Abstract
Despite growing literature on pregnancy in women with perinatally-acquired HIV infection (PHIV), little is known regarding HIV and reproductive health outcomes postpartum. We describe pregnancy, reproductive, and HIV care outcomes for 2 years postpartum among pregnant women with PHIV who delivered in a large urban health system in Atlanta, Georgia, USA from 2011–2016. We reviewed medical records of women with PHIV to estimate retention in HIV care (two HIV care visits or viral load measurements >90 days apart) and viral suppression (<200 copies/mL) at 12 and 24 months postpartum. Among 22 pregnant women with PHIV, 13 (59%) had a CD4 count of less than 300 cells/mm3 at the time of antenatal care entry; most (n = 13, 59%) women achieved viral suppression at time of delivery. Three quarters of women attended a postpartum HIV primary care visit, within an average of 193 (range 17–727) days. Only 4 (20%) women were retained and 3 (15%) virally suppressed at 12 postpartum, and 2 (12%) were retained and none virally suppressed at 24 months. Despite the unique challenges they face, multidisciplinary efforts are needed to engage women with PHIV during pregnancy and facilitate the transition to sustained HIV primary care in the postpartum period.
Keywords: HIV care continuum; postpartum; Georgia, USA; perinatal HIV infection
Introduction
Perinatal HIV transmission has sharply declined over the last 20 years with continued improvement in antiretroviral therapy (ART) and widespread adoption of multiple risk reduction practices (Joint United Nations Programme on HIV/AIDS, 2015). Children with perina-tally-acquired HIV infection (PHIV) now have access to more potent and tolerable treatment options resulting in increased survival into adulthood (Agwu & Fairlie, 2013). Close to 5,000 individuals with PHIV live in the United States; the majority are over 13 years old (Centers for Disease Control and Prevention [CDC], 2014).
Unfortunately, adolescents and young adults with PHIV have low rates of viral suppression (34%–68%) (Agwu, Fleishman, Rutstein, Korthuis, & Gebo, 2013; Dollfus et al., 2010; Van Dyke et al., 2011), high rates of AIDS diagnoses (Foster et al., 2009), and frequent drug resistance (Agwu & Fairlie, 2013; Hogg et al., 2006; Wang et al., 2012), which ultimately lead to AIDS and non-AIDS complications (Agwu et al., 2013; Dollfus et al., 2010; Foster et al., 2009; Van Dyke et al., 2011) and increased risk of death (Hogg et al., 2006; Wang et al., 2012). Adolescents face unique challenges, which may result in poor retention in HIV care (Farmer et al., 2014) and low ART adherence (Agwu & Fairlie, 2013; Foster et al., 2009). As resistance develops, available ART options become fewer, more complex, and may have greater side effects, which further challenges adherence (Foster et al., 2009).
Despite poor HIV outcomes, pregnancy rates among women with PHIV are similar to the general population (Neilan et al., 2017). Pregnancy provides an opportunity for increased interactions with the healthcare system, and potentially improved ART adherence due to motivation to reduce vertical transmission risk (Mellins et al., 2008). However, poor continuity in postpartum HIV care has been demonstrated among HIV-infected women overall, with retention in care at one year postpartum ranging from 37% to 60% (Adams, Brady, Michael, Yehia, & Momplaisir, 2015; Rana, Gillani, Fla-nigan, Nash, & Beckwith, 2010; Siddiqui, Bell, Sangi-Haghpeykar, Minard, & Levison, 2014; Swain et al., 2016). While data are emerging on pregnancy in women with PHIV (Agwu, Jang, Korthuis, Araneta, & Gebo, 2011; Badell, Kachikis, Haddad, Nguyen, & Lind-say, 2013; Buchberg et al., 2015; Jao et al., 2012), little is known regarding postpartum HIV outcomes.
Methods
We conducted a retrospective cohort study of women with PHIV who delivered between January 2011 and August 2016 at Grady Memorial Hospital, a large, urban, publically-funded hospital in Atlanta, Georgia. Women and HIV-exposed babies most commonly receive HIV care after delivery at the Ryan-White funded Grady Infectious Diseases Program. The study received Emory University Institutional Review Board and the Grady Research Oversight Committee approvals (#86122).
