Abstract
Background and Purpose
Initial studies support the use of strength training (ST) as a safe and effective intervention after stroke. Our previous work shows that relatively aggressive, higher intensity ST translates into large effect sizes for paretic and non-paretic leg muscle volume, myostatin expression, and maximum strength post-stroke. An unanswered question pertains to how our unique ST model for stroke impacts skeletal muscle endurance (SME). Thus, we now report on ST-induced adaptation in the ability to sustain isotonic muscle contraction.
Methods
Following screening and baseline testing, hemiparetic stroke participants were randomized to either ST or an attention-matched stretch control group (SC). Those in the ST group trained each leg individually to muscle failure (20 repetition sets, 3× per week for 3 months) on each of three pneumatic resistance machines (leg press, leg extension, and leg curl). Our primary outcome measure was SME, quantified as the number of submaximal weight leg press repetitions possible at a specified cadence. The secondary measures included one-repetition maximum strength, 6-minute walk distance (6MWD), 10-meter walk speeds, and peak aerobic capacity (VO2 peak).
Results
ST participants (N = 14) had significantly greater SME gains compared with SC participants (N = 16) in both the paretic (178% versus 12%, P < .01) and non-paretic legs (161% versus 12%, P < .01). These gains were accompanied by group differences for 6MWD (P < .05) and VO2 peak (P < .05).
Conclusion
Our ST regimen had a large impact on the capacity to sustain submaximal muscle contraction, a metric that may carry more practical significance for stroke than the often reported measures of maximum strength.
Keywords: Stroke recovery, stroke rehabilitation, strength training, exercise, endurance
Introduction
Paretic-side skeletal muscle abnormalities contribute to poor strength, fitness, and function, with serious implications for both disability and ongoing cardiometabolic risk after stroke.1–4 Our group designed a unique strength training (ST) model for stroke, showing great potential for reversing both paretic and non-paretic-side skeletal muscle wasting.5 These ST-induced adaptations carry high clinical relevance, resulting in altered tissue composition and improved insulin sensitivity to affect whole body metabolic health.5,6
Of the trials completed to date after stroke, success of ST interventions has often been gauged by impact on maximum strength.5,7–17 Although measures of one-repetition maximum (1-RM) and peak torque generating capacity strength are clearly important measures through which to evaluate the effectiveness of a training stimulus, the ability to sustain submaximal muscle contraction may be even more relevant to everyday function.18,19 This is especially true in stroke disability, wherein activities of daily living are more often contingent upon submaximal sustainment than maximal exertion.20–23 For this reason, it would seem logical to target and design interventions with an eye toward maximizing skeletal muscle endurance (SME) in addition to maximum strength. To our knowledge, the general concept of training for SME has not yet emerged in stroke rehabilitation.
Hence, our objective was to apply an ST intervention tailored for muscle endurance gains after stroke. We sought to assess bilateral capacity for improving maintenance of submaximal muscle contraction at a specified cadence. In addition, we determined whether our endurance-based ST regimen for stroke changed other widely reported functional metrics, some of which do not consistently respond to more standard ST regimens.7,17 The designated primary assessment (SME) was compared between ST and stretch control (SC) groups in both the paretic and non-paretic legs across a 3-month period. Likewise, the chosen secondary functional measures (1-RM, 6-minute walk distance [6MWD], VO2 peak, and 10-meter walk speeds [10MWS]) were assessed and compared between groups. We hypothesized that ST would show between-group superiority and large effect sizes for SME, and that secondary functional measures would also improve based on the unique features of the training protocol.
Materials and Methods
Subjects
Recruits came from the University of Maryland Medical System and the Baltimore VA Medical Center referral networks. Patients with chronic hemiparesis (>6 months post-stroke) were identified and invited to participate after completion of all standard physical therapy. Potential participants presented with mild to moderate hemiparetic gait and preserved capacity for ambulation either with or without an assistive device. This study was approved by the institutional review board for research involving humans at the University of Maryland, Baltimore. Written informed consent was obtained from each participant.
Screening
Baseline evaluation included a medical history and examination, ensuring that all specified entry criteria were met. Additionally, a physician-supervised treadmill tolerance test at no incline was first performed to assess gait safety and to select walking velocity for subsequent peak exercise testing as previously described.24,25 Participants minimized handrail support, and a gait belt was worn for safety. For the graded treadmill screening test, all participants who achieved adequate exercise intensities without signs of myocardial ischemia or other contraindications for participating in exercise training were deemed suitable for safe entry.
