Expanded Abstract
Purpose
Costly surveillance and treatment of bladder cancer can lead to financial toxicity (FT), a treatment-related financial burden. Our objective was to define the prevalence of FT among bladder cancer patients and identify delays in care and its effect on health-related quality of life (HRQOL).
Methods
We identified bladder cancer patients in the UNC Health Registry/Cancer Survivorship Cohort. FT was defined as agreement with having “to pay more for medical care than you can afford.” HRQOL was measured using general and cancer-specific validated questionnaires. Statistical analyses were performed using Fisher’s exact and student t-tests.
Results
138 bladder cancer patients were evaluated. Median age was 66.9 years, 75% male, and 89% white. Thirty-three participants (24%) endorsed FT. Participants who were younger (p=0.02), black (p=0.01), reported less than a college degree (p=0.01) and had non-invasive disease (p=0.04) were more likely to report FT. On multivariable analysis, only age was a significant predictor of FT. Patients who endorsed FT were more likely to report delaying care (39% vs. 23%, p=0.07) due to inability to take time off work or afford general expenses. Patients with FT reported worse physical and mental health on general HRQOL questionnaires (p=0.03 and <0.01, respectively) and lower cancer–specific HRQOL (p=0.01), in both physical (p=0.01) and functional well–being (p=0.05).
Conclusions
FT is a major concern among bladder cancer patients. Younger patients were more likely to experience FT. Those who endorsed FT experienced delays in care and poorer HRQOL, suggesting that treatment costs should have an important role in medical decision-making.
Implications for Clinical Practice
Until recently, the costs of cancer treatment were described on a societal rather than individual level. The negative impact of individual treatment costs have garnered recent attention. FT is an adverse event, similar to nausea or fatigue. FT has been associated with tangible processes and outcomes, including delay in care and poorer HRQOL. In our study, age was an important predictor of FT with younger patients more commonly impacted, likely related to loss of work-related income and lack of universal healthcare coverage such as Medicare. Given that FT is related to delays in care and poor QOL (both linked to negative oncologic outcomes), cancer treatment costs should be part of shared-decision making. Although national guidelines have only begun to emphasize the importance of financial discussions, most clinicians do not routinely discuss treatment costs with their patients, despite patient interest and improved patient satisfaction associated with these discussions. Oncology patients in particular could benefit from comprehensive discussions which involve not only treatment efficacy and side effects, but also cost comparisons. FT can be addressed in other ways as well, including close scrutiny of surveillance practices. Risk-stratified algorithms tailor these surveillance procedures to avoid the unnecessary cost of expensive diagnostic testing which offer little benefit. Risk-based surveillance, financial counselors, and comprehensive provider-patient discussions all contribute to combatting FT among our patients. We must accept the responsibility of providing our patients with high value care, of which FT plays an increasingly important role.
Keywords: Urinary bladder neoplasms, cost of cancer care, outcomes research, health care costs, quality of life, decision making
Introduction
The United States’ healthcare system prioritizes cutting-edge technology and innovative pharmaceuticals, the costs of which have been progressively redirected to individual patients. The maintenance of our high quality of care and use of expensive treatments must be balanced with patients’ quality of life (QOL), which can be negatively impacted by financial stress. Interest in this problem has been growing since 2013, when the phrase financial toxicity (FT) was coined. FT, defined as treatment-related financial distress, has been particularly relevant in the field of oncology, which often requires expensive treatments and long-term surveillance.1
FT is a particular concern for patients with bladder cancer because of both its high prevalence2 and significant cost. Bladder cancer is the most expensive cancer from diagnosis to death3,4 due to its long-term survival and ongoing surveillance.5 Surveillance includes imaging and cystoscopy at frequent intervals over years, contributing up to 60% of the cost of bladder cancer care.5 In addition to the direct costs of care, indirect costs of cancer treatment, such as time away from work6 also contribute to the burden of treatment. The negative impact of FT has garnered national attention, from features on CBS’ 60 Minutes7 to articles in the Washington Post8 and the Wall Street Journal.9
Cancer patients are 2.65 times more likely to declare bankruptcy than those without cancer.10 Beyond obvious monetary consequences, FT can also have negative long-term effects on cancer outcomes. FT requiring bankruptcy has recently been linked to early mortality among cancer patients.11 Patients reporting FT also exhibit medication non-adherence, skip doctor appointments, and decline necessary procedures to off-set costs.1
While the prevalence and impact of FT have been studied in many common cancers, such as breast12 and lung13 cancers, the effects of FT on bladder cancer patients remain unknown. The objective of our study was to 1) assess the prevalence of FT and associated patient-level factors among bladder cancer patients, 2) evaluate patient-reported delays in care and the reasons for those delays, and 3) examine the relationship between FT and health-related quality of life (HRQOL). To our knowledge, this study is the first to evaluate the prevalence and impact of FT in the bladder cancer population.
