Abstract
Background:
Exposure to perfluoroalkyl substances (PFASs) may increase risk for metabolic diseases; however, epidemiologic evidence is lacking at the present time. Pregnancy is a period of enhanced tissue plasticity for the fetus and the mother and may be a critical window of PFAS exposure susceptibility.
Objective:
We evaluated the associations between PFAS exposures and metabolic outcomes in pregnant women.
Methods:
We analyzed 1,240 pregnant women from the Spanish INMA [Environment and Childhood Project (INfancia y Medio Ambiente)] birth cohort study (recruitment period: 2003–2008) with measured first pregnancy trimester plasma concentrations of four PFASs (in nanograms/milliliter). We used logistic regression models to estimate associations of PFASs ( and categorized into quartiles) with impaired glucose tolerance (IGT) and gestational diabetes mellitus (GDM), and we used linear regression models to estimate associations with first-trimester serum levels of triglycerides, total cholesterol, and C-reactive protein (CRP).
Results:
Perfluorooctane sulfonate (PFOS) and perfluorohexane sulfonate (PFHxS) were positively associated with IGT (137 cases) [OR per (95% CI: 1.06, 3.78) and ( 95% CI: 0.99, 2.76), respectively]. PFOS and PFHxS associations with GDM (53 cases) were in a similar direction, but less precise. PFOS and perfluorononanoate (PFNA) were negatively associated with triglyceride levels [percent median change per (95% CI: , ) and percent median change per (95% CI: , , respectively], whereas perfluorooctanoate (PFOA) was positively associated with total cholesterol [percent median change per (95% CI: 0.01%, 2.54%)]. PFASs were not associated with CRP in the subset of the population with available data ().
Conclusions:
Although further confirmation is required, the findings from this study suggest that PFAS exposures during pregnancy may influence lipid metabolism and glucose tolerance and thus may impact the health of the mother and her child. https://doi.org/10.1289/EHP1062
Introduction
Perfluoroalkyl substances (PFASs) have been used in many applications since the 1950s, including industrial applications and consumer products (Casals-Casas and Desvergne 2011). PFASs bioaccumulate in the food chain and in animal and human tissues, and exposure persists in the environment and living organisms for years (Wang et al. 2014). The routes of human exposure to PFASs include diet (animal and plant-based foods), migration from packaged foods, drinking water, and inhalation of indoor dust (Wang et al. 2014). Detectable blood levels of PFASs have been reported in pregnant women in Spain (Manzano-Salgado et al. 2015) and in other European (Gebbink et al. 2015), North American (Kato et al. 2014), Asian (Okada et al. 2013), and African (Hanssen et al. 2010) regions. PFAS exposure may pass from mother to child through the placenta (Manzano-Salgado et al. 2015) and through breast milk (Mogensen et al. 2015).
Evidence from epidemiological studies suggests that exposures to PFASs and to other endocrine-disrupting chemicals (EDCs) (i.e., synthetic substances that have been shown to alter the function of the endocrine system in intact organisms) may contribute to obesity (de Cock and van de Bor 2014), lipid alterations (Kabir et al. 2015), diabetes (Taylor et al. 2013), and to autoimmune diseases and inflammation (Kuo et al. 2012). A study of female CD-1 mice reported that mice exposed to low doses of perfluorooctanoate (PFOA) in utero had higher serum insulin and leptin concentrations at 21–33 wk of age than controls (Hines et al. 2009). Rats exposed to PFOA or perfluorooctane sulfonate (PFOS) were reported to have lower total serum cholesterol levels and to show evidence of liver toxicity compared with controls (Lau et al. 2007). Experimental evidence further supports that PFOA and PFOS exposures may alter inflammatory responses and the production of cytokines (DeWitt et al. 2012) that play a role in the pathogenesis of metabolic diseases (Caër et al. 2017).
Few epidemiological studies have evaluated associations between PFAS exposures and metabolic outcomes, and findings have been inconclusive. A cross-sectional study of 571 adults in Taiwan reported that PFOS was positively associated with prevalent diabetes, but associations with other PFAS compounds were negative (inverse) (Su et al. 2016). A prospective cohort study of 258 pregnant women in the United States reported a positive association between serum PFOA concentrations and gestational diabetes mellitus (GDM); associations of other PFASs were positive but close to the null (Zhang et al. 2015). However, a prospective study of 1,274 pregnant women in Canada reported limited evidence of a positive association with GDM for plasma perfluorohexane sulfonate (PFHxS) (significant for the second vs. first quartile comparison only) and no evidence of associations for PFOA or PFOS (Shapiro et al. 2016). Similarly, a recent study of 604 pregnant Faroese women that used a multiple-pollutant modeling approach found no association of PFAS exposures with GDM (Valvi et al. 2017). Further, a Norwegian study of PFASs and serum lipid levels in second-trimester samples from 891 pregnant women reported that PFOS was positively associated with total cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL), and that PFOA, PFHxS, and three other PFASs were also positively associated with HDL (Starling et al. 2014). Finally, a Danish study that examined PFOS, PFOA, and total cholesterol in serum samples collected from 854 women during the 30th week of gestation reported positive associations for both PFASs (Skuladottir et al. 2015).
Exposure during pregnancy may affect the mother and may also affect her child during gestation and in later life (Bach et al. 2015; Casals-Casas and Desvergne 2011). However, until now, only a few studies with relatively small populations have examined the potential role of PFAS exposures on metabolic outcomes at pregnancy (Starling et al. 2014; Skuladottir et al. 2015; Zhang et al. 2015). Therefore, we evaluated associations of exposures in early pregnancy to PFOS, PFOA, and the emerging PFAS compounds PFHxS and PFNA with metabolic outcomes including impaired glucose tolerance (IGT) and GDM, and with serum levels of triglycerides, total cholesterol, and C-reactive protein (CRP), in a cohort of Spanish women.
Methods
Study Population and Data Collection
The population-based birth cohort study INMA [INfancia y Medio Ambiente (Environment and Childhood)] recruited 2,150 pregnant women in the Spanish regions of Valencia (), Sabadell (), and Gipuzkoa () between 2003 and 2008 (Guxens et al. 2012). Women were enrolled during the first trimester of pregnancy at the primary health care center or hospital of each region and afterwards were followed at the third trimester of pregnancy and at birth. The inclusion criteria were of age, no assisted reproduction, a singleton pregnancy, intention to deliver at the reference hospital, and no communication handicap. Because the main focus of the INMA studies is on child health, plasma PFAS analysis was limited to mother–child pairs who had archived plasma samples from pregnancy and information on child health outcomes at 4 y of age (). Of these, 1,240 mothers who also had information about at least one of the metabolic outcomes of interest were included in the present analysis (58% of those initially enrolled) (see Figure S1). Investigators who performed the PFAS analyses did not have information on the women’s metabolic outcomes, and those who assessed the metabolic outcomes did not have information on PFAS concentrations. To assess potential selection bias, we performed a comparison of main characteristics between pregnant women included in and excluded from analysis.
Nonfasting blood samples were collected from the women at the first-trimester prenatal visit, and interview-based questionnaires administered by trained research staff were used to collect information about sociodemographic (including age, education, occupation, and parity history) and lifestyle characteristics (including alcohol consumption and smoking during pregnancy) at first- and third-trimester prenatal visits.
All participants provided written informed consent. The study was approved by the hospital ethics committees of each participating region.
Exposure Assessment of PFAS
Plasma was separated from blood samples collected at approximately 13 wk of gestation (), aliquoted into cryotubes, and stored at until analysis at the Institute for Occupational Medicine, Rheinisch-Westfälische Technische Hochschule (RWTH) Aachen University, Germany, as previously described (Manzano-Salgado et al. 2015). Plasma concentrations of PFASs were determined using column-switching high performance liquid chromatography (HPLC) (Agilent 1100 Series HPLC) coupled to tandem mass spectrometry (Sciex API 3000 LC/MS/MS System). The limit of quantification (LOQ) was for PFOS, PFOA, and PFHxS, and for PFNA.