Outcomes Testing
SME
Endurance for both paretic and non-paretic legs was assessed individually on a leg press device that allowed for unilateral movement (Keiser K-300, Fresno CA). Using a standardized protocol, the objective was to determine the maximum number of leg press repetitions possible at 70% of 1-RM according to a fixed metronome cadence (60 bpm, 0°–90°). The non-paretic leg was always assessed first, followed by separate testing on the paretic leg. Participants were instructed to perform the concentric and eccentric movements of the leg press in a highly controlled manner, moving out with one metronome click and back with the subsequent metronome click, repeating as many times as possible until either complete muscle failure or disruption of the specified cadence. In this way, we could ascertain the capacity of the quadriceps to sustain controlled contraction on the paretic and non-paretic sides, both before and after the interventions. The 3-month post-test used the same absolute level of resistance (70% of baseline 1-RM), enabling us to properly gauge the enhanced efficiency of sustaining leg press movement with the original pretraining level of resistance. This is somewhat analogous to studies assessing gait economy on a treadmill, during which the same absolute treadmill speed (i.e., level of work) is utilized for both baseline and post-training testing.26 The number of repetitions at the required cadence was the outcome value compared across time and between groups for both the paretic and non-paretic legs.
1-RM
The 1-RM strength testing was conducted separately on each side to account for sizeable strength discrepancies between legs as previously described.5 Briefly, the participants were positioned with knees at 90° on the seated leg press machine. The participants were then instructed on technique and breathing prior to commencing with the first of several trials geared toward arriving at maximum strength. Ideally, the 1-RM value was arrived at within eight trials, two of which were utilized as warm-up sets with lighter weights of multiple repetitions (10 and 5 repetitions for Trials 1 and 2, respectively). For Trials 3–8, one-repetition attempts were progressively increased and separated by 2-minute rest periods, until such time that an inability to move a weight was demonstrated, after which a regression back to 1-RM was required.5
6MWD
The 6-minute walk is an ambulation distance representative of what might be required for community-based activity of daily living (ADL) tasks, having been shown as an effective measurement method for gait endurance.27 The participants used assistive devices when necessary. They were instructed to cover as much distance as they could over a flat 100-foot walking surface demarcated by traffic cones during the 6-minute time period.
Peak Oxygen Consumption (VO2 Peak)
Treadmill testing with open circuit spirometry was conducted to measure peak aerobic capacity. This was done using a previously described treadmill testing protocol for stroke survivors.28
10MWS
To gauge walking speed over a shorter distance, we conducted standard 10-m walk tests at both self-selected walking speed and fastest comfortable walking speed before and after training as previously described.24 Standardized instructions and commands were utilized during these short distance walking assessments.
Randomization
Initially, participants were randomized to either the ST or SC control group following baseline testing using a blocked allocation schema and a computer-based pseudorandom number generator. Separate blocked randomizations were performed according to age (<65 versus ≥ 65 yrs.) and ratio of leg press 1-RM strength in the paretic leg to 1-RM strength in the non-paretic leg (<.64 versus ≥ .64). A ratio of .64 represented the median leg press strength ratio of stroke participants tested in our facility. The ratio was used to make the intervention groups more equal in terms of strength and stroke-related strength reduction on the paretic side. Randomization was partially confounded toward the end of the study by the need to assign the entire final group of participants (n = 4) to ST based on an uneven discontinuation rate and to achieve more even final group numbers. This did not cause any inequities between groups for participant characteristics.
Intervention Protocols (3 Months)
ST Group
The ST program consisted of three sessions per week of bilateral training for the lower extremities. This was accomplished with exercises performed on three Keiser K-300 air-powered machines utilizing pneumatic resistance (leg extension, leg curl, and leg press). The leg extension machine trained the quadriceps (knee extensor) muscle group. The leg curl machine trained the hamstring (knee flexor) group. Finally, the Keiser leg press machine provided stimulus to both muscle groups through closed-chain kinetic effort. Because of the large discrepancy in strength and function between the paretic and non-paretic legs of these stroke patients, we exercised the legs separately on each Keiser machine, thus insuring the maximum degree of stimulus on each side.
Participants performed two sets of 20 repetitions on each leg and each machine (20 × 2 × 3 = 120 repetitions per session), enabling development of both strength and endurance. For each unilateral set, the resistance was set to a level that would produce muscle failure between the 10th and 15th repetitions. After the first instance of muscle failure, we slowly lowered the resistance a bit at a time, such that the full set of 20 could be completed. Most often, participants would experience two to three instances of muscle failure per set, with some variability based on energy level and perception of effort on any particular day. Each set exposed participants to both a front-end component designed to maximize 1-RM strength and a latter portion targeting muscle endurance change. Each ST session of 120 repetitions, split between legs and machines, took approximately 45 minutes to complete.