Materials and Methods
We performed a cross sectional study of 138 bladder cancer patients identified within the University of North Carolina Health Registry/Cancer Survivorship Cohort (HR/CSC), an incident-prevalent cohort of oncology patients recruited from August 2010 to August 2016. To be eligible for the HR/CSC, patients were required to be an English- or Spanish-speaking adult, aged 18 or older with a North Carolina mailing address and an upcoming oncology appointment in the UNC Health Care System.
After screening for eligibility, patients were recruited in-person during their visit to UNC and informed consent was obtained at that time. A baseline questionnaire was administered, typically within two weeks of enrollment, by computer-assisted telephone interview that lasted approximately one hour. The baseline questionnaire was extensive, including information on demographics, previous healthcare access and services, diagnosis and treatment, FT, and HRQOL. Among this cohort, we identified patients with pathologically-confirmed, primary cancer of the bladder. Enrolled HR/CSC patients were clinically annotated with diagnostic pathology and first course of treatment data via the UNC Hospital Tumor Registry and linked to more extensive clinical data in the Carolina Data Warehouse which gathers information from the electronic medical record (EMR). Additional patient details were manually abstracted from the EMR, including important treatment information (e.g. cycles of chemotherapy, intravesical treatments).
We defined FT as selecting “agree” or “strongly agree” with the following statement “you have to pay more for medical care than you can afford,” found on the patient satisfaction questionnaire (PSQ-18) and used in other FT analyses. 14 We examined the association between baseline FT and HRQOL using both general and cancer-specific scales, using the Functional Assessment of Cancer Therapy (FACT),15 including a bladder cancer-specific FACT, and Patient-Reported Outcomes Measurement Information System (PROMIS)16 questionnaires.
Statistical Analysis
Descriptive statistics were used to summarize all study variables, and patients were categorized into two groups based on FT endorsement. Fisher’s exact tests and student’s t-tests were used to evaluate differences in demographic, diagnostic, and treatment characteristics between FT groups. All analyses were completed using SAS version 9.3 (Cary, NC). This study was reviewed and approved by the University of North Carolina Institutional Review Board.
Results
Among 144 bladder cancer patients enrolled in HR/CSC, n=138 (96%) completed the baseline questionnaire and were included in the analysis. Median age was 66.9 years, 75% were male, 89% were white, and 66% had less than a college degree (Table 1). Approximately half of patients had a bladder cancer stage of cT2 or higher.
Table 1.
