Abstract
The intervertebral disc is a unique avascular organ that supports axial skeleton flexion and rotation. The high proteoglycan content of the nucleus pulposus tissue, present at the center of the disc, is pivotal for its mechanical function, distribution of compressive loads. Chronic low back pain, a prevalent and costly condition, is strongly associated with disc degeneration. Degenerated discs exhibit high levels of inflammatory cytokines, matrix catabolizing enzymes, and an overall reduction in proteoglycan content. Although the cytokine profile of diseased discs has been widely studied, little is known of what initiates and drives inflammation and subsequent low back pain. Recent studies by Albert and colleagues have shown that anaerobic bacteria are present in a high percentage of painful, herniated discs and long-term treatment with antibiotics resolves symptoms associated with chronic low back pain. It is thought that these anaerobic bacteria in the disc may stimulate inflammation though toll-like receptors to further exacerbate disc degeneration. Despite the promise and novelty of this theory, there are other possible inflammatory mediators that need careful consideration. The metabolic environment associated with diabetes and atypical matrix degradation products also have the ability to activate many of the same inflammatory pathways as seen during microbial infection. It is therefore imperative that the research community must investigate the contribution of all possible drivers of inflammation to address the wide spread problem of discogenic chronic low back pain.
Introduction
Understanding the intervertebral disc (IVD) is necessary to address the serious global health problem of low back pain. Low back pain (LBP) is a profoundly debilitating and increasingly prevalent condition. It is currently the worldwide leading cause of disability. This condition is responsible for 58.2 million years lived with disability in 1990, 83 million in 2010, and an economic burden conservatively estimated at 85 billion dollars in 2005 alone (Buchbinder et al., 2013; Martin et al., 2008). Although LBP is a complex problem without one clear etiology, there is a strong association between LBP and disc degeneration. A study reviewing the MRIs of patients with persistent LBP showed disc degeneration in 87% of participants (Arnbak et al., 2015). Additionally, patients with severely degenerate discs are 3.2 times more likely to suffer from LBP (Livshits et al., 2011). Despite the strong link between disc degeneration and pain, degeneration is a normal consequence of aging that routinely does not cause discomfort, even with a corresponding reduction in mechanical function (Cheung et al., 2009; Dreischarf et al., 2014). Learning more about the healthy IVD and identifying the underlying pathology that distinguishes painful from non-painful degenerate discs will simultaneously generate new therapeutic targets for discogenic LBP and help continue to elucidate the mechanisms of disc degeneration. Developing effective treatments for painful disc degeneration will ameliorate the suffering of countless individuals.
Discs are compressible structures found sandwiched between each pair of adjacent vertebral bodies. This pattern of rigid bone separated by spongy disc enables the vertebral column both to distribute compressive loads and provide structural support to the axial skeleton, while still allowing for flexion and rotation. The IVD comprises three distinct tissues. The annulus fibrosus (AF), a concentric ring structure of organized lamellar collagen, laterally incases the proteoglycan rich inner nucleus pulposus (NP). The third component, two cartilaginous endplates, acts as the interface between the central portion of the disc and the neighboring vertebral bodies. Hydrophilic proteoglycan of the NP enables it to resist compressive loads through osmotic pressurization. The tensile strength of the AF prevents the gelatinous NP from extruding laterally from the disc space. The disc is the largest avascular tissue in the human body. While outer annulus has limited vascular supply, inner annulus and NP is completely devoid of any blood vessels. Consequently, metabolic support of much of the IVD is dependent on the endplates facilitating diffusion of nutrients and waste products between the disc and the adjacent vertebral body circulation. The central role of the NP in disc function makes its study pivotal to understanding disease pathogenesis, and thus much of the current research focuses on how the NP changes during development and degeneration.
Like chondrocytes, NP cells inhabit a hypoxic niche, but equating NP cells to chondrocytes is analogous to calling dolphins fish; both animals have fins, but they have very different evolutionary origins. NP cells are a unique, dynamic, and diverse cell type. They are the only derivative from the embryonic notochord. They consist of two morphologically distinct populations on a continuum of differentiation, a group of larger vacuolated cells resembling notochordal cells and a second group of smaller cells resembling chondrocytes (Chen et al., 2005; Risbud and Shapiro, 2011; Risbud et al., 2015; Trout et al., 1982).
The origin, environment, and function of healthy NP cells are reflected in their gene expression profile: sonic hedgehog (shh), bradchyry, hypoxia-inducible factor-1 alpha (HIF-1α), glucose transporter-1 (GLUT-1), carbonic anhydrase 12 (CA12), aggrecan (agg) and collagen II (col II) at a ratio >20, keratin 18/19, and cluster of differentiation 24 (CD24) (Risbud et al., 2015). Shh and bradchyry are reflective of NP’s notochordal origin (Mwale et al., 2004; Winkler et al., 2014). HIF-1α and GLUT-1 both represent an adaption of NP cells to survive in the metabolically demanding environment of the disc (Agrawal et al., 2007). Due to the hypoxic niche necessitating a reliance on glycolysis, CA12 maybe necessary for disc pH regulation (Power et al., 2011). Aggrecan, collagen 2, and keratin18/19 are structural components of the NP. Aggracan’s predominance is necessary to create osmotic pressure to resist compression. The NP niche also puts unique osmotic requirements on its cells, which respond by using Tonicity-Responsive Enhancer Binding Protein (TonEBP) to maintain viability and function (Johnson et al., 2014). As animals age, a dwindling population of vacuolated cells remains in the disc possibly due to their differentiation into morphologically distinct cells. The remaining vacuolated cells are thought to maintain overall cell viability and inhibit endothelial cell invasion (Cornejo et al., 2015; Erwin et al., 2011). Although this differentiation is a normal part of maturation, loading and injury can accelerate the process (Purmessur et al., 2013; Yang et al., 2009). Thus, understanding the function and phenotype of healthy NP can both help identify diseased discs and inform possible routes to treatment.
