Abstract
Background:
In older adults, selenium status has been positively associated with cognitive function. We recently reported a positive association between maternal selenium status in pregnancy and children’s cognitive function at 1.5 y.
Objective:
We followed up the children to assess if prenatal and childhood selenium status was associated with cognitive abilities at 5 and 10 y.
Methods:
This longitudinal cohort study was nested in Maternal and Infant Nutrition Interventions in Matlab (MINIMat), a population-based, randomized supplementation trial in pregnancy in rural Bangladesh. Selenium in maternal blood [erythrocyte fraction (Ery-Se) at baseline] and in child hair and urine was measured using inductively coupled plasma mass spectrometry. Children’s cognition at 5 and 10 y was assessed using the Wechsler Preschool and Primary Scale of Intelligence™ and the Wechsler Intelligence Scale for Children®, respectively. In total, 1,408 children were included.
Results:
Multivariable-adjusted linear regression analyses showed that prenatal selenium status was positively associated with children’s cognitive function at 5 and 10 y. An increase in maternal Ery-Se from the fifth to the 95th percentile [median: hemoglobin (Hb)] was associated with an increase in full developmental score of 3.5 [95% confidence interval (CI): 0.1, 7.0], corresponding to 0.16 standard deviation (SD) at 5 y, and 8.1 (95% CI: 3.8, 13), corresponding to 0.24 SD at 10 y. In addition, urine and hair selenium concentrations at 5 and 10 y of age were positively associated with cognitive function at 10 y, although associations were inverse for concentrations . Some associations were slightly stronger for girls than for boys.
Conclusions:
Measures of prenatal and childhood (below the 98th percentile) selenium status were associated with higher cognitive function scores at 5 and 10 y of age. https://doi.org/10.1289/EHP1691
Introduction
Selenium deficiency has been associated with cognitive decline in the elderly (Akbaraly et al. 2007; Gao et al. 2007; Rita Cardoso et al. 2014). Animal studies suggest that selenium supplementation may prevent dopamine loss, degeneration of neurons, and lipid peroxidation in the brain induced by exposure to various pro-oxidative toxicants (al-Deeb et al. 1995; Imam et al. 1999; Zafar et al. 2003). These findings suggest that selenium may help maintain cognitive function. Selenium functions as an essential component of antioxidant systems such as glutathione peroxidases and thioredoxin reductases, and it is also incorporated into iodothyronine deiodinases, which are involved in thyroid hormone metabolism (Roman et al. 2014). All of these systems are important for brain function as well as for neurodevelopment. However, little is known about the potential impact of poor selenium status on neurodevelopment and if there are critical early-life windows for effects of selenium on brain function later in life.
In animal studies, selenium deficiency during gestation affected markers of neurological development in rat offspring (Mitchell et al. 1998), and maternal selenium supplementation was negatively associated with anxiety-like behavior in pups and positively associated with cognitive function in adulthood (Laureano-Melo et al. 2015). In a Chinese study (), cord blood selenium concentrations were positively associated with children’s neonatal behavioral neurological assessments scores (reflexes, passive tone, active tone, behavior, and general assessment) at 3 d of age (Yang et al. 2013). In addition, we recently showed that maternal erythrocyte selenium (Ery-Se) in late pregnancy was positively associated with children’s cognitive function at 1.5 y of age in rural Bangladesh (; Skröder et al. 2015). Subsequently, similar associations were indicated between maternal plasma selenium in pregnancy and Polish children’s psychomotor functions within the first years of life (; Polanska et al. 2016). A study evaluating potential effects of polychlorinated biphenyls, lead, and mercury on neuromotor function in Inuit preschool children (; Després et al. 2005) reported no observed beneficial effect of blood selenium on the outcomes. However, these children all had very high concentrations of blood selenium [whole blood average concentration of , corresponding to intakes above the upper limit of for 4–8-y-old children (IOM 2000)]. Thus, there are clear gaps in knowledge about both beneficial intake levels of selenium at different ages and potential effects of early-life selenium on outcomes in older children. In the present study, we followed up Bangladeshi children who were previously assessed at 1.5 y of age to determine whether prenatal and concurrent selenium exposures were associated with cognitive function at 5 and 10 y of age.
Methods
Study Area and Subjects
The study area and participants have been described in detail in our previous publications (Hamadani et al. 2011; Kippler et al. 2012; Skröder et al. 2015; Gustin et al. 2017; Rahman et al. 2016). Briefly, the study area is Matlab, a rural area situated southeast of Dhaka, in Bangladesh. In this area, the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), has been collecting information via a health and demographic surveillance system (HDSS) over the past 60 y. Community health workers visit all households in Matlab on a monthly basis and collect information about vital events such as births, deaths, and in- and out-migration; this information is used to continuously update the HDSS. The present cohort was nested in a population-based randomized maternal and infant nutrition intervention study (MINIMat; trial number ISRCTN16581394, http://www.isrctn.com/ISRCTN16581394) that aimed to assess the effects of food and micronutrient supplementation during pregnancy (Persson et al. 2012). Women were recruited in early pregnancy [at approximately gestational week (GW) 8] between November 2001 and October 2003 and were randomly assigned to begin one of three daily micronutrient supplements at the 14-wk visit: iron and folic acid; iron and folic acid [the standard supplement for pregnant women recommended by the World Health Organization (WHO 2002)]; or iron and folic acid plus 13 additional micronutrients (including of selenium in the form of sodium selenite). Micronutrient supplementation was combined with food supplementation ( of energy and protein per day, ) that women were randomly invited to begin at enrollment (early supplementation, GW9) or at the time of their choosing (usual care, typically initiated at approximately GW20), for a total of six intervention groups (Persson et al. 2012). We have previously reported that maternal selenium status in late pregnancy (GW30) was similar for women in all six supplement groups (; Skröder et al. 2015). Eligibility criteria for enrollment in MINIMat were viable fetus and gestational age at the first visit to the icddr,b health care facility (approximately GW8–10; both assessed by ultrasound examination), no severe illness, and written consent for participation (Persson et al. 2012).
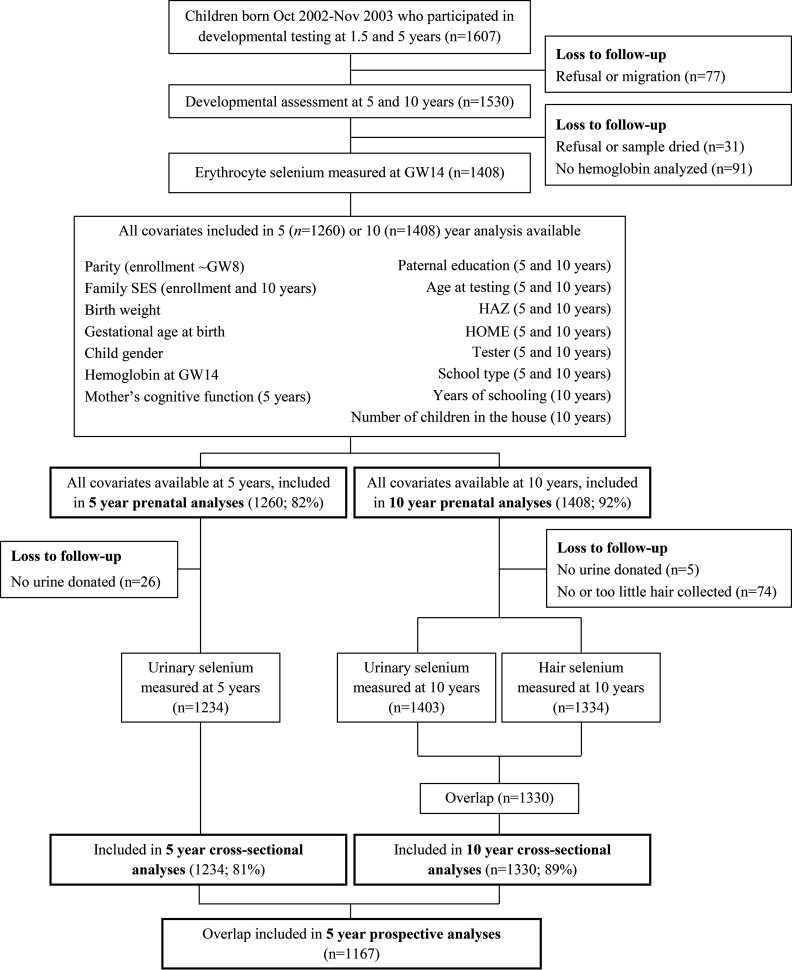
For the present study, we invited children who were born between October 2002 and November 2003, who participated in the developmental follow-up at 1.5 and 5 y of age, and who were alive and registered as residents in the study area at 10 y of age (). Of these, 1,530 (95%) agreed to participate (Figure 1). The main reasons for not participating were out-migration and parents’ refusal. In total, 1,408 children had data for maternal selenium in erythrocytes at GW14 and hemoglobin (Hb; required for adjustment of selenium concentrations in erythrocytes) and complete outcome and covariate data (described below) at 5 or 10 y. Longitudinal analyses of associations with prenatal selenium included 1,260 children with outcomes measured at 5 y and 1,408 children with outcomes measured at 10 y. Out of these children, cross-sectional analyses included those who had selenium concentrations measured in urine at 5 y () or in urine and hair at 10 y (; Figure 1). Reasons for missing selenium measurement at any time point included refusal (to provide urine, blood, or hair), no hemoglobin measurement, or an inadequate blood or hair sample for analysis. Longitudinal analyses of associations between selenium concentrations at 5 y and outcomes at 10 y included 1,167 children with all of the abovementioned measurements. In our previous study, we used selenium concentrations in blood collected at GW30 (Skröder et al. 2015), but because more women had donated a blood sample at GW14, we chose to analyze the GW14 samples for the present study, which resulted in a much larger sample size.
Figure 1.