Demographic and Clinical Characteristics and Financial Toxicity Among Bladder Cancer Patients
| Overall N= (%) | Financial toxicity, n=33 (24%) | No financial toxicity, n=105 (76%) | p | ||
|---|---|---|---|---|---|
| Age (years) | <65 | 61 (44%) | 21 (64%) | 40 (38%) | 0.02 |
| ≥65 | 77 (56%) | 12 (36%) | 65 (62%) | ||
| Gender | Female | 34 (25%) | 8 (24%) | 26 (25%) | 1.00 |
| Male | 104 (75%) | 25 (76%) | 79 (75%) | ||
| Race | Black | 14 (10%) | 8 (24%) | 6 (6%) | 0.01 |
| Hispanic | 1 (1%) | 0 (0%) | 1 (1%) | ||
| White | 123 (89%) | 25 (76%) | 98 (93%) | ||
| BMI (kg/m2) | 18.5–24.9 | 41 (30%) | 8 (24%) | 33 (31%) | 0.55 |
| 25–29.9 | 52 (38%) | 11 (33%) | 41 (39%) | ||
| 30–34.9 | 24 (17%) | 8 (24%) | 16 (15%) | ||
| >35 | 21 (15%) | 6 (18%) | 15 (14%) | ||
| Marital Status | Single | 14 (10%) | 3 (9%) | 11 (10%) | 0.95 |
| Married/living with partner | 95 (69%) | 22 (69%) | 73 (70%) | ||
| Divorced/separated/widowed | 28 (20%) | 7 (22%) | 21 (20%) | ||
| Education | Eighth grade or less | 4 (3%) | 1 (3%) | 3 (3%) | 0.01 |
| Some high school | 9 (7%) | 4 (12%) | 5 (4%) | ||
| High school degree/GED | 45 (33%) | 13 (39%) | 32 (31%) | ||
| Some college or technical school | 33 (24%) | 11 (33%) | 22 (21%) | ||
| Some graduate/masters | 28 (20%) | 4 (12%) | 24 (23%) | ||
| Completed postgraduate/professional | 19 (14%) | 0 (0%) | 19 (18%) | ||
| Insurance | Medicaid | 10 (7%) | 3 (9%) | 7 (7%) | 0.75 |
| Medicare only | 19 (14%) | 5 (16%) | 14 (14%) | ||
| Private | 100 (74%) | 22 (69%) | 78 (76%) | ||
| Self Pay | 6 (4%) | 2 (6%) | 4 (4%) | ||
| Work for Pay | Yes | 38 (27%) | 11 (33%) | 27 (26%) | 0.38 |
| No | 100 (73%) | 22 (67%) | 78 (74%) | ||
| Bladder Cancer Clinical T Stage | Non-invasive (Tis, Ta, T1) | 69 (51%) | 21 (68%) | 48 (46%) | 0.04 |
| Invasive (T2–T4) | 66 (49%) | 10 (32%) | 56 (54%) | ||
| Previous intravesical chemotherapy | Yes | 42 (30%) | 12 (36%) | 30 (29%) | 0.40 |
| No | 96 (70%) | 21 (64%) | 75 (71%) | ||
| Postoperative readmissions | Yes | 29 (33%) | 6 (27%) | 23 (34%) | 0.61 |
| No | 60 (67%) | 16 (73%) | 44 (66%) | ||
| Charlson Comorbidity Index | Median (IQR) | 5 (4, 7) | 4 (3, 6) | 5 (4, 7) | 0.02 |
| Time from cancer diagnosis (yr) | Median (IQR) | 0.4 (0.1, 1.2) | 0.5 (0.3, 1.0) | 0.4 (0.1, 1.3) | 0.93 |
| Distance traveled (miles) | Median (IQR) | 62 (32, 119) | 62 (33, 121) | 62 (31, 117) | 0.35 |
| Number of prior cystoscopies | Median (IQR) | 1 (1, 2) | 1 (1, 1) | 1 (1, 2) | 0.59 |
| Number of prior TURBTs | Median (IQR) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 0.14 |
Thirty-three participants (24%) endorsed FT. Participants who were younger (p=0.02), black (p=0.01), had less than a college degree (p=0.01), and a lower Charlson comorbidity index (p=0.02) were more likely to endorse FT (Table 1). As education increased, the percentage of FT decreased. Although insurance status was not a statistically significant predictor of FT (p=0.75), a linear relationship between insurance type and FT was observed. Those with private insurance were least likely to report FT (22%) followed by Medicare (26%), Medicaid (30%) and self pay (33%).
