Abstract
Introduction
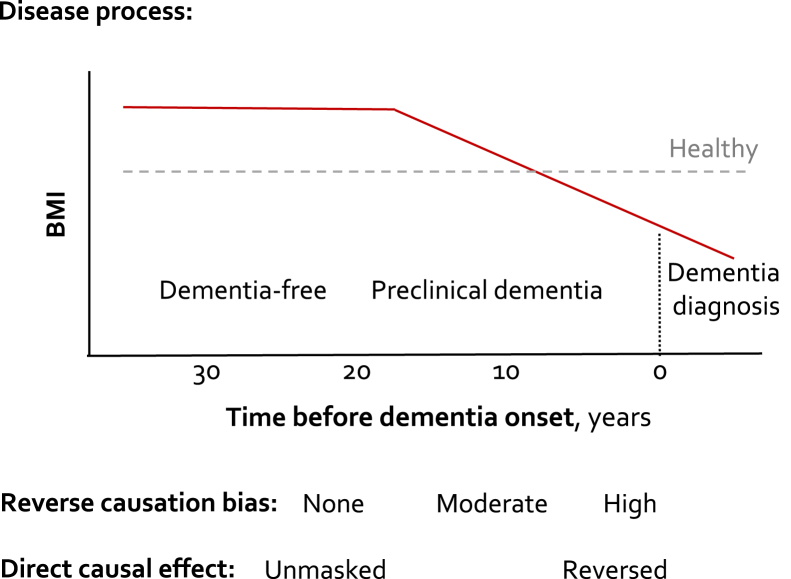
Higher midlife body mass index (BMI) is suggested to increase the risk of dementia, but weight loss during the preclinical dementia phase may mask such effects.
Methods
We examined this hypothesis in 1,349,857 dementia-free participants from 39 cohort studies. BMI was assessed at baseline. Dementia was ascertained at follow-up using linkage to electronic health records (N = 6894). We assumed BMI is little affected by preclinical dementia when assessed decades before dementia onset and much affected when assessed nearer diagnosis.
Results
Hazard ratios per 5-kg/m2 increase in BMI for dementia were 0.71 (95% confidence interval = 0.66–0.77), 0.94 (0.89–0.99), and 1.16 (1.05–1.27) when BMI was assessed 10 years, 10-20 years, and >20 years before dementia diagnosis.
Conclusions
The association between BMI and dementia is likely to be attributable to two different processes: a harmful effect of higher BMI, which is observable in long follow-up, and a reverse-causation effect that makes a higher BMI to appear protective when the follow-up is short.
Keywords: Body mass index, Dementia, Cohort study, Bias, Obesity
Highlights
-
•
Data from 1.3 million adults from 39 prospective cohort studies were pooled for the analyses.
-
•
Higher BMI was associated with increased risk of dementia when the follow-up was long.
-
•
When follow-up was short, lower BMI was linked to increased dementia risk probably due to reverse causation.
1. Introduction
The costs of dementia are enormous and increasing globally [1]. Current clinical guidelines for dementia prevention view obesity as one of the modifiable risk factors [2], [3], but the evidence is based on a relatively limited number of observational studies and the findings are mixed [4], [5], [6], [7], [8], [9], [10], [11], [12]. The most recent meta-analysis, including 4 studies and 16,282 participants, suggested a 1.4-fold increased risk of dementia in the obese [9]. The largest study in the field, published after the inclusion date for the meta-analysis, found no increase in dementia incidence among the obese [13]. On the contrary, higher body mass index (BMI) was linked to lower dementia risk.
The reasons for this discordance in findings are unclear. One possibility is that the observed association between BMI and dementia is attributable to two processes: one is a direct association between higher BMI and increased dementia risk, and the other is an association confounded by weight loss during the preclinical dementia phase, which leads a harmful exposure to appear protective via reverse causation (Fig. 1). This hypothesis is supported by the fact that clinical diagnosis of dementia is often preceded by a long (20–30 years) preclinical phase [14], [15], [16], [17] during which cardiometabolic changes, including weight loss, are common [5], [6], [18], [19]. Thus, lower BMI close to dementia onset might be a consequence of preclinical disease rather than a cause of dementia. The investigations [4], [5], [6], [7] supporting this two-process hypothesis are based on small numbers (N < 3000) and thus vulnerable to random errors. A further limitation is that these studies did not directly seek to determine the etiological phase at exposure measurement by stratifying the analyses by the length of follow-up between the assessment of BMI and dementia onset.
Fig. 1.
Conceptual model: Effect of reverse causation (preclinical disease reduces weight) on BMI at different etiological periods before dementia diagnosis. Abbreviation: BMI, body mass index.