Flow chart for recruitment into the present study. GW, gestational week; HAZ, height-for-age z-score; HOME, modified version of Home Observation for Measurement of the Environment; SES, socioeconomic status.
The project was approved by the research and ethical review committees at icddr,b as well as by the Regional Ethical Review Board, Stockholm, Sweden, and was conducted in accordance with the Helsinki Declaration. The mothers gave their written consent at recruitment to MINIMat and again before the developmental testing of the children at 5 and 10 y of age (Hamadani et al. 2011; Persson et al. 2012).
Element Analyses
Erythrocyte selenium (Ery-Se) has been suggested to be a suitable biomarker of long-term selenium status (Ashton et al. 2009) and was used as such in both our previous study (follow-up at 1.5 y; Skröder et al. 2015) and the present study. Mothers’ blood samples were collected in Li-heparin tubes at GW14. Blood collection was performed at health care facilities in 2001–2003, whereupon the samples were transferred to the hospital laboratory for separation of plasma and erythrocytes. Selenium in erythrocytes (stored at to ) was measured during 2014–2015 using inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7700x, Agilent Technologies) with an octopole reaction system operated in hydrogen mode (Lu et al. 2015). Before the analyses, approximately of erythrocytes were diluted 1:25 in an alkali solution [2% (wt:vol) 1-butanol, 0.05% (wt:vol) ethylenediaminetetraacetic acid (EDTA), 0.05% (wt:vol) Triton X-100, 1% (wt:vol) ammonium hydroxide ( and internal standard; Sigma-Aldrich]. The samples were vortex mixed, sonicated for 5 min, and centrifuged at for 2 min [MSE centrifuge, Super Minor, MSE UK]. The limit of detection (LOD) was , and no samples were below this limit. Quality control was performed by including commercial reference materials for blood (Seronorm™ Trace Elements Whole Blood L-1, lot 1103128, and L-2, lot 1103129), and the obtained results were in good accord with the recommended values (see Table S1). Some of the blood samples included in the present study () were analyzed in 2007 (after being stored frozen at or since collection in 2001–2003) via ICP-MS following acid digestion; details of this method and of the related quality control have been described in detail elsewhere (Kippler et al. 2009). The results from the two analytical methods were highly correlated, although the selenium concentrations from the alkali method were consistently 10% lower than those from the acidic method (Lu et al. 2015). Therefore, to improve the comparability of results across both methods, selenium concentrations derived using the acidic method were multiplied by 0.90. To adjust for differences in hematocrit, Ery-Se was expressed in micrograms per gram Hb (Skröder et al. 2015; Stefanowicz et al. 2013). In addition to selenium, we also measured concentrations of arsenic, cadmium, lead, zinc, and manganese in erythrocytes as described in detail elsewhere (Kippler et al. 2009; Lu et al. 2015).
We recently reported that selenium concentrations in hair correlated positively with concentrations in erythrocytes (Spearman’s rank correlation coefficient of 0.54 for samples representing the time of blood collection) in children from the same study population (Skröder et al. 2017). Therefore, we also used hair selenium concentrations to assess selenium status at the 10-y follow-up.
Hair samples were cut from the occipital part of the children’s heads using 18/8 stainless steel scissors and were transported in high-quality paper envelopes to Karolinska Institutet (Stockholm, Sweden) for analyses (Skröder et al. 2017). A sample of of hair (closest to the scalp) was washed in 2% Triton X-100 solution for 1 h, rinsed 10 times with deionized water, and dried for 24 h at room temperature (). Samples (approximately ) were weighed close to an alpha source (placed inside the analytical balance) to decrease static electricity by neutralizing the material. Then, samples were digested in a microwave digestion system (UltraCLAVE, Milestone Inc.) with of concentrated nitric acid (Scharlau Trace Analysis Grade; Scharlab) and deionized water for 30 min at and a pressure of . After cooling to a temperature below , the digested solutions were diluted with deionized water to an acid concentration of 20% and were analyzed using ICP-MS (Agilent 7700x, Agilent Technologies). Selenium concentrations measured in a reference hair sample (NCSZC81002b, China National Analysis Center for Iron and Steel) were consistent with the recommended value [means of and , respectively (Skröder et al. 2017)]. We also repeatedly measured an in-house hair sample (from multiple residents of the Faroe Islands, homogenized in liquid nitrogen in a cryogenic homogenizing system) to assess measurement consistency over the year it took to analyze all of the study hair samples (). The LOD was , and no samples had a concentration below this value. In addition, we analyzed the concentration of mercury in hair as described elsewhere (Gustin et al. 2017).
Urinary selenium (U-Se) is a measure of recent intake (within 3 d; Hawkes et al. 2008), in contrast to Ery-Se, which is a marker of average intake over approximately 2–3 months owing to the long life span of erythrocytes (Nève 1995), and hair selenium, which represents average intake over approximately one month per centimeter counting from the scalp (Lemire et al. 2009; Skröder et al. 2017). However, because urine was the only available biological medium at 5 y, we measured selenium in urine samples collected at both 5 and 10 y of age. Children’s urine was collected at 5 and 10 y of age in combination with home interviews (Hamadani et al. 2011). All samples were frozen and were transported to Karolinska Institutet for analysis. The samples were diluted 1:10 in 1% nitric acid, and the selenium concentrations were measured using ICP-MS as described above for erythrocytes and as previously described for urine in younger children from the same study population (Skröder Löveborn et al. 2016). All samples were above the LOD ( at 5 y and at 10 y). Quality control was performed by including commercial reference materials for urine [Seronorm™ Trace Elements Urine Blank OK4636, Seronorm™ Trace Elements Urine NO2525, Seronorm™ Trace Elements Urine 1011644, Seronorm™ Trace Elements Urine 1011645 and National Institute of Standards and Technology (NIST) Standard Reference Material® 2670a], and the results for these samples were in line with recommended values (see Table S1). We also measured concentrations of arsenic, cadmium, and lead in urine at both 5 and 10 y, and we measured concentrations of manganese in drinking water collected at the 10-y follow-up (Kippler et al. 2012, 2016). All concentrations in urine were adjusted to the average specific gravity (1.012) to compensate for variations in dilution (Nermell et al. 2008).
Outcome Assessment
Children’s cognitive function at 5 y was assessed using the third edition of the Wechsler Preschool and Primary Scale of Intelligence™ (WPPSI-III) at the nearest health care facility (Wechsler 2002). WPPSI-III was slightly modified for use in Bangladeshi children (Hamadani et al. 2011). We used seven subtests of WPPSI-III: information (0–34 points), vocabulary (0–43 points), and comprehension (0–38 points) were summed to form the verbal score. Block design (0–40 points), matrix reasoning (0–29 points), and picture completion (0–32 points) formed the performance score. The verbal and performance scores were then summed together with the processing speed (Coding; 0–65 points), resulting in the full developmental score. Seven testers were trained for conducting the WPPSI-III and were rotated across the four health care facilities to minimize tester-related bias. A supervisor rated 10% of all tests and found adequate interobserver reliability (interobserver reliability ; Kippler et al. 2012).
At 10 y, the Wechsler Intelligence Scale for Children®, fourth edition (WISC-IV), was used as described previously (Rahman et al. 2016). This test was translated to the Bengali language and was culturally adapted to fit the present population with slight changes in the questions. Four female psychologists were trained for six weeks on the included tests before the children’s assessment. Interrater reliabilities were measured between each tester and trainer, and training continued until agreement was achieved (Rahman et al. 2016). The test generates four scales: verbal comprehension (based on vocabulary, information, and comprehension), perceptual reasoning (based on block design, picture concept, and matrix reasoning), working memory (based on digit span and arithmetic), and processing speed (based on coding and symbol search). Higher score for processing speed indicates faster response time. In addition, the full developmental score, which represents the child’s general intellectual ability, was calculated as the sum of the raw scores of the subscales. We used the raw scores from each test to avoid systematic differences between our study population and most populations used as the basis for standardization.
Covariates
Information about the mothers’ background characteristics was obtained at enrollment in MINIMat [maternal age, body mass index (BMI), education, parity; obtained at approximately GW8] or from the HDSS [socioeconomic factors such as assets and housing used to derive the socioeconomic status (SES)]. Family SES was assessed via a wealth index based on information on items such as family ownership of assets, housing structure, and dwelling characteristics (Gwatkin et al. 2000). This information was updated at the follow-up at 10 y. Maternal and paternal education was expressed as the number of years of formal schooling and was updated at the follow-ups at both 5 and 10 y. In addition, Raven’s Coloured Progressive Matrices™ test was performed when mothers brought their children to the health care facilities at the 5-y follow-up and was used to assess maternal nonverbal IQ in terms of abstract logical reasoning (Hamadani et al. 2011;Raven et al. 2003; ). To assess the quality and quantity of children’s stimulation at home, we used a modified version of Home Observation for Measurement of the Environment (HOME) at 5 and 10 y (Bradley et al. 2003; Caldwell 1967). The HOME consists of questions regarding responsibility, encouragement of maturity, emotional climate, learning materials and opportunities, enrichment, family companionship, family integration, and physical environment. The questions were translated into the Bengali language, and seven questions were dropped because the mothers could not understand them owing to cultural irrelevance. Children’s birth anthropometry was measured by the attending nurse, or by trained paramedics for those having home delivery, using standard methods (Persson et al. 2012). At each developmental testing taking place at the health care facilities, the children’s height was measured using a stadiometer (seca 214, Leicester Height Measure; seca), and weight was measured using a digital scale (Tanita HD–318; Tanita). These measures were converted to age- and gender-standardized z-scores (height-for-age, HAZ; weight-for-age, WAZ; BMI-for-age, BAZ) using the WHO growth references (de Onis et al. 2007; WHO 2010). At the follow-up at 5 y, information on the type of school [none, primary, Madrasa (Islamic), kindergarten, Maktab, or nonformal] was collected, and this information was updated at the follow-up at 10 y [none, public primary, Madrasa, nongovernmental organization (NGO; nonprofit private), or English medium (private)]. Finally, at the 10-y follow-up, we also collected information about the number of years of children’s formal education and the number of children in the family; we also measured children’s Hb in peripheral blood (finger prick) using a HemoCue photometer (Hemocue AB).
