, 1,032 had a clinic examination and 502 underwent an in-home examination which included cognitive testing. We excluded 39 women for indeterminate or missing cognitive data. An additional 98 women with prevalent dementia at baseline (defined by a modified-MMSE (mMMSE) score of <20 (range 0-26), self-reported dementia, and/or already taking AD medications) and 45 women with no contrast sensitivity data were excluded. This led to a final sample for this analysis of 1,352 women.
The study was approved by the institutional review boards at each clinic as well as the coordinating center of University of California, San Francisco and California Pacific Medical Center. All participants provided written informed consent.
Contrast Sensitivity and Other Ophthalmological Assessments
Contrast sensitivity, a functional measure of low contrast vision, was assessed using a VISTECH VCTS 6500 wall chart and light meter. The VISTECH system presents a series of sine wave gratings at calibrated levels of contrast. A light meter was used to measure and enable standardized illumination (target 50 to 70 foot Lamberts given that this was an older population), and sites were instructed to test subjects in areas of uniform illumination. The testing distance was 10 feet from the chart with the middle of the chart around eye level. Subjects were tested with their glasses on if they wore glasses for distance; no other correction was made for potential refractive error. Study subjects were instructed to report whether a grating was visible, and if visible, the orientation of the grating. At the 10-foot distance the spatial frequencies tested on each line are 1.5, 3, 6, 12 and 18 cycles per degree. The highest spatial frequency that subjects could accurately report in each line was scored. The averages of each line were plotted against a contrast sensitivity curve, to yield a score between 0 (worst) and 150 (best). For our primary analysis, we averaged contrast sensitivity between eyes; in a sensitivity analysis we also examined contrast sensitivity in the worst eye. Self-reported age-related macular degeneration (AMD), glaucoma and cataract were evaluated in other sensitivity analyses.
MCI/Dementia Adjudication and Cognitive Assessments
Ten years after visual contrast sensitivity testing in each eye participants underwent a battery of cognitive tests. This included an assessment of global cognition (3MS), verbal memory (California Verbal Learning Test II or CVLT II, short form), executive function (Trails B, Digit Span Forward, Digit Span Backward), and semantic memory (category and verbal fluency).7 Dementia and Mild Cognitive Impairment (MCI) diagnosis was determined using a two-stage process. First, participants were screened for cognitive impairment using the expanded neuropsychological test battery. Those who screened negative were considered cognitively normal. Those who screened positive were further clinically adjudicated by a panel of clinical experts. Criteria for a diagnosis of dementia were based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV)9 and that of MCI were based on a modified Peterson criteria.10 Further details on dementia/MCI diagnosis in this cohort have been published elsewhere.7, 11
Other Measurements
Each participant completed a questionnaire and was asked at the Year 10 study visit (baseline for this analysis) about self-reported health, smoking status, and alcohol use in the past 30 days. Women were also queried about a physician diagnosis of medical conditions including myocardial infarction, stroke, diabetes, and hypertension. A modified version of the Mini-Mental State Examination (mMMSE) was administered.12 Depressive symptoms were evaluated using the Geriatric Depression Scale (GDS).13 Body mass index (BMI) was calculated using measures of body weight and height. Participants were queried about race/ethnicity and education at the time of initial SOF enrollment.
Statistical Analysis
For the primary analysis, multivariable logistic regression was used to analyze whether contrast sensitivity at baseline was associated with the development of MCI/dementia 10 years later. A multivariable model adjusting for factors that could potentially relate to both contrast sensitivity and cognition and based on prior literature, including age, race, education, BMI, history of stroke, myocardial infarction, hypertension, or diabetes, current smoking, current alcohol use (any drinks in the last 30 days), mMMSE, and GDS was also conducted. For the primary analysis, average contrast sensitivity of both eyes was used. Additional sensitivity analyses were performed by analyzing contrast sensitivity from the worst eye only. For secondary analyses, we also analyzed whether cognitive tests were different among quartiles of contrast sensitivity at baseline using a general linear regression model. Non-linear modeling was examined using Lowess and cubic splines. An alpha of <0.05 was considered statistically significant. SAS (Version 9.4) was used for the final analysis.
RESULTS
Baseline Characteristics
Of the 1352 women, the mean age was 77.7 years (SD 3.3) at the baseline visit for this analysis, 11.8% were African-American, and mean education was 12.9 years (SD 2.5). Ten years later, 536 (39.6%) women had developed MCI/dementia. At baseline, the MCI/dementia group was slightly older, more likely to be African-American, reported a lesser amount of alcohol use in the last month, had a higher rate of depression, a lower education level, and a slightly lower mMMSE. Table 1 reports baseline characteristics based on quartile of contrast sensitivity. Baseline cognitive testing with mMMSE and Trails B showed slightly lower scores in women with lower baseline visual contrast sensitivity.