SC Group
The control group performed 45 minutes of supervised stretching exercises on raised padded tables. Stretching and range of motion exercises are used in physical therapy after stroke, and are commonly prescribed as continued outpatient exercises upon completion of rehabilitation care. Thus, we viewed our stretching program as a representative component of usual care. Our experience showed that participants enjoyed the stretching program and were compliant with attendance. The standardized stretching regimen consisted of a battery of passive and active stretching exercises primarily aimed at the lower extremity musculature. Stroke participants kept a log book of stretching exercise parallel to that used in the ST group to balance potential motivation and self-efficacy effects these records may impart. The ST and SC groups were matched for level of research staff attention by means of this control group model, and potential influence on outcomes stemming from regular travel to and from our research center was also accounted for using SC controls.
Adverse events were monitored to determine whether training or testing resulted in negative changes to any category of general health or function. This would include but not be limited to issues pertaining to musculoskeletal health, vital signs, blood sugar, acute illness, or any changes to overall health status as determined through structured participant questioning prior to each training session. We did not formally evaluate changes in tone or spasticity.
Data Analysis
Baseline participant characteristic values (mean ± standard deviation) were compared between groups using an independent t-test. Categorical baseline variables (ratios) were compared between groups using the Fisher’s exact test. Repeated-measures analysis of variance (two factors, time × group) was used to predict the values of outcome variables across time, detecting significant two-way interactions for changes in outcomes over 3 months. Repeated values are mean ± standard errors with a two-tailed P value of .05 required for significance. Within-group changes were assessed for significance with a paired t-test. The IBM SPSS 22 statistical package (Armonk, New York) was used for all baseline and longitudinal data analyses.
Results
Subjects
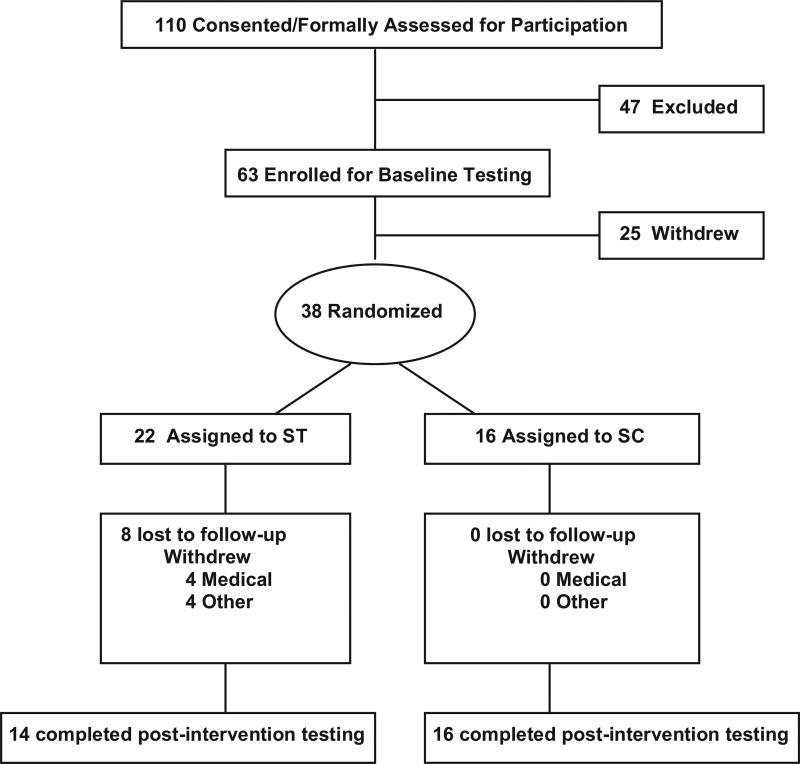
Of 30 who completed, 14 were ST and 16 were SC. At baseline, there were no significant differences between groups for age, latency, gender, race, weight, or body mass index (Table 1). Likewise, there were no statistically significant baseline differences between groups for the primary outcome variable (SME = number of repetitions at 70% of 1-RM) or any of the secondary functional measures (6MWD, 10MWT, VO2 peak, and 1-RM). All the physical and functional characteristics of the participants in both groups are summarized in Table 1. As depicted in Figure 1, there were eight who were lost to follow-up in the ST group and none in the SC group. Dropouts in ST resulted from either medical reasons unrelated to study procedures or compliance issues. Among those who completed the study, there were no serious adverse events resulting from either intervention. Both ST and SC were generally well tolerated and adequately adhered to (>85% of sessions attended).
Table 1.