Patients with non-invasive bladder cancer were more likely to report FT than those with invasive bladder cancer (30% vs. 15%; p=0.04). Time since diagnosis, distance travelled, whether the patient works for pay, and previous treatment were not associated with FT. In a multivariable model including age, stage, and comorbidity, older age retained its significant association with lower odds of FT (OR = 0.29, 95% CI 0.13, 0.65). However, stage (OR = 0.45, 95% CI 0.18, 1.12) and comorbidity (OR = 0.99, 95% CI 0.78, 1.26) were not independently associated with FT.
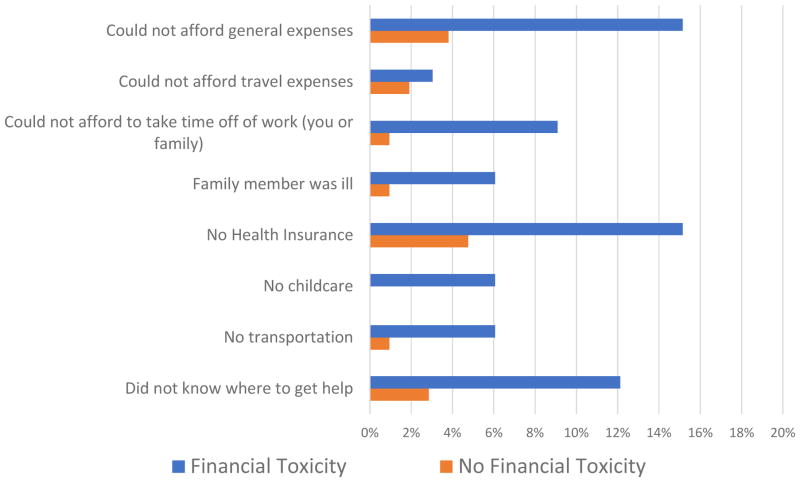
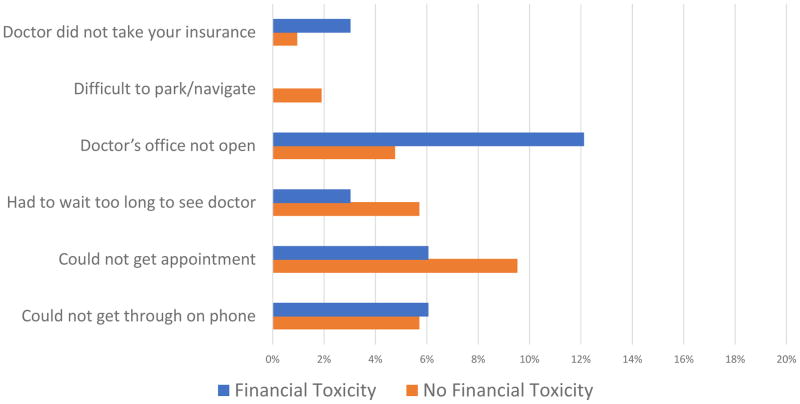
Patients who endorsed FT were more likely to report delaying care (39% vs. 23%) although this did not reach statistical significance (p=0.07). Patient-level and hospital/clinic level factors causing delay in care are illustrated in Figures 1 and 2, respectively. Patients reporting FT were more likely to delay care due to patient-level factors such as inability to take time off work (p=0.04) and inability to afford general expenses (p=0.04).
Figure 1.
Patient-Level Factors Causing Delay in Care, With and Without Financial Toxicity
Figure 2.
Hospital/Clinic-Level Factors Causing Delay in Care, With and Without Financial Toxicity
With regard to general HRQOL using the PROMIS questionnaires, patients with FT had worse physical and mental health scores compared to those without FT (p=0.03 and p<0.01, respectively) (Table 2). Patients who endorsed FT reported significantly lower general cancer–specific QOL (72 vs. 82; p=0.01) as well as physical well–being (20 vs. 23; p=0.01) and lower functional well–being (15 vs. 18; p=0.05). No differences in social well–being, emotional well–being or bladder–cancer specific QOL were noted.