Table 1.
Baseline characteristics of women by quartile of baseline contrast sensitivity (N=1352)
| Lowest quartile (1.4-22.0) (N=340) |
2nd quartile (22.1-28.9) (N=338) |
3rd quartile (28.9-40.8) (N=338) |
Highest quartile (40.9-147.0) (N=336) |
p-value | |
|---|---|---|---|---|---|
| Age, mean (SD), y | 78.7 (3.6) | 77.5 (3.2) | 77.6 (3.3) | 77.1 (3.0) | <0.0001 |
| African American, % | 12.9% | 14.2% | 11.5% | 8.6% | 0.048 |
| Education, mean (SD), y | 12.9 (2.6) | 12.6 (2.5) | 13.0 (2.4) | 13.1 (2.6) | 0.14 |
| Self-reported health (exc/good), % | 84.7% | 88.2% | 86.4% | 87.8% | 0.36 |
| Body Mass Index, mean (SD), kg/m2 | 27.2 (4.8) | 27.8 (4.8) | 27.5 (4.6) | 28.0 (5.0) | 0.12 |
| Stroke, % | 3.8% | 2.4% | 3.9% | 1.8% | 0.26 |
| Hypertension, % | 40.0% | 39.4% | 34.3% | 29.5% | 0.002 |
| Myocardia Infarction, % | 3.2% | 3.0% | 0.9% | 2.1% | 0.13 |
| Diabetes, % | 5.9% | 8.0% | 2.7% | 3.6% | 0.021 |
| Smoking, % | 2.9% | 4.1% | 2.4% | 3.3% | 0.86 |
| Alcohol use in the past month, % | 44.4% | 45.0% | 49.6% | 42.0% | 0.82 |
| Depression (GDS>=6), % | 7.7% | 4.1% | 5.6% | 4.2% | 0.10 |
| Geriatrics Depression Score, mean (SD) | 1.8 (2.3) | 1.5 (2.0) | 1.6 (2.1) | 1.4 (1.8) | 0.19 |
| mMMSE (0-26), mean (SD) | 24.9 (1.4) | 24.9 (1.3) | 25.0 (1.3) | 25.2 (1.1) | 0.012 |
| Trails B, mean (SD) | 149.1 (80.7) | 136.2 (64.0) | 127.1 (51.5) | 125.8 (55.9) | <0.0001 |
Abbreviations: GDS, Geriatrics Depression Score; mMMSE, modified Mini-Mental State Examination
Decreased vision contrast sensitivity predicts the development of MCI/Dementia over 10 years
Mean contrast sensitivity in the cohort was 32.9 (SD 16.1) and the range was 1.4 to 147 (on a scale of 0 to 150 with 150 being the best performance). There was an increased risk of MCI/dementia with lower baseline contrast sensitivity (p<0.0001 for linear trend), with an OR of 2.16 (CI 1.58 to 2.96) for women in the lowest quartile of contrast sensitivity compared with women in the highest quartile (Table 2 and Figure 1). For every 10-point decrease in contrast sensitivity, there was an increased risk of developing MCI/dementia (unadjusted odds ratio (OR) 1.21, 95% confidence interval (CI) 1.13 to 1.31). In a model adjusting for baseline age, race and education, the odds of MCI/dementia was modestly attenuated but remained significant (OR=1.15, 95% CI 1.07 to 1.24, for every 10 point decrease in contrast sensitivity). Additional adjustment for depression score, alcohol use, diabetes, hypertension and baseline cognitive score led to similar results (OR=1.15; 95%CI 1.06-1.24). Analysis of a model using contrast sensitivity in the worst eye (rather than averaging both eyes) did not significantly change in the results (Table 2). Examination of the association between contrast sensitivity and dementia using non-linear modeling, including Lowess and cubic splines, showed no substantial improvement of the fit compared to linear modeling.
Table 2.