Participant characteristics by group (mean ± SD)
| Variable | ST (n = 14) | SC (n = 16) | P value |
|---|---|---|---|
| Age (years) | 57 ± 14 | 55 ± 9 | .65 |
| Stroke latency (years) | 5 ± 4 | 6 ± 5 | .43 |
| Gender (M:F) | 10:4 | 11:5 | 1.00 |
| Race (B:W) | 9:5 | 11:5 | .72 |
| Weight (kg) | 85 ± 19 | 90 ± 17 | .45 |
| BMI (kg/m2) | 28 ± 5 | 31 ± 8 | .18 |
| SSWS 10 m (mph) | 1.8 ± .7 | 1.9 ± .6 | .49 |
| 6MWD (ft) | 974 ± 282 (n = 13) | 1057 ± 451 | .57 |
| Peak VO2 (mL/kg/min) | 19.5 ± 6 | 18.8 ± 49 (n = 15) | .69 |
| 1-RM leg press (lbs, pneumatic) | 295 ± 124 | 388 ± 203 | .15 |
| Endurance (number of repetitions at 70% 1-RM) | 15 ± 9 | 16 ± 11 | .80 |
| AFO use (Y:N) | 6:8 | 3:13 | .24 |
| Assistive device use (Y:N) | 5:9 | 4:12 | .69 |
Abbreviations: 1-RM, one repetition maximum; 6MWD, 6-minute walk distance; AFO, ankle foot orthosis; BMI, body mass index; B:W, black:white; M:F, male:female; SC, stretch control; SD, standard deviation; SSWS, self-selected walking speed; ST, strength training; VO2, oxygen consumption; Y:N, yes:no.
Figure 1.
Flow diagram. Abbreviation: ST, strength training; SC, stretch control group.
Effects of ST versus SC on SME
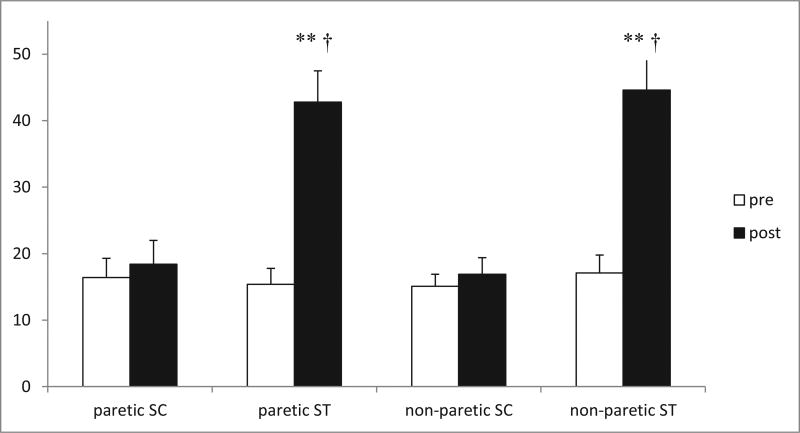
Two-way repeated-measures analysis showed that ST participants (n = 14) had significantly greater SME gains than SC participants (N = 15) (P < .001). As shown in Figure 2, ST group SME changes of 178% in the paretic leg and 161% in the non-paretic leg were greater than the 12% SC group changes observed for both the paretic and nonparetic legs (P < .01). Beyond the two-way interaction results, within-group analysis showed that generalized lower extremity stretching exercises, travel to the medical center, and attention matching in SC were not sufficient to impact SME for either the paretic or non-paretic leg (P = NS), with only ST having a within-group effect (P < .01).
Figure 2.
Bar graph depicting change in endurance (number of repetitions of submaximal weight at a specified cadence) with training in ST (n = 14) versus SC (n = 15). A significant time × group interaction (†P < .001) indicated that change in ST was statistically significantly greater than change in SC. **Denotes significant within-group change for SC (P < .001). Values are mean ± SE. The y-axis represents the number of repetitions at specified cadence. Abbreviations: SC, stretch control; SE, standard error; ST, strength training.
Effects of ST versus SC on 6MWD
The changes in 6MWD recorded for ST (+14%) and SC (0%) were statistically different by 2 × 2 repeated-measures analysis of variance (P = .011), indicating that ST significantly improved practical walking endurance and was greater than the change observed in SC (Table 2). Within-group analysis showed that only the ST group experienced significant intergroup improvement subsequent to the applied training regimen (P = .018).
Table 2.