This review aims to synthesize current research describing pathology of degenerative disc disease, and discuss new theories concerning drivers of painful disc disease. We will in particular focus on the exciting yet controversial theory that subclinical bacterial infection is a driver of painful disc disease, while additionally exploring complementary and alternative theories of inflammation initiation.
Characteristics of the degenerating disc
Studying the inflammatory cytokine profile of degenerating discs has informed much of what we know about degenerative disc disease. During the degenerative process, NP and AF cells and then additionally invading immune cells secrete elevated levels of tumor necrosis factor alpha (TNFα), interleukin-1β (IL-1β), IL-6 and IL-17. These cytokines stimulate production of additional cytokines leading to matrix metalloproteinase (MMP) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) mediated extracellular matrix destruction, and altering synthetic and biophysical properties of NP cells (Maidhof et al., 2014). IL-1β and TNFα are the two most widely studied cytokines in the pathogenesis of disc degeneration. IL-1β is synthesized in an inactive pro-IL-1β form and activated by caspase-1 before secretion. IL-1β binds to IL-1β receptor that acts through myeloid differentiation primary response gene 88 (MYD88) to induce expression of matrix-degrading enzymes (Ellman et al., 2012; Risbud and Shapiro, 2014). IL-1β and its receptor expression increase corresponding to degradation severity, and the action of IL-1β interferes with aggrecan and collagen 2 transcription and translation (Le Maitre et al., 2005). On the other hand, TNFα is synthesized as a type II transmembrane protein. TNF-α-converting enzyme (TACE) cleaves the membrane bound portion to generate secreted TNFα. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) pathways are the two primary downstream targets of TNFα signaling in disc cells (Risbud and Shapiro, 2014). Importantly, in addition to transcriptional induction of several catabolic mediators, TNF-α promotes ADAMTS-5 processing and activation by elevating cell surface levels of SDC4, a heparan sulfate proteoglycan (Wang et al., 2011). A recent study has also shown the critical contribution of SDC4 in controlling TNF-α mediated transcriptional induction of MMP-3 (Wang et al., 2014). In addition to their well described catabolic functions, higher levels of inflammatory cytokines TNF-α and IL-6 have been shown to cause cell death in DRG neurons (Murata et al., 2011). Moreover, IL-6 and IL-8 are correlated with painful degenerate discs (Burke et al., 2002). Both IL-1β and TNFα also upregulate nerve growth factor (NGF), with IL-1β additionally inducing the expression of brain-derived neurotrophic factor (BDNF), and substance P (Abe et al., 2007; García-Cosamalón et al., 2010; Purmessur et al., 2008). Thus, not only could these neurotrophic factors cause pain by DRG sensitization and through retrograde signaling, but substance P has been shown to up regulate synthesis of inflammatory cytokines (IL-6, IL-8, and TNFα) further exacerbating to progression of disc degeneration (Kepler et al., 2015, 2012). While it is evident that inflammatory phenotype characterizes degenerate discs; little is known about how the inflammation is initiated and sustained to give rise to chronic LBP in this widespread disease.
Subclinical bacterial infection of the spinal motion segment as a novel initiator of LBP
There is growing interest and controversy in the notion that subclinical anaerobic bacterial infection could play a role in symptomatic disc degeneration. This subclinical infection is in contrast to LBP from overt discitis, where 80 percent of patients present with elevations in erythrocyte sedimentation rate, and 50 percent of patients present with a fever (Carragee et al., 1997; Sapico and Montgomerie, 1979). Changes in endplate radiological appearance may lead to clues to identifying patients who are suffering from bacteria mediated LBP. Modic et al. used MRI to detect changes in vertebral bone marrow associated with degenerative disc disease; Type 1 changes were described as decreased signal intensity on T1-weighted images with increased signal intensity on T2-weighted images, and Type 2 changers were described as increased signal intensity on T1-weighted images with slightly increased intensity on T2-weighted images (Modic et al., 1988). Fat appears bright and increases the signal intensity in T1 images, whereas water increases in the signal intensity on T2 images. Consequently the painful Type 1 changes are associated with edema in the vertebral bodies. More recently, Jensen et al. showed that these same Modic Type 1 changes are strongly correlated with LBP (Jensen et al., 2008). In 2013, Albert et al. proposed that anaerobic bacteria, predominantly Propionibacterium acnes (p. acnes), might be responsible for these radiologic changes associated with LBP. The discs in their study infected with anerobic bacteria were significantly more likely to have Modic Type 1 changes than both discs infected with aerobic bacteria and discs with no detectable infection (Hanne B. Albert et al., 2013). Any time a bacterial culture is taken from a surgical sample, there is a chance of environmental contamination, which could have affected Albert et al.’s results. However, they found a difference between the aerobic and anaerobic bacteria groups. Moreover, other studies have also found anaerobic bacteria in nucleus material, thus adding strength to the notion that the hypoxic conditions in the inner AF and NP may be preferable for survival and colonization of anerobic microorganisms (Agarwal et al., 2010; Fritzell et al., 2004).