Metabolic Outcomes
In Spain, the routine clinical examination for GDM in pregnant women is performed at 24–28 wk of gestation according to the guidelines recommended by the Spanish Group of Diabetes and Pregnancy. Women whose physicians considered them to be at high risk for GDM [based on age, body mass index (BMI), personal/family history of diabetes, or previous pregnancy complications] were given a oral glucose challenge test (OGCT), with blood glucose values measured after 1 h. Women with a postchallenge blood glucose on the OGCT completed a 3-h oral glucose tolerance test (OGTT) 2–3 wk after the first screening test, with blood glucose concentrations measured at baseline and 1, 2, and 3 h after the glucose challenge. Results of the OGTT are routinely used to classify women as having GDM if two or more of the baseline or postchallenge blood glucose concentrations exceed National Diabetes Data Group (NDDG) reference values (Expert Committee on the Diagnosis and Classification of Diabetes Mellitus 2003) and as having IGT only (without GDM) if one or fewer of the four blood glucose concentrations exceeds NDDG reference values. We extracted information about IGT and GDM diagnosis from the medical records. Information about GDM was available for women from all three INMA subcohorts, but information about IGT only (i.e., IGT in women not diagnosed with GDM) was available in the medical records for women in the Sabadell and Gipuzkoa cohorts. Our a priori hypothesis was that PFAS exposures may interfere with insulin signaling, glucose homeostasis, or both (Yan et al. 2015, Qiu et al. 2016), which could result in elevated blood glucose levels as well as in GDM. Therefore, we used one set of logistic regression models to estimate associations between PFASs and GDM (with noncases defined as all women without a diagnosis of GDM, regardless of whether they had IGT), and a second set of models to estimate associations between PFASs and IGT, where all women diagnosed with GDM or IGT were classified as cases, and all remaining women were classified as noncases. In addition, we performed a sensitivity analysis in which we repeated the logistic regression models of associations with IGT after excluding women from the Valencia subcohort because Valencia women with IGT but not GDM would have been misclassified as noncases owing to missing IGT information.
Total cholesterol and triglycerides were determined in maternal nonfasting serum collected at approximately 13 wk of gestation using colorimetric enzymatic methods. Measurements were performed at the Bizkaia Basque Country Public Health Laboratory for the Gipuzkoa and Sabadell subcohorts and at the General Biochemistry Laboratory of La Fe Hospital (Valencia) for the Valencia subcohort. CRP levels were measured in first-trimester pregnancy serum by immunoturbidimetry at the Consulting Químico Sanitario (CQS) Laboratory for the Gipuzkoa subcohort and at the Echevarne Sabadell Laboratory for the Sabadell subcohort. CRP levels were not measured in the Valencia subcohort.
Other Covariates
We considered a wide range of potential confounders based on previous literature related to PFAS exposures (Berg et al. 2014; Manzano-Salgado et al. 2016; Sagiv et al. 2015) and/or metabolic outcomes in pregnant women (Halldorsson et al. 2012; Perng et al. 2014; Ryckman et al. 2015; Skuladottir et al. 2015; Starling et al. 2014; Taylor et al. 2013; Wild et al. 2015; Zhang et al. 2015). These confounders included subcohort (Valencia, Sabadell, Gipuzkoa), country of birth, age, gestational week at blood extraction, parity, previous breastfeeding duration, marital status (living with the child’s father, other situation), education, social class [based on occupation according to the International Standard Classification of Occupations–88: I and II, professionals and managers; III, other nonmanual and skilled; IV and V, semiskilled and unskilled manual (http://www.ilo.org/public/english/bureau/stat/isco/isco88/index.htm)], occupation at first prenatal visit, smoking during pregnancy, physical activity [in metabolic equivalents of task (METs) per hour per day], and prepregnancy BMI (kilograms/meters squared; based on prepregnancy self-reported weight divided by measured squared height at first prenatal visit). We further considered the potential confounding role of the women’s diet in the past three months before the blood extraction for PFAS analysis; this was assessed at the first prenatal visit using a 101-item food frequency questionnaire (FFQ) previously validated in Spanish adults (Vioque et al. 2013). From the FFQ, we obtained information on total energy (kilocalories/day), alcohol (grams/day), fish and red meat intake (categorized in tertiles by servings/week), and the relative Mediterranean Diet Score (rMED), a proxy measure of diet quality based on the extent of adherence to a Mediterranean diet (Fernández-Barrés et al. 2016).
Statistical Analysis
We substituted maternal PFAS concentration values that were below the LOQ with LOQ/4. We calculated the Spearman correlation coefficients between PFASs. PFAS concentrations, triglycerides, and CRP levels had right-skewed distributions and therefore were .
We used generalized additive models (GAMs) to examine the linearity of the relationships between continuous PFAS concentrations and the metabolic outcomes. Because some of the associations were not linear (i.e., a p-gain in the GAM ; see Table S1), we show effect estimates for both continuous PFASs and per quartile of PFAS exposure. We used logistic regression to estimate associations between PFAS concentrations and dichotomous outcomes (i.e., IGT and GDM), and we used linear regression models to estimate associations with continuous outcomes (serum cholesterol, triglycerides, and CRP concentrations). We estimated all associations using a basic model adjusted for subcohort only and using a fully adjusted model with additional covariates. We tested for heterogeneity among subcohorts by including an interaction term () in the fully adjusted models (data not shown). All interaction terms were nonsignificant (); therefore, we report coefficients from analyses of the overall population only.
The analysis population included 1,240 women with information about GDM/IGT and 1,194 women with information about serum lipid levels (see Figure S1). Analyses of associations with serum CRP levels were limited to 651 women from the Sabadell and Gipuzkoa subcohorts because CRP was not measured in the Valencia subcohort. The fully adjusted models included slightly fewer observations owing to missing data for additional covariates (). Because of the low proportion of observations with missing covariate data, we performed complete case analyses.
Covariates included in the multivariable-adjusted models were selected using directed acyclic graphs (DAGs, data not shown) including subcohort, women’s country of birth, previous breastfeeding, parity history, prepregnancy BMI, and gestational week at blood collection. Other potential confounders were evaluated and retained if adjustment caused a change in effect estimates for PFASs and the study outcomes; thus, the fully adjusted models also included physical activity and rMED.
We performed sensitivity analyses of associations between PFASs and IGT after excluding women from the Valencia subcohort (because of missing information about their IGT status). In addition, we estimated associations with all metabolic outcomes using multiple-pollutant models adjusted simultaneously for all four PFAS compounds.
To facilitate the interpretation of effect estimates for continuous outcomes, coefficients are expressed as relative percent change in medians (Barrera-Gómez and Basagaña 2015). The level of statistical significance was set to a two-sided . All analyses were conducted using STATA statistical software (version 12.0; StataCorp LLC).
Results
Study Population Characteristics
Nearly all of the women were born in Spain (93.2%), and most were working during the first pregnancy trimester (73.6%); approximately half were of lower social class (IV–V: 46.9%) and nulliparous (56.1%) (Table 1). Most women had never breastfed (60.7%) and did not report smoking during pregnancy (68.6%), and 18.5% and 7.9% were classified as overweight and obese, respectively, based on their BMI before pregnancy (Table 1). Compared with women excluded from the present analysis, those who were included were slightly older (mean age 31.9 y vs. 31.4 y) and were more likely to be born in Spain (93.2% versus 86.5%), to have a university education (35.1% versus 29.2%), to be of higher social class (Class I–II: 24% vs. 17%), and to have lower average total energy intake ( vs. ) (Table 1).
Table 1.