Odds of developing MCI/dementia over 10 years by quartile of baseline contrast sensitivity (N=1352)
| Unadjusted OR (95% CI) |
Base Model† OR (95% CI) |
Base + baseline mMMSE OR (95% CI) |
Base Model + Covariates¶ OR (95% CI) |
|
|---|---|---|---|---|
| Contrast sensitivity average | ||||
| Lowest quartile | 2.16 (1.58, 2.96) | 1.75 (1.26, 2.43) | 1.70 (1.22, 2.36) | 1.71 (1.22, 2.38) |
| 2nd quartile | 1.58 (1.15, 2.17) | 1.39 (1.00, 1.92) | 1.34 (0.97, 1.87) | 1.38 (0.99, 1.93) |
| 3rd quartile | 1.38 (1.00, 1.90) | 1.27 (0.91, 1.75) | 1.25 (0.90, 1.73) | 1.27 (0.91, 1.78) |
| Highest quartile | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| p-for-trend | <0.0001 | 0.0008 | 0.002 | 0.002 |
| Contrast sensitivity worst eye | ||||
| Lowest quartile | 1.98 (1.44, 2.70) | 1.58 (1.14, 2.19) | 1.57 (1.13, 2.19) | 1.59 (1.14, 2.23) |
| 2nd quartile | 1.37 (1.00, 1.88) | 1.22 (0.88, 1.69) | 1.19 (0.85, 1.65) | 1.23 (0.88, 1.71) |
| 3rd quartile | 1.36 (0.99, 1.86) | 1.17 (0.85, 1.62) | 1.20 (0.86, 1.66) | 1.23 (0.88, 1.71) |
| Highest quartile | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| p-for-trend | <0.0001 | 0.008 | 0.011 | 0.010 |
| Contrast sensitivity average | ||||
| Excluding glaucoma (N=1205) | ||||
| Lowest quartile | 2.10 (1.50, 2.92) | 1.67 (1.18, 2.37) | 1.60 (1.13, 2.28) | 1.64 (1.15, 2.34) |
| 2nd quartile | 1.56 (1.12, 2.17) | 1.37 (0.97, 1.92) | 1.30 (0.92, 1.83) | 1.36 (0.96, 1.93) |
| 3rd quartile | 1.43 (1.03, 2.00) | 1.31 (0.93, 1.84) | 1.28 (0.91, 1.81) | 1.31 (0.92, 1.86) |
| Highest quartile | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| p-for-trend | <0.0001 | 0.005 | 0.012 | 0.008 |
| Excluding AMD (N=1226) | ||||
| Lowest quartile | 2.15 (1.54, 3.00) | 1.73 (1.23, 2.45) | 1.66 (1.17, 2.36) | 1.68 (1.18, 2.39) |
| 2nd quartile | 1.68 (1.20, 2.34) | 1.46 (1.04, 2.05) | 1.40 (1.00, 1.98) | 1.44 (1.02, 2.04) |
| 3rd quartile | 1.45 (1.04, 2.01) | 1.33 (0.95, 1.85) | 1.31 (0.93, 1.84) | 1.33 (0.94, 1.87) |
| Highest quartile | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| p-for-trend | <0.0001 | 0.002 | 0.005 | 0.004 |
| Excluding cataract (N=425) | ||||
| Lowest quartile | 2.09 (1.16, 3.79) | 1.80 (0.96, 3.36) | 1.79 (0.96, 3.37) | 1.76 (0.93, 3.36) |
| 2nd quartile | 2.00 (1.17, 3.43) | 1.69 (0.97, 2.96) | 1.66 (0.95, 2.92) | 1.68 (0.95, 2.97) |
| 3rd quartile | 1.47 (0.87, 2.50) | 1.38 (0.80, 2.38) | 1.38 (0.80, 2.39) | 1.44 (0.82, 2.50) |
| Highest quartile | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| p-for-trend | 0.004 | 0.036 | 0.041 | 0.051 |
Abbreviations: mMMSE, modified Mini-Mental State Examination; AMD, age-related macular degeneration
Adjusted for baseline age, race, education
Adjusted for alcohol use, history of diabetes, history of hypertension, Geriatrics Depression score, and baseline mMMSE
Figure 1.
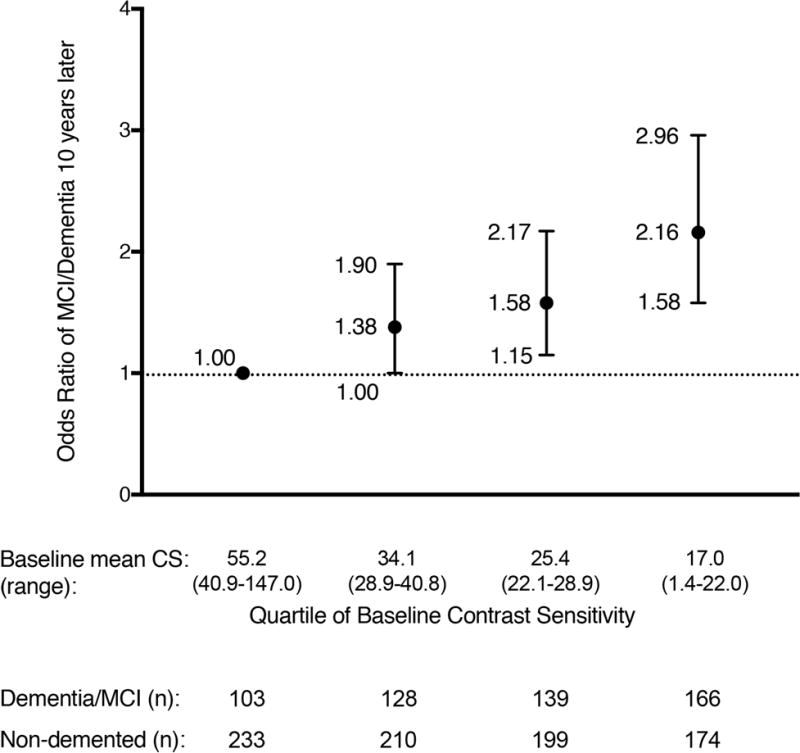
Odds of MCI/dementia at 10 years by baseline quartile of vision contrast sensitivity
Glaucoma and age-related macular degeneration (AMD) reduce contrast sensitivity and have been previously associated with dementia.14-18 The association between contrast sensitivity and MCI/dementia remained after excluding for women with baseline self-reported glaucoma (p<0.0001 for trend in OR per quartile) or excluding baseline self-reported AMD (p<0.0001 for trend in OR per quartile) (Table 2). Cataract can also reduce contrast sensitivity. Excluding women with self-reported cataract (N=425 analyzed), the association between contrast sensitivity and MCI/dementia remained (p<0.004 for the trend in the OR per quartile for the unadjusted model), although the sample size was substantially smaller (Table 2).