Statistical Analysis
Statistical analyses were performed using Stata (version 12, StataCorp). A p-value was considered statistically significant. Because U-Se values at 5 and 10 y of age were somewhat right skewed, Spearman correlation was used to assess bivariate associations between continuous variables (Ery-Se, U-Se, hair selenium, maternal IQ, age, BMI, parity, maternal and paternal education, SES, Hb, gestational age and weight at birth, HOME, HAZ, WAZ, and BAZ), whereas associations between categorical variables [gender, tester, type of school, paternal job status, and selenium concentrations above or below cut-offs (see below)] were assessed using the Mann-Whitney U-test or the Kruskal-Wallis, chi-squared, or Fisher’s exact test. The seven testers at the 5-y follow-up were grouped into three categories based on scoring owing to a low number of children scored by some of the testers.
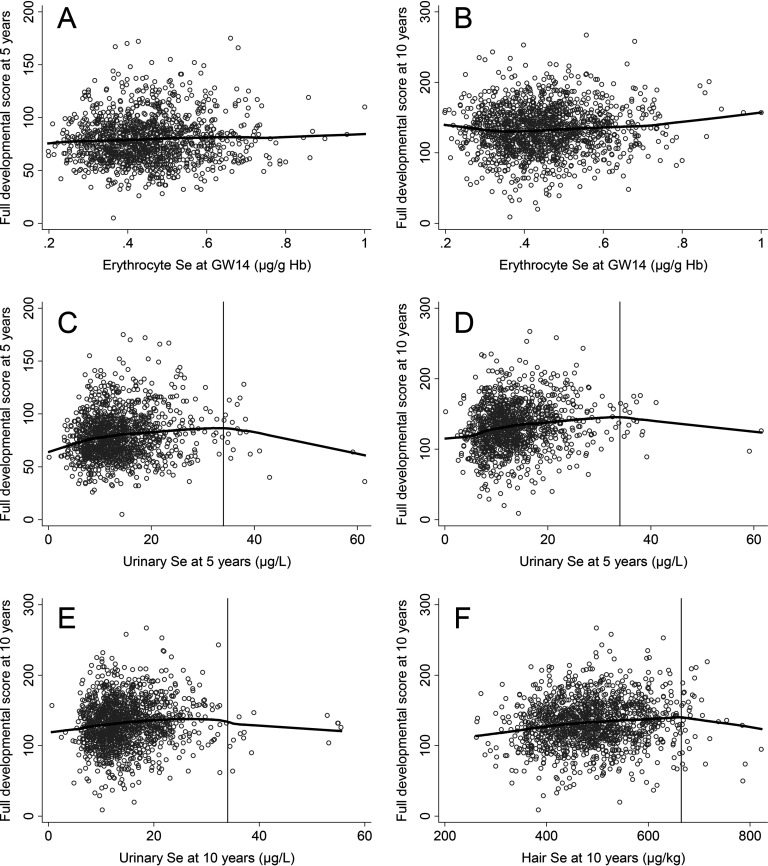
We visually examined all evaluated associations by plotting the outcomes against selenium concentrations measured in the different types of samples. Associations that appeared to be more or less linear were modeled using multivariable-adjusted linear regression: specifically, associations between prenatal selenium concentrations (Ery-Se) and verbal and performance scores at 5 y (see Figure S1); between prenatal selenium concentrations (Ery-Se) and verbal comprehension, working memory, perceptual reasoning, and processing speed at 10 y (see Figure S1); and between prenatal selenium concentrations (Ery-Se) and full developmental score at 5 and 10 y (Figure 2; see also Figure S1). Associations that appeared to be nonlinear were modeled using multivariable-adjusted linear spline models: specifically, associations between 5-y U-Se and all outcomes at 5 and 10 y (Figure 2; see also Figure S2); between 10-y U-Se and all outcomes at 10 y (Figure 2; see also Figure S3); and between 10-y hair selenium and all outcomes at 10 y (Figure 2; see also Figure S2). Spline models included single knots at for both 5- and 10-y U-Se and at for 10-y hair selenium, which were generally consistent with the inflection points for the exposure–outcome curves for all outcomes (see Figures S2, S3, and S4). The choice of linear or linear spline models was also based on the of the models, and differences between models were assessed with the F-test for models including the full developmental score. The linear spline regression models had significantly higher values for the associations between U-Se at 5 y and full developmental score at 5 y () and between hair selenium at 10 y and full developmental score at 10 y (). For U-Se at or 10 y and full developmental score at 10 y, there were no statistical differences between the linear spline regression models and the linear models ( and 0.33, respectively); thus, we chose to show the results from the linear spline regression analyses for ease of presentation.
Figure 2.
Scatter plots with smoothed lowess lines for prenatal and childhood selenium (Se) and full developmental score (raw score) at 5 and 10 y. (A) Erythrocyte Se at gestational week (GW) 14 [ hemoglobin (Hb)] and full developmental score at 5 y, (B) erythrocyte Se at GW14 ( Hb) and full developmental score at 10 y, (C) urinary Se at 5 y () and full developmental score at 5 y, (D) urinary Se at 5 y ( ) and full developmental score at 10 y, (E) urinary Se at 10 y () and full developmental score at 10 y, (F) hair Se at 10 y () and full developmental score at 10 y. The vertical lines in the lower panels represent the turning point used for the knot in the linear spline regression analyses ( for urinary Se at 5 and 10 y and for hair Se at 10 y).
We used complete subject analyses and built the regression models using backward elimination. In the initial step, we included covariates that are known to influence any of the outcomes (gender and age; a priori) and variables that correlated (Spearman, Mann-Whitney, Kruskal-Wallis, chi-squared, or Fisher’s exact test ) with selenium concentrations at any time point (GW14, 5 y, or 10 y) and any of the outcomes at 5 or 10 y (eight outcomes in total). The initial covariates selected were gender and child age at testing, maternal age, BMI, parity, maternal and paternal education, paternal job status, and SES (all collected at enrollment); Hb at GW14; gestational age and weight at birth; birth order at 5 y, tester, HOME, type of school attended, HAZ, WAZ, and BAZ (all collected at 5 and 10 y); SES, years of schooling, Hb, and number of children in the family (all collected at 10 y). We then used backward elimination of covariates that did not improve the models and eliminated variables that were not significant (), starting with the variable with the highest p-value. The eliminations continued as long as they increased the model for at least one of the outcomes assessed with that model. Two separate multivariable-adjusted models were built for predicting full developmental scores at 5 and 10 y, using Ery-Se at GW14 as the exposure variable. In the model for full developmental score at 5 y, the initial set of potential covariates included all the variables listed above that were collected at enrollment, birth, and at 5 y. For the model at 10 y, variables collected at enrollment, birth, and at 10 y were included. When two potential covariates were highly correlated (), we selected the covariate that resulted in the greatest improvement of the model to reduce the potential for collinearity. We did not adjust for maternal education because it was highly correlated with SES () and paternal education (), and adjusting for paternal versus maternal education resulted in a higher value.
Thus, primary models (Model 1) for outcomes assessed at 5 y, including either longitudinal associations with prenatal selenium (Ery-Se at GW14) or cross-sectional associations with U-Se at 5 y, were adjusted for gender and age at testing, parity at enrollment (number of children), family SES at enrollment (scale from to 5), maternal Hb at GW14 (grams/deciliter), birth weight (grams); and, based on information obtained at the 5-y examination, tester (5 categories), HOME score (continuous), HAZ (continuous), school type (none/primary/madrasa/kindergarten/maktab/nonformal), mothers’ cognitive function (Raven’s Coloured and Progressive Matrices™ test, continuous), and paternal education (years of formal schooling).
The longitudinal associations of prenatal selenium and outcomes assessed at 5 y were additionally adjusted for erythrocyte manganese and zinc at GW14 (micrograms/kilogram; Model 2). Because these elements were not measured at 5 y, there was no Model 2 for the cross-sectional analyses at 5 y. In an additional step (Model 3), associations with outcomes at 5 y of age were additionally adjusted for arsenic, cadmium, and lead concentrations in erythrocytes at GW14 (micrograms/kilogram, for the longitudinal analyses) or in urine at 5 y (micrograms per liter, for the cross-sectional analyses) because the antioxidative effects of selenium might be affected by these pro-oxidant neurotoxicants (Bergkvist et al. 2010; Hamadani et al. 2011; Kippler et al. 2012). We did not adjust any model for the nutritional intervention groups (one arm of which included a multiple micronutrient capsule that contained selenium) because we used the selenium concentrations measured at baseline (GW14), that is to say, before the supplementation started.
The primary models for outcomes measured at 10 y (Model 1) and associations with exposures at all three time points (i.e., longitudinal associations with prenatal Ery-Se or with U-Se at 5 y of age and cross-sectional associations with U-Se or hair selenium at 10 y of age) included gender and age at testing; gestational age at birth (in weeks); mothers’ cognitive function at 5 y; and, based on information obtained at the 10-y examination, number of children in the family, tester (3 categories), HOME score, HAZ, school type (none/madrasa/NGO/primary/English medium), SES at 10 y (scale from to 10), years of schooling, and paternal education. Model 2 was additionally adjusted for either erythrocyte manganese and zinc at GW14 (for longitudinal associations with prenatal selenium) or water manganese measured at 10 y (micrograms per liter; for cross-sectional associations with U-Se or hair selenium at 10 y). Model 3 included all Model 1 covariates plus either erythrocyte arsenic, cadmium, and lead concentrations at GW14 (micrograms per kilogram; for longitudinal associations), or urinary arsenic, cadmium, and lead concentrations at 5 y (micrograms per liter; for longitudinal associations with U-Se at 5 y), or hair mercury (micrograms per kilogram) and urinary arsenic, cadmium, and lead concentrations at 10 y (micrograms per liter; for cross-sectional associations). We checked that the residuals of all regression models were normally distributed using quantile-quantile plots and residual versus fitted plots.