Sociodemographic and clinical data were collected from antenatal care entry until 24 months postpartum via standardized collection from electronic medical records. Mode of infection was determined through patient report. Adequacy of antenatal care was determined using the validated Kessner Index (adequate, intermediate, inadequate) (Kotelchuck, 1994). For infants, birth outcomes, infant HIV testing, confirmed infections, and number of infant pediatric follow-up visits were recorded from birth to discharge from the HIV clinic.
Retention in HIV care was defined as two HIV care visits or viral load measurements, greater than 90 days apart in one year, at 12 and 24 months postpartum. Viral suppression was considered achieved if the last HIV RNA viral load was ≤ 200 copies/mL in the 12 and 24-month periods, respectively. Viral suppression or retention was required at 12 months to be considered suppressed/retained at 24 months.
Results
Demographic, pregnancy, and delivery characteristics
We identified 22 pregnant women with PHIV whose average age was 21 (range 16–29) years and were majority (21, 96%) African-American; for 14 (64%), this was their first pregnancy (Table 1). Nearly two thirds (13, 59%) were in HIV care before pregnancy (10 within the same health system as their obstetric care), but only 29% were on ART at pregnancy diagnosis. At entry into antenatal care, women were at an average of 16 weeks gestational age (range 8–34 weeks); the average CD4 cell count was 297.2 (range 9–730) cells/mm3, 8 women (36%) were virally suppressed, and 6 (27%) had documented ART resistance mutations. All women received ART during pregnancy, and among those who were not receiving ART at pregnancy diagnosis, ART was initiated at an average of 16 weeks gestational age (range 10–27 weeks). Overall, protease inhibitor-containing (n = 15, 68%) or integrase inhibitor-containing (n = 3, 14%) regimen were used. Nearly three quarters (n = 16, 73%) of women received regimens consisting of multiple pills twice daily.
Table 1.
Demographic and clinical characteristics of pregnant women with perinatally-acquired HIV infection, Atlanta, Georgia, 2011–2016 (n = 22).
| Variable | Frequency (%) or mean (range) |
|---|---|
| Age, years | 21.0 (16–29) |
| Race/ethnicity | |
| African-American (non-Hispanic) | 21 (95.5%) |
| Hispanic | 1 (4.5%) |
| Years since HIV diagnosis | 17.9 (2–25) |
| In HIV care at pregnancy diagnosis | 13 (59.1%) |
| On antiretroviral therapy at pregnancy diagnosis | 6 (28.6%) |
| Gestational age at antenatal care entry (weeks) | 16 (8–34) |
| CD4 cell count at antenatal care entry, cells/mm3 | 297.2 (9–730) |
| Viral suppression at antenatal care entry | 8 (36.4%) |
| Antiretroviral regimen (anchor drug) | |
| PI-containing regimen | 5 (68.2%) |
| INSTI-containing regimen | 3 (13.6%) |
| NNRTI-containing regimen | 1 (4.6%) |
| INSTI + PI-containing regimen | 2 (9.1%) |
| INSTI+PI + NNRTI-containing regimen | 1 (4.6%) |
| Antiretroviral regimen (backbone drug) | |
| TDF-containing regimen | 10 (45.5%) |
| ZDV-containing regimen | 10 (45.5%) |
| TDF + ZDV-containing regimen | 2 (9.1%) |
| Number of antenatal care visits | 8 (2–14) |
| Adequacy of antenatal care (Kessner Index) | |
| Adequate | 7 (32%) |
| Intermediate | 11 (50%) |
| Inadequate | 2 (9%) |
| Gestational age at delivery (weeks) | 38.2 (35.1–40.3) |
| Viral load at delivery | |
| HIV RNA <1000 copies/mL | 17 (77.3%) |
| HIV RNA <200 copies/mL | 13 (59.1%) |
| Mode of delivery | |
| Vaginal delivery | 8 (36.4%) |
| Cesarean section | 14 (63.6%) |
| Cesarean section for high HIV viral load | 6 (42.9%) |
| Perinatal transmission of HIV to infant | 1 (4.5%) |
| Birth weight of infant (grams) | 2753 (1701–3620) |
| Apgar score (1 min) | 7.8 (3–9) |
| Apgar score (5 min) | 8.8 (7–9) |
| Neonatal ICU admission | 4 (18.2%) |
| Contraceptive plan at delivery | 20 (90.9%) |
| DMPA | 8 (36.4%) |
| Oral contraceptive | 2 (9.1%) |
| Hormone IUD | 2 (9.1%) |
| Implant | 6 (27.3%) |
| Tubal ligation | 1 (4.5%) |
| Condoms | 1 (4.5%) |
| Contraception provided at time of delivery | 15 (68.2%) |
| Repeat pregnancy during follow-up period | 6 (28.6%) |
| Attended postpartum obstetric follow-up visit | 13 (59.1%) |
| Infant follow-up | |
| Infant age at last follow-up visit (months) | 15.0 (9–21) |
| Number of visits | 6.1 (3–8) |
| Average time to postpartum HIV primary care visit (days) | 192.5 (17–727) |
| HIV primary care visit within 90 days | 6 (27.3%) |
| HIV primary care visit within 180 days | 3 (13.6%) |
| No postpartum HIV primary care visit | 5 (22.7%) |
| Disruption in ART postpartum | 19 (86.4%) |
Abbreviations: DMPA, depot medroxyprogesterone acetate; INSTI, integrase inhibitor; IUD, intra-uterine device; NNRTI, non-nucleoside reverse-transcriptase inhibitors; PI, protease inhibitor; TDF, Tenofovir disoproxil fumarate; ZDV, zidovudine.