Decreased contrast sensitivity is associated with worse cognitive performance 10 years later
As a secondary analysis, we examined whether baseline contrast sensitivity averaged from both eyes was associated with cognitive performance 10 years later (Table 3). In an unadjusted model, lower baseline contrast sensitivity was associated with a lower performance in the 3MS, Trails B, CVLT (10 minute recall), digit span backward, verbal fluency and category fluency. Contrast sensitivity was not associated with digit span forward. After further adjustment for other dementia risk factors, including alcohol use, diabetes, hypertension and depression, the associations were attenuated but remained significant for Trails B and CVLT and were borderline significant for 3MS (p=0.05).
Table 3.
Performance on cognitive testing 10 years later by quartile of baseline contrast sensitivity
| Unadjusted Mean (SE) |
Base Model† mean (SE) |
Base + baseline mMMSE mean (SE) |
Base + Covariates¶ mean (SE) |
||
|---|---|---|---|---|---|
| 3MS | Lowest quartile | 85.6 (0.55) | 86.4 (0.54) | 86.5 (0.52) | 86.5 (0.52) |
| 2nd quartile | 87.2 (0.55) | 87.4 (0.53) | 87.5 (0.52) | 87.4 (0.52) | |
| 3rd quartile | 88.5 (0.55) | 88.3 (0.53) | 88.3 (0.52) | 88.3 (0.52) | |
| Highest quartile | 89.0 (0.55) | 88.3 (0.54) | 88.1 (0.53) | 88.3 (0.52) | |
| p-value | <0.0001 | 0.033 | 0.06 | 0.05 | |
| Trails B | Lowest quartile | 217.4 (8.6) | 209.7 (8.6) | 209.5 (8.5) | 208.7 (8.5) |
| 2nd quartile | 193.5 (8.1) | 191.7 (7.9) | 190.8 (7.9) | 190.6 (7.9) | |
| 3rd quartile | 179.8 (7.9) | 180.3 (7.8) | 180.6 (7.7) | 180.3 (7.7) | |
| Highest quartile | 157.0 (8.0) | 165.2 (7.9) | 166.0 (7.9) | 166.5 (7.9) | |
| p-value | <0.0001 | 0.002 | 0.002 | 0.004 | |
| CVLT Delayed Recall | Lowest quartile | 4.6 (0.15) | 4.8 (0.15) | 4.8 (0.15) | 4.8 (0.15) |
| 2nd quartile | 5.1 (0.15) | 5.1 (0.15) | 5.1 (0.14) | 5.1 (0.14) | |
| 3rd quartile | 5.3 (0.15) | 5.2 (0.14) | 5.2 (0.14) | 5.2 (0.14) | |
| Highest quartile | 5.6 (0.15) | 5.5 (0.15) | 5.4 (0.14) | 5.4 (0.14) | |
| p-value | <0.0001 | 0.017 | 0.028 | 0.034 | |
| Digit Span Forward | Lowest quartile | 7.2 (0.12) | 7.2 (0.12) | 7.2 (0.12) | 7.2 (0.12) |
| 2nd quartile | 7.3 (0.12) | 7.3 (0.12) | 7.3 (0.12) | 7.4 (0.12) | |
| 3rd quartile | 7.5 (0.12) | 7.5 (0.12) | 7.5 (0.12) | 7.5 (0.12) | |
| Highest quartile | 7.5 (0.12) | 7.5 (0.12) | 7.5 (0.12) | 7.5 (0.12) | |
| p-value | 0.22 | 0.37 | 0.45 | 0.48 | |
| Digit Span Backward | Lowest quartile | 5.3 (0.11) | 5.4 (0.11) | 5.4 (0.11) | 5.4 (0.11) |
| 2nd quartile | 5.3 (0.11) | 5.4 (0.11) | 5.4 (0.11) | 5.4 (0.11) | |
| 3rd quartile | 5.7 (0.11) | 5.6 (0.11) | 5.6 (0.11) | 5.6 (0.11) | |
| Highest quartile | 5.9 (0.11) | 5.8 (0.11) | 5.7 (0.11) | 5.7 (0.11) | |
| p-value | 0.001 | 0.037 | 0.07 | 0.07 | |
| Verbal Fluency | Lowest quartile | 10.2 (0.23) | 10.3 (0.23) | 10.3 (0.22) | 10.3 (0.22) |
| 2nd quartile | 10.4 (0.23) | 10.5 (0.22) | 10.5 (0.22) | 10.5 (0.22) | |
| 3rd quartile | 10.8 (0.23) | 10.8 (0.22) | 10.8 (0.22) | 10.8 (0.22) | |
| Highest quartile | 11.2 (0.23) | 11.1 (0.23) | 11.1 (0.22) | 11.1 (0.22) | |
| p-value | 0.009 | 0.06 | 0.09 | 0.10 | |
| Category Fluency | Lowest quartile | 10.1 (0.20) | 10.2 (0.20) | 10.2 (0.20) | 10.3 (0.20) |