In our previous study of prenatal selenium status (measured at GW30, in contrast with GW14 in the present analysis) and measures of cognitive function at 1.5 y of age in children from the same study population (Bayley Scales of Infant Development® and measures of comprehensive and expressive language development based on principles of the MacArthur Communicative Development Inventory; ), we noted some evidence of variation in associations between girls and boys (Skröder et al. 2015). Therefore, we also performed a qualitative comparison of associations stratified by gender in the present analysis. In addition, we assessed whether there were any statistical differences between the estimates for girls and boys using the Wald test.
Results
General Characteristics and Selenium Status
The main characteristics of the mothers and children included in the study, by girls and boys () or by hair selenium above and below (), are shown in Table 1 (see also Table S2 for U-Se above and below () at 5 () and 10 y (). The mean ages at developmental testing were 5.4 (5th–95th percentile: 5.3–6.4) and 9.5 (9.4–9.7) y. In general, the children were lean and short, with approximately 33% being stunted [ of HAZ, WHO cut-off (WHO 2010)] at 5 y and 28% at 10 y, and approximately 40% were underweight ( of WAZ, WHO cut-off) at both 5 and 10 y of age. Girls appeared to be more underweight at both 5 and 10 y (45% vs. 40% underweight girls vs. boys, respectively, at both 5 and 10 y). In addition, girls scored lower than boys in the cognitive subtests at 10 y, but there was no difference in the scores at 5 y. At 5 y, 288 of the 1,408 children (20%; 127 girls and 161 boys) did not attend any school; at 10 y, only 8 children (0.6%; 5 girls and 3 boys) did not go to school.
Table 1.
Main characteristics of mothers and children included in the study by girls and boys () and hair selenium below and above ().
| Characteristic | All() | Girls() | Boys () | p-Valuea | Hair Se () | Hair Se () | p-Valuea | |
|---|---|---|---|---|---|---|---|---|
| Mothers | ||||||||
| Parity at GW8b | 1,408 | 0.30 | 0.17 | |||||
| SES at GW8 | 1,408 | 0.67 | 0.11 | |||||
| Ery-Se at GW14 ( Hb) | 1,408 | 0.41 | 0.012 | |||||
| Ery-Zn at GW14 () | 1,408 | 0.70 | 0.62 | |||||
| Ery-Mn at GW14 () | 1,408 | 0.66 | 0.62 | |||||
| Ery-As at GW14 () | 1,408 | 0.55 | 0.43 | |||||
| Ery-Cd at GW14 () | 1,408 | 0.12 | 0.68 | |||||
| Ery-Pb at GW14 () | 1,408 | 0.59 | 0.77 | |||||
| Raven’s score at 5 y | 1,408 | 0.28 | 0.24 | |||||
| Education at 5 y (y) | 1,408 | 0.73 | 0.049 | |||||
| Education at 10 y (y) | 1,408 | 0.63 | 0.10 | |||||
| Paternal educationat 5 y | 1,399 | 0.20 | 0.045 | |||||
| Paternal education at 10 y | 1,408 | 0.42 | 0.075 | |||||
| Children at birth | ||||||||
| Birth weight (g) | 1,327 | 0.88 | ||||||
| Gestational age at birth (wk) | 1,408 | 0.012 | 0.027 | |||||
| Children at 5 years | ||||||||
| Age (y) | 1,408 | 0.42 | 0.41 | |||||
| HAZ (z-score) | 1,408 | 0.77 | 0.17 | |||||
| HOME | 1,344 | 0.37 | 0.0024 | |||||
| Tester (%) | 1,408 | 0.45 | 0.81 | |||||
| Tester 1 | 8 | 9 | 7 | 8 | 6 | |||
| Tester 2 | 78 | 77 | 79 | 78 | 76 | |||
| Tester 3 | 14 | 14 | 14 | 14 | 18 | |||
| School type (%) | 1,408 | 0.059 | 0.16 | |||||
| None | 20 | 18 | 22 | 20 | 29 | |||
| Primary | 23 | 23 | 23 | 23 | 26 | |||
| Madrasa | 5 | 4 | 4 | 5 | 6 | |||
| Kindergarten | 6 | 6 | 6 | 6 | 12 | |||
| Maktab | 29 | 27 | 31 | 29 | 21 | |||
| Nonformal | 17 | 20 | 14 | 17 | 6 | |||
| U-Se () | 1,357 | 0.033 | ||||||
| U-As () | 1,357 | 0.66 | 0.33 | |||||
| U-Cd () | 1,357 | 0.058 | 0.46 | |||||
| U-Pb () | 1,357 | 0.31 | 0.92 | |||||
| Full developmental score | 1,408 | 0.29 | 0.46 | |||||
| Verbal score | 1,408 | 0.57 | 0.54 | |||||
| Performance score | 1,408 | 0.69 | 0.39 | |||||
| Children at 10 y | ||||||||
| Age (y) | 1,408 | 0.96 | 0.86 | |||||
| HAZ (z-score) | 1,408 | 0.33 | 0.24 | |||||
| HOME | 1,408 | 0.0063 | 0.15 | |||||
| Tester (%) | 1,408 | 0.14 | 0.59 | |||||
| Tester 1 | 26 | 25 | 28 | 27 | 18 | |||
| Tester 2 | 25 | 27 | 23 | 25 | 32 | |||
| Tester 3 | 24 | 22 | 25 | 23 | 26 | |||
| Tester 4 | 25 | 26 | 24 | 25 | 24 | |||
| School type (%) | 1,408 | 0.93 | ||||||
| None | 10 | 5 | 15 | 11 | 9 | |||
| Madrasa | 3 | 3 | 3 | 3 | 0 | |||
| NGO | 77 | 84 | 70 | 77 | 79 | |||
| Primary | 9 | 7 | 12 | 9 | 12 | |||
| English medium | 1 | 1 | 0 | 0 | 0 | |||
| Years of schooling | 1,408 | 0.14 | ||||||
| SES | 1,408 | 0.98 | 0.055 | |||||
| U-Se () | 1,403 | 0.0088 | ||||||
| U-As () | 1,403 | 0.96 | 0.47 | |||||
| U-Cd () | 1,403 | 0.078 | 0.13 | |||||
| U-Pb () | 1,403 | 0.39 | 0.54 | |||||
| Hair Se () | 1,334 | |||||||
| Hair Hg () | 1,334 | 0.083 | 0.037 | |||||
| Water Mn () | 1,408 | 0.90 | 0.84 | |||||
| Full developmental score | 1,408 | 0.16 | 0.32 | |||||
| Verbal comprehension | 1,408 | 0.043 | 0.29 | |||||
| Perceptual reasoning | 1,408 | 0.53 | ||||||
| Working memory | 1,408 | 0.38 | ||||||
| Processing speedc | 1,408 | 0.63 |
Note: Ery-As, erythrocyte arsenic; Ery-Cd, erythrocyte cadmium; Ery-Mn, erythrocyte manganese; Ery-Pb, erythrocyte lead; Ery-Se, erythrocyte selenium; Ery-Zn, erythrocyte zinc; GW, gestational week; Hair Hg, hair mercury; Hair Se, hair selenium; HAZ, height-for-age z-score; HOME, quality and quantity of children’s stimulation at home assessed using a modified version of Home Observation for Measurement of the Environment; NGO, nongovernmental organization; SES, socioeconomic status assessed via a wealth index based on information on family ownership of e.g. assets, housing structure, and dwelling characteristics (Gwatkin DR et al. 2000); U-As, urinary arsenic; U-Cd, urinary cadmium; U-Pb, urinary lead; U-Se, urinary selenium; Water Mn, water manganese.
Mann-Whitney U-test, chi-squared, or Fisher’s exact test.
GW8 corresponds to enrollment into Maternal and Infant Nutrition Interventions in Matlab (MINIMat).
Higher processing speed indicates faster response time.
The girls had approximately 10% lower mean U-Se concentrations at both 5 and 10 y, and they had slightly lower mean hair selenium at 10 y (Table 1). In bivariate analyses, selenium concentrations at all time points were correlated with each other (Table 2). Ery-Se at GW14 correlated with several of the outcomes at both 5 and 10 y, and U-Se at 5 y correlated with all outcomes measured at both 5 and 10 y. Similarly, both U-Se and hair selenium at 10 y correlated with all outcomes at 10 y (Table 2).
Table 2.
Spearman correlations [ (p-value)] for selenium measurements at gestational week 14, 5 y, and 10 y ().
| Ery-Se GW14 | U-Se 5 y | U-Se 10 y | Hair Se 10 y | |
|---|---|---|---|---|
| Hair Se 10 y | 0.13 () | 0.19 () | 0.23 () | 1.0 |
| U-Se 10 y | 0.069 (0.012) | 0.26 ( ) | ||
| U-Se 5 y | 0.14 () | |||
| Full developmental score 5 y | 0.049 (0.080) | 0.13 () | NA | NA |
| Verbal score 5 y | 0.033 (0.24) | 0.11 () | NA | NA |
| Performance score 5 y | 0.056 (0.048) | 0.10 () | NA | NA |
| HAZ 5 y | 0.019 (0.50) | 0.15 () | NA | NA |
| WAZ 5 y | (0.19) | 0.10 () | NA | NA |
| Full developmental score 10 y | 0.058 (0.031) | 0.17 () | 0.11 () | 0.12 () |
| Verbal comprehension 10 y | 0.025 (0.35) | 0.14 () | 0.12 () | 0.13 () |
| Perceptual reasoning 10 y | 0.050 (0.063) | 0.18 () | 0.10 () | 0.10 () |
| Working memory 10 y | 0.052 (0.090) | 0.12 () | 0.078 (0.0047) | 0.11 () |
| Processing speed 10 ya | 0.066 (0.013) | 0.11 () | 0.057 (0.039) | 0.053 (0.053) |
| HAZ 10 y | (0.6) | 0.13 () | 0.11 () | 0.071 (0.0099) |
| WAZ 10 y | (0.93) | 0.13 () | 0.094 () | 0.087 (0.0015) |
Note: Ery-Se, erythrocyte selenium; GW, gestational week; Hair Se, hair selenium; HAZ, height-for-age z-score; NA, not applicable; U-Se, urinary selenium; WAZ, weight-for-age z-score.