The hypothesis that subclinical bacterial infection can cause LBP is further supported by the ability of antibiotic treatment to resolve back pain in patients with Type 1 Modic changes. In a double-blind randomized clinical controlled trial, Albert et al. found that a 100 day course of amoxicillin-clavulanate resulted in significant improvement in both disability and pain of patients suffering from LBP (Hanne B Albert et al., 2013). This is one of the most strikingly successful experimental treatments for LBP to date, but along with the possible shortcomings of their earlier discussed study, there are possible confounding effects of amoxicillin-clavulanate. There is some evidence suggesting that clavulanate has anti-inflammatory action in the treatment of ulcerative colitis, and analgesic properties during morphine withdrawal in mice, which may lead to a misinterpretation of this clinical study (Casellas et al., 1998; Hajhashemi and Dehdashti, 2014). It is important to note that treating back pain with long term antibiotics also raises global health concerns by exacerbating the already serious problem of antibiotic resistance (Roca et al., 2015). Despite these limitations, subclinical bacterial infection is a promising new theory of LBP. Additional investigation into the underlying mechanisms linking anaerobic bacterial infection to LBP could enhance our collective understanding the disease and identify treatments directly targeting the pathways contributing to painful disc degeneration.
TLR signaling as one of the important links between disc inflammation and LBP
Relevant to the discussion above, activation of toll-like receptor signaling (TLR) may possibly link subclinical bacterial infection with inflammation induced LBP. Toll-like receptors are an integral part of the innate immune system. They recognize common microbial components and induce inflammatory cytokine production. These cytokines initiate the innate immunological response while also instructing the development of acquired antigen-specific immunity (Takeda and Akira, 2005). TLRs 1/2/4/6 are expressed by disc cells in a degeneration dependent manner making this signaling cascade relevant to disease pathogenesis (Klawitter et al., 2014; Rajan et al., 2012). Similar to IL-1 signaling, both TLR 2 and TLR 4 utilize the MyD88 adaptor protein to initiate downstream signaling (Kawai et al., 1999). Upon ligand engagement, MyD88 recruits members of the interleukin-1 receptor-associated kinase (IRAK) family that activate TNF receptor-associated factor 6 (TRAF6) (Kawagoe et al., 2008) which in turn signals through tumor growth factor beta activated kinase 1 and then through NF-κB and MAPK signaling (Adhikari et al., 2007).
In the IVD, cytokines and TLR constitute a feedforward loop whereby inflammatory cytokines stimulate expression of TLRs, and TLR signaling in turn triggers the production of inflammatory cytokines promoting degradation. Lipopolysaccharide (LPS), a component of gram-negative bacterial cell walls, engages TRL4 triggering the expression of IL-1β, TNFα, IL-6 as well as nitric oxide (NO) (Rajan et al., 2012). Inhibition of MyD88 attenuates this LPS induced inflammatory gene expression (Ellman et al., 2012). It is interesting to note that p. acnes is a gram positive bacteria and is not expected to stimulate LPS sensitive TLR4, but instead been shown to signal through pathogen-associated molecular patters (PAMPs) - TLR2 axis (Zähringer et al., 2008). However, it is important to note that direct stimulation of TLR4 is not the only way to amplify TLR4 dependent inflammatory response; in other human tissues, inoculation with p. acnes has been shown to increase TLR4 expression and to sensitize TLR4 through lymphocyte antigen 96 (MD-2) upregulation (Jugeau et al., 2005; Romics et al., 2004). The inflammatory environment in degenerated discs potentiates TRL mediated responses; stimulation with IL-1β or TNFα increases the expression of TLR1/4 mRNA, and significantly increased the transcriptional and translational expression of TLR2 (Klawitter et al., 2014). In addition to TLR signaling, subclinical bacterial infection may stimulate caspase-1 mediated IL-1β activation though NACHT, LRR, and PYD domainscontaining protein 3 (NLRP3). Expression levels of NLRP3, caspase-1, and IL-1β were all found to be higher in degenerate discs (Chen et al., 2015). Although the possible link between subclinical bacterial infection to persistent inflammation and painful disc degeneration warrants further investigation, bacteria are only one of possible ways to activate inflammation in the disc. Even something as seemingly straightforward as mechanical loading can have complex signal mediated actions on disc disease. Along with other effects, mechanical loading upregulates TNFα IL-6, TLR2 and TLR4 expression (Gawri et al., 2014). It is important to recognize the complexity IVD degeneration to adequately treat this debilitating condition. The influence of extracellular metabolic stress and matrix breakdown products on disc inflammation cannot be neglected.