| 2nd quartile | 10.6 (0.20) | 10.6 (0.20) | 10.6 (0.20) | 10.6 (0.20) | |
| 3rd quartile | 10.7 (0.20) | 10.7 (0.19) | 10.7 (0.19) | 10.7 (0.19) | |
| Highest quartile | 10.8 (0.20) | 10.7 (0.20) | 10.7 (0.20) | 10.7 (0.20) | |
| p-value | 0.035 | 0.29 | 0.32 | 0.43 |
Abbreviations: 3MS, Modified Mini-Mental State Examination; CVLT, California Verbal Learning Test
Adjusted for baseline age, race, education
Adjusted for alcohol use, history of diabetes, history of hypertension, Geriatrics Depression score, and baseline mMMSE
DISCUSSION
In a cohort of 1,352 community-dwelling older women, reduced contrast sensitivity was associated with a greater risk of development of MCI/dementia within 10 years. These associations were independent of other known risk factors of MCI/dementia, such as age, education, smoking, or race. These associations were not significantly affected by adjusting for a self-described history of glaucoma, AMD or cataract. These findings support the use of contrast sensitivity testing as part of a multimodal panel to identify healthy aging adults at highest risk for dementia. These findings also support the hypothesis that neurodegeneration or dysfunction in the visual system may parallel or precede the cortical or subcortical degeneration that leads to clinical dementia.
Cross-sectional studies have previously demonstrated that patients with dementia exhibit impairment in visual contrast sensitivity. Using frequency doubling technology to assess contrast sensitivity in the visual field, Risacher et al (2013) observed reduced contrast sensitivity in subjects with Alzheimer’s or MCI compared to healthy controls.4 Similar observations were seen in AD patients using different methods to evaluate contrast sensitivity.3, 19-23 Because contrast sensitivity depends on brain networks that can be affected in AD and other dementias, one explanation for these observations is that the association could be secondary to widespread neurodegenerative changes in later stage dementia or even difficulties in performing and reporting the task. An early analysis from the SOF cohort, published in 2004, reported an association between high-contrast visual acuity and reduced performance on the Mini-Mental State Examination (MMSE) and functional decline, but this was before adjudication for MCI/dementia in the cohort was completed.24 Contrast sensitivity is a more sensitive tool than visual acuity testing to identify sub-clinical impairment of visual function.25 In patients with glaucoma, changes in contrast sensitivity can be detected earlier than changes in visual acuity,26 and in patients with AMD changes in contrast sensitivity correlate better with real-world function and prognosis.27 Using a contrast sensitivity-based cutoff as a measure of poor visual function, Fischer et al showed that poor visual function was associated with future reduced MMSE scores and a self-reported history of dementia 10 years later in a separate community-dwelling cohort of aging individuals.28 We now show that reduced contrast sensitivity precedes development of dementia at 10 years of longitudinal follow-up in a well-phenotyped, prospective, community-based cohort.