Characteristics of pregnant women included and excluded from the analysis.
| Characteristic | Included ()a | Excluded ()a | p-Valueb | ||
|---|---|---|---|---|---|
| or % | or % | ||||
| Sociodemographic factors | |||||
| Subcohort | |||||
| Gipuzkoa | 322 | 25.9% | 316 | 34.7% | |
| Sabadell | 411 | 33.1% | 246 | 27.1% | |
| Valencia | 507 | 40.9% | 348 | 38.2% | |
| Country of birth | |||||
| Spanish | 1,151 | 93.2% | 761 | 86.5% | |
| Others | 84 | 6.8% | 119 | 13.5% | |
| Missing | 5 | 0.4% | 30 | 3.3% | |
| Maternal age (y) | 1,237 | 695 | 0.04 | ||
| Missing | 3 | 0.2% | 215 | 24% | |
| Parity | |||||
| Nulliparous | 695 | 56.1% | 473 | 53.7% | 0.28 |
| Multiparous | 543 | 43.9% | 407 | 46.3% | |
| Missing | 2 | 0.2% | 66 | 7.2% | |
| Breastfeeding history | |||||
| None | 751 | 60.7% | 531 | 60.3% | 0.46 |
| 0–6 months | 213 | 17.1% | 156 | 17.8% | |
| 274 | 22.2% | 193 | 21.9% | ||
| Missing | 2 | 0.2% | 30 | 3.2% | |
| Education | |||||
| Primary education or less | 282 | 22.8% | 281 | 31.9% | |
| Secondary education | 521 | 42.1% | 341 | 38.8% | |
| University degree | 434 | 35.1% | 257 | 29.2% | |
| Missing | 3 | 0.2% | 31 | 3.4% | |
| Social class | |||||
| I–II: Professionals and managers | 297 | 23.9% | 150 | 17% | |
| III: Skilled manual/nonmanual | 361 | 29.1% | 207 | 23.5% | |
| IV–V: Semiskilled/unskilled | 582 | 46.9% | 525 | 59.5% | |
| Lifestyle factors | |||||
| Pre-BMI ()c | |||||
| Underweight | 54 | 4.3% | 47 | 5.4% | 0.63 |
| Normal | 858 | 69.2% | 615 | 69.9% | |
| Overweight | 229 | 18.5% | 152 | 17.3% | |
| Obese | 99 | 7.9% | 65 | 7.4% | |
| Smoking during pregnancy | |||||
| Never smoked | 841 | 68.6% | 507 | 65.6% | 0.35 |
| Smoked until 1st trimester | 187 | 15.2% | 125 | 16.2% | |
| Smoked until 3rd trimester | 198 | 16.1% | 141 | 18.2% | |
| Missing | 14 | 1.1% | 137 | 15% | |
| Alcohol intake at 1st trimester [GM (GSD)] | 1,234 | 0.3 (5.2) | 900 | 0.3 (4.8) | 0.27 |
| Missing | 6 | 0.5% | 10 | 1.1% | |
| Physical activity during 1st trimester (METs/hour/day) | 1,235 | 866 | 0.97 | ||
| Missing | 5 | 0.4% | 44 | 4.8% | |
| Relative Mediterranean diet score | |||||
| Low | 389 | 31.7% | 281 | 32.9% | 0.97 |
| Middle | 551 | 44.9% | 376 | 44.1% | |
| High | 286 | 23.3% | 197 | 23.1% | |
| Missing | 14 | 1.1% | 56 | 6.1% | |
| Total energy intake (kcal/day) | 1,234 | 869 | |||
| Missing | 6 | 0.5% | 41 | 4.5% | |
Note: BMI, body mass index; GM, geometric mean; GSD, geometric standard deviation; MET, metabolic equivalent of task; SD, standard deviation.
Some covariates include fewer observations because of missing values.
p-Value for the comparison between participants included and not included in analysis; Chi-squared test for percentage values comparisons and Student’s t-test for media values comparisons.
Classification according to World Health Organization (WHO) criteria.
The overall prevalences of GDM and IGT (including GDM cases) were 4.3% and 11%, respectively (Table 2). The INMA-Sabadell subcohort had the highest prevalence of IGT (17%), and the INMA-Valencia subcohort had the highest prevalence of GDM (5.3%). INMA study participants who were excluded from the present analysis were less likely to be diagnosed with GDM (2.6%) or IGT (6.6%) than women who were included. Compared with women who were excluded, women included in the present analysis had similar mean cholesterol ( vs. ) and geometric mean CRP concentrations ( for both groups), but slightly lower geometric mean triglyceride concentrations ( vs. , ). Women from the INMA-Valencia subcohort had higher average cholesterol and triglycerides than women from the other two cohorts (Table 3).
Table 2.
Prevalence [(%)] of gestational diabetes mellitus and impaired glucose tolerance in pregnant women from the INMA birth cohort study.
| Prevalence status | All INMA subcohorts | INMA-Valencia | INMA-Sabadell | INMA-Gipuzkoa | Women excluded from analysis (all INMA subcohorts) | p-Valuea |
|---|---|---|---|---|---|---|
| GDM | ||||||
| No | 1,187 (95.7) | 480 (94.7) | 397 (96.6) | 310 (96.3) | 886 (97.4) | 0.05 |
| Yes | 53 (4.3) | 27 (5.3) | 14 (3.4) | 12 (3.7) | 24 (2.6) | |
| IGTb | ||||||
| No | 1,103 (88.9) | 480 (94.7)c | 323 (83.0) | 300 (87.2) | 850 (93.4) | 0.02 |
| Yes | 137 (11.1) | 27 (5.3)c | 66 (17.0) | 44 (12.8) | 60 (6.6) |
Note: GDM, gestational diabetes mellitus; IGT, impaired glucose tolerance; INMA, Environment and Childhood Project (INfancia y Medio Ambiente).
Chi-squared test p-value comparing prevalences between participants included and excluded from analysis.
Cases include women diagnosed with GDM (all subcohorts) and women diagnosed with IGT (Sabadell and Gipuzkoa subcohorts only).
IGT cases and noncases in the Valencia cohort are classified based on GDM only because IGT data were not available for this subcohort.
Table 3.
Serum lipids and C-reactive protein in pregnant women from the INMA birth cohort study.
| Serum lipid/protein levels | Min–Max | Mean (SD) | P25 | P50 | P75 | |
|---|---|---|---|---|---|---|
| Cholesterol (mg/dL)a | ||||||
| All subcohorts | 1,194 | 97–324 | 195 (33) | 174 | 192 | 214 |
| INMA-Valencia | 479 | 119–324 | 201 (33) | 180 | 199 | 220 |
| INMA-Sabadell | 398 | 105–283 | 190 (31) | 170 | 188 | 208 |
| INMA-Gipuzkoa | 317 | 97–321 | 193 (34) | 171 | 188 | 211 |
| Triglycerides (mg/dL)a | ||||||
| All subcohorts | 1194 | 35–374 | 105 (42.8) | 76 | 96 | 126 |
| INMA-Valencia | 479 | 38–374 | 111 (45) | 81 | 103 | 136 |
| INMA-Sabadell | 398 | 35–333 | 107 (44) | 77 | 96 | 127 |
| INMA-Gipuzkoa | 317 | 40–250 | 94 (35) | 69 | 86 | 112 |
| CRP (mg/dL)b | ||||||
| All subcohorts | 651 | 0.03–6.5 | 0.6 (0.7) | 0.2 | 0.4 | 0.7 |
| INMA-Valencia | NA | NA | NA | NA | NA | NA |
| INMA-Sabadell | 329 | 0.02–6.5 | 0.7 (0.7) | 0.2 | 0.3 | 0.6 |
| INMA-Gipuzkoa | 322 | 0.03–5.4 | 0.6 (0.7) | 0.2 | 0.3 | 0.6 |
Note: CRP, C-reactive protein; INMA, Environment and Childhood Project (INfancia y Medio Ambiente); Max, maximum value; Min, minimum value; NA, not available; P25, percentile 25; P50, percentile 50; P75, percentile 75; SD, standard deviation.
Data for cholesterol and triglycerides were missing for a total of 46 women from Valencia (), Sabadell (), and Gipuzkoa ().
Data for CRP were missing for a total of 589 women from Valencia () and Sabadell ().
PFOS and PFOA concentrations were quantified in all samples analyzed, and PFHxS and PFNA concentrations were below the LOQ in 3.7% and 0.65% of samples, respectively (Table 4). PFOS had the highest geometric mean concentration, followed by PFOA, PFNA, and PFHxS. PFOA and PFNA were the most highly correlated PFASs (Spearman’s ), followed by PFOA and PFHxS (). For other pairs of PFASs, the correlation coefficients ranged from 0.44 to 0.52.
Table 4.
Plasma PFAS concentrations (nanograms/milliliter) in pregnant women participating in the INMA birth cohort study ().
| PFAS | a(%) | GM (GSD) | Min | Percentiles | Max | ||||
|---|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | |||||
| PFOA | 0 | 2.31 (1.71) | 0.28 | 0.96 | 1.63 | 2.35 | 3.30 | 5.23 | 31.64 |
| PFOS | 0 | 5.77 (1.61) | 0.28 | 2.52 | 4.51 | 6.05 | 7.81 | 11.35 | 38.58 |
| PFHxS | 46 (3.71) | 0.55 (1.96) | 0.05 | 0.24 | 0.40 | 0.58 | 0.82 | 1.39 | 11.00 |
| PFNA | 8 (0.65) | 0.64 (1.75) | 0.03 | 0.28 | 0.49 | 0.65 | 0.90 | 1.49 | 5.51 |
Note: GM, geometric mean; GSD, geometric standard deviation; INMA, Environment and Childhood Project (INfancia y Medio Ambiente); LOQ, limit of quantification; Max, maximum value; Min, minimum value; PFAS, perfluoroalkyl substance; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate.