Higher processing speed indicates faster response time.
Prenatal Selenium and Children’s Cognitive Function at 5 and 10 y
In the multivariable-adjusted linear regression analyses, Ery-Se at GW14 was positively associated with all outcomes at 5 and 10 y (Table 3), but associations were stronger for the outcomes measured at 10 y than for those measured at 5 y. None of the associations for the outcomes at 5 y was markedly affected by adjusting for essential elements (manganese and zinc in erythrocytes at GW14; Model 2). The estimates for full developmental score, perceptual reasoning, and processing speed at 10 y increased by 18–42% after adjusting for the essential elements; however, the estimates for working memory and verbal comprehension were very similar after the adjustment (Table 3). When adjusting Model 1 for toxic elements (erythrocyte arsenic, cadmium, and lead at GW14; Model 3), the estimates at both 5 and 10 y were not markedly affected.
Table 3.
Multivariable-adjusted regression analyses (linear for Ery-Se, linear spline with knot at for U-Se) for selenium status in early life and cognitive function in childhood.
| Outcomes | Ery-Se GW14 (per Hb) | U-Se at 5 y (, per ) | U-Se at 5 y (, per ) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | B (95% CI) | p-Value | ||||
| 5 ya | |||||||||
| Model 1 | 1,260 | 1,214 | 20 | ||||||
| Full developmental score | 0.94 (0.027, 1.9) | 0.044 | 1.1 (, 2.8) | 0.19 | (, 0.13) | 0.054 | |||
| Verbal score | 0.47 (, 0.95) | 0.054 | 0.51 (, 1.4) | 0.26 | (, 0.17) | 0.059 | |||
| Performance score | 0.27 (, 0.62) | 0.13 | 0.033 (, 0.69) | 0.92 | (, 0.15) | 0.062 | |||
| Model 2 | 1,260 | ||||||||
| Full developmental score | 0.97 (, 2.1) | 0.094 | NA | NA | |||||
| Verbal score | 0.41 (, 1.0) | 0.17 | NA | NA | |||||
| Performance score | 0.32 (, 0.76) | 0.14 | NA | NA | |||||
| Model 3 | 1,260 | 1,214 | 20 | ||||||
| Full developmental score | 0.99 (0.0051, 2.0) | 0.049 | 1.2 (, 3.0) | 0.17 | (, 0.72) | 0.071 | |||
| Verbal score | 0.46 (, 0.98) | 0.079 | 0.67 (, 1.6) | 0.15 | (, 0.86) | 0.11 | |||
| Performance score | 0.25 (, 0.63) | 0.19 | 0.056 (, 0.73) | 0.87 | (, 0.29) | 0.073 | |||
| 10 yb | |||||||||
| Model 1 | 1,408 | 1,149 | 18 | ||||||
| Full developmental score | 2.2 (1.0, 3.3) | 2.5 (, 5.0) | 0.053 | 1.1 (, 14) | 0.87 | ||||
| Verbal comprehension | 0.50 (0.12, 0.89) | 0.010 | 0.49 (, 1.3) | 0.25 | (, 2.8) | 0.50 | |||
| Perceptual reasoning | 0.53 (0.079, 0.98) | 0.021 | 1.2 (0.18, 2.2) | 0.020 | 3.1 (, 8.1) | 0.23 | |||
| Working memory | 0.38 (0.14, 0.62) | 0.002 | 0.34 (, 0.86) | 0.20 | (, 2.5) | 0.92 | |||
| Processing speedc | 0.75 (0.29, 1.2) | 0.001 | 0.52 (, 1.5) | 0.32 | (, 4.8) | 0.89 | |||
| Model 2 | 1,408 | ||||||||
| Full developmental score | 2.6 (1.2, 3.9) | NA | NA | ||||||
| Verbal comprehension | 0.50 (0.051, 0.95) | 0.029 | NA | NA | |||||
| Perceptual reasoning | 0.75 (0.22, 1.3) | 0.006 | NA | NA | |||||
| Working memory | 0.39 (0.11, 0.67) | 0.006 | NA | NA | |||||
| Processing speedc | 0.94 (0.40, 1.5) | 0.001 | NA | NA | |||||
| Model 3 | 1,408 | 1,149 | 18 | ||||||
| Full developmental score | 2.2 (1.0, 3.4) | 2.5 (, 5.1) | 0.061 | 1.0 (, 14) | 0.88 | ||||
| Verbal comprehension | 0.51 (0.10, 0.92) | 0.014 | 0.52 (, 1.4) | 0.24 | (, 3.0) | 0.53 | |||
| Perceptual reasoning | 0.56 (0.081, 1.0) | 0.022 | 1.2 (0.18, 2.2) | 0.021 | 3.2 (, 8.3) | 0.23 | |||
| Working memory | 0.37 (0.12, 0.62) | 0.004 | 0.25 (, 0.78) | 0.35 | (, 2.4) | 0.84 | |||
| Processing speedc | 0.78 (0.30, 1.3) | 0.002 | 0.53 (, 1.6) | 0.32 | (, 4.8) | 0.87 | |||
Note: CI, confidence interval; Ery-Se, erythrocyte selenium; GW, gestational week; Hb, hemoglobin; NA, not applicable; U-Se, urinary selenium.
Analyses of outcomes at 5 y are adjusted as follows: Model 1 is adjusted for children’s gender, parity and family socioeconomic status (SES) at enrollment, birth weight, Hb at GW14, age at testing, height-for-age z-score (HAZ), modified Home Observation for Measurement of the Environment score (HOME), testers, school type, mothers’ cognitive function, and paternal education (all assessed at 5-y follow-up). Model 2 is Model 1 further adjusted for erythrocyte zinc and manganese at GW14 (prenatal analyses). There is no Model 2 for the concurrent analyses because there were no exposure markers for zinc or manganese available at 5. Model 3 is Model 1 further adjusted for erythrocyte cadmium, lead, and arsenic at GW14 (prenatal analyses) or urinary arsenic, cadmium, and lead at 5 y (cross-sectional analyses).
Analyses of outcomes at 10 y are adjusted as follows: Model 1 is adjusted for children’s gender, gestational age at birth, age at testing, number of children in the family, HAZ, HOME, testers, school type and years of schooling, family SES, and paternal education (all assessed at 10 y), and mothers’ cognitive function assessed at 5 y. Model 2 is Model 1 further adjusted for erythrocyte zinc and manganese at GW14 (prenatal analyses). There is no Model 2 for the longitudinal analyses with U-Se at 5 years because there were no exposure markers for zinc or manganese available at 5 y. Model 3 is Model 1 further adjusted for erythrocyte cadmium, lead, and arsenic at GW14 (prenatal analyses) or urinary arsenic, cadmium, and lead at 5 y (longitudinal analyses with U-Se at 5 y) or urinary arsenic, cadmium, lead, and hair mercury at 10 y (cross-sectional analyses).
Higher processing speed indicates faster response time.
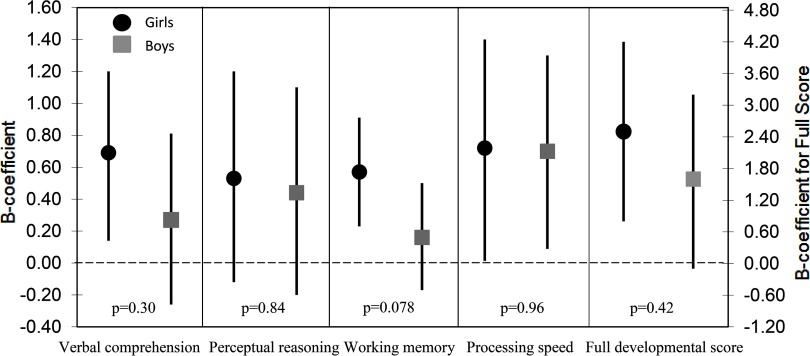
When stratifying Model 1 by gender, we found positive associations for both girls and boys. The estimates for the outcomes at 5 y were marginally stronger for the girls, although they were not significantly different from the estimates for the boys ( for all outcomes; see Figure S5). The estimates were also marginally stronger for the girls for all outcomes at 10 y; however, the 95% confidence intervals were largely overlapping, and there was an indicated statistical difference in the estimates for working memory only (; Figure 3).
Figure 3.
Estimates (B-coefficient) and 95% confidence interval (CI) (straight line) for associations between all outcomes at 10 y and erythrocyte selenium (Se) [per hemoglobin (Hb)] at gestational week (GW) 14, stratified by gender ( girls and 737 boys). p-Value for difference between estimates (Wald test). Adjustments: gestational age at birth, age at testing, mothers’ cognitive function assessed at 5 y, number of children in the family, height-for-age z-score (HAZ), modified version of Home Observation for Measurement of the Environment score (HOME), testers, school type and years of schooling, family socioeconomic status (SES), and paternal education (all assessed at 10 y).