Impaired contrast sensitivity can be caused by injury throughout the visual system, including the cornea, anterior chamber, lens, vitreous, retina, optic nerve, anterior visual pathways, posterior visual pathways, and higher level cortical processing. We attempted to evaluate whether specific ocular pathologies in select localizations on the causal pathway, including self-reported glaucoma, AMD and cataract, could have contributed to the observed association between contrast sensitivity and MCI/dementia and cognitive performance but did not observe any association. However, as these conditions were based on self-report, our ability to address this possibility was limited. Furthermore, as a number of other ophthalmological conditions were not formally assessed in these subjects and the majority of the cohort did not undergo detailed ophthalmological study evaluations, this dataset does not allow for localization of where in the visual system the impaired contrast sensitivity in these women originates. On the clinical level, the inclusion of patients without a detailed ophthalmologic evaluation could also be considered a strength of this study when considering the potential application of visual contrast sensitivity as a screening tool. The use of existing refraction also reflects a practical, real-world approach that is not confounded by a requirement that patients undergo additional testing.
The presence of reduced contrast sensitivity years before clinical onset of dementia suggests that this association is not simply a consequence of later-stage dementia and that reduced contrast sensitivity can precede clinical onset of dementia and may even precede frank cortical or subcortical neurodegeneration in some cases. The mechanistic underpinnings of impaired visual function in the setting of early-stage neurodegenerative diseases remains unclear and may be multifactorial. Retinal neuron loss occurs in patients with AD,29, 30 and retinal thinning can be non-invasively detected in living individuals with mild-cognitive impairment and AD.31 Retinal amyloid deposition has been reported in AD patients,32 but several follow-up studies did not confirm this pathology.33, 34 Other areas of the visual system also degenerate in AD, such as the lateral geniculate nucleus and visual cortex, and could contribute to impaired contrast sensitivity.35-37 Microvascular disease, a risk factor for both dementia and chronic vision loss, may also play a causal role in our observed findings.38, 39
Alzheimer’s disease is the most common cause of dementia in aging adults and is associated with an early selective decline in memory function. Hippocampal volume has been associated with retinal nerve fiber layer thickness on optical coherence tomography in healthy elderly subjects without demonstrated dementia.40 The MCI/dementia adjudication in our study was made clinically, and there was no collection of CSF biomarkers or neuroimaging to evaluate for amyloid/tau or other pathologies. If we assume that Alzheimer’s disease is the most common cause of dementia in our cohort, and if reduced low contrast acuity was specific for predicting Alzheimer’s pathology, it might be expected that there would be a selective association between low contrast acuity and tests of short-term memory function. However, in this analysis, low contrast sensitivity was associated with reduced future cognitive performance in several domains, including those involved in memory, executive functioning and language. Future studies will be helpful in clarifying whether contrast sensitivity is most useful in predicting specific causes of dementia. New methods of testing visual function are rapidly being developed, including those that allow for repeated measures in home-based settings.20 Such measures may improve the accuracy of contrast sensitivity testing and improve its ability to predict MCI/dementia. Technological advances in visual function testing may also increase accessibility and the generalizability of this strategy for dementia risk screening.
Our study has important limitations. The SOF cohort included mostly white women. In future studies it will be invaluable to analyze whether the association between contrast sensitivity and future MCI/dementia is generalizable to men and to people of other racial/ethnic and geographic backgrounds. Our study population was also relatively old (mean age 78 years old at the baseline for this analysis and nearly 88 years old at the time of dementia adjudication), and these results may not apply in younger adults. The 39% conversion rate to dementia that was observed in our cohort is consistent with other studies of dementia incidence in the oldest old age group.11, 41-43 MCI/dementia adjudication in our study was made clinically, and future analyses could benefit from the addition of CSF biomarkers or neuroimaging to support more specific dementia pathologies, as well as additional longitudinal assessments of cognitive performance to analyze associations of visual contrast sensitivity with rates of cognitive decline. There may also be unmeasured biases in sample selection due to mortality, loss to follow-up or inability to assess for MCI/dementia. Localization of contrast sensitivity deficits to particular ocular pathologies in future studies will also be important for understanding potential mechanisms driving the observed association between contrast sensitivity impairment and future dementia.
In summary, these data support an association between impaired contrast sensitivity and future MCI/dementia. If these observations are confirmed and validated, contrast sensitivity has the potential to provide a non-invasive way of identifying aging adults at greatest risk of developing dementia, which could be incorporated as part of a multimodal risk assessment.
Acknowledgments
The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, R01 AG027576, and R01 AG026720. Dr. Yaffe also receives additional funding from NIA K24AG031155.
Footnotes
Author Contributions:
MW, JMG, LYL, YO, AJG, SC and KY contributed to the conception and design of the study; MW, JMG, LYL, KS, KP, SC and KY contributed to the acquisition and analysis of data; MW, JMG, LYL, KY contributed to drafting the text and preparing the figures.
Potential Conflicts of Interest: No conflicts to report.