Damage-associated molecular patters (DAMPs) as novel mediators of chronic inflammation and LBP
In addition to the drivers of inflammation described above, some endogenous molecules and their atypical cleavage products have the ability to stimulate sterile inflammation in the disc. This family of molecules, collectively called damage-associated molecular patters (DAMPs), include fragments of commonly found extracellular matrix components such as hyaluronan and fibronectin as well as high-mobility group protein B1 (HMGB1), a nuclear factor for chromatin assembly. Hyaluronic acid fragments (12 to 24 mer) have been shown to enhance pro-inflammatory cytokine production (IL-1β, IL-6, and IL-8) and matrix degrading enzymes (MMP-1, MMP-3, and MMP-13) leading to an inflammatory environment and matrix breakdown. These effects are mediated by TLR2 signaling and can be inhibited by high molecular weight hyaluronan (Quero et al., 2013). The TLR2 signaling cascade activated by hyaluronan fragments is the same MyD88-IRAK-TRAF6 dependent cascade activated by both LPS and CpG sequence DNA binding (Kawagoe et al., 2008; Scheibner et al., 2006). It is interesting to note that by binding TLR2 instead of TRL4 or CD44, hyaluronan fragments stimulate NP cells in a manner that more closely resembles macrophages than chondrocytes, hinting at the unique nature and perhaps origin of these cells (Campo et al., 2012, 2010; Scheibner et al., 2006).
HMGB1 is another DAMP that is receiving attention in the disc field. HMGB1 is both passively released from necrotic cells and secreted by monocytes. It likely exerts its inflammatory function by binding to RAGE receptors (Scaffidi et al., 2002; Voll et al., 2008). Delivery of anti-HMGB1 reduces pain and TNF expression after application of NP tissue to DRG in rat study (Otoshi et al., 2011). Fibronectin fragments are another possible inflammatory mediator in disc degeneration. Fibronectin fragments are absent in infant cadaveric human IVDs, but increase in concentration with aging and severity of disc degeneration (Ruel et al., 2014). Delivery of 30 kDa fibronectin fragments into rabbit lumbar discs induced a degenerative phenotype similar to spontaneous human disc degeneration. Comparing their results to a PBS-injected sham, fibronectin fragment injection caused a significant decrease in disc height and a significant reduction in proteoglycan synthesis (Liu et al., 2013). These effects may be due to increase in MMP-9 and MMP-13 along with the simultaneous decrease in collagen 2 and aggracan expression observed in rabbit NP cells in vitro (Anderson et al., 2005). In contrast to signaling through RAGE and TLR, fibronectin fragments exert their catabolic effects through integrin α5β1 (Xia and Zhu, 2011). The enhanced catabolism caused by fibronectin fragments could also be a result of their out competing CCN2 for α5β1 binding. Noteworthy, CCN2 causes context dependent effects in the IVD, promoting aggrecan expression and inhibiting the catabolic effects of IL-1β when bound to α5β1 and otherwise, possibly through HSPGs, promoting catabolism (Tran et al., 2014). Thus, atypical matrix cleavage products may interfere with disc homeostasis pushing the homeostatic balance more towards catabolism.
Systemic metabolic syndrome as a driver of disc inflammation and LBP
Obesity and diabetes are two epidemics in Western society that increase the risk of developing painful disc degeneration. Beyond the greater forces that the spinal motion segments of an obese individual experience, diabetes creates a niche environment that can drive disc degeneration. Advanced glycation end products (AGEs) associated with diabetes can accelerate disc degeneration by inducing catabolism and promoting inflammation. Although diabetes and obesity are linked, recent research has attempted to isolate the effects of diabetes by comparing both obese Sprague-Dawley rats to diabetic obese UCD-T2DM rats while using lean rats as an additional control. The diabetic rats alone had an elevated AGE concentration, which was associated with diminished glycosaminoglycan and water content of discs. These compositional changes resulted an experimentally consistent decrease in disc mechanics (Fields et al., 2015). In a separate study, Tsai and colleagues showed that treatment with AGEs of both rat and human NP cells caused a dose dependent increase in MMP-2 and extracellular signal-related kinases (ERK) activity, and elevation in advanced AGE-specific receptor (RAGE) mRNA expression, which all could lead to enhanced NF-κB activation (Tsai et al., 2014). Both the anti-inflammatory pentosan-polysulfate and AGE-inhibitor pyridoxamine, FDA approved compounds, are able to reduce the deleterious effects streptozotocin induced diabetes on disc disease in mice (Illien-Junger et al., 2013). Independent of the aberrant reactions caused by hyperglycemia leading to AGE production, elevated blood glucose concentration, common to diabetes, has been shown to inhibit NP cell proliferation in vitro, which could further alter cellular homeostasis in the disc (Johnson et al., 2008). Taken together these studies suggest that diabetes, obesity and their metabolic consequences may encourage disc degeneration.