Table 2.
Financial Toxicity and Health Related Quality of Life
| Overall, mean (SD) | Financial toxicity, mean (SD) n=33 (24%) |
No financial toxicity, mean (SD) n=105 (76%) |
p | |
|---|---|---|---|---|
| FACT-GP1, Total score | 79 (18) | 72 (21) | 82 (17) | 0.01 |
| FACT-GP1, Physical well being | 22 (5) | 20 (6) | 23 (5) | 0.01 |
| FACT-GP1, Social/family well being | 21 (6) | 19 (7) | 21 (6) | 0.11 |
| FACT-GP1, Emotional well being | 19 (5) | 18 (5) | 20 (5) | 0.21 |
| FACT-GP1, Functional well being | 17 (8) | 15 (9) | 18 (8) | 0.05 |
| FACT-BL2, Bladder cancer specific | 33 (7) | 32 (8) | 34 (6) | 0.41 |
| PROMIS3 Global Physical Health T-Score | 46 (10) | 43 (11) | 47 (9) | 0.03 |
| PROMIS3 Global Mental Health T-Score | 50 (10) | 46 (10) | 51 (9) | <0.01 |
FACT-GP: Functional Assessment of Cancer Therapy- General Population
FACT-BL: Functional Assessment of Cancer Therapy- Bladder Cancer
PROMIS: Patient-Reported Outcomes Measurement Information System
Discussion
Herein, we report the first study that investigates the prevalence and impact of FT among bladder cancer patients. We observed that nearly one-quarter of bladder cancer patients in our institutional bladder cancer cohort endorsed treatment-related financial distress. Patients reporting FT were more likely to be younger, black, have less than a college degree, and a non-invasive cancer diagnosis, although stage was not associated with FT on multivariable analysis. Moreover, delay of care and poorer HRQOL, both general and cancer-specific, were associated with patients endorsing FT.
Financial toxicity in oncology patients has been studied in a number of different settings since 2010, when attention shifted toward the impact of increasing costs on patient-reported outcomes. Until 2014, studies of FT have used three different measurements: subjective, objective, and monetary. This inconsistency has likely resulted in the wide variability in FT prevalence found among studies. A recent review reported a FT prevalence range of 16–73%.17 In order to address this variation, a validated patient-reported outcome measure, the Comprehensive Score for Financial Toxicity (COST),18 was developed in 2014. Although our study was initiated prior to implementation of the COST questionnaire, the prevalence observed in the current study (24%) was within the range of studies using similar measurements of FT.14,19, 20,21
Working-aged patients have consistently reported higher rates of FT,10,19,20,22,23 a finding that was supported in the current study based on age, but not corroborated based on self-reported work status. Two main factors likely contribute to this finding: the loss of work-related income in working-aged patients23 and the lack of universal access to healthcare prior to age 65, when United States residents are first eligible for Medicare benefits. Although insurance was not a statistically significant predictor of FT, there was a clear association between level of insurance and FT. Those with private and Medicare insurance had the lowest rates of FT compared to those with Medicaid and self pay. Additionally, we found that race and education were associated with FT, which have been previously reported,14,19,24 although time since diagnosis, distance traveled and previous treatment were not associated with FT in our study.
Although history of cancer treatment was not associated with FT, stage did appear to be predictive on bivariable analysis, with more non-invasive bladder cancer patients reporting FT than invasive cancer. However, stage was not an independent predictor of FT on multivariable analysis. To our knowledge, only one other study has assessed the relationship between FT and cancer stage.25 In their general cancer population, more advanced cancer was associated with FT, contrary to our findings. The conflicting findings are likely a result of multiple factors. First, our study included an incident/prevalent cohort whereas other studies may represent more prevalent cases, which could influence results. Second, differences in treatment for non-invasive and invasive cancer vary based on site. Non-invasive bladder cancer, unlike many other non-invasive malignancies, requires intensive and costly surveillance and intravesical treatments. Patients presenting to the multidisciplinary cancer clinic often present with BCG-refractory disease, suggesting that these patients may have undergone more intensive surveillance and treatments in the past. Differences in treatment and surveillance may impact cost and result in FT.5 Other FT studies included a larger proportion of advanced cancer patients and intentionally excluded stage from their analyses, potentially missing an association between stage and FT.19,20 Furthermore, our sample size was small, and therefore may have missed an association between stage and FT.