The LOQ was for PFHxS, PFOS, PFOA and for PFNA.
Associations between PFAS Exposures and Metabolic Outcomes
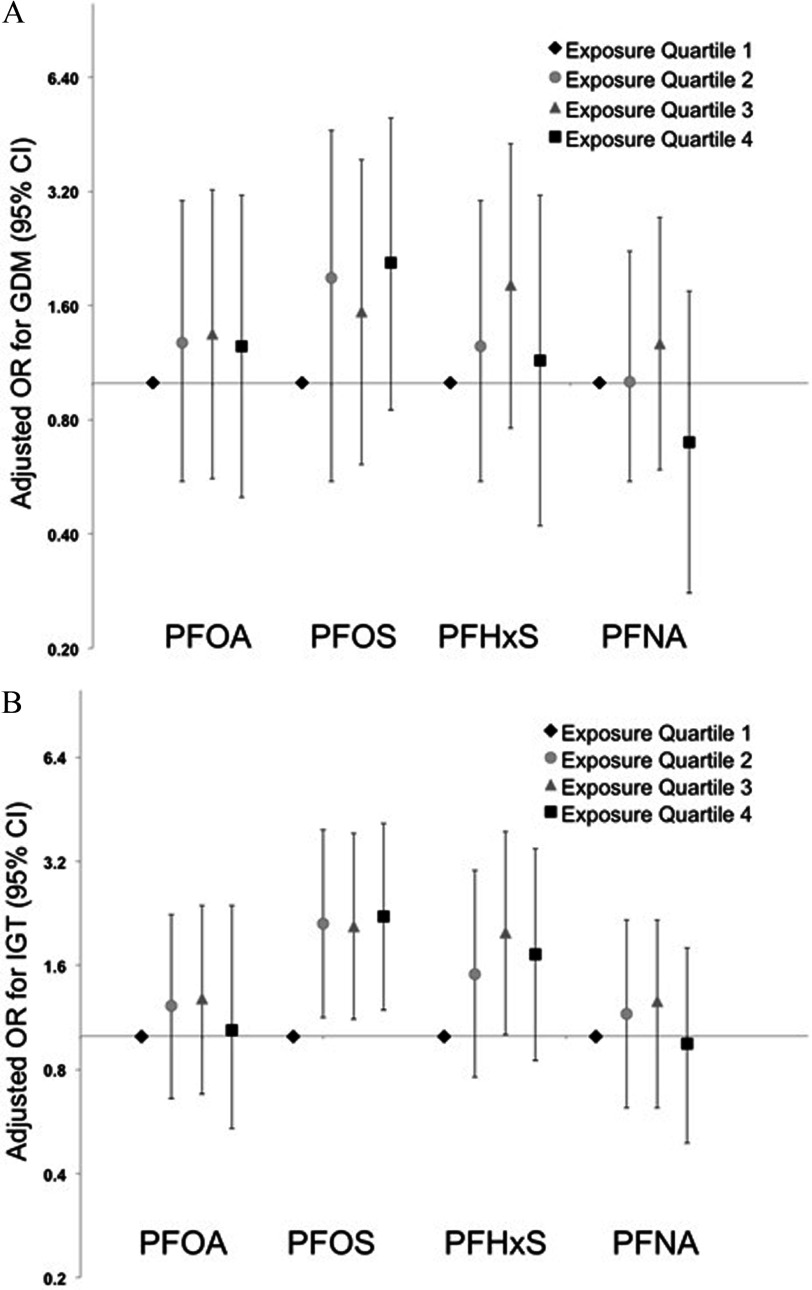
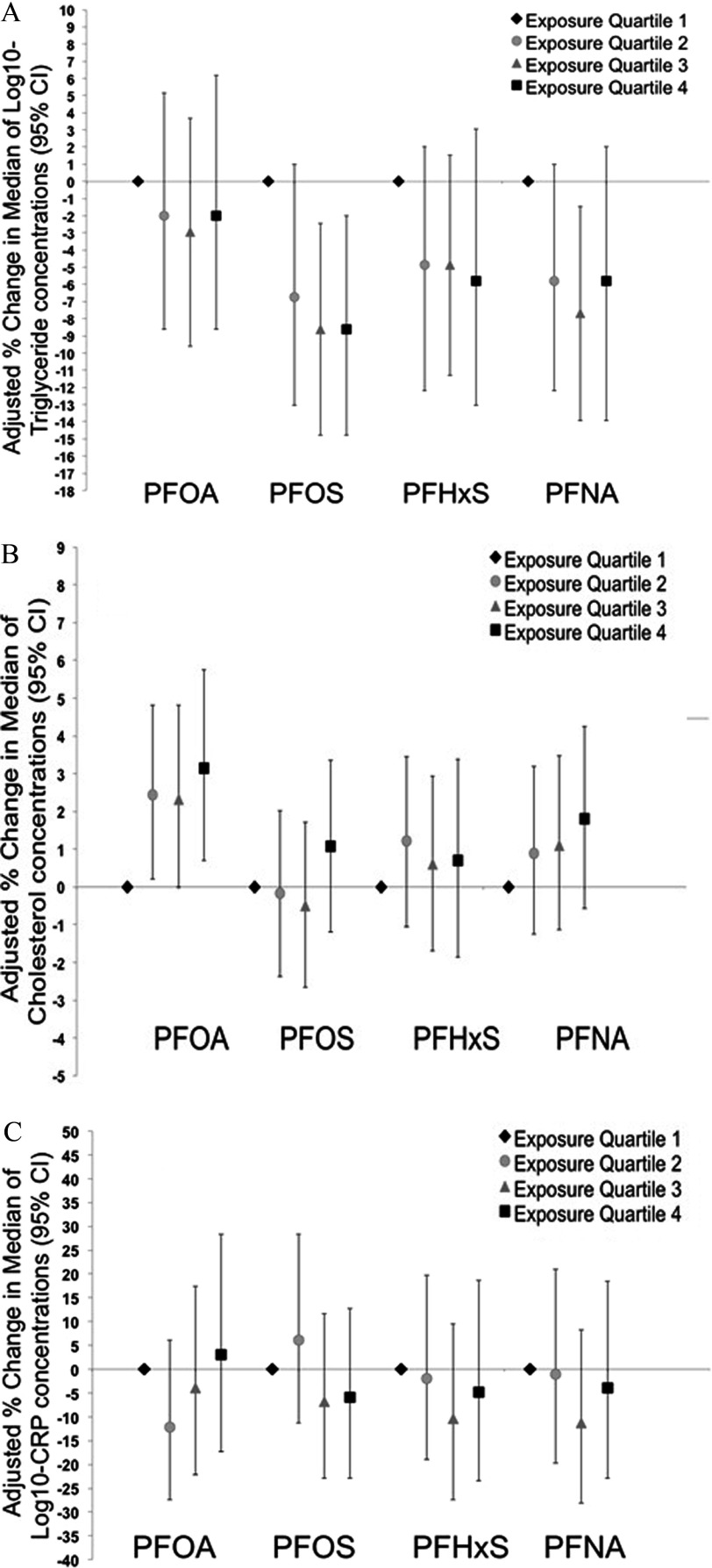
Association estimates from basic and fully adjusted models were generally consistent, with a nonsignificant positive association between PFOS and GDM [ cases; adjusted OR for a 1-unit increase in (95% CI: 0.93, 6.18)], without a clear monotonic dose–response trend (Table 5 and Figure 1A). PFHxS was also positively associated with GDM, although ORs were closer to the null and were not significant. ORs for IGT ( cases) were more precise, with a positive association with PFOS [OR for a 1-unit increase in (95% CI: 1.06, 3.78)] that was similar for all quartiles relative to the lowest quartile (Table 5 and Figure 1B). As for GDM, PFHxS was also positively associated with IGT, although ORs were closer to the null than for PFOS. Associations between PFASs and IGT were generally consistent with the main analyses when women from the Valencia cohort were excluded, although the estimates were less precise ( observations and 108 cases only) (Table 5). All four PFASs were negatively associated with serum triglyceride concentrations, although associations were close to the null for PFOA and somewhat stronger for PFOS [percent change in the median per unit increase in (95% CI: , )] and PFNA [percent change per unit increase in (95% CI: , )] than for PFHxS, and estimated associations were similar for all quartiles relative to the lowest quartile (Table 6 and Figure 2A). Associations between PFASs and triglycerides were nonlinear for PFHxS and PFNA (see Table S1). Serum cholesterol levels were positively associated with PFOA [percent change in median per unit increase in (95% CI: 0.01, 2.54%)], with similar effect estimates for all quartiles (Table 6 and Figure 2B). However, associations between total cholesterol and the other PFASs were essentially null. Associations between PFASs and CRP were based on only 640 observations (because of missing CRP data for Valencia women), and the results were imprecise and inconclusive (Table 6 and Figure 2C).