Urinary Selenium at 5 y and Children’s Cognitive Function at 5 and 10 y
In the linear spline regression analyses using U-Se at 5 y as a marker of selenium status, we found positive associations for all outcomes at 5 y up to the spline knot at , although they were not statistically significant (Table 3). Additionally adjusting for toxic elements (arsenic, cadmium, and lead in urine at 5 y) did not improve the associations between U-Se and the outcomes (Model 3, Table 3). Above the spline knot, the associations with outcomes at 5 y were inverse. However, there were only 20 children with such high U-Se concentrations. When stratifying the analyses (Model 1) by gender, we found no statistical difference between the estimates below the spline knot for girls and boys for full developmental score (), performance score (), or verbal score (), or above the spline knot for any outcome ( for all outcomes).
Concerning the outcomes at 10 y of age, we found significant, positive associations between U-Se at 5 y (up to the spline knot at ) and perceptual reasoning (Table 3). This association did not change markedly when adjusting for toxic elements (urinary arsenic, cadmium, and lead at 5 y; Model 3, Table 3). After stratifying by gender, we found essentially no differences between girls and boys below the spline knot (p for difference between estimates: full developmental score, ; verbal comprehension, ; processing speed, ; perceptual reasoning, ; working memory, ); or above the spline knot (full developmental score, ; verbal comprehension, ; processing speed, ; perceptual reasoning, ; working memory, ).
Selenium Status and Children’s Cognitive Function at 10 y
In the multivariable-adjusted linear spline regression analyses with both selenium status and outcomes at 10 y of age, we found positive associations between hair selenium (below the spline knot at ) and full developmental score, verbal comprehension, and working memory (Table 4). Additionally adjusting for water manganese did not change the estimates markedly (Table 4, Model 2). Furthermore, adjusting Model 1 for concurrent exposure to toxic elements (urinary arsenic, cadmium, and lead and hair mercury; Model 3), did not affect the estimates for hair selenium ( change for all outcomes). Above the spline knot, we found significant inverse associations for full developmental score, working memory, and processing speed, even though there were only 34 children with such high concentrations. When using U-Se instead of hair selenium, we found associations in the same directions as for hair selenium below and above the spline knot at U-Se , although they were not significant (Table 4).
Table 4.
Multivariable-adjusted linear spline regression analyses for concurrent selenium status (urinary and hair selenium) and cognitive function at 10 years ().
| Outcomes | U-Se at 10 y, (per , ) | U-Se at 10 y, (per , ) | Hair Se at 10 y, (per , ) | Hair Se at 10 y, (per , ) | ||||
|---|---|---|---|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | B (95% CI) | p-Value | B (95% CI) | p-Value | |
| 10 y | ||||||||
| Model 1 | ||||||||
| Full developmental score | 1.2 (, 3.7) | 0.36 | (, 6.2) | 0.40 | 2.2 (0.46, 3.9) | 0.013 | (, ) | 0.011 |
| Verbal comprehension | 0.50 (, 1.3) | 0.24 | (, 1.2) | 0.19 | 0.79 (0.23, 1.4) | 0.006 | (, 1.7) | 0.18 |
| Perceptual reasoning | 0.44 (, 1.4) | 0.38 | (, 1.8) | 0.25 | 0.60 (, 1.3) | 0.080 | (, 1.6) | 0.15 |
| Working memory | 0.10 (, 0.62) | 0.70 | (, 1.7) | 0.62 | 0.41 (0.054, 0.76) | 0.024 | (, ) | 0.006 |
| Processing speeda | 0.13 (, 1.1) | 0.81 | 0.77 (, 5.1) | 0.73 | 0.38 (, 1.1) | 0.28 | (, ) | 0.014 |
| Model 2 | ||||||||
| Full developmental score | 1.0 (, 3.6) | 0.42 | (, 6.3) | 0.40 | 2.1 (0.34, 3.8) | 0.019 | (, ) | 0.012 |
| Verbal comprehension | 0.47 (, 1.3) | 0.27 | (, 1.2) | 0.19 | 0.76 (0.19, 1.3) | 0.009 | (, 1.7) | 0.19 |
| Perceptual reasoning | 0.43 (, 1.4) | 0.39 | (, 1.8) | 0.25 | 0.60 (, 1.3) | 0.083 | (, 1.6) | 0.15 |
| Working memory | 0.070 (, 0.59) | 0.79 | (, 1.7) | 0.63 | 0.38 (0.024, 0.74) | 0.036 | (, ) | 0.007 |
| Processing speeda | 0.074 (, 1.1) | 0.88 | 0.80 (, 5.2) | 0.72 | 0.33 (, 1.0) | 0.35 | (1.4, ) | 0.015 |
| Model 3 | ||||||||
| Full developmental score | 2.1 (, 4.8) | 0.12 | (, 6.9) | 0.47 | 2.1 (0.41, 3.9) | 0.015 | (, ) | 0.010 |
| Verbal comprehension | 0.78 (, 1.7) | 0.082 | (, 1.4) | 0.23 | 0.76 (0.19, 1.3) | 0.009 | (, 1.5) | 0.16 |
| Perceptual reasoning | 0.69 (, 1.7) | 0.19 | (, 2.0) | 0.29 | 0.59 (, 1.3) | 0.087 | (, 1.5) | 0.14 |
| Working memory | 0.33 (, 0.88) | 0.24 | (, 1.8) | 0.68 | 0.41 (0.052, 0.76) | 0.025 | (, ) | 0.006 |
| Processing speeda | 0.31 (, 1.4) | 0.56 | 0.91 (, 5.3) | 0.68 | 0.38 (, 1.1) | 0.28 | (, ) | 0.014 |
Note: Model 1 has been adjusted as follows: children’s gender, age at testing, number of children in the family, gestational age at birth, height-for-age z-score (HAZ), modified version of Home Observation for Measurement of the Environment score (HOME), testers, school type and years of schooling, family socioeconomic status (SES), and paternal education (all assessed at 10 y), and mothers’ cognitive function assessed at 5 y. Model 2 is Model 1 further adjusted for water manganese at 10 y. Model 3 is Model 1 further adjusted for toxic element (arsenic, cadmium, and lead in children’s urine and mercury in children’s hair at 10 y). CI, confidence interval; Hair Se, hair selenium; U-Se, urinary selenium.
Higher processing speed indicates faster response time.
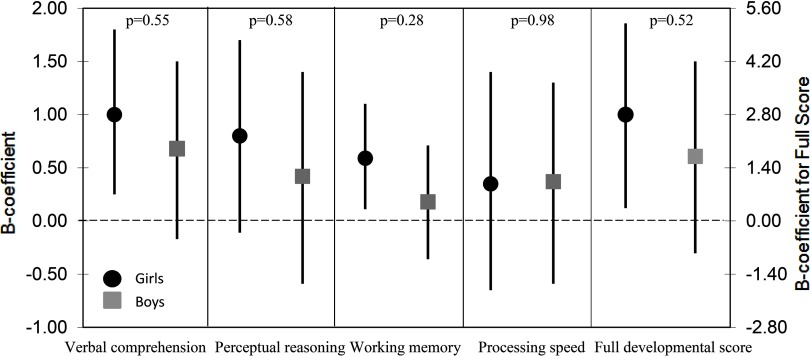
When stratifying Model 1 by gender, we found positive associations for both girls and boys (below the spline knot at ), although the estimates were generally stronger for the girls (Figure 4). In particular, the estimate for the association between hair selenium and working memory was 0.49 [95% confidence interval (CI): 0.11, 1.1] for the girls, compared with 0.18 (95% CI: , 0.72) for the boys. However, differences in estimates were not significant below the spline knot (Figure 4) or above the spline knot ( for all outcomes).
Figure 4.
Estimates (B-coefficient) and 95% confidence interval (CI) (straight line) for associations between all outcomes at 10 y and hair selenium (Se; below spline knot at , per ) at 10 y, stratified by gender ( girls and 659 boys). p-Value for difference between estimates (Wald test). Adjustments: gestational age at birth, age at testing, mothers’ cognitive function assessed at 5 y, number of children in the family, height-for-age z-score (HAZ), modified version of Home Observation for Measurement of the Environment score (HOME), testers, school type and years of schooling, family socioeconomic status (SES), and paternal education (all assessed at 10 y).
Discussion
To our knowledge, this large longitudinal study is the first to assess the impact of both prenatal and childhood selenium status on child development. Together with our previous findings of a positive association between maternal selenium status in pregnancy (Ery-Se) and language comprehension and psychomotor development at 1.5 y of age in a subset of 750 children from the same cohort (Skröder et al. 2015), the present results provide evidence that adequate selenium status is important for long-term brain development. We found positive associations between maternal Ery-Se in early pregnancy () and the outcomes at 10 y in particular, where an increase from the 5th to the 95th percentile (median Hb, corresponding to ) was associated with an estimated mean increase in full developmental score of 8.1 points (95% CI: 3.8, 13), corresponding to an increase of 0.24 SD. For the cross-sectional evaluation at 10 y (), a linear spline model with a single knot (at ; 98th percentile) revealed that an increase from the 5th to the 95th percentile in hair selenium was associated with an average increase in full developmental score of 5.9 points (95% CI: 1.3, 11), corresponding to 0.18 SD. The direction of the association changed from positive to negative for children with higher hair selenium concentrations (), although estimates for this group were based on only 34 children. Similar patterns were found for children tested at 5 y of age; however, the short-term exposure biomarker (U-Se) used at 5 y resulted in more imprecise associations. Taken together, the results indicate that both prenatal and childhood selenium status influence brain development. The findings seem robust even after adjustment for multiple potential confounders. The estimated selenium-related mean increases in some outcome scores were higher in girls than in boys, consistent with a greater potential benefit in girls, although the estimates were imprecise and the differences were small.