Regardless of the association between stage and FT, financial distress clearly impacts quality of life, health behaviors and outcomes. Prior research has reported a negative association between FT and HRQOL in cancer patients. 21,22,24,26 Our study supported this inverse relationship, revealing that bladder cancer patients who endorsed FT reported poorer HRQOL on both general and cancer-specific questionnaires and in the majority of subcategories of QOL. HRQOL differences between FT and no FT were significant. On average, patients with FT had a lower FACT-GP total score (−10), PROMIS global physical health score (−4), and PROMIS global mental health score (−5). Minimally important differences range from 3 to 7 for the FACT-GP15 and 5 (half the standard deviation of 10) for PROMIS,16 suggesting that these HRQOL differences are meaningful. A 2010 study of cancer patients using National Health Interview Survey data found that patients endorsing FT were also more likely to delay or forgo care and reduce prescription medications, dental care, eyeglasses, and mental health care.19 Our findings support these results in that bladder cancer patients who endorse FT were also more likely to delay care, although this did not reach statistical significance (p=0.07), possibly due to sample size. In order to reveal possible interventions for this problem, we evaluated reasons for delaying care. We examined hospital/clinic-level factors such as ability to make an appointment, and patient-level factors such as lack of transportation. Among 14 potential reasons for delay in care, two were associated with FT: the inability to afford general expenses and the inability to miss work. These findings reflect that the most significant factors contributing to FT are likely high out-of-pocket treatment-related costs and the loss of income related to missed work or decreased employment in working-aged patients.27
Negative health behaviors such as delayed care can have worrisome consequences, including poor outcomes. Our study supports prior research that draws an association between FT and negative oncologic outcomes.1,10,11,21,22,24,26 For a holistic approach to cancer treatment, urologists have a responsibility to communicate costs to patients and include them in shared decision-making. The importance of cost discussions has recently been incorporated into multiple organizations’ guidelines, including the American Society of Clinical Oncology.28 Unfortunately, most clinicians do not discuss treatment costs with their patients, despite the patients’ desire to discuss the topic.29 Although many barriers exist (i.e. time, embarrassment, cost education), honest discussions regarding treatment expenses is a tangible step that can improve patient satisfaction and decrease OOP costs,29 and thus possibly mitigate some of the adverse effects of FT. Ideally, patient-physician interactions should be organized into 3 parts: 1) discussion regarding treatment options 2) financial counseling and 3) shared decision-making.
Another mechanism to address FT related to bladder cancer involves scrutiny of our surveillance practices. Among non-invasive bladder cancer patients, a large portion of the costs of care is related to extensive follow-up surveillance with expensive cystoscopy procedures and imaging.5 On average, CT urography costs $356 (ranging from $270 to $467) while cystoscopy costs $208 (ranging from $166 to $258).30 Costs may be surpassed by charges, often opaque, leading to rapid accumulation of expenses given stringent surveillance strategies. In 2016, the American Urologic Association revised their guidelines to create a risk-based strategy which relaxes surveillance among patients with low risk disease. However, guidelines for follow up care continue to be largely based on expert opinion and lack strong evidence. The creation of thoughtful, efficient follow-up algorithms offers a potential opportunity to improve patient care through diminishing direct out-of-pocket costs and inconvenience (e.g. missed work) related to multiple yearly visits. Although there are benefits to close surveillance of bladder cancer, future studies must evaluate the optimal timing and benefits of follow up surveillance in order to reduce the competing risk of FT.