The purpose of the present analyses was to investigate the BMI-dementia association using raw unpublished data from over 1.3 million adults from Europe, the United States, and Asia. To separate direct and biased associations, we stratified the analysis by duration of follow-up. We assumed that BMI is little affected by preclinical dementia when the BMI assessment is long before dementia onset and is considerably affected when BMI is assessed nearer the diagnosis.
2. Methods
2.1. Study population
We searched the Individual-Participant Data Meta-analysis in Working Populations (IPD-Work) consortium [20], [21], the Inter-University Consortium for Political and Social Research (www.icpsr.umich.edu/icpsrweb/ICPSR), and the UK Data Service (http://ukdataservice.ac.uk) to identify eligible large-scale prospective cohort studies for which data on BMI and dementia were available. We included 39 prospective cohort studies from Europe, the United States, and Asia (Appendix 1), which comprised a total of 1,349,857 participants with no history of dementia; were population based with BMI assessed from all participants before the ascertainment of dementia; recorded hospital-treated dementia or dementia deaths; and had accrued a minimum of 3 years of follow-up.
2.2. Measurements
Height and weight at baseline were measured in 11 studies and self-reported in 28 (Appendix 1, avaliable in the online Supplementary Materials). BMI was calculated as weight in kilograms divided by height in meters squared. We assessed the following baseline covariates because they are known to be associated with BMI and dementia risk: education/socioeconomic position (harmonized into high, intermediate, and low), smoking (current smoker vs. other), and prevalent cardiometabolic disease (coronary heart disease, stroke, and diabetes; one or more vs. none) [2], [3].
We obtained information about dementia status at follow-up from national death and hospital admission registries, reimbursements for medical treatment of dementia, and surveys on physician-diagnosed diseases, although the exact definition varied between the studies (Appendix 1). We used all underlying diagnoses in electronic medical records. In line with the National Health Service's recommendations, dementias were denoted by International Classification of Diseases (10th revision) codes ICD-10 F00, F01, F03, G30, and G31 [22], [23].
2.3. Statistical analysis
We used a two-step individual-participant data meta-analysis including study-specific analyses in the first step and pooling the study-specific estimates in the second. In each study, we performed Cox regression to generate hazard ratios and accompanying 95% confidence intervals (CIs) for the association between BMI and dementia. In the National Health Interview Survey (NHIS), the National Health and Nutrition Survey (NHANES), and the Health and Retirement Study (HRS) [24], [25], [26], [27], appropriate sampling weights were used. As one study was based on twins [28], individual observations were not independent, and we therefore used Cox regression with cluster information to get robust standard error estimates applying Breslow's method for handling ties. In all studies, each participant was followed up from the date of BMI assessment to the first record of dementia, death, or the end of follow-up.
In the basic Cox model, BMI was included as a continuous variable to allow inclusion of studies with few dementia cases (minimum 10). As in previous large-scale pooled analyses [29], [30], [31], hazard ratios were expressed per 5-unit (kg/m2) increase in BMI, which represents the increase in relative risk associated with moving up one BMI category (e.g., from healthy weight to overweight or from overweight to obese). Age at BMI assessment, sex, and ethnicity were included as covariates. Additional adjustments were undertaken for education/socioeconomic status, smoking, and prevalent cardiometabolic disease.
To study reverse causation bias, we excluded the first years of follow-up [32], using a standard procedure in epidemiology, which involves increasing the length of time (follow-up) between measurement of the exposure and the outcome, thus reducing the likelihood that BMI levels are affected by preclinical dementia. Initially, we excluded the first 5 years of follow-up and then progressively increased the exclusion period in 5-year increments up to an exclusion period of 20 years. We expected these analyses to provide estimates increasingly less affected by reverse causation. In a second set of analyses, we examined the BMI-dementia association by follow-up time. To ensure sufficient numbers in these stratified analyses, we divided follow-up time into three 10-year categories: <10 years, 10–20 years, and >20 years after baseline. The first category includes BMI measurements during the preclinical phase of dementia, whereas in the last category, most BMI assessments are likely to be before this stage.
Studies with dementia death as outcome are vulnerable to ascertainment bias if the exposure (BMI) is associated with survival. For example, participants who died before the age of late-onset dementia (65 years) have less opportunity to receive a diagnosis of dementia than those who live longer. In contrast, participants dying around the median age of dementia recording (85 years in this study), have increased opportunities to be diagnosed with dementia. To study this bias, we examined whether BMI was associated with mortality before the age of 65 years and mortality after the age of 85 years [33]. In addition, we repeated the main analyses excluding dementia cases ascertained from death registries only.