Table 5.
Odds ratios (95% CI) for the associations of PFASs with gestational diabetes mellitus and impaired glucose tolerance.
| PFAS (ng/mL) | GDM 53 cases, 1,161 noncases | IGT (all subcohorts)a 135 cases, 1,079 noncases | IGT (Sabadell and Gipzukoa only)b108 cases, 611 noncases | |||||
|---|---|---|---|---|---|---|---|---|
| cases/noncases | Basic modelc OR (95% CI) | Fully adjustedd OR (95% CI) | cases/noncases | Basic modelc OR (95% CI) | Fully adjustedd OR (95% CI) | cases/noncases | Fully adjustedd OR (95% CI) | |
| PFOA | ||||||||
| Q1: | 10/295 | Reference | Reference | 21/284 | Reference | Reference | 17/169 | Reference |
| Q2: | 14/292 | 1.46 (0.63, 3.37) | 1.28 (0.55, 3.02) | 34/272 | 1.30 (0.72, 2.35) | 1.22 (0.66, 2.25) | 27/152 | 1.17 (0.57, 2.41) |
| Q3: | 15/287 | 1.62 (0.70, 3.78) | 1.35 (0.56, 3.23) | 41/261 | 1.37 (0.76, 2.48) | 1.28 (0.68, 2.39) | 34/145 | 1.22 (0.58, 2.58) |
| Q4: 3.30 to 31.64 | 14/287 | 1.53 (0.64, 3.69) | 1.25 (0.50, 3.13) | 39/262 | 1.15 (0.63, 2.12) | 1.04 (0.54, 2.39) | 30/145 | 0.86 (0.39, 1.89) |
| Per increase | 53/1161 | 1.34 (0.74, 2.44) | 1.20 (0.62, 2.30) | 135/1079 | 1.24 (0.83, 1.86) | 1.24 (0.78, 1.94) | 108/611 | 1.19 (0.69, 2.03) |
| PFOS | ||||||||
| Q1: | 8/298 | Reference | Reference | 18/288 | Reference | Reference | 16/175 | Reference |
| Q2: | 15/289 | 1.89 (0.79, 4.53) | 1.89 (0.77, 4.64) | 35/269 | 2.26 (1.24, 4.12) | 2.11 (1.13, 3.94) | 27/146 | 1.95 (0.97, 3.95) |
| Q3: | 13/291 | 1.64 (0.67, 4.03) | 1.54 (0.61, 3.87) | 40/264 | 2.22 (1.22, 4.02) | 2.08 (1.12, 3.86) | 32/144 | 1.92 (0.96, 3.85) |
| Q4: 7.81 to 38.58 | 17/283 | 2.21 (0.93, 5.21) | 2.07 (0.85, 5.01) | 42/258 | 2.36 (1.31, 4.25) | 2.22 (1.19, 4.13) | 33/146 | 2.02 (1.00, 4.07) |
| Per increase | 53/1161 | 2.57 (1.05, 6.25) | 2.40 (0.93, 6.18) | 135/1079 | 2.06 (1.16, 3.68) | 1.99 (1.06, 3.78) | 108/611 | 1.98 (0.96, 4.09) |
| PFHxS | ||||||||
| Q1: | 9/293 | Reference | Reference | 15/287 | Reference | Reference | 11/168 | Reference |
| Q2: | 13/292 | 1.44 (0.61, 3.45) | 1.25 (0.51, 3.03) | 24/281 | 1.61 (0.82, 3.16) | 1.51 (0.76, 3.02) | 18/133 | 1.85 (0.81, 4.22) |
| Q3: | 19/283 | 2.29 (0.99, 5.29) | 1.81 (0.76, 4.28) | 42/260 | 2.28 (1.19, 4.35) | 1.99 (1.01, 3.90) | 30/133 | 1.88 (0.82, 4.31) |
| Q4: 0.82 to 11.00 | 12/293 | 1.63 (0.62, 4.27) | 1.15 (0.42, 3.12) | 54/251 | 2.15 (1.11, 4.18) | 1.72 (0.85, 3.49) | 49/177 | 1.84 (0.79, 4.28) |
| Per increase | 53/1161 | 2.06 (0.99, 4.24) | 1.58 (0.73, 3.44) | 135/1079 | 1.89 (1.19, 3.02) | 1.65 (0.99, 2.76) | 108/611 | 1.51 (0.85, 2.68) |
| PFNA | ||||||||
| Q1: | 14/289 | Reference | Reference | 20/283 | Reference | Reference | 9/109 | Reference |
| Q2: | 13/295 | 0.99 (0.46, 2.17) | 1.01 (0.62, 2.23) | 29/279 | 1.08 (0.58, 1.99) | 1.16 (0.62, 2.17) | 23/151 | 1.64 (0.69, 3.87) |
| Q3: | 17/285 | 1.40 (0.66, 2.98) | 1.27 (0.59, 2.73) | 46/256 | 1.41 (0.58, 1.99) | 1.26 (0.68, 2.33) | 38/169 | 1.50 (0.65, 3.48) |
| Q4: 0.90 to 5.51 | 9/292 | 0.74 (0.31, 1.82) | 0.70 (0.28, 1.75) | 40/261 | 0.96 (0.81, 2.62) | 0.95 (0.49, 1.80) | 38/182 | 1.36 (0.58, 3.19) |
| Per increase | 53/1161 | 0.93 (0.46, 1.89) | 0.85 (0.40, 1.80) | 135/1079 | 1.08 (0.67, 1.74) | 0.95 (0.57, 1.60) | 108/611 | 1.06 (0.57, 1.96) |
Note: CI, confidence interval; GDM, gestational diabetes mellitus; IGT, impaired glucose tolerance; OR, odds ratio; PFAS, perfluoroalkyl substance; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; Q1–Q4, quartiles 1 through 4.
IGT cases include women diagnosed with GDM from all 3 subcohorts, plus women diagnosed with IGT only (without GDM) from the Sabadell and Gipzukoa subcohorts.
Sensitivity analysis of women diagnosed with IGT (without GDM) from the Sabadell and Gipzukoa subcohorts only.
Basic models are adjusted for subcohort only.
Fully adjusted models include subcohort, country of birth, prepregnancy body mass index, previous breastfeeding, parity, gestational week at blood extraction, physical activity, and relative Mediterranean Diet Score.
Figure 1.
Adjusted odds ratios (ORs) [95% confidence intervals (CIs)] for the associations between quartile-specific perfluoroalkyl substances (PFAS)-exposure groups and gestational diabetes mellitus (GDM) (A) and impaired glucose tolerance (IGT) (B). All models are adjusted for subcohort, country of birth, prepregnancy body mass index, previous breastfeeding, parity, gestational week at blood extraction, physical activity, and relative Mediterranean Diet Score (rMED). See Table 4 for corresponding numeric data. PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate.
Table 6.