The women attended an average of 8 (range 2–14) antenatal care visits and per the Kessner Index, antenatal care was adequate for 7 (32%), intermediate for 11 (50%), and inadequate for 2 (9%) women. The average gestational age at delivery was 38.2 (range 35.1–40.3) weeks. At time of delivery, 13 (59%) women were virally suppressed; 14 (64%) deliveries were by Cesarean section (6 [43%] were due to high third trimester viral load or ART non-adherence). The infants weighed an average of 2753 grams (range 1701–3620), with Apgar scores of 7.8 (range 3–9) at one minute and 8.8 (range 7–9) at five minutes. Four infants required admission to the neonatal ICU: two for prematurity, one for small size for gestational age, and one for hypoglycemia. One case of perinatal HIV transmission occurred to the infant of a woman who had inadequate antenatal care and presented after 40 weeks gestation with prolonged rupture of membranes and HIV viral load of 26,950 copies/ml. She received intrapartum zidovudine and had a Cesarean section, but the infant was positive for HIV-1 via DNA testing on the day of birth. The infant initiated ART and was virally suppressed at 12 months of age.
Postpartum contraception and repeat pregnancy
Eighteen (18%) women reported a contraceptive plan at the time of delivery; 19 (68%) received a contraception method other than condoms upon discharge, including 41% receiving either sterilization (1 women) or a long-acting reversible contraceptive method (8 women). Six (29%) women had a subsequent pregnancy during an average 40 months of follow-up; average time from delivery to conception was 16.9 (range 2.2–35.4) months and 4 (67%) occurred within 2 years of delivery.
Postpartum follow-up care
Thirteen women (59%) attended their postpartum obstetric visit within 90 days. To date, 3 HIV-exposed but uninfected infants were still receiving follow-up HIV testing. Of the remaining infants, there were an average of 6.1 (range 3.9–8.4) well child visits until an average 15 (range 9–21) months of age. Twelve infants were discharged from clinic after completing HIV testing, 5 were lost to follow-up, and 1 moved out of state.
After delivery, the women transitioned care from the obstetric clinic at the main campus of Grady Memorial Hospital to the Grady Infectious Diseases Program, which provides outpatient non-obstetric HIV care, located just 2 miles apart. All of the women intended to follow up within this healthcaresystem. Women with PHIV attended their first HIV primary care visit within an average 193 (range 17–727) days; 5 (23%) women did not attend a postpartum HIV primary care visit. Ten (46%) women continued postpartum HIV care with pediatric HIV providers, 4 (18%) saw adult HIV providers, and 3 (14%) only received HIV care with an obstetrician during a repeat pregnancy. Disruption in ART in the postpartum period was documented in the medical record for 19 (86%) women. At 12 months postpartum, only 4 (20%) women were retained in HIV care, and 3 (15%) were virally suppressed; at 24 months postpartum, only 2 (12%) were retained and none were virally suppressed (Figure 1).
Figure 1.