References
- 1.International AsD. Policy Brief for Heads of Government: The Global Impact of Dementia 2013-2050. London: 2013. [Google Scholar]
- 2.Cormack FK, Tovee M, Ballard C. Contrast sensitivity and visual acuity in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2000 Jul;15(7):614–20. doi: 10.1002/1099-1166(200007)15:7<614::aid-gps153>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Crow RW, Levin LB, LaBree L, Rubin R, Feldon SE. Sweep visual evoked potential evaluation of contrast sensitivity in Alzheimer’s dementia. Invest Ophthalmol Vis Sci. 2003 Feb;44(2):875–8. doi: 10.1167/iovs.01-1101. [DOI] [PubMed] [Google Scholar]
- 4.Risacher SL, Wudunn D, Pepin SM, et al. Visual contrast sensitivity in Alzheimer’s disease, mild cognitive impairment, and older adults with cognitive complaints. Neurobiol Aging. 2013 Apr;34(4):1133–44. doi: 10.1016/j.neurobiolaging.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzo M, Anderson SW, Dawson J, Nawrot M. Vision and cognition in Alzheimer’s disease. Neuropsychologia. 2000;38(8):1157–69. doi: 10.1016/s0028-3932(00)00023-3. [DOI] [PubMed] [Google Scholar]
- 6.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995 Mar 23;332(12):767–73. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 7.Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011 Nov;70(5):722–32. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoyama JS, Evans DS, Coppola G, Kramer JH, Tranah GJ, Yaffe K. Genetic modifiers of cognitive maintenance among older adults. Hum Brain Mapp. 2014 Sep;35(9):4556–65. doi: 10.1002/hbm.22494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Association. AP. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) 4th. Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- 10.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999 Mar;56(3):303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 11.Yaffe K, Middleton LE, Lui LY, et al. Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch Neurol. 2011 May;68(5):631–6. doi: 10.1001/archneurol.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espeland MA, Rapp SR, Robertson J, et al. Benchmarks for designing two-stage studies using modified mini-mental state examinations: experience from the Women’s Health Initiative Memory Study. Clin Trials. 2006;3(2):99–106. doi: 10.1191/1740774506cn140oa. [DOI] [PubMed] [Google Scholar]
- 13.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 14.Baker ML, Wang JJ, Rogers S, et al. Early age-related macular degeneration, cognitive function, and dementia: the Cardiovascular Health Study. Arch Ophthalmol. 2009 May;127(5):667–73. doi: 10.1001/archophthalmol.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayer AU, Ferrari F, Erb C. High occurrence rate of glaucoma among patients with Alzheimer’s disease. Eur Neurol. 2002;47(3):165–8. doi: 10.1159/000047976. [DOI] [PubMed] [Google Scholar]
- 16.Helmer C, Malet F, Rougier MB, et al. Is there a link between open-angle glaucoma and dementia? The Three-City-Alienor cohort. Ann Neurol. 2013 Aug;74(2):171–9. doi: 10.1002/ana.23926. [DOI] [PubMed] [Google Scholar]
- 17.Keenan TD, Goldacre R, Goldacre MJ. Associations between age-related macular degeneration, Alzheimer disease, and dementia: record linkage study of hospital admissions. JAMA Ophthalmol. 2014 Jan;132(1):63–8. doi: 10.1001/jamaophthalmol.2013.5696. [DOI] [PubMed] [Google Scholar]
- 18.Yochim BP, Mueller AE, Kane KD, Kahook MY. Prevalence of cognitive impairment, depression, and anxiety symptoms among older adults with glaucoma. J Glaucoma. 2012 Apr-May;21(4):250–4. doi: 10.1097/IJG.0b013e3182071b7e. [DOI] [PubMed] [Google Scholar]
- 19.Bassi CJ, Solomon K, Young D. Vision in aging and dementia. Optom Vis Sci. 1993 Oct;70(10):809–13. doi: 10.1097/00006324-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Cronin-Golomb A. Vision in Alzheimer’s disease. Gerontologist. 1995 Jun;35(3):370–6. doi: 10.1093/geront/35.3.370. [DOI] [PubMed] [Google Scholar]
- 21.Gilmore GC, Levy JA. Spatial contrast sensitivity in Alzheimer’s disease: a comparison of two methods. Optom Vis Sci. 1991 Oct;68(10):790–4. doi: 10.1097/00006324-199110000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Gilmore GC, Whitehouse PJ. Contrast sensitivity in Alzheimer’s disease: a 1-year longitudinal analysis. Optom Vis Sci. 1995 Feb;72(2):83–91. doi: 10.1097/00006324-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Hutton JT, Morris JL, Elias JW, Poston JN. Contrast sensitivity dysfunction in Alzheimer’s disease. Neurology. 1993 Nov;43(11):2328–30. doi: 10.1212/wnl.43.11.2328. [DOI] [PubMed] [Google Scholar]