Basic functional measures before and after training in ST and SC
| Outcome/Training group | Pretraining | Post-training | Within-group P value | Between-group P value |
|---|---|---|---|---|
| 6MWD—ST (ft) (n = 13) | 974 ± 78 | 1106 ± 103 | .018* | .011*** |
| 6MWD—SC (ft) (n = 16) | 1057 ± 113 | 1054 ± 109 | .923 | |
| VO2 peak—ST (mL/kg/min) (n = 14) | 19.5 ± 1.5 | 20.7 ± 1.6 | .062 | .038*** |
| VO2 peak—SC (mL/kg/min) (n = 15) | 18.6 ± 1.1 | 18.2 ± 1.1 | .373 | |
| 10 MWS–SSWS—ST (mph) (n = 14) | 1.8 ± .2 | 1.9 ± .2 | .118 | .275 |
| 10 MWS–SSWS—SC (mph) (n = 16) | 1.9 ± .2 | 2.0 ± .2 | .609 | |
| 10 MWS–FCWS—ST (mph) (n = 13) | 2.5 ± .2 | 2.8 ± .3 | .033* | .251 |
| 10 MWS–FCWS—SC (mph) (n = 14) | 2.7 ± .2 | 2.8 ± .2 | .261 | |
| 1-RM par. ST (lbs, pneumatic) (n = 14) | 295 ± 33 | 421 ± 44 | <.001** | <.001*** |
| 1-RM par. SC (lbs, pneumatic) (n = 16) | 388 ± 51 | 399 ± 47 | .453 | |
| 1-RM non-par. ST (lbs, pneumatic) (n = 14) | 452 ± 47 | 545 ± 48 | .003* | .017*** |
| 1-RM non-par. SC (lbs, pneumatic) (n = 15) | 548 ± 36 | 564 ± 34 | .330 |
Abbreviations: 1-RM, one repetition maximum; 6MWD, 6-minute walk distance; FCWS, fastest comfortable walking speed; MWS, meter walk speed; non-par, non-paretic.; par., paretic; SC, stretch control; SE, standard error; SSWS, self-selected walking speed; ST, strength training. Values are mean±SE.
Significant within group (P < .05);
significant within group (P < .01);
significant between groups (P < .05).
Effects of ST versus SC on VO2 Peak
There was a time × group interaction in VO2 peak, with a 6% increase in ST and a 2% decline for SC (P < .05) (Table 2). The within-group change for VO2 peak trended toward significance in the ST group (P = .062).
Effects of ST versus SC on 10MWT
In contrast to 6MWD, neither of the shorter distance 10-meter walking measures (self-selected or fastest comfortable) showed time by group interactions over the 6-month intervention period (P = .275 and P = .251, respectively) (Table 2). In the case of self-selected walking time, there were also no within-group changes observed for either ST (P = .118) or SC (P = .609). However, there was a within-group change for fastest comfortable walking speed in ST (P = .033), thus showing some impact on shorter distance walking capacity.
Effects of ST versus SC on 1-RM Strength
As expected, there were significantly greater gains in leg press 1-RM strength for ST compared with SC in both the paretic (43% versus 3%) and non-paretic (21% versus 3%) legs (P = .001 and P = .003, respectively, Table 2). Additionally, within-group analyses showed that the SC group did not produce any change in maximum leg press strength, in contrast to ST.
Discussion
This paper provides unique focus on an understudied aspect of stroke rehabilitation. Both the novel intervention and primary outcome were tailored toward determination of the potential for SME gains in chronic stroke, a population whose compromised functional status makes endurance questions especially relevant. Although maximum strength likely has its place for facilitating everyday function in those with neurologic disability,7 it is logically less of a prominent factor than sustainment of repeated submaximal muscle contractions.23 Coordinated multi-joint movements that make up everyday tasks like walking are dependent on repetitive submaximal contractions and are less associated with the explosive one time movements represented by a 1-RM strength test.22 Importantly, the magnitude of SME gains produced by our ST treatment group (150 + %) was unexpected and suggestive of newly acquired potential for altering sustainment of everyday activities. None of the other outcome measures showed comparable effect sizes. Clearly, different approaches to studying how SME translates into community and home-based function should be considered, as the current study does not address that issue. In fact, many research-related functional assessments may be limited in discerning how endurance characteristics impact activities of daily living, functional confidence, and motivation for pursuing a more active lifestyle.
Past work from our laboratory and other groups has provided ample rational for utilizing ST as a tool for proper rehabilitation and health maintenance in hemiparetic stroke.5,6,12,13,15,16,29 This has been independent of the newly formulated questions pertaining to SME. Stroke skeletal muscle, particularly on the paretic side, is markedly degraded,1,4,30 leading to a myriad of clinical problems in the categories of strength, function, and metabolic health relevant to ongoing vascular event risk.23,31 For example, we know from our studies involving serial computed tomography measurements of the thigh that muscle wasting becomes severe to the point of causing 24% reductions in paretic-side muscle volume.4 Similarly, muscle biopsy studies reveal profound degradations in fiber type distributions, fiber size, capillary density, and molecular characteristics after stroke.30,32–38 Collectively, post-stroke skeletal muscle abnormalities have come to be viewed as a primary driver of dysfunction and general health risk after stroke,1 providing strong rationale for the application of evidence-based ST models in this population.