Percentage of median change (95% CI) for associations of PFASs (categorized into quartiles or continuous ) with (milligrams/deciliter), total cholesterol (milligrams/deciliter) and protein (milligrams/deciliter).
| PFAS (ng/mL) | (mg/dL) | Total cholesterol (mg/dL) | protein (mg/dL) | |||
|---|---|---|---|---|---|---|
| Basic modela % change (95% CI) | Fully adjustedb% change (95% CI) | Basic modela% change (95% CI) | Fully adjustedb% change (95% CI) | Basic modela% change (95% CI) | Fully adjustedb% change (95% CI) | |
| PFOA | ||||||
| Q1: | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2: | (, 3.05) | (, 5.13) | 4.85 (, 10.1) | 2.43 (0.20, 4.81) | (, 5.13) | (, 6.18) |
| Q3: | (, ) | (, 6.13) | 3.70 (, 9.18) | 2.33 (, 4.81) | (, 10.5) | (, 17.3) |
| Q4: 3.30 to 31.64 | (, 0.38) | (, 6.18) | 5.24 (, 10.9) | 3.15 (0.70, 5.76) | (, 10.0) | 3.05 (, 28.4) |
| Per increase | (, ) | (, 1.42) | 0.90 (, 2.14) | 1.26 (0.01, 2.54) | (, 7.31) | 2.86 (, 14.3) |
| PFOS | ||||||
| Q1: | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2: | (, ) | (, 1.01) | (, 4.30) | (, 2.02) | 5.13 (, 23.7) | 6.18 (, 28.4) |
| Q3: 0 | (, ) | (, ) | (, 4.99) | (, 1.71) | (, 10.5) | (, 11.6) |
| Q4: 7.81 to 38.58 | (, ) | (, ) | 2.79 (, 8.04) | 1.08 (, 3.36) | (, 11.2) | (, 12.7) |
| Per increase | (, ) | (, ) | 1.17 (, 2.59) | 0.88 (, 2.37) | (, ) | (, 3.35) |
| PFHxS | ||||||
| Q1: | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2: | (, ) | (, 2.02) | 2.95 (, 8.22) | 1.21 (, 3.45) | (, 7.2) | (, 19.7) |
| Q3: | (, 3.05) | (, 2.02) | 3.06 (, 8.53) | 0.60 (, 2.94) | (, 8.42) | (, 9.42) |
| Q4: 0.82 to 11.00 | (, 1.40) | (, 3.05) | 3.70 (, 9.62) | 0.70 (, 3.38) | (, 13.0) | (, 18.7) |
| Per increase | (, ) | (, 1.45) | 0.43 (, 1.91) | (, 1.45) | (, 3.05) | (, 4.40) |
| PFNA | ||||||
| Q1: | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2: | (, ) | (, 1.01) | 0.85 (, 6.15) | 0.90 (, 3.19) | 1.01 (, 21.4) | (, 20.9) |
| Q3: | (, ) | (, ) | 4.12 (, 9.56) | 1.11 (, 3.47) | (, 8.30) | (, 8.33) |
| Q4: 0.90 to 5.51 | (, ) | (, 2.02) | 3.61 (, 9.23) | 1.81 (, 4.24) | (, 10.6) | (, 18.5) |
| Per increase | (, ) | (, ) | 0.55 (, 1.82) | 0.46 (, 1.70) | (, 7.88) | 1.22 (, 12.3) |
Note: Effect estimates from the linear regression models expressed as percent change in medians per increase in exposure. CI, confidence interval; OR, odds ratio; PFAS, perfluoroalkyl substance; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; Q1–Q4, quartiles 1 through 4.
Basic models are adjusted for subcohort only.
Fully adjusted models include subcohort, country of birth, prepregnancy body mass index, previous breastfeeding, parity, gestational week at blood extraction, physical activity, and relative Mediterranean Diet Score.
Figure 2.
Adjusted percent changes [95% confidence intervals (CIs)] in median concentrations of (A), total cholesterol (B), and protein (C) per quartile PFAS-exposure group. All models are adjusted for subcohort, country of birth, prepregnancy body mass index, previous breastfeeding, parity, gestational week at blood extraction, physical activity, and relative Mediterranean Diet Score (rMED). See Table 5 for corresponding numeric data. CRP, C-reactive protein; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate.
Sensitivity Analyses
In the multivariate-adjusted models including all four PFAS compounds, associations with the odds for GDM and IGT were strengthened for PFOS and attenuated for PFHxS (see Table S2). Further, the positive association between PFOA and serum total cholesterol levels increased in magnitude [percent change in median per unit increase in (95% CI: 0.20, 3.93%)], whereas associations of PFOS and PFNA with triglyceride levels were attenuated (see Table S3).
Discussion
Findings from this large study provide evidence for associations between PFAS exposures and metabolic outcomes in pregnant women. Plasma PFOS concentrations were positively associated with both IGT and GDM, although estimates for GDM were based on fewer cases and were less precise, and ORs were comparable for all quartiles relative to the lowest exposure category. PFHxS was also positively associated with both outcomes, although ORs were closer to the null than those for PFOS. Further, PFOA concentrations were associated with a slight increase in nonfasting serum cholesterol levels, whereas PFOS and PFNA concentrations were associated with small decreases in serum triglyceride levels. In multipollutant models adjusted for all PFAS compounds, the associations between PFOS concentrations and the odds for IGT or GDM and those between PFOA and cholesterol levels were strengthened, whereas associations for PFHxS and PFNA with the study outcomes were attenuated. Moreover, we did not find evidence for associations between PFAS exposures and serum CRP levels, although this analysis was based on only a subset of the study population (), which may in part explain the imprecise coefficients.
To our knowledge, only three previous epidemiologic studies, with median concentrations of PFOS, PFOA, or both that were two to three times as high as those in our population, have evaluated the associations between PFAS concentrations and risk for GDM. In a prospective cohort study of 258 U.S. women, PFOA concentrations were significantly associated with self-reported GDM, whereas ORs for PFOS, PFNA, and four additional PFASs were close to the null (Zhang et al. 2015). In a prospective study of Canadian women, PFHxS was positively associated with IGT (49 cases vs. 1,102 women with normal blood glucose), but the association was nonlinear and was strongest for the second versus first quartile (Shapiro et al. 2016). In addition, there was only a weak positive association between PFHxS and GDM (44 cases), and there was no evidence of positive associations between either outcome and PFOA or PFOS. In a study of pregnant Faroese women (49 GDM cases vs. 555 women without GDM), no clear association with GDM was found for PFOS, PFOA, PFHxS, PFNA, perfluorodecanoic acid (PFDA), or overall PFAS exposure (Valvi et al. 2017). In the present study, associations derived using a multipollutant model that included all four PFASs were substantially attenuated for PFHxS and IGT, whereas associations between PFOS and IGT were comparable to those found using the single-pollutant models. Although we found some evidence of a positive association between PFOS and GDM, our findings for GDM (including null findings for other PFASs) were imprecise owing to the small number of cases () and should therefore be interpreted with caution. In addition, women were evaluated for IGT and GDM at their physician’s discretion, which may have resulted in some women being misclassified as noncases for both outcomes. Moreover, we did not have information on IGT (without GDM) for women from the Valencia subcohort, although ORs for associations between IGT and PFOS and PFHxS when Valencia women were excluded were generally consistent with those from the primary analysis.
Studies using animal models have indicated that PFOS and PFOA may interfere with the phosphatidylinositol 3-kinase-serine/threonine protein kinase (PI3K-AKT) signaling pathway, which plays an important role in the metabolic actions of insulin (Yan et al. 2015). PFOA exposure decreased expression of the PI3-AKT signaling pathway in rats (Yan et al. 2015), and in vitro experiments using human hepatoma HepG2 cells suggested that PFOS exposure may also inhibit AKT activation, leading to insulin resistance (Qiu et al. 2016). Therefore, the associations of PFOS and IGT observed in our study could be causal and require further confirmation in other populations. Such confirmation is critical because GDM is associated with short- and long-term adverse outcomes in the mother and in her offspring, including an increased risk of fetal macrosomia (i.e., high birth weight) and hyperinsulinemia, as well as with higher risks of cesarean section and hypertensive disorders in the mother (Kampmann et al. 2015). Moreover, women diagnosed with GDM are more likely to develop type 2 diabetes, metabolic syndrome, and cardiovascular disease after pregnancy, and their children are at increased risk of childhood obesity and glucose intolerance (Kampmann et al. 2015).