Conclusion
The clinical success of antibiotic use for treating low back pain is exciting yet controversial. Recent data suggests that anaerobic p. acnes could induce its inflammatory effects on the intervertebral disc through TLR signaling. Despite this clinical success, focusing only on bacteria as a driver of inflammation would miss the effects of aberrant mechanical loading, metabolic syndromes such as diabetes, and endogenous atypical cleavage products that initiate many of the same downstream effects as bacteria. An effective treatment for painful disc degeneration is likely to be comprehensive. Understanding the contribution and interplay between all inflammatory drivers will lead to a responsible and effective treatment for low back pain.
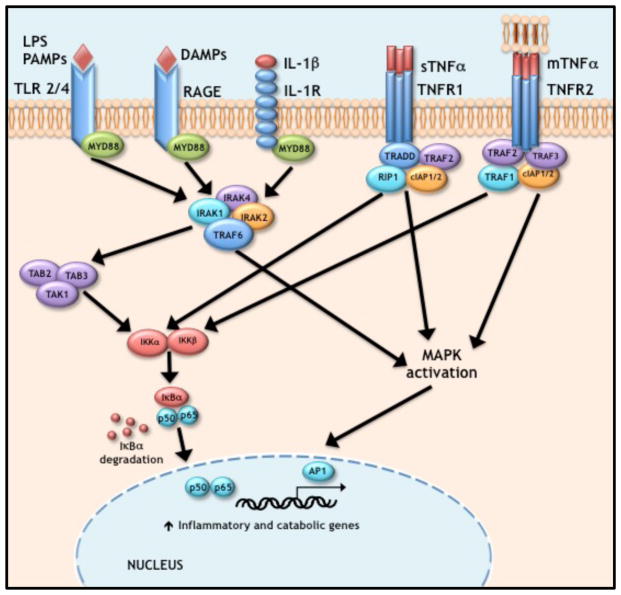
Figure 1. Signaling pathways driving inflammation.
TRL, RAGE, and IL-1 all initiate their inflammatory effects though MYD88 with subsequent action though members of the IRAK family that activate TRAF6. TNF-α can simulate the cell in both its soluble and membrane bound form by activating TNFR1 and TNFR2 respectively. TNFR1 ligand binding results in a conformational change leading to the recruitment of TRADD, RIP1, TRAF2, and CIAP1/2. Ligand binding to TNFR2 results in the recruitment of similar down stream factors. All five of these receptor pathways converge on NF-κβ and MAPK activation to induce their inflammatory and catabolic effects.
References
- Abe Y, Akeda K, An HS, Aoki Y, Pichika R, Muehleman C, Kimura T, Masuda K. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine (Phila Pa 1976) 2007;32:635–42. doi: 10.1097/01.brs.0000257556.90850.53. [DOI] [PubMed] [Google Scholar]
- Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–26. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- Agarwal VJ, Golish R, Kondrashov D, Alamin TF. Results of Bacterial Culture from Surgically Excised Intervertebral Disc in 52 Patients Undergoing Primary Lumbar Microdiscectomy at a Single Level. Spine J. 2010;10:S45–S46. [Google Scholar]
- Agrawal A, Guttapalli A, Narayan S, Albert TJ, Shapiro IM, Risbud M. V Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol. 2007;293:C621–31. doi: 10.1152/ajpcell.00538.2006. [DOI] [PubMed] [Google Scholar]
- Albert HB, Lambert P, Rollason J, Sorensen JS, Worthington T, Pedersen MB, Norgaard HS, Vernallis A, Busch F, Manniche C, Elliott T. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J. 2013;22:690–696. doi: 10.1007/s00586-013-2674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert HB, Sorensen JS, Christensen BS, Manniche C. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized clinical controlled trial of efficacy. Eur Spine J. 2013;22:697–707. doi: 10.1007/s00586-013-2675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DG, Li X, Balian G. A fibronectin fragment alters the metabolism by rabbit intervertebral disc cells in vitro. Spine (Phila Pa 1976) 2005;30:1242–6. doi: 10.1097/01.brs.0000164097.47091.4c. [DOI] [PubMed] [Google Scholar]
- Arnbak B, Jensen TS, Egund N, Zejden A, Horslev-Petersen K, Manniche C, Jurik AG. Prevalence of degenerative and spondyloarthritis-related magnetic resonance imaging findings in the spine and sacroiliac joints in patients with persistent low back pain. Eur Radiol. 2015 doi: 10.1007/s00330-015-3903-0. [DOI] [PubMed] [Google Scholar]
- Buchbinder R, Blyth FM, March LM, Brooks P, Woolf AD, Hoy DG. Placing the global burden of low back pain in context. Best Pract Res Clin Rheumatol. 2013;27:575–89. doi: 10.1016/j.berh.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Burke JG, Watson RWG, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- Campo GM, Avenoso A, Campo S, D’Ascola A, Nastasi G, Calatroni A. Small hyaluronan oligosaccharides induce inflammation by engaging both toll-like-4 and CD44 receptors in human chondrocytes. Biochem Pharmacol. 2010;80:480–90. doi: 10.1016/j.bcp.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Campo GM, Avenoso A, D’Ascola A, Prestipino V, Scuruchi M, Nastasi G, Calatroni A, Campo S. Hyaluronan differently modulates TLR-4 and the inflammatory response in mouse chondrocytes. Biofactors. 2012;38:69–76. doi: 10.1002/biof.202. [DOI] [PubMed] [Google Scholar]
- Carragee EJ, Kim D, van der Vlugt T, Vittum D. The clinical use of erythrocyte sedimentation rate in pyogenic vertebral osteomyelitis. Spine (Phila Pa 1976) 1997 doi: 10.1097/00007632-199709150-00005. [DOI] [PubMed] [Google Scholar]