Several systematic reviews have established ST as a safe and effective means for addressing post-stroke weakness, with mixed results for commonly utilized functional outcome measures.8–10,39 More recently, our group preliminarily established the utility of an ST intervention model for inducing clinically relevant skeletal muscle tissue changes.5 Importantly, these adaptations in chronically disabled stroke survivors occur in the absence of concomitant increases in muscle spasticity, which had been a prior misconception driving avoidance of this particular therapy model. The chief distinguishing feature between our groups’ training approach and that of others is the deliberate intent to affect muscle endurance changes through higher repetition training sets. Specifically, our training sessions consisted of 120 repetitions for the lower extremities, typically producing an initial instance of muscle failure between the 10th and 15th repetitions of each 20-repetition unilateral set. Following the initial failure, the weight would be lowered just enough to achieve a few more repetitions, repeating until a full 20 repetitions were achieved. Sessions were progressed by advancing initial training weight as participants became able to reach repetition 15 without lowering. This training strategy was unique in the context of stroke rehabilitation, resulting in an unprecedented and relatively intense training stimulus, ideal for causing the large effect sizes in SME. We now propose that this training approach receive strong consideration in terms of clinical application, based in part on robust effects for endurance.
To our knowledge, the only reference made to muscle endurance in the context of ST trials for stroke came in a 2010 study by Lee et al.15 These investigators studied the effects of progressive resistance training and high-intensity cycling on “muscle performance,” with muscle endurance represented as more of a secondary outcome, having been conducted on the same day and in close proximity to potentially confounding muscle power tests. Other methodological differences compared with the current study included endurance testing at 90% of 1-RM (limiting the baseline number of baseline achievable repetitions to between 4 and 6 repetitions), an assessment period of only 30 seconds, and no cadence specification. In addition, training sets per session (n = 2) involved just eight repetitions per set. Despite these potential confounders related to assessment of muscle endurance capacity, this study did see improvement in the number of repetitions participants could perform at 90% 1-RM during a 30-second time period. The current study builds on these results, providing what we consider to be a unique foundation for understanding the extent to which SME can be improved after stroke using a relatively short training period. Prior to the current study, it was unclear how strength and muscle tissue adaptations translated into the capacity for sustained muscle contraction. The answer now seems to be that it has quite a large impact, but the mechanisms remain unclear and fall beyond the scope of the current study.
To summarize, this controlled study demonstrates what added emphasis on training for muscle endurance can do to improve submaximal muscle contraction sustainment. Having a standardized SC group ruled out the possibility that any of the observed improvements in muscle endurance resulted from staff attention or travel to and from the training center. Study limitations included small sample size and heterogeneity in participant disability level. Resource constraints prevented assessor blinding in all cases, representing a potential confounder limiting interpretation of results.
Future studies should consider the broader public health impact of muscle endurance gains of this magnitude. For example, detailed physical activity monitoring, broader functional assessment batteries, and quality of life assessments could shed additional insight on the broader significance of muscle endurance gains. Also, some attention could be paid to whether increasing training sets beyond 20 repetitions would result in even better ST-induced endurance changes. Hybrid approaches combining ST with aerobic training models could also be considered in the context of direct comparison studies for muscle endurance. In general, future work should determine the full impact of endurance improvements, including how endurance changes influence free-living physical activity patterns and community-based functional capacity.
Acknowledgments
We thank all of our loyal stroke survivors for their commitment to regular testing and exercise training visits. We also acknowledge the dedication of our outstanding research staff and their commitment to participant safety, treatment fidelity, and the general satisfaction of participants.
Sources of funding: Dr. Ivey was supported by a VA Rehabilitation Research and Development (RR&D) Merit Award and VA ORH funding. Dr. Ryan was supported by a VA Senior Research Career Scientist Award. Dr. Prior was supported by a VA Career Development Award and K23-AG040775 (NIH and American Federation for Aging Research). The authors also wish to acknowledge support from the VA RR&D Maryland Exercise and Robotics Center of Excellence (MERCE), Department of Veterans Affairs and Veterans Affairs Medical Center, Baltimore Geriatric Research, Education and Clinical Center (GRECC), and the National Institute on Aging (NIA) Claude D. Pepper Older Americans Independence Center (P30-AG028747).