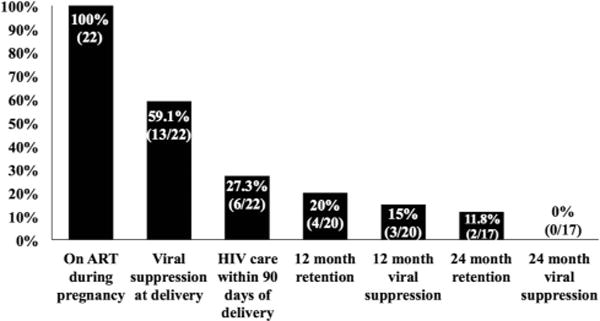
HIV care continuum during pregnancy and two years postpartum for women with perinatally-acquired HIV infection (n = 22). Changes in denominator are due to the fact that by the time of analysis, only 20 of the women were are least 12 months postpartum, and only 17 of the women were at least 24 months postpartum.
Discussion
This is the first report of long-term retention in care and viral suppression among postpartum women with PHIV. Retention in care among this cohort of 22 women with PHIV was startlingly low with 20% and 12% retained at 12 and 24 months postpartum, respectively. While previous studies have reported low retention in HIV care among postpartum HIV-infected women despite high levels of healthcare engagement during pregnancy (Adams et al., 2015; Boehme et al., 2014; Buchberg et al., 2015; Mellins et al., 2008; Rana et al., 2010; Sha et al., 2011; Siddiqui et al., 2014; Swain et al., 2016), retention and viral suppression estimates for this population of postpartum women with PHIV are even lower than these previous reports. While women achieved viral suppression at time of delivery at rates similar to other US populations of women with PHIV (Agwu et al., 2011; Badell et al., 2013; Jao et al., 2012; Phillips et al., 2011), only 15% were suppressed at one year and none at two years postpartum, highlighting the challenges with optimizing HIV care in this growing population and the need for novel interventions.
Individuals with PHIV have poor HIV outcomes during and after adolescence. This period often coincides with the transition out of pediatric care, where disruption in social support, new provider-patient relationship, possibly a new clinic site, insurance changes, and psychosocial changes associated with adolescence, may contribute to poor visit and medication adherence, virologic failure, and immune deterioration (Agwu et al., 2013; Hussen et al., 2015). For women, who often become pregnant precisely during this transition period, the additional complex postpartum challenges of new motherhood, caring for an infant, financial and insurance challenges, risk of postpartum depression, and switch between obstetric and HIV primary care providers, add compounding barriers during an already vulnerable period. While the transition from obstetric to HIV primary care occurred within the same health care system for this group of women, it still involved a new provider, clinic staff, and location. When added to the above-mentioned difficulties of the early postpartum period, and the fact that nearly three quarters of these women newly initiated ART during pregnancy, compounding obstacles to engagement and retention in long term HIV care are evident in this population.
In this cohort, repeat pregnancy occurred in nearly one-third of women, commonly within 2 years of delivery. Unfortunately, a few women received postpartum HIV care only as part of care occurring during subsequent pregnancy. While our data did not capture pregnancy intention, the frequency and timing of repeat pregnancy underscores the importance of incorporating reproductive health care into primary care during the postpartum period, both for women’s health and further prevention of perinatal HIV transmission.
Our data show that despite frequent interactions with healthcare providers during pregnancy and high rates of pediatric follow-up for the infants, women with PHIV have low retention in HIV care and viral suppression after delivery. Women with PHIV have greater ART drug resistance, complex psychosocial issues, and increased HIV-related morbidity (Agwu & Fairlie, 2013). In our cohort, 73% of women with PHIV received an ART regimen consisting of multiple pills in multiple daily doses, with resistance concerns likely contributing to these complex regimens. The additional responsibilities of motherhood and transition from obstetric to HIV care could further disrupt treatment, resulting in further accumulation of resistanace, virologic failure, and HIV-related complications.
There are limitations to this study. As a retrospective chart review, we relied on the accuracy and scope of electronic medical records; some socioeconomic and partner/caregiver characteristics could not be described, such health insurance, housing, employment, HIV disclosure, and involvement of partner. While this analysis is limited by its small sample size of PHIV followed at a single US center and thus may not generalizable to postpartum PHIV women in other settings, we nonetheless report on one of the largest US cohorts of pregnant women with PHIV. Nonetheless, due to the overall low numbers of women with PHIV, we did not directly compare women with PHIV and behaviorally acquired HIV, and could not assess factors associated with outcomes due to very low retention and viral suppression rates.