Very few prospective studies have assessed the impact of prenatal selenium status on child cognition. Following our previous study (Skröder et al. 2015), a Polish study () reported that maternal plasma selenium during the first trimester (, ) was positively associated with motor development at 1 y of age and with language development and cognitive function (borderline significant) at 2 y of age (Polanska et al. 2016). Similarly, a Greek study () showed a borderline positive association between maternal U-Se () and children’s cognition (McCarthy Scales of Children’s Abilities) at 4 y of age (Kippler et al. 2016). However, a recent Spanish study suggested an inverse relationship between serum selenium in the first trimester () and child neuropsychological development at approximately 1 y of age (Bayley Scales of Infant Development; ) above (Amorós et al. 2017). Moreover, a U.S. study found no association between fairly high Ery-Se concentrations during pregnancy (; ) and cognitive function at 7.7 y (Oken et al 2016). Considering the marked variation in selenium levels across studies, there appears to be some consistency concerning the importance of selenium for brain development at lower selenium levels. However, further studies are warranted to evaluate potential susceptibility factors.
There are also a few cross-sectional studies concerning childhood selenium status and cognition. In Bangladesh, a positive association was found between blood selenium and motor function in 9.6-y-old children (; Parvez et al. 2011). A Chinese study of 927 newborns showed a positive association between cord serum selenium and scores in the neonatal behavioral neurological assessment at 3 d of age, but only up to , after which the association turned inverse (; Yang et al. 2013). This pattern suggests that the range of selenium intake that is beneficial for child development may be narrow, as it is for selenium toxicity in general, although this has not been studied extensively (Jablonska and Vinceti 2015; Vinceti et al. 2014). We also observed indications of inverse associations in the cross-sectional analyses of selenium in hair and urine and the different measures of cognitive function at 5 and 10 y in the children with the highest selenium concentrations. However, there were few children above the turning points of hair selenium at (range to ; ) and U-Se of (range to approximately ; and 18, at 5 and 10 y, respectively). Despite the generally low selenium levels in the study area (see below), there may well be a risk of excess selenium intake through supplementation, particularly because people may not be aware of the narrow therapeutic index of selenium. However, further studies are needed to determine the potential for toxic levels of different chemical forms of selenium intake through supplementation or even through diet and whether safe levels of intake may vary among different populations.
We assessed prenatal selenium status based on selenium concentrations in maternal erythrocytes, which, like hair selenium, represents average intake over a longer time period (approximately 2–3 months; Skröder et al. 2017) than plasma selenium (a few weeks; Nève 1995) or urine (a few days; Hawkes et al. 2008). For a subsample of the study population () we previously measured plasma selenium in the beginning of the second trimester (Li et al. 2008), and we found a strong correlation with selenium in the erythrocytes [, (Skröder et al. 2015)]. In that sample, approximately 60% had concentrations , indicating selenium deficiency (Fairweather-Tait et al. 2011), which is in accord with the reported low selenium content in the agricultural soil in Bangladesh (Ahsan et al. 2009). Consequently, we did not have sufficient numbers of highly exposed women to evaluate potential toxic effects of such selenium levels. The children in the present cohort appeared to have somewhat higher selenium status than their mothers, based on the urine and erythrocyte concentrations measured in another subsample of 488 mother–child (9 y of age) pairs (Skröder Löveborn et al. 2016).
One of the suggested mechanisms for a positive effect of selenium on child cognition is protection against oxidative stress through the antioxidative properties of selenoproteins. Selenium plays an important role in the protection of neurons to a large extent through selenoprotein P, which is a major contributor to selenium content in the brain (Takemoto et al. 2010; Schweizer and Fradejas-Villar 2016). In addition, antioxidative glutathione peroxidases have been shown to be highly active in glial cells (Damier et al. 1993; Power and Blumbergs 2009), and thioredoxin reductases appear important for cerebellar function (Schweizer and Fradejas-Villar 2016). In addition, selenium is essential for thyroid function (Roman et al. 2014), which is important for child development.
Strengths of this study include the longitudinal study design with measurements of a wide range of selenium levels both prenatally and at 5 and 10 y, as well as cognitive assessment at both 5 and 10 y. Essentially no women reported smoking or alcohol consumption. Furthermore, we had a large sample size, and we measured multiple exposures using ICP-MS. A limitation of this study is that we had no plasma selenium measured in the children, which could have simplified comparisons of selenium status with other populations.
Conclusion
Our findings show that adequate selenium status in both fetal life and childhood may be beneficial for children’s cognitive function. We also found evidence of adverse effects in children with the highest selenium levels, based on small numbers of observations. Considering the high prevalence of inadequate selenium intake throughout the world (Fairweather-Tait et al. 2011), the results are likely relevant for other populations. The effect size may be of little clinical relevance on an individual level because the impact of selenium on cognitive abilities was small compared with the effects of factors such as SES, schooling, parental IQ, and stimulation at home. However, on a population level, even small decreases are of importance with respect to the proportion of children falling below the limit for intellectual disability. It is also important to stress that selenium deficiency, unlike some of the other abovementioned factors, is preventable, and that the effects of early-life selenium deficiency may persist into adulthood.
Supplemental Material
Acknowledgments
We thank all participating women and children and everyone involved in the cognitive testing and collection of samples and data. We also thank M. Levi, M. Grandér, B. Palm, B. Nermell, S. Ahmed, S.M. Rahman, and K. Gustin at Karolinska Institutet for measurements of trace elements. All authors read and approved the final manuscript. This work was supported by grants from the Swedish Research Council (grants 521-2013-2269 and 2015-03206), the Swedish Research Council Formas (grant 210-2013-751), the Swedish International Development Cooperation Agency (grant SWE-186), the European Commission [Public health impact of long-term, low-level mixed element exposure in susceptible population strata (PHIME)], and Karolinska Institutet. The Maternal and Infant Nutrition Interventions in Matlab (MINIMat) supplementation trial in pregnancy was funded by UNICEF, Sida, the U.K. Medical Research Council, the Swedish Research Council, the U.K. Department for International Development, the International Centre for Diarrhoeal Disease Research, Bangladesh, the Global Health Research Fund (Japan), the Child Health and Nutrition Research Initiative, Uppsala University, and the U.S. Agency for International Development.
References
- Ahsan DA, DelValls TA, Blasco J. 2009. Distribution of arsenic and trace metals in the floodplain agricultural soil of Bangladesh. Bull Environ Contam Toxicol 82(1):11–15, PMID: 18696001, 10.1007/s00128-008-9502-x. [DOI] [PubMed] [Google Scholar]
- Akbaraly TN, Hininger-Favier I, Carrière I, Arnaud J, Gourlet V, Roussel AM, et al. 2007. Plasma selenium over time and cognitive decline in the elderly. Epidemiology 18(1):52–58, PMID: 17130689, 10.1097/01.ede.0000248202.83695.4e. [DOI] [PubMed] [Google Scholar]
- al-Deeb S, al-Moutaery K, Bruyn GW, Tariq M. 1995. Neuroprotective effect of selenium on iminodipropionitrile-induced toxicity. J Psychiatry Neurosci 20(3):189–192, PMID: 7786879. [PMC free article] [PubMed] [Google Scholar]
- Amorós R, Murcia M, Ballester F, Broberg K, Iñiguez C, Rebagliato M, et al. 2017. Selenium status during pregnancy: influential factors and effects on neuropsychological development among Spanish infants. Sci Total Environ 1:610–611:741–749, PMID: 28822941, 10.1016/j.scitotenv.2017.08.042. [DOI] [PubMed] [Google Scholar]
- Ashton K, Hooper L, Harvey LJ, Hurst R, Casgrain A, Fairweather-Tait SJ. 2009. Methods of assessment of selenium status in humans: A systematic review. Am J Clin Nutr 89(6):2025S–2039S, PMID: 19420095, 10.3945/ajcn.2009.27230F. [DOI] [PubMed] [Google Scholar]
- Bergkvist C, Kippler M, Hamadani JD, Grandér M, Tofail F, Berglund M, et al. 2010. Assessment of early-life lead exposure in rural Bangladesh. Environ Res 110(7):718–724, PMID: 20656285, 10.1016/j.envres.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Caldwell BM, Corwyn RF. 2003. The child care home inventories: Assessing the quality of family child care homes. Early Child Res Q 18(3):294–309, 10.1016/S0885-2006(03)00041-3. [DOI] [Google Scholar]
- Caldwell BM. 1967. Descriptive evaluations of child development and of developmental settings. Pediatrics 40(1):46–54, PMID: 6028898. [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F. 1993. Glutathione peroxidase, glial cells and Parkinson's disease. Neuroscience 52(1):1–6, PMID: 8433802, 10.1016/0306-4522(93)90175-F. [DOI] [PubMed] [Google Scholar]