References
- 1.Hafer-Macko CE, Ryan AS, Ivey FM, et al. Skeletal muscle changes after hemiparetic stroke and potential beneficial effects of exercise intervention strategies. J Rehabil Res Dev. 2008;45:261–272. doi: 10.1682/jrrd.2007.02.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivey FM, Macko RF, Ryan AS, et al. Cardiovascular health and fitness after stroke. Top Stroke Rehabil. 2005;12:1–16. doi: 10.1310/GEEU-YRUY-VJ72-LEAR. [DOI] [PubMed] [Google Scholar]
- 3.Ivey FM, Gardner AW, Dobrovolny CL, et al. Unilateral impairment of leg blood flow in chronic stroke patients. Cerebrovasc Dis. 2004;18:283–289. doi: 10.1159/000080353. [DOI] [PubMed] [Google Scholar]
- 4.Ryan AS, Buscemi A, Forrester L, et al. Atrophy and intramuscular fat in specific muscles of the thigh: associated weakness and hyperinsulinemia in stroke survivors. Neurorehabil Neural Repair. 2011;25:865–872. doi: 10.1177/1545968311408920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan AS, Ivey FM, Prior S, et al. Skeletal muscle hypertrophy and muscle myostatin reduction after resistive training in stroke survivors. Stroke. 2011;42:416–420. doi: 10.1161/STROKEAHA.110.602441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivey FM, Ryan AS. Resistive training improves insulin sensitivity after stroke. J Stroke Cerebrovasc Dis. 2014;23:225–229. doi: 10.1016/j.jstrokecerebrovasdis.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wist S, Clivaz J, Sattelmayer M. Muscle strengthening for hemiparesis after stroke: a meta-analysis. Ann Phys Rehabil Med. 2016;59:114–124. doi: 10.1016/j.rehab.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Pak S, Patten C. Strengthening to promote functional recovery poststroke: an evidence-based review. Top Stroke Rehabil. 2008;15:177–199. doi: 10.1310/tsr1503-177. [DOI] [PubMed] [Google Scholar]
- 9.Lexell J, Flansbjer UB. Muscle strength training, gait performance and physiotherapy after stroke. Minerva Med. 2008;99:353–368. [PubMed] [Google Scholar]
- 10.Morris SL, Dodd KJ, Morris ME. Outcomes of progressive resistance strength training following stroke: a systematic review. Clin Rehabil. 2004;18:27–39. doi: 10.1191/0269215504cr699oa. [DOI] [PubMed] [Google Scholar]
- 11.Patten C, Lexell J, Brown HE. Weakness and strength training in persons with poststroke hemiplegia: rationale, method, and efficacy. J Rehabil Res Dev. 2004;41:293–312. doi: 10.1682/jrrd.2004.03.0293. [DOI] [PubMed] [Google Scholar]
- 12.Flansbjer UB, Lexell J, Brogardh C. Long-term benefits of progressive resistance training in chronic stroke: a 4-year follow-up. J Rehabil Med. 2012;44:218–221. doi: 10.2340/16501977-0936. [DOI] [PubMed] [Google Scholar]
- 13.Flansbjer UB, Miller M, Downham D, et al. Progressive resistance training after stroke: effects on muscle strength, muscle tone, gait performance and perceived participation. J Rehabil Med. 2008;40:42–48. doi: 10.2340/16501977-0129. [DOI] [PubMed] [Google Scholar]
- 14.Ouellette MM, LeBrasseur NK, Bean JF, et al. High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke. 2004;35:1404–1409. doi: 10.1161/01.STR.0000127785.73065.34. [DOI] [PubMed] [Google Scholar]
- 15.Lee MJ, Kilbreath SL, Singh MF, et al. Effect of progressive resistance training on muscle performance after chronic stroke. Med Sci Sports Exerc. 2010;42:23–34. doi: 10.1249/MSS.0b013e3181b07a31. [DOI] [PubMed] [Google Scholar]
- 16.Hill TR, Gjellesvik TI, Moen PM, et al. Maximal strength training enhances strength and functional performance in chronic stroke survivors. Am J Phys Med Rehabil. 2012;91:393–400. doi: 10.1097/PHM.0b013e31824ad5b8. [DOI] [PubMed] [Google Scholar]
- 17.Severinsen K, Jakobsen JK, Pedersen AR, et al. Effects of resistance training and aerobic training on ambulation in chronic stroke. Am J Phys Med Rehabil. 2014;93:29–42. doi: 10.1097/PHM.0b013e3182a518e1. [DOI] [PubMed] [Google Scholar]
- 18.McNeil CJ, Rice CL. Fatigability is increased with age during velocity-dependent contractions of the dorsiflexors. J Gerontol A Biol Sci Med Sci. 2007;62:624–629. doi: 10.1093/gerona/62.6.624. [DOI] [PubMed] [Google Scholar]
- 19.Reuben DB, Magasi S, McCreath HE, et al. Motor assessment using the NIH toolbox. Neurology. 2013;80:S65–S75. doi: 10.1212/WNL.0b013e3182872e01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuppuswamy A, Clark E, Rothwell J, et al. Limb heaviness: a perceptual phenomenon associated with poststroke fatigue? Neurorehabil Neural Repair. 2016;30:360–362. doi: 10.1177/1545968315597071. [DOI] [PubMed] [Google Scholar]