Our study was not without limitations. The cross-sectional study design did not allow for an assessment of FT over time or to assess for causal relationships. Longitudinal assessment would likely reveal important details about how FT impacts patients with this disease, particularly for bladder cancer which draws many parallels to chronic disease. Our study was also limited by the lack of a validated assessment tool (e.g. COST), which as previously mentioned was developed after the initiation of our study. Furthermore, this study was conducted in a single institution, and therefore, a larger multi-institutional study would be needed to establish generalizability of results. Finally, we lacked comprehensive diagnostic and treatment data, which we attempted to address through retrospective chart review although not all were followed exclusively at our institution.
Despite these limitations, our study has several strengths. As the first evaluation of FT among bladder cancer patients, our findings represent an opportunity to evaluate mechanisms to streamline costs and incorporate the effects of financial distress in shared decision-making. Our detailed abstraction allowed for individual confirmation of each patient’s diagnosis and treatment. Our sample of bladder cancer patients was also consistent with national trends of bladder cancer patients, with the exception of a higher proportion of white patients. Our results will need to be externally validated in a larger, more nationally representative patient population.
Conclusions
In conclusion, we found that FT was a major concern for bladder cancer survivors and was associated with delays in care and poorer HRQOL. The etiology of FT is likely related to both missed work opportunities and high out-of-pocket healthcare costs. Cost communication should be a topic included in treatment discussions to improve shared decision-making conversations. Furthermore, optimizing surveillance intervals could reduce extraneous out-of-pocket costs for patients, decrease financial burden and improve patient outcomes.
Abbreviations
- FT
Financial toxicity
- HRQOL
health-related quality of life
- QOL
quality of life
- HR/CSC
Health Registry/Cancer Survivorship Cohort
- EMR
electronic medical record
- PSQ-18
Patient Satisfaction Questionnaire-18
- FACT
Functional Assessment of Cancer Therapy
- PROMIS
Patient-Reported Outcomes Measurement Information System
- OOP
out of pocket
Footnotes
Conflicts of Interest: None
Presentations: This research was presented at:
The 17th Annual Meeting of the Society of Urologic Oncology (San Antonio, TX 11/30/2016 – 12/2/2016)
The 81st Annual Meeting of the Southeastern Section of the AUA (Austin, TX 3/23/2017 – 3/26/2017)
The 2017 Annual Meeting of the American Urological Association (Boston, MA 5/12/17 – 5/16/17).
Funding: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zafar SY, et al. The Financial Toxicity of Cancer Treatment: A Pilot Study Assessing Out-of-Pocket Expenses and the Insured Cancer Patient’s Experience. The Oncologist. 2013;18:381–390. doi: 10.1634/theoncologist.2012-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2013 Incidence and Mortality Web-based Report. Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2016. [Google Scholar]
- 3.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. PharmacoEconomics. 2003;21:1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 4.Mossanen M, Gore JL. The burden of bladder cancer care: direct and indirect costs. Curr Opin Urol. 2014;24:487–491. doi: 10.1097/MOU.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 5.Avritscher EBC, et al. Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology. 2006;68:549–553. doi: 10.1016/j.urology.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 6.McDougall J, Ramsey S, Shih Y. Financial Toxicity: A Growing Concern among Cancer Patients in the United States. 2014 [Google Scholar]
- 7.Stahl, Lesley, correspondent. “The Cost of Cancer Drugs.” 60 Minutes. Bonin, Richard. CBS. Oct 05. 2014.