- de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. 2007. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85(9):660–667, PMID: 18026621, 10.1590/S0042-96862007000900010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després C, Beuter A, Richer F, Poitras K, Veilleux A, Ayotte P, et al. 2005. Neuromotor functions in Inuit preschool children exposed to Pb, PCBs, and Hg. Neurotoxicol Teratol 27(2):245–257, PMID: 15734276, 10.1016/j.ntt.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, et al. 2011. Selenium in human health and disease. Antioxid Redox Signal 14(7):1337–1383, PMID: 20812787, 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- Gao S, Jin Y, Hall KS, Liang C, Unverzagt FW, Ji R, et al. 2007. Selenium level and cognitive function in rural elderly Chinese. Am J Epidemiol 165(8):955–965, PMID: 17272290, 10.1093/aje/kwk073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin K, Tofail F, Mehrin F, Levi M, Vahter M, Kippler M. 2017. Methylmercury exposure and cognitive abilities and behavior at 10 years of age. Environ Int 102:97–105, PMID: 28216013, 10.1016/j.envint.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Gwatkin DR, Rustein S, Johnson K, Pande RP, Wagstaff A. 2000. Socio-economic Differences in Health, Nutrition, and Population in Bangladesh. Washington, DC:World Bank. [Google Scholar]
- Hamadani JD, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M, et al. 2011. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: A population-based cohort study. Int J Epidemiol 40(6):1593–1604, PMID: 22158669, 10.1093/ije/dyr176. [DOI] [PubMed] [Google Scholar]
- Hawkes WC, Richter BD, Alkan Z, Souza EC, Derricote M, Mackey BE, et al. 2008. Response of selenium status indicators to supplementation of healthy North American men with high-selenium yeast. Biol Trace Elem Res 122(2):107–121, PMID: 18193397, 10.1007/s12011-007-8066-7. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Newport GD, Islam F, Slikker W Jr, Ali SF. 1999. Selenium, an antioxidant, protects against methamphetamine-induced dopaminergic neurotoxicity. Brain Res 818(2):575–578, PMID: 10082851, 10.1016/S0006-8993(98)01311-0. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine Panel on Dietary Antioxidants and Related Compounds (IOM). 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids: A Report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Washington, DC:National Academy Press. [Google Scholar]
- Jablonska E, Vinceti M. 2015. Selenium and human health: Witnessing a Copernican revolution? J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 33(3):328–368, PMID: 26074278, 10.1080/10590501.2015.1055163. [DOI] [PubMed] [Google Scholar]
- Kippler M, Bottai M, Georgiou V, Koutra K, Chalkiadaki G, Kampouri M, et al. 2016. Impact of prenatal exposure to cadmium on cognitive development at preschool age and the importance of selenium and iodine. Eur J Epidemiol 31(11):1123–1134, PMID: 27147065, 10.1007/s10654-016-0151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippler M, Goessler W, Nermell B, Ekstrom EC, Lonnerdal B, El Arifeen S, et al. 2009. Factors influencing intestinal cadmium uptake in pregnant Bangladeshi women–a prospective cohort study. Environ Res 109(7):914–921, PMID: 19646688, 10.1016/j.envres.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Kippler M, Tofail F, Hamadani JD, Gardner RM, Grantham-McGregor SM, Bottai M, et al. 2012. Early-life cadmium exposure and child development in 5-year-old girls and boys: a cohort study in rural Bangladesh. Environ Health Perspect 120(10):1462–1468, PMID: 22759600, 10.1289/ehp.1104431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureano-Melo R, Império GE, da Silva-Almeida C, Kluck GE, Cruz Seara Fde A, da Rocha FF, et al. 2015. Sodium selenite supplementation during pregnancy and lactation promotes anxiolysis and improves mnemonic performance in Wistar rats' offspring. Pharmacol Biochem Behav 138:123–132, PMID: 26364924, 10.1016/j.pbb.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Lemire M, Mergler D, Huel G, Passos CJ, Fillion M, Philibert A, et al. 2009. Biomarkers of selenium status in the Amazonian context: Blood, urine and sequential hair segments. J Expo Sci Environ Epidemiol 19(2):213–222, PMID: 18446187, 10.1038/jes.2008.14. [DOI] [PubMed] [Google Scholar]
- Li L, Ekström EC, Goessler W, Lönnerdal B, Nermell B, Yunus M, et al. 2008. Nutritional status has marginal influence on the metabolism of inorganic arsenic in pregnant Bangladeshi women. Environ Health Perspect 116(3):315–321, PMID: 18335097, 10.1289/ehp.10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Kippler M, Harari F, Grandér M, Palm B, Nordqvist H, et al. 2015. Alkali dilution of blood samples for high throughput ICP-MS analysis-comparison with acid digestion. Clin Biochem 48(3):140–147, PMID: 25498303, 10.1016/j.clinbiochem.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Nicol F, Beckett GJ, Arthur JR. 1998. Selenoprotein expression and brain development in preweanling selenium- and iodine-deficient rats. J Mol Endocrinol 20(2):203–210, PMID: 9584835, 10.1677/jme.0.0200203. [DOI] [PubMed] [Google Scholar]
- Nermell B, Lindberg AL, Rahman M, Berglund M, Persson LA, El Arifeen S, et al. 2008. Urinary arsenic concentration adjustment factors and malnutrition. Environ Res 106(2):212–218, PMID: 17900556, 10.1016/j.envres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Nève J. 1995. Human selenium supplementation as assessed by changes in blood selenium concentration and glutathione peroxidase activity. J Trace Elem Med Biol 9(2):65–73, PMID: 8825978, 10.1016/S0946-672X(11)80013-1. [DOI] [PubMed] [Google Scholar]
- Oken E, Rifas-Shiman SL, Amarasiriwardena C, Jayawardene I, Bellinger DC, Hibbeln JR, et al. 2016. Maternal prenatal fish consumption and cognition in mid childhood: Mercury, fatty acids, and selenium. Neurotoxicol Teratol 57:71–78, PMID: 27381635, 10.1016/j.ntt.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, et al. 2011. Arsenic exposure and motor function among children in Bangladesh. Environ Health Perspect 119(11):1665–1670, PMID: 21742576, 10.1289/ehp.1103548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson LA, Arifeen S, Ekström EC, Rasmussen KM, Frongillo EA, Yunus M. 2012. Effects of prenatal micronutrient and early food supplementation on maternal hemoglobin, birth weight, and infant mortality among children in Bangladesh: The MINIMat randomized trial. JAMA 307(19):2050–2059, PMID: 22665104, 10.1001/jama.2012.4061. [DOI] [PubMed] [Google Scholar]
- Polanska K, Krol A, Sobala W, Gromadzinska J, Brodzka R, Calamandrei G, et al. 2016. Selenium status during pregnancy and child psychomotor development-Polish Mother and Child Cohort study. Pediatr Res 79(6):863–869, PMID: 26885758, 10.1038/pr.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JH, Blumbergs PC. 2009. Cellular glutathione peroxidase in human brain: Cellular distribution, and its potential role in the degradation of Lewy bodies in Parkinson's disease and dementia with Lewy bodies. Acta Neuropathol 117(1):63–73, PMID: 18853169, 10.1007/s00401-008-0438-3. [DOI] [PubMed] [Google Scholar]
- Rahman SM, Kippler M, Tofail F, Bolte S, Hamadani JD, Vahter M. 2016. Manganese in drinking water and cognitive abilities and behavior at 10 years of age: A prospective cohort study . Environ Health Perspect 125(5):057003, PMID: 28564632, 10.1289/EHP631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JC, Court JH. 2003. Manual for Raven’s Progressive Matrices and Vocabulary Scales. San Antonio, TX:Harcourt Assessment. [Google Scholar]
- Rita Cardoso B, Silva Bandeira V, Jacob-Filho W, Franciscato Cozzolino SM. 2014. Selenium status in elderly: Relation to cognitive decline. J Trace Elem Med Biol 28(4):422–426, PMID: 25220532, 10.1016/j.jtemb.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Roman M, Jitaru P, Barbante C. 2014. Selenium biochemistry and its role for human health. Metallomics 6(1):25–54, PMID: 24185753, 10.1039/c3mt00185g. [DOI] [PubMed] [Google Scholar]
- Schweizer U, Fradejas-Villar N. 2016. Why 21? The significance of selenoproteins for human health revealed by inborn errors of metabolism. FASEB J 30(11):3669–3681, PMID: 27473727, 10.1096/fj.201600424. [DOI] [PubMed] [Google Scholar]
- Skröder Löveborn H, Kippler M, Lu Y, Ahmed S, Kuehnelt D, Raqib R, et al. 2016. Arsenic metabolism in children differs from that in adults. Toxicol Sci 152(1):29–39, PMID: 27056082, 10.1093/toxsci/kfw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skröder HM, Hamadani JD, Tofail F, Persson LÅ, Vahter ME, Kippler MJ. 2015. Selenium status in pregnancy influences children's cognitive function at 1.5 years of age. Clin Nutr 34(5):923–930, PMID: 25444556, 10.1016/j.clnu.2014.09.020. [DOI] [PubMed] [Google Scholar]
- Skröder H, Kippler M, Nermell B, Tofail F, Levi M, Rahman S, et al. 2017. Major limitations in using element concentrations in hair as biomarkers of exposure to toxic and essential trace elements in children. Environ Health Perspect 125(6):067021, PMID: 28669939, 10.1289/EHP1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanowicz FA, Talwar D, O'Reilly DS, Dickinson N, Atkinson J, Hursthouse AS, et al. 2013. Erythrocyte selenium concentration as a marker of selenium status. Clin Nutr 32(5):837–842, PMID: 23391458, 10.1016/j.clnu.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Takemoto AS, Berry MJ, Bellinger FP. 2010. Role of selenoprotein P in Alzheimer's disease. Ethn Dis 20(suppl1):S192–S195, PMID: 20521393. [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Mandrioli J, Borella P, Michalke B, Tsatsakis A, Finkelstein Y. 2014. Selenium neurotoxicity in humans: Bridging laboratory and epidemiologic studies. Toxicol Lett 230(2):295–303, PMID: 24269718, 10.1016/j.toxlet.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 2002. Wechsler Preschool and Primary Scale of Intelligence™– Third Edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- WHO (World Health Organization). Global Database on Child Growth and Malnutrition. http://www.who.int/nutgrowthdb/about/introduction/en/index5.html [accessed 20 October 2017].
- WHO. 2002. Standards for maternal and neonatal care: Iron and folate supplementation. http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/iron_folate_supplementation.pdf [accessed 2 May 2013].
- Yang X, Yu X, Fu H, Li L, Ren T. 2013. Different levels of prenatal zinc and selenium had different effects on neonatal neurobehavioral development. Neurotoxicology 37:35–39, PMID: 23570748, 10.1016/j.neuro.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Zafar KS, Siddiqui A, Sayeed I, Ahmad M, Salim S, Islam F. 2003. Dose-dependent protective effect of selenium in rat model of Parkinson's disease: Neurobehavioral and neurochemical evidences. J Neurochem 84(3):438–446, PMID: 12558963, 10.1046/j.1471-4159.2003.01531.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.