A previous cross-sectional study of Norwegian women () reported a positive association between PFOS and total cholesterol and weaker positive associations for all but one of the other six PFASs evaluated (Starling et al. 2014). PFOS and other PFASs were also associated with higher HDL cholesterol, a component of total cholesterol that is generally considered to be beneficial to health. PFOS and PFOA were also positively associated with serum total cholesterol levels in late pregnancy (approximately 30 wk) in a large Danish cohort () that did not evaluate associations with HDL or other PFASs (Skuladottir et al. 2015). In the present study, we measured PFAS exposures and total cholesterol (but not HDL) at an earlier stage of pregnancy (approximately 13 wk), and we found evidence of a weak positive association between total cholesterol and PFOA, whereas associations with other PFASs were essentially null. Differences in the timing of exposure and outcome measurements and differences in exposure ranges (which were higher for PFOS in both previous studies, and for PFOA in the Danish study, compared with the present study) may have contributed to inconsistencies among the studies. In addition, individual PFAS concentrations were moderately to highly correlated in all three study populations, which makes it difficult to attribute associations to specific individual PFAS compounds. A variety of noncausal mechanisms may also contribute to, or explain, inconsistent findings, including random error, selection bias, confounding, and differences in susceptibility related to coexposures, comorbid conditions, or other factors. We found evidence of negative associations with triglyceride levels, particularly for PFOS and PFNA, although effect estimates were similar for all quartiles above the reference level, and they were attenuated in the multipollutant adjusted model. Starling et al. (2014) examined seven PFASs and triglycerides in Norwegian women, but the results did not support clear dose–response relationships overall [although there were statistically significant associations for the fourth vs. first quartile of perfluoroundecanoic acid (PFUnDA) and the second vs. first quartile of perfluoroheptane sulfonate (PFHpS)]. In an in vitro study, PFASs with 4–12 carbons (including the compounds we studied) activated the human peroxisome proliferator-activated receptor () in transiently transfected COS-1 cells (Wolf et al. 2012). regulates fatty acid uptake and metabolism, and its activation may reduce plasma triglycerides and increase HDL cholesterol levels (Shah et al. 2010). However, additional observational and experimental studies are needed to confirm associations and to investigate possible PPAR-dependent or independent mechanisms for effects of PFASs on lipid metabolism.
It has been proposed that PFAS exposures may affect health by increasing inflammation (Lau et al. 2007), and serum CRP is a well-established clinical marker of systemic inflammation. However, information on CRP was available for only a subset of the study population, and the overall results were imprecise and inconclusive. Larger studies integrating inflammatory markers beyond CRP are needed to evaluate any potential role of inflammation in PFAS-related metabolic effects.
Compared with INMA participants who were excluded from the present analysis (because of missing information for PFASs or the study outcomes), women who were included were more likely to have been born in Spain, to have higher levels of education and social class, and to be older. Included women also had a higher prevalence of IGT and GDM and somewhat lower triglyceride serum levels and total energy intake. In addition, a previous analysis of the INMA cohort showed that women born outside Spain had lower average PFAS concentrations than women born in Spain (Manzano-Salgado et al. 2016). Therefore, findings for the women included in the present analysis may not be representative of the INMA cohort population overall. Further, cross-sectional associations between PFAS and total cholesterol and triglycerides might be explained by reverse causation if, for example, PFAS pharmacokinetics are affected by lipid levels, or if PFAS and lipid concentrations are both influenced by a common factor. Total cholesterol and triglycerides were measured in nonfasting samples collected from the pregnant women in our study population, which may have resulted in values that were not representative of the usual lipid levels in some women. Although our study had a number of strengths, we cannot rule out the potential influence of random error, bias due to uncontrolled confounding, outcome misclassification, or other methodological issues that might produce misleading results, and it will be important for our findings to be confirmed in additional study populations. Strengths of this study include the assessment of PFAS exposures using plasma biomarkers. The long elimination half-lives of PFOA, PFOS and PFHxS in human blood are estimated to be from 3 to 9 y (Olsen et al. 2007); thus, one single PFAS measurement may reflect chronic exposure. Moreover, we measured PFAS concentrations early in pregnancy, which may reduce the likelihood of confounding due to physiological changes of gestation, such as changes in the glomerular filtration rate (Verner et al. 2015). The large sample size and the broad list of measured confounders are other important strengths of this study.
Conclusion
Our findings suggest that PFAS exposures may influence lipid metabolism and glucose tolerance during pregnancy. To our knowledge, this is the largest study of associations between PFAS exposures and multiple metabolic outcomes in pregnant women to date. Our findings require confirmation but are worthy of further exploration, given their potential implications for the short- and long-term health of mothers and their children.
Supplemental Material
Acknowledgments
The authors are grateful to all the participants for their generous collaboration. A full roster of the Environment and Childhood Project (INfancia y Medio Ambiente) (INMA) project investigators can be found at http://www.proyectoinma.org/presentacion-inma/listado-investigadores/en listado-investigadores.html.
This study was funded in part by grants from the European Union (FP7-ENV-2011 cod 282957 and HEALTH.2010.2.4.5-1), the Instituto de Salud Carlos III, the Spanish Ministry of Health (Red INMA G03/176; CB06/02/0041; FIS-PI12/01890, FIS-PI041436, FIS- PI081151, FIS-PI06/0867, FIS-PS09/00090; FIS-FEDER: 03/1615, 04/1509, 04/1112, 04/1931, 05/1079, 05/1052, 06/1213, 07/0314, 09/02647, 11/01007, 11/02591, 11/02038, 13/1944, 13/2032, 14/00891, and 14/01687; Miguel Servet-FEDER CP11/0178, MS13/00054, and CPII16/00051; and PFIS-FI14/00099), Generalitat Valenciana (FISABIO: UGP 15-230, UGP-15-244, and UGP-15-249), the Department of Health of the Basque Government (2005111093 and 2009111069), the Provincial Government of Gipuzkoa (DFG06/004 and DFG08/001), the Generalitat de Catalunya-CIRIT (1999SGR 00241), and the National Institutes of Health/National Institute of Environmental Health Sciences (grant number ES021477). This study has been reviewed and approved by the accredited committees of the following institutions: The Municipal Institute of Sanitary Assistance of Barcelona, La Fe University Hospital of Valencia, and The Donostia Hospital.