- Casellas F, Borruel N, Papo M, Guarner F, Antolin M, Videla S, Malagelada JR. Antiinflammatory effects of enterically coated amoxicillin-clavulanic acid in active ulcerative colitis. Inflamm Bowel Dis. 1998;4:1–5. doi: 10.1097/00054725-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Chen J, Yan W, Setton La. Molecular phenotypes of notochordal cells purified from nucleus pulposus via fluorescence-activated cell sorting. Eur Cells Mater. 2005;10:16. [Google Scholar]
- Chen ZH, Jin SH, Wang MY, Jin XL, Lv C, Deng YF, Wang JL. Enhanced NLRP3, caspase-1, and IL-1β levels in degenerate human intervertebral disc and their association with the grades of disc degeneration. Anat Rec (Hoboken) 2015;298:720–6. doi: 10.1002/ar.23059. [DOI] [PubMed] [Google Scholar]
- Cheung KMC, Karppinen J, Chan D, Ho DWH, Song YQ, Sham P, Cheah KSE, Leong JCY, Luk KDK. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine (Phila Pa 1976) 2009;34:934–940. doi: 10.1097/BRS.0b013e3181a01b3f. [DOI] [PubMed] [Google Scholar]
- Cornejo MC, Cho SK, Giannarelli C, Iatridis JC, Purmessur D. Soluble factors from the notochordal-rich intervertebral disc inhibit endothelial cell invasion and vessel formation in the presence and absence of pro-inflammatory cytokines. Osteoarthr Cartil. 2015;23:487–496. doi: 10.1016/j.joca.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreischarf M, Albiol L, Rohlmann A, Pries E, Bashkuev M, Zander T, Duda G, Druschel C, Strube P, Putzier M, Schmidt H. Age-Related Loss of Lumbar Spinal Lordosis and Mobility – A Study of 323 Asymptomatic Volunteers. PLoS One. 2014;9:e116186. doi: 10.1371/journal.pone.0116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman MB, Kim JS, An HS, Chen D, KCR, An J, Dittakavi T, van Wijnen AJ, Cs-Szabo G, Li X, Xiao G, An S, Kim SG, Im HJ. Toll-like receptor adaptor signaling molecule MyD88 on intervertebral disk homeostasis: in vitro, ex vivo studies. Gene. 2012;505:283–90. doi: 10.1016/j.gene.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin WM, Islam D, Inman RD, Fehlings MG, Tsui FWL. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: implications for the mechanisms of intervertebral disc degeneration. Arthritis Res Ther. 2011;13:R215. doi: 10.1186/ar3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields AJ, Berg-Johansen B, Metz LN, Miller S, La B, Liebenberg EC, Coughlin DG, Graham JL, Stanhope KL, Havel PJ, Lotz JC. Alterations in intervertebral disc composition, matrix homeostasis and biomechanical behavior in the UCD-T2DM rat model of type 2 diabetes. J Orthop Res. 2015 doi: 10.1002/jor.22807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzell P, Bergstrom T, Welinder-Olsson C. Detection of bacterial DNA in painful degenerated spinal discs in patients without signs of clinical infection. Eur Spine J. 2004;13:702–706. doi: 10.1007/s00586-004-0719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cosamalon J, del Valle ME, Calavia MG, Garcia-Suarez O, Lopez-Muniz A, Otero J, Vega JA. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat. 2010;217:1–15. doi: 10.1111/j.1469-7580.2010.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawri R, Rosenzweig DH, Krock E, Ouellet Ja, Stone LS, Quinn TM, Haglund L. High mechanical strain of primary intervertebral disc cells promotes secretion of inflammatory factors associated with disc degeneration and pain. Arthritis Res Ther. 2014;16:R21. doi: 10.1186/ar4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajhashemi V, Dehdashti K. Antinociceptive effect of clavulanic acid and its preventive activity against development of morphine tolerance and dependence in animal models. Res Pharm Sci. 2014;9:315–321. [PMC free article] [PubMed] [Google Scholar]
- Illien-Junger S, Grosjean F, Laudier DM, Vlassara H, Striker GE, Iatridis JC. Combined anti-inflammatory and anti-AGE drug treatments have a protective effect on intervertebral discs in mice with diabetes. PLoS One. 2013;8:e64302. doi: 10.1371/journal.pone.0064302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TS, Karppinen J, Sorensen JS, Niinimaki J, Leboeuf-Yde C. Vertebral endplate signal changes (Modic change): A systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J. 2008;17:1407–1422. doi: 10.1007/s00586-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WEB, Stephan S, Roberts S. The influence of serum, glucose and oxygen on intervertebral disc cell growth in vitro: implications for degenerative disc disease. Arthritis Res Ther. 2008;10:R46. doi: 10.1186/ar2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZI, Shapiro IM, Risbud MV. Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: evolving role of TonEBP. Matrix Biol. 2014;40:10–6. doi: 10.1016/j.matbio.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugeau S, Tenaud I, Knol AC, Jarrousse V, Quereux G, Khammari A, Dreno B. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105–13. doi: 10.1111/j.1365-2133.2005.06933.x. [DOI] [PubMed] [Google Scholar]