- 24.Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc. 2004 Dec;52(12):1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x. [DOI] [PubMed] [Google Scholar]
- 25.Marmor MF. Contrast sensitivity versus visual acuity in retinal disease. Br J Ophthalmol. 1986 Jul;70(7):553–9. doi: 10.1136/bjo.70.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velten IM, Korth M, Horn FK, Budde WM. Temporal contrast sensitivity with peripheral and central stimulation in glaucoma diagnosis. Br J Ophthalmol. 1999 Feb;83(2):199–205. doi: 10.1136/bjo.83.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander MF, Maguire MG, Lietman TM, Snyder JR, Elman MJ, Fine SL. Assessment of visual function in patients with age-related macular degeneration and low visual acuity. Arch Ophthalmol. 1988 Nov;106(11):1543–7. doi: 10.1001/archopht.1988.01060140711040. [DOI] [PubMed] [Google Scholar]
- 28.Fischer ME, Cruickshanks KJ, Schubert CR, et al. Age-Related Sensory Impairments and Risk of Cognitive Impairment. J Am Geriatr Soc. 2016 Oct;64(10):1981–7. doi: 10.1111/jgs.14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanks JC, Schmidt SY, Torigoe Y, Porrello KV, Hinton DR, Blanks RH. Retinal pathology in Alzheimer’s disease. II. Regional neuron loss and glial changes in GCL. Neurobiol Aging. 1996 May-Jun;17(3):385–95. doi: 10.1016/0197-4580(96)00009-7. [DOI] [PubMed] [Google Scholar]
- 30.Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med. 1986 Aug 21;315(8):485–7. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- 31.Paquet C, Boissonnot M, Roger F, Dighiero P, Gil R, Hugon J. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2007 Jun 13;420(2):97–9. doi: 10.1016/j.neulet.2007.02.090. [DOI] [PubMed] [Google Scholar]
- 32.Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011 Jan;54(Suppl 1):S204–17. doi: 10.1016/j.neuroimage.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho CY, Troncoso JC, Knox D, Stark W, Eberhart CG. Beta-amyloid, phospho-tau and alpha-synuclein deposits similar to those in the brain are not identified in the eyes of Alzheimer’s and Parkinson’s disease patients. Brain Pathol. 2014 Jan;24(1):25–32. doi: 10.1111/bpa.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schon C, Hoffmann NA, Ochs SM, et al. Long-term in vivo imaging of fibrillar tau in the retina of P301S transgenic mice. PLoS One. 2012;7(12):e53547. doi: 10.1371/journal.pone.0053547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armstrong RA. Visual field defects in Alzheimer’s disease patients may reflect differential pathology in the primary visual cortex. Optom Vis Sci. 1996 Nov;73(11):677–82. doi: 10.1097/00006324-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Hof PR, Cox K, Morrison JH. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer’s disease: I. Superior frontal and inferior temporal cortex. J Comp Neurol. 1990 Nov 01;301(1):44–54. doi: 10.1002/cne.903010105. [DOI] [PubMed] [Google Scholar]
- 37.Leuba G, Saini K. Pathology of subcortical visual centres in relation to cortical degeneration in Alzheimer’s disease. Neuropathol Appl Neurobiol. 1995 Oct;21(5):410–22. doi: 10.1111/j.1365-2990.1995.tb01078.x. [DOI] [PubMed] [Google Scholar]
- 38.Sohn EH, van Dijk HW, Jiao C, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016 May 10;113(19):E2655–64. doi: 10.1073/pnas.1522014113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaffe K, Vittinghoff E, Pletcher MJ, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014 Apr 15;129(15):1560–7. doi: 10.1161/CIRCULATIONAHA.113.004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casaletto KB, Ward ME, Baker NS, et al. Retinal thinning is uniquely associated with medial temporal lobe atrophy in neurologically normal older adults. Neurobiol Aging. 2017 Mar;51:141–7. doi: 10.1016/j.neurobiolaging.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucca U, Tettamanti M, Logroscino G, et al. Prevalence of dementia in the oldest old: the Monzino 80-plus population based study. Alzheimers Dement. 2015 Mar;11(3):258–70.e3. doi: 10.1016/j.jalz.2014.05.1750. [DOI] [PubMed] [Google Scholar]
- 42.Fratiglioni L, Viitanen M, von Strauss E, Tontodonati V, Herlitz A, Winblad B. Very old women at highest risk of dementia and Alzheimer’s disease: incidence data from the Kungsholmen Project, Stockholm. Neurology. 1997 Jan;48(1):132–8. doi: 10.1212/wnl.48.1.132. [DOI] [PubMed] [Google Scholar]
- 43.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann Neurol. 2010 Jan;67(1):114–21. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]