References
- Bach CC, Bech BH, Brix N, Nohr EA, Bonde JPE, Henriksen TB. 2015. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: a systematic review. Crit Rev Toxicol 45(1):53–67, PMID: 25372700, 10.3109/10408444.2014.952400. [DOI] [PubMed] [Google Scholar]
- Barrera-Gómez J, Basagaña X. 2015. Models with transformed variables. Epidemiology 26(2):e16–e17, PMID: 25643111, 10.1097/EDE.0000000000000247. [DOI] [PubMed] [Google Scholar]
- Berg V, Nøst TH, Huber S, Rylander C, Hansen S, Veyhe AS, et al. 2014. Maternal serum concentrations of per- and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environ Int 69:58–66, PMID: 24815340, 10.1016/j.envint.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Caër C, Rouault C, Le Roy T, Poitou C, Aron-Wisnewsky J, Torcivia A, et al. 2017. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci Rep 7(1):3000, PMID: 28592801, 10.1038/s41598-017-02660-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals-Casas C, Desvergne B. 2011. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol 73:135–162, PMID: 21054169, 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- de Cock M, van de Bor M. 2014. Obesogenic effects of endocrine disruptors, what do we know from animal and human studies?. Environ Int 70:15–24, PMID: 24879368, 10.1016/j.envint.2014.04.022. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. 2012. Immunotoxicity of perfluorinated compounds: Recent developments. Toxicol Pathol 40(2):300–311, PMID: 22109712, 10.1177/0192623311428473. [DOI] [PubMed] [Google Scholar]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. 2003. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 26(suppl1):S5–S20, PMID: 12502614. [DOI] [PubMed] [Google Scholar]
- Fernández-Barrés S, Romaguera D, Valvi D, Martínez D, Vioque J, Navarrete-Muñoz EM, et al. 2016. Mediterranean dietary pattern in pregnant women and offspring risk of overweight and abdominal obesity in early childhood: the INMA birth cohort study. Pediatr Obes 11(6):491–499, PMID: 26763767, 10.1111/ijpo.12092. [DOI] [PubMed] [Google Scholar]
- Gebbink WA, Glynn A, Berger U. 2015. Temporal changes (1997–2012) of perfluoroalkyl acids and selected precursors (including isomers) in Swedish human serum. Environ Pollut 199:166–173, PMID: 25660070, 10.1016/j.envpol.2015.01.024. [DOI] [PubMed] [Google Scholar]
- Guxens M, Ballester F, Espada M, Fernández MF, Grimalt JO, Ibarluzea J, et al. 2012. Cohort profile: The INMA–INfancia y Medio Ambiente–(Environment and Childhood) Project. Int J Epidemiol 41(4):930–940, PMID: 21471022, 10.1093/ije/dyr054. [DOI] [PubMed] [Google Scholar]
- Halldorsson TI, Rytter D, Haug LS, Bech BH, Danielsen I, Becher G, et al. 2012. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect 120(5):668–673, PMID: 22306490, 10.1289/ehp.1104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen L, Röllin H, Odland J, Moe M, Sandanger T. 2010. Perfluorinated compounds in maternal serum and cord blood from selected areas of South Africa: results of a pilot study. J Environ Monit 12(6):1355–1361, PMID: 20424796, 10.1039/b924420d. [DOI] [PubMed] [Google Scholar]
- Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. 2009. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol 304(1–2):97–105, PMID: 19433254, 10.1016/j.mce.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Kabir ER, Rahman MS, Rahman I. 2015. A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol 40(1):241–258, PMID: 26164742, 10.1016/j.etap.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Kampmann U, Madsen LR, Skajaa GO, Iversen DS, Moeller N, Ovesen P. 2015. Gestational diabetes: a clinical update. World J Diabetes 6(8):1065–1072, PMID: 26240703, 10.4239/wjd.v6.i8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wong L, Chen A, Dunbar C, Webster GM, Lanphear BP, et al. 2014. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in multiethnic cohort of Cincinnati, Ohio pregnant women during 2003–2006. Environ Sci Technol 48(16):9600–9608, PMID: 25026485, 10.1021/es501811k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C-H, Yang S-N, Kuo P-L, Hung C-H. 2012. Immunomodulatory effects of environmental endocrine disrupting chemicals. Kaohsiung J Med Sci 28(7 suppl): S37–S42, PMID: 22871600, 10.1016/j.kjms.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. 2007. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99(2):366–394, PMID: 17519394, 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Martinez D, Ibarluzea J, et al. 2016. Variability of perfluoroalkyl substance concentrations in pregnant women by socio-demographic and dietary factors in a Spanish birth cohort. Environ Int 92–93:357–365, PMID: 27132161, 10.1016/j.envint.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Manzano-Salgado CB, Casas M, Lopez-Espinosa M-J, Ballester F, Basterrechea M, Grimalt JO, et al. 2015. Transfer of perfluoroalkyl substances from mother to fetus in a Spanish birth cohort. Environ Res 142:471–478, PMID: 26257032, 10.1016/j.envres.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jørgensen E. 2015. Breastfeeding as an exposure pathway for perfluorinated alkylates. Environ Sci Technol 49(17):10466–10473, PMID: 26291735, 10.1021/acs.est.5b02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada E, Kashino I, Matsuura H, Sasaki S, Miyashita C, Yamamoto J, Ikeno T, et al. 2013. Temporal trends of perfluoroalkyl acids in plasma samples of pregnant women in Hokkaido, Japan, 2003–2011. Environ Int 60:89–96, PMID: 24013022, 10.1016/j.envint.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng W, Rifas-Shiman SL, Rich-Edwards JW, Stuebe AM, Oken E. 2014. Inflammation and weight gain in reproductive-aged women. Ann Hum Biol 43(1):91–95, PMID: 25510294, 10.3109/03014460.2014.968619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu T, Chen M, Sun X, Cao J, Feng C, Li D, et al. 2016. Perfluorooctane sulfonate-induced insulin resistance is mediated by protein kinase B pathway. Biochem Biophys Res Commun 477(4):781–785, PMID: 27363333, 10.1016/j.bbrc.2016.06.135. [DOI] [PubMed] [Google Scholar]
- Ryckman K, Spracklen C, Smith C, Robinson J, Saftlas A. 2015. Maternal lipid levels during pregnancy and gestational diabetes: A systematic review and meta-analysis. BJOG 122(5):643–651, PMID: 25612005, 10.1111/1471-0528.13261. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Rifas-Shiman SL, Webster TF, Mora AM, Harris MH, Calafat AM, et al. 2015. Sociodemographic and perinatal predictors of early pregnancy per- and polyfluoroalkyl substance (PFAS) concentrations. Environ Sci Technol 49(19):11849–11858, PMID: 26333069, 10.1021/acs.est.5b02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Rader DJ, Millar JS. 2010. The effect of PPAR-alpha agonism on apolipoprotein metabolism in humans. Atherosclerosis 210(1):35–40, PMID: 20005515 10.1016/j.atherosclerosis.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Shapiro GD, Dodds L, Arbuckle TE, Ashley-Martin J, Ettinger AS, Fisher M, et al. 2016. Exposure to organophosphorus and organochlorine pesticides, perfluoroalkyl substances, and polychlorinated biphenyls in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: The MIREC Study. Environ Res 147:71–81, PMID: 26852007, 10.1016/j.envres.2016.01.040. [DOI] [PubMed] [Google Scholar]
- Skuladottir M, Ramel A, Rytter D, Haug LS, Sabaredzovic A, Bech BH, et al. 2015. Examining confounding by diet in the association between perfluoroalkyl acids and serum cholesterol in pregnancy. Environ Res 143(Pt A):33–38, PMID: 26432473, 10.1016/j.envres.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Starling AP, Engel SM, Whitworth KW, Richardson DB, Stuebe AM, Daniels JL, et al. 2014. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environ Int 62:104–112, PMID: 24189199, 10.1016/j.envint.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TC, Kuo CC, Hwang JJ, Lien GW, Chen MF, Chen PC. 2016. Serum perfluorinated chemicals, glucose homeostasis and the risk of diabetes in working-aged Taiwanese adults. Environ Int 88:15–22, PMID: 26700417, 10.1016/j.envint.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Taylor KW, Novak RF, Anderson HA, Birnbaum LS, Blystone C, Devito M, et al. 2013. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect 121(7):774–783, PMID: 23651634, 10.1289/ehp.1205502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvi D, Oulhote Y, Weihe P, Dalgård C, Bjerve KS, Steuerwald U, et al. 2017. Gestational diabetes and offspring birth size at elevated environmental pollutant exposures. Environ Int 107:205–215, PMID: 28753482, 10.1016/j.envint.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner M-A, Loccisano AE, Morken N-H, Yoon M, Wu H, McDougall R, et al. 2015. Associations of perfluoroalkyl substances (PFAS) with lower birth weight: an evaluation of potential confounding by glomerular filtration rate using a physiologically based pharmacokinetic model (PBPK). Environ Health Perspect 123(12):1317–1324, PMID: 26008903, 10.1289/ehp.1408837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vioque J, Navarrete-Muñoz E-M, Gimenez-Monzó D, García-de-la-Hera M, Granado F, Young IS, et al. 2013. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr J 12(1):26, PMID: 23421854, 10.1186/1475-2891-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Buck RC, Hungerbühler K. 2014. Global emission inventories for C4-C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: Production and emissions from quantifiable sources. Environ Int 70:62–75, PMID: 24932785, 10.1016/j.envint.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Wild R, Weedin EA, Wilson D. 2015. Dyslipidemia in pregnancy. Cardiol Clin 33(2):209–215, 10.1016/j.ccl.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, Schmid JE, Lau C, Abbott BD. 2012. Activation of mouse and human peroxisome proliferator-activated receptor-alpha (PPARα) by perfluoroalkyl acids (PFAAs): Further investigation of C4-C12 compounds. Reprod Toxicol 33(4):546–551, PMID: 22107727, 10.1016/j.reprotox.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Yan S, Zhang H, Zheng F, Sheng N, Guo X, Dai J. 2015. Perfluorooctanoic acid exposure for 28 days affects glucose homeostasis and induces insulin hypersensitivity in mice. Sci Rep 5:11029, PMID: 26066376, 10.1038/srep11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Sundaram R, Maisog J, Calafat AM, Barr DB, Buck Louis GM. 2015. A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil Steril 103(1):184–189, PMID: 25450302, 10.1016/j.fertnstert.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.