- Kawagoe T, Sato S, Matsushita K, Kato H, Matsui K, Kumagai Y, Saitoh T, Kawai T, Takeuchi O, Akira S. Sequential control of Toll-like receptordependent responses by IRAK1 and IRAK2. Nat Immunol. 2008;9:684–91. doi: 10.1038/ni.1606. [DOI] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–22. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kepler CK, Markova DZ, Hilibrand AS, Vaccaro AR, Risbud MV, Albert TJ, Anderson DG. Substance P Stimulates Production of Inflammatory Cytokines in Human Disc Cells. Spine J. 2012;12:S109. doi: 10.1097/BRS.0b013e3182a42bc2. [DOI] [PubMed] [Google Scholar]
- Kepler CK, Markova DZ, Koerner JD, Mendelis J, Chen C-M, Vaccaro AR, Risbud MV, Albert TJ, Anderson DG. Substance P Receptor Antagonist Suppresses Inflammatory Cytokine Expression in Human Disc Cells. Spine (Phila Pa 1976) 2015 doi: 10.1097/BRS.0000000000000954. [DOI] [PubMed] [Google Scholar]
- Klawitter M, Hakozaki M, Kobayashi H, Krupkova O, Quero L, Ospelt C, Gay S, Hausmann O, Liebscher T, Meier U, Sekiguchi M, Konno SI, Boos N, Ferguson SJ, Wuertz K. Expression and regulation of toll-like receptors (TLRs) in human intervertebral disc cells. Eur Spine J. 2014;30:1878–1891. doi: 10.1007/s00586-014-3442-4. [DOI] [PubMed] [Google Scholar]
- Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–45. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HF, Zhang H, Qiao GX, Ning B, Hu YL, Wang DC, Hu YG. A novel rabbit disc degeneration model induced by fibronectin fragment. Joint Bone Spine. 2013;80:301–6. doi: 10.1016/j.jbspin.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Livshits G, Popham M, Malkin I, Sambrook PN, Macgregor AJ, Spector T, Williams FMK. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis. 2011;70:1740–1745. doi: 10.1136/ard.2010.137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidhof R, Jacobsen T, Papatheodorou A, Chahine NO. Inflammation induces irreversible biophysical changes in isolated nucleus pulposus cells. PLoS One. 2014;9:1–11. doi: 10.1371/journal.pone.0099621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BI, Deyo Ra, Mirza SK, Turner Ja, Comstock Ba, Hollingworth W, Sullivan SD. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–664. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–9. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- Murata Y, Rydevik B, Nannmark U, Larsson K, Takahashi K, Kato Y, Olmarker K. Local application of interleukin-6 to the dorsal root ganglion induces tumor necrosis factor-α in the dorsal root ganglion and results in apoptosis of the dorsal root ganglion cells. Spine (Phila Pa 1976) 2011;36:926–32. doi: 10.1097/BRS.0b013e3181e7f4a9. [DOI] [PubMed] [Google Scholar]
- Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 8:58–63. doi: 10.22203/ecm.v008a06. discussion 63–4, 2004. [DOI] [PubMed] [Google Scholar]
- Otoshi K, Kikuchi S, Kato K, Sekiguchi M, Konno S. Anti-HMGB1 neutralization antibody improves pain-related behavior induced by application of autologous nucleus pulposus onto nerve roots in rats. Spine (Phila Pa 1976) 2011;36:E692–8. doi: 10.1097/BRS.0b013e3181ecd675. [DOI] [PubMed] [Google Scholar]
- Power KA, Grad S, Rutges JPHJ, Creemers LB, van Rijen MHP, O’Gaora P, Wall JG, Alini M, Pandit A, Gallagher WM. Identification of cell surface-specific markers to target human nucleus pulposus cells: expression of carbonic anhydrase XII varies with age and degeneration. Arthritis Rheum. 2011;63:3876–86. doi: 10.1002/art.30607. [DOI] [PubMed] [Google Scholar]
- Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res Ther. 2008;10:R99. doi: 10.1186/ar2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purmessur D, Guterl CC, Cho SK, Cornejo MC, Lam YW, Ballif BA, Laudier JCI, Iatridis JC. Dynamic pressurization induces transition of notochordal cells to a mature phenotype while retaining production of important patterning ligands from development. Arthritis Res Ther. 2013;15:R122. doi: 10.1186/ar4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quero L, Klawitter M, Schmaus A, Rothley M, Sleeman J, Tiaden AN, Klasen J, Boos N, Hottiger MO, Wuertz K, Richards PJ. Hyaluronic acid fragments enhance the inflammatory and catabolic response in human intervertebral disc cells through modulation of toll-like receptor 2 signalling pathways. Arthritis Res Ther. 2013;15:R94. doi: 10.1186/ar4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan NE, Bloom O, Maidhof R, Stetson N, Sherry BB, Levine M, Chahine NO. Toll-Like Receptor 4 (TLR4) Expression and Stimulation in a Model of Intervertebral Disc Inflammation and Degeneration. Spine (Phila Pa 1976) 2012;38:1. doi: 10.1097/BRS.0b013e31826b71f4. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Schoepflin ZR, Mwale F, Kandel RA, Grad S, Iatridis JC, Sakai D, Hoyland JA. Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J Orthop Res. 2015;33:283–93. doi: 10.1002/jor.22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbud MV, Shapiro IM. Notochordal cells in the adult intervertebral disc: new perspective on an old question. Crit Rev Eukaryot Gene Expr. 2011;21:29–41. doi: 10.1615/critreveukargeneexpr.v21.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca I, Akova M, Baquero F, Carlet J, Cavaleri M, Coenen S, Cohen J, Findlay D, Gyssens I, Heure OE, Kahlmeter G, Kruse H, Laxminarayan R, Liebana E, Lopez-Cerero L, MacGowan a, Martins M, Rodriguez-Bano J, Rolain J-M, Segovia C, Sigauque B, Taconelli E, Wellington E, Vila J. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015;6:22–29. doi: 10.1016/j.nmni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romics L, Dolganiuc A, Kodys K, Drechsler Y, Oak S, Velayudham A, Mandrekar P, Szabo G. Selective priming to Toll-like receptor 4 (TLR4), not TLR2, ligands by P. acnes involves up-regulation of MD-2 in mice. Hepatology. 2004;40:555–64. doi: 10.1002/hep.20350. [DOI] [PubMed] [Google Scholar]
- Ruel N, Markova DZ, Adams SL, Scanzello C, Cs-Szabo G, Gerard D, Shi P, Anderson DG, Zack M, An HS, Chen D, Zhang Y. Fibronectin fragments and the cleaving enzyme ADAM-8 in the degenerative human intervertebral disc. Spine (Phila Pa 1976) 2014;39:1274–9. doi: 10.1097/BRS.0000000000000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapico FL, Montgomerie JZ. Pyogenic vertebral osteomyelitis: report of nine cases and review of the literature. Rev Infect Dis. 1979;1:754–76. doi: 10.1093/clinids/1.5.754. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan Fragments Act as an Endogenous Danger Signal by Engaging TLR2. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Tran CM, Schoepflin ZR, Markova DZ, Kepler CK, Anderson DG, Shapiro IM, Risbud MV. CCN2 suppresses catabolic effects of interleukin-1β through α5β1 and αVβ3 integrins in nucleus pulposus cells: implications in intervertebral disc degeneration. J Biol Chem. 2014;289:7374–7387. doi: 10.1074/jbc.M113.526111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout JJ, Buckwalter Ja, Moore KC, Landas SK. Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell. 1982;14:359–369. doi: 10.1016/0040-8166(82)90033-7. [DOI] [PubMed] [Google Scholar]
- Tsai TT, Ho NYJ, Lin YT, Lai PL, Fu TS, Niu CC, Chen LH, Chen WJ, Pang JHS. Advanced glycation end products in degenerative nucleus pulposus with diabetes. J Orthop Res. 2014;32:238–44. doi: 10.1002/jor.22508. [DOI] [PubMed] [Google Scholar]
- Voll RE, Urbonaviciute V, Herrmann M, Kalden JR. High mobility group box 1 in the pathogenesis of inflammatory and autoimmune diseases. Isr Med Assoc J. 2008;10:26–8. [PubMed] [Google Scholar]
- Wang J, Markova D, Anderson DG, Zheng Z, Shapiro IM, Risbud MV. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286:39738–49. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang H, Yang H, Li J, Cai Q, Shapiro IM, Risbud MV. Tumor Necrosis Factor-α- and Interleukin-1β-Dependent Matrix Metalloproteinase-3 Expression in Nucleus Pulposus Cells Requires Cooperative Signaling via Syndecan 4 and Mitogen-Activated Protein Kinase-Nuclear Factor κB Axis: Implications in Inflammatory D. Am J Pathol. 2014;184:1–13. doi: 10.1016/j.ajpath.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler T, Mahoney EJ, Sinner D, Wylie CC, Dahia CL. Wnt signaling activates Shh signaling in early postnatal intervertebral discs, and re-activates Shh signaling in old discs in the mouse. PLoS One. 2014;9:e98444. doi: 10.1371/journal.pone.0098444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Zhu Y. Fibronectin fragment activation of ERK increasing integrin α5 and β1 subunit expression to degenerate nucleus pulposus cells. J Orthop Res. 2011;29:556–561. doi: 10.1002/jor.21273. [DOI] [PubMed] [Google Scholar]
- Yang F, Leung VYL, Luk KDK, Chan D, Cheung KMC. Injury-induced sequential transformation of notochordal nucleus pulposus to chondrogenic and fibrocartilaginous phenotype in the mouse. J Pathol. 2009;218:113–21. doi: 10.1002/path.2519. [DOI] [PubMed] [Google Scholar]
- Zahringer U, Lindner B, Inamura S, Heine H, Alexander C. TLR2 - promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology. 2008;213:205–24. doi: 10.1016/j.imbio.2008.02.005. [DOI] [PubMed] [Google Scholar]