- 8.Johnson Carolyn. Washington Post. Apr 8, 2016. [Web. Accessed: 10 February 2017]. The Burden of Cancer isn’t Just Cancer. [Google Scholar]
- 9.Ward L. Wall Street Journal. Feb 17, 2016. [Accessed: 19 June 2017]. The High Cost of Cancer Care May Take Physical and Emotional Toll on Patients. [Google Scholar]
- 10.Ramsey S, et al. Washington State Cancer Patients Found To Be At Greater Risk For Bankruptcy Than People Without A Cancer Diagnosis. Health Aff (Millwood) 2013 doi: 10.1377/hlthaff.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramsey SD, et al. Financial Insolvency as a Risk Factor for Early Mortality Among Patients With Cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34:980–986. doi: 10.1200/JCO.2015.64.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arozullah AM, et al. The financial burden of cancer: estimates from a study of insured women with breast cancer. J Support Oncol. 2004;2:271–278. [PubMed] [Google Scholar]
- 13.Pisu M, et al. Economic hardship of minority and non-minority cancer survivors 1 year after diagnosis: another long-term effect of cancer? Cancer. 2015;121:1257–1264. doi: 10.1002/cncr.29206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knight TG, et al. Financial toxicity in adults with cancer: Adverse outcomes and potential areas of intervention. J Clin Oncol. 2016;34:6624–6624. [Google Scholar]
- 15.Cella DF, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol Off J Am Soc Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 16.Cella D, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon LG, Merollini KMD, Lowe A, Chan RJ. A Systematic Review of Financial Toxicity Among Cancer Survivors: We Can’t Pay the Co-Pay. Patient - Patient-Centered Outcomes Res. 2016:1–15. doi: 10.1007/s40271-016-0204-x. [DOI] [PubMed] [Google Scholar]
- 18.de Souza JA, et al. The Development of a Financial Toxicity Patient-Reported Outcome in Cancer The COST Measure. Cancer. 2014;120:3245–3253. doi: 10.1002/cncr.28814. [DOI] [PubMed] [Google Scholar]
- 19.Kent EE, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care?: Cancer-Related Financial Problems. Cancer. 2013;119:3710–3717. doi: 10.1002/cncr.28262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khera N. Reporting and grading financial toxicity. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32:3337–3338. doi: 10.1200/JCO.2014.57.8740. [DOI] [PubMed] [Google Scholar]
- 21.Kale HP, Carroll NV. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer. 2016;122:283–289. doi: 10.1002/cncr.29808. [DOI] [PubMed] [Google Scholar]
- 22.de Oliveira C, et al. Patient time and out-of-pocket costs for long-term prostate cancer survivors in Ontario, Canada. J Cancer Surviv Res Pract. 2014;8:9–20. doi: 10.1007/s11764-013-0305-7. [DOI] [PubMed] [Google Scholar]
- 23.Finkelstein EA, Tangka FK, Trogdon JG, Sabatino SA, Richardson LC. The personal financial burden of cancer for the working-aged population. Am J Manag Care. 2009;15:801–806. [PubMed] [Google Scholar]
- 24.Yabroff KR, et al. Financial Hardship Associated With Cancer in the United States: Findings From a Population-Based Sample of Adult Cancer Survivors. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34:259–267. doi: 10.1200/JCO.2015.62.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimman M, Jan S, Yip CH, et al. The ACTION Study Group. Catastrophic health expenditure and 12-month mortality associated with cancer in Southeast Asia: results from a longitudinal study in eight countries. BMC Med. 2015;13 doi: 10.1186/s12916-015-0433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenn KM, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. 2014;10:332–338. doi: 10.1200/JOP.2013.001322. [DOI] [PubMed] [Google Scholar]
- 27.Sharp L, Timmons A. Pre-diagnosis employment status and financial circumstances predict cancer-related financial stress and strain among breast and prostate cancer survivors. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2016;24:699–709. doi: 10.1007/s00520-015-2832-4. [DOI] [PubMed] [Google Scholar]
- 28.Meropol NJ, et al. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27:3868–3874. doi: 10.1200/JCO.2009.23.1183. [DOI] [PubMed] [Google Scholar]
- 29.Shih Y-CT, Chien C-R. A review of cost communication in oncology: Patient attitude, provider acceptance, and outcome assessment. Cancer. 2017;123:928–939. doi: 10.1002/cncr.30423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halpern JA, Chughtai B, Ghomrawi H. Cost-effectiveness of Common Diagnostic Approaches for Evaluation of Asymptomatic Microscopic Hematuria. JAMA Intern Med. 2017;177:800–807. doi: 10.1001/jamainternmed.2017.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]