In conclusion, with high rates of resistance and fewer available treatment options, individuals with PHIV already have more challenges achieving optimal HIV care. The lives of young women with PHIV, struggling with emotional and psychosocial development associated with adolescence, stigma of HIV infection, and burden of chronic illness, are further complicated by the physical, mental, social, and financial challenges of pregnancy and infant care (Phillips et al., 2011). Pregnancy offers a time for interventions to optimize the transition to postpartum HIV primary care for women with PHIV. Efforts should be made to engage women with PHIV during pregnancy to facilitate the transition to sustained HIV primary care in the postpartum period.
Acknowledgments
The authors thank Grady Health System patients, clinicians, and staff. Drs. Susan Davis, Emily Grossniklaus, and Jessica Tarleton and Ms. Jeronia Blue who contributed to building the dataset.
Funding
This work was supported by the National Institutes for Health [grant number 1K23AI114407, ANS].
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author.
ORCID
Christina M. Meade http://orcid.org/0000-0003-1233-4334
References
- Adams JW, Brady KA, Michael YL, Yehia BR, Momplaisir FM. Postpartum engagement in HIV care: An important predictor of long-term retention in care and viral suppression. Clinical Infectious Diseases Advance Access. 2015;61(12):1880–1887. doi: 10.1093/cid/civ678. [DOI] [PubMed] [Google Scholar]
- Agwu AL, Fairlie L. Antiretroviral treatment, management challenges and outcomes in perinatally HIV-infected adolescents. Journal of the International AIDS Society. 2013;16(1):18579. doi: 10.7448/IAS.16.1.18579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agwu AL, Fleishman JA, Rutstein R, Korthuis PT, Gebo K. Changes in advanced immunosuppression and detectable HIV viremia among perinatally HIV-infected youth in the multisite United States HIV research network. Journal of the Pediatric Infectious Diseases Society. 2013;2(3):215–223. doi: 10.1093/jpids/pit008. [DOI] [PubMed] [Google Scholar]
- Agwu AL, Jang SS, Korthuis PT, Araneta MRG, Gebo KA. Pregnancy incidence and outcomes differ in vertically and behaviorally HIV-infected youth in a multi-site clinical cohort. JAMA : The Journal of the American Medical Association. 2011;305(5):468–470. doi: 10.1001/jama.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badell ML, Kachikis A, Haddad LB, Nguyen ML, Lindsay M. Comparison of pregnancies between perinatally and sexually HIV-infected women: An observational study at an urban hospital. Infectious Diseases in Obstetrics and Gynecology. 2013;2013:1–6. doi: 10.1155/2013/301763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme AK, Davies SL, Moneyham L, Shrestha S, Schumacher S, Kempf MC. A qualitative study on factors impacting HIV care adherence among postpartum HIV-infected women in the rural southeastern USA. AIDS Care. 2014;26(5):574–581. doi: 10.1080/09540121.2013.844759. [DOI] [PubMed] [Google Scholar]
- Buchberg MK, Fletcher FE, Vidrine DJ, Levison J, Peters MY, Hardwicke R, Bell TK. A mixed-methods approach to understanding barriers to postpartum retention in care among low-income, HIV-infected women. AIDS Patient Care and STDs. 2015;29(3):126–132. doi: 10.1089/apc.2014.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Diagnoses of HIV infection in the United States and dependent areas 2014 [Google Scholar]
- Dollfus C, Le Chenadec J, Faye A, Blanche S, Briand N, Rouzioux C. Long-term outcomes in adolescents perinatally infected with HIV-1 and followed up since birth in the French perinatal cohort (EPF/ANRS CO10) Clinical Infectious Diseases. 2010;51(2):214–224. doi: 10.1086/653674. [DOI] [PubMed] [Google Scholar]
- Farmer C, Yehia BR, Fleishman JA, Rutstein R, Mathews WC, Nijhawan A, Agwu AL. Factors associated with retention among non–perinatally HIV-infected youth in the HIV research network. Journal of the Pediatric Infectious Diseases Society. 2014;5(1):39–46. doi: 10.1093/jpids/piu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster C, Judd A, Tookey P, Tudor-Williams G, Dunn D, Shingadia D, Lyall H. Young people in the United Kingdom and Ireland with perinatally acquired HIV: The pediatric legacy for adult services. AIDS Patient Care and STDs. 2009;23(3):159–166. doi: 10.1089/apc.2008.0153. [DOI] [PubMed] [Google Scholar]