- 21.Rybar MM, Walker ER, Kuhnen HR, et al. The stroke-related effects of hip flexion fatigue on over ground walking. Gait Posture. 2014;39:1103–1108. doi: 10.1016/j.gaitpost.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyngstrom AS, Kuhnen HR, Kirking KM, et al. Functional implications of impaired control of submaximal hip flexion following stroke. Muscle Nerve. 2014;49:225–232. doi: 10.1002/mus.23886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Billinger SA, Arena R, Bernhardt J, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2532–2553. doi: 10.1161/STR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 24.Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke. 2005;36:2206–2211. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]
- 25.Macko RF, Ivey FM, Forrester LW. Task-oriented aerobic exercise in chronic hemiparetic stroke: training protocols and treatment effects. Top Stroke Rehabil. 2005;12:45–57. doi: 10.1310/PJQN-KAN9-TTVY-HYQH. [DOI] [PubMed] [Google Scholar]
- 26.Macko RF, DeSouza CA, Tretter LD, et al. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients. A preliminary report. Stroke. 1997;28:326–330. doi: 10.1161/01.str.28.2.326. [DOI] [PubMed] [Google Scholar]
- 27.Lord SE, McPherson K, McNaughton HK, et al. Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? Arch Phys Med Rehabil. 2004;85:234–239. doi: 10.1016/j.apmr.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Dobrovolny CL, Ivey FM, Rogers MA, et al. Reliability of treadmill exercise testing in older patients with chronic hemiparetic stroke. Arch Phys Med Rehabil. 2003;84:1308–1312. doi: 10.1016/s0003-9993(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 29.Bale M, Strand LI. Does functional strength training of the leg in subacute stroke improve physical performance? A pilot randomized controlled trial. Clin Rehabil. 2008;22:911–921. doi: 10.1177/0269215508090092. [DOI] [PubMed] [Google Scholar]
- 30.Hafer-Macko CE, Yu S, Ryan AS, et al. Elevated tumor necrosis factor-alpha in skeletal muscle after stroke. Stroke. 2005;36:2021–2023. doi: 10.1161/01.STR.0000177878.33559.fe. [DOI] [PubMed] [Google Scholar]
- 31.Billinger SA, Coughenour E, Mackay-Lyons MJ, et al. Reduced cardiorespiratory fitness after stroke: biological consequences and exercise-induced adaptations. Stroke Res Treat. 2012;2012:959120. doi: 10.1155/2012/959120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frontera WR, Grimby L, Larsson L. Firing rate of the lower motoneuron and contractile properties of its muscle fibers after upper motoneuron lesion in man. Muscle Nerve. 1997;20:938–947. doi: 10.1002/(sici)1097-4598(199708)20:8<938::aid-mus2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Hachisuka K, Umezu Y, Ogata H. Disuse muscle atrophy of lower limbs in hemiplegic patients. Arch Phys Med Rehabil. 1997;78:13–18. doi: 10.1016/s0003-9993(97)90003-4. [DOI] [PubMed] [Google Scholar]
- 34.Landin S, Hagenfeldt L, Saltin B, et al. Muscle metabolism during exercise in hemiparetic patients. Clin Sci Mol Med. 1977;53:257–269. doi: 10.1042/cs0530257. [DOI] [PubMed] [Google Scholar]
- 35.Scelsi R, Lotta S, Lommi G, et al. Hemiplegic atrophy. Morphological findings in the anterior tibial muscle of patients with cerebral vascular accidents. Acta Neuropathol. 1984;62:324–331. doi: 10.1007/BF00687615. [DOI] [PubMed] [Google Scholar]
- 36.De Deyne PG, Hafer-Macko CE, Ivey FM, et al. Muscle molecular phenotype after stroke is associated with gait speed. Muscle Nerve. 2004;30:209–215. doi: 10.1002/mus.20085. [DOI] [PubMed] [Google Scholar]
- 37.Daugaard JR, Richter EA. Relationship between muscle fibre composition, glucose transporter protein 4 and exercise training: possible consequences in non-insulin-dependent diabetes mellitus. Acta Physiol Scand. 2001;171:267–276. doi: 10.1046/j.1365-201x.2001.00829.x. [DOI] [PubMed] [Google Scholar]
- 38.Prior SJ, McKenzie MJ, Joseph LJ, et al. Reduced skeletal muscle capillarization and glucose intolerance. Microcirculation. 2009;16:203–212. doi: 10.1080/10739680802502423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris JE, Eng JJ. Strength training improves upper-limb function in individuals with stroke: a meta-analysis. Stroke. 2010;41:136–140. doi: 10.1161/STROKEAHA.109.567438. [DOI] [PubMed] [Google Scholar]