- Hogg RS, Bangsberg DR, Lima VD, Alexander C, Bonner S, Yip B, Harrigan PR. Emergence of drug resistance is associated with an increased risk of death among patients first starting HAART. PLoS Medicine. 2006;3(9):e356. doi: 10.1371/journal.pmed.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussen SA, Chahroudi A, Boylan A, Camacho-Gonzalez AF, Hackett S, Chakraborty R. Transition of youth living with HIV from pediatric to adult-oriented healthcare: A review of the literature. Future Virology. 2015;9(10):921–929. doi: 10.2217/fvl.14.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao J, Sigel K, Chen KT, Rodriquez-Caprio G, Posada R, Shust G, Sperling RS. Small for gestational age birth outcomes in pregnant women with perinatally acquired HIV. AIDS (London, England) 2012;26(7):855–859. doi: 10.1097/QAD.0b013e328351f6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) 2015 progress report on the global plan towards the elimination of new HIV Infections among children and keeping mothers alive 2015 [Google Scholar]
- Kotelchuck M. The adequacy of prenatal care utilization index: Its US distribution and association with low birthweight. American Journal of Public Health. 1994;84(9):1486–1489. doi: 10.2105/ajph.84.9.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellins CA, Chu C, Malee K, Allison S, Smith R, Harris L, Larussa P. Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care. 2008;20(8):958–968. doi: 10.1080/09540120701767208. [DOI] [PubMed] [Google Scholar]
- Neilan AM, Karalius B, Patel K, Van Dyke RB, Abzug MJ, Agwu AL, Ciaranello AL. Association of risk of viremia, immunosuppression, serious clinical events, and mortality with increasing age in perinatally human immunodeficiency virus–infected youth. JAMA Pediatrics. 2017;171(5):450–460. doi: 10.1001/jamapediatrics.2017.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips UK, Rosenberg MG, Dobroszycki J, Katz M, Sansary J, Golatt MA, Abadi J. Pregnancy in women with perinatally acquired HIV-infection: Outcomes and challenges. AIDS Care. 2011;23(9):1076–1082. doi: 10.1080/09540121.2011.554643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana AI, Gillani FS, Flanigan TP, Nash BT, Beckwith CG. Follow-up care among HIV-infected pregnant women in Mississippi. Journal of Women’s Health. 2010;19(10):1863–1867. doi: 10.1089/jwh.2009.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha BE, Tierney C, Cohn SE, Sun X, Coombs RW, Frenkel LM, Stek A. Postpartum viral load rebound in HIV-1–infected women treated with highly active antiretroviral therapy: AIDS clinical trials group protocol A5150. HIV Clinical Trials. 2011;12(1):9–23. doi: 10.1310/hct1201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui R, Bell T, Sangi-Haghpeykar H, Minard C, Levison J. Predictive factors for loss to postpartum follow-up among low income HIV-infected women in Texas. AIDS Patient Care and STDs. 2014;28(5):248–253. doi: 10.1089/apc.2013.0321. [DOI] [PubMed] [Google Scholar]
- Swain CA, Smith LC, Nash D, Pulver WP, Gordon D, Bian F, McNutt LA. Postpartum human immunodeficiency virus care among women diagnosed during pregnancy. Obstetrics & Gynecology. 2016;128(1):44–51. doi: 10.1097/AOG.0000000000001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke RB, Patel K, Siberry GK, Burchett SK, Spector SA, Chernoff MC, Seage GR. Antiretroviral treatment of US children with perinatally acquired HIV infection: Temporal changes in therapy between 1991 and 2009 and predictors of immunologic and virologic outcomes. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011;57(2):165–173. doi: 10.1097/QAI.0b013e318215c7b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xing H, Ruan Y, Liao L, Zhou H, Chen B, Shao Y. Effect of viral load and drug resistance on mortality among Chinese HIV-infected patients receiving antiretroviral treatment. Antivirals and Antiretrovirals. 2012;4(3):60–65. doi: 10.4172/jaa.1000047. [DOI] [Google Scholar]