We used SAS (version 9.4) or Stata (MP version 13.1) to analyze the study-specific data. In the second step of the analysis, we used meta-analysis to combine study-specific estimates. To provide conservative estimates and take into account that the associations are not necessarily similar across cohort studies, we present the summary hazard ratios from the random-effect analysis. We examined heterogeneity of the study-specific estimates with the I2 statistic (higher values denote greater heterogeneity). We used Stata (MP, version 13.1) to compute the meta-analysis.
3. Results
Descriptive characteristics for each cohort study are provided in Appendix 1. The weighted mean follow-up across studies was 16.1 years and ranged from 4.3 to 37.7 years. Over the 21,798,141 person-years at risk in 1,349,857 participants, 6894 incident dementia cases were recorded. Overall, we observed an inverse association between BMI and dementia. The age-, sex-, and ethnicity-adjusted hazard ratio per 5-unit increase in BMI was 0.87 (95% CI = 0.82–0.93). An I2 statistic of 65.9% indicated significant heterogeneity in the study-specific hazard ratios (P < .0001). To further examine this association, we undertook four sets of analyses.
3.1. Incremental exclusions to the follow-up
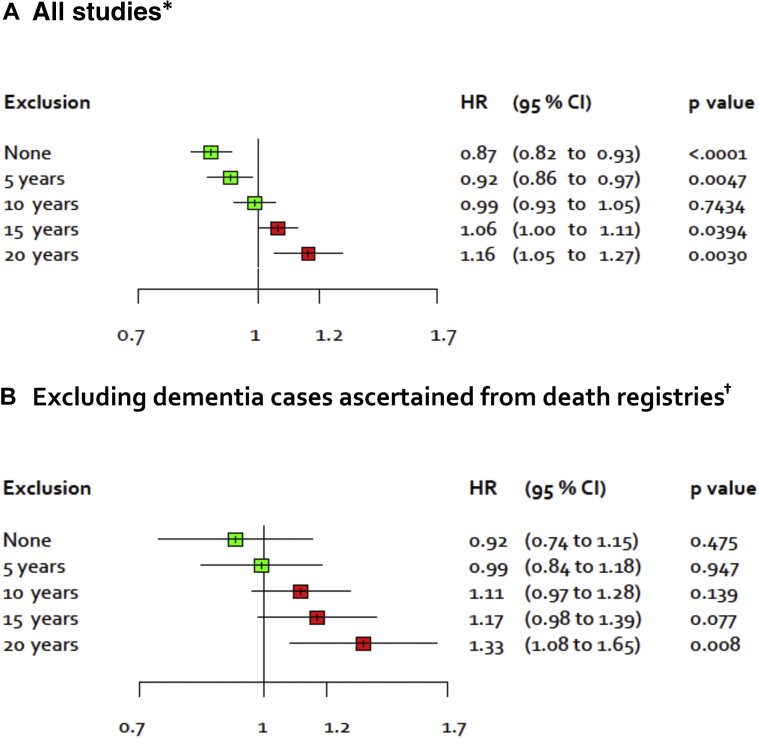
We performed a detailed analysis of incremental exclusions to the follow-up period (Table 1). To assess BMI before the preclinical phase, we excluded up to 20 years of the follow-up (the numbers of participants and dementia cases in these analyses are shown in Appendix 1). With the progressive exclusions of follow-up, the magnitude of the BMI-dementia association changed in a stepwise manner such that the hazard ratio for higher BMI and reduced dementia risk was first attenuated to the null and then increased significantly above 1.0, suggesting higher BMI to be associated with greater risk of dementia (Fig. 2A, study-specific estimates in Appendix 2). The age-, sex-, and ethnicity-adjusted hazard ratios per 5-unit increase in BMI were 0.87, 0.92, 0.99, 1.06, and 1.16 after excluding the first 0, 5, 10, 15, and 20 years of follow-up. The last estimate, which provides evidence of an increased risk of dementia associated with higher BMI in models excluding the first 20 years of follow-up, was robust to further adjustments for potential confounders, including education/socioeconomic status, smoking, and cardiometabolic disease at baseline (Table 2). Furthermore, in these analyses, there was no evidence of heterogeneity in the study-specific effect estimates. Galbraith plots in Appendix 3 confirmed that no single study or studies drove these associations.
Table 1.
Numbers and mean ages for the analytic samples
| Exclusion of follow-up | Number of studies | N (total) | N (dementia cases) | Mean age at BMI assessment among dementia cases | Mean age at dementia diagnosis | Difference between mean ages at dementia and at BMI assessment |
|---|---|---|---|---|---|---|
| None | 39 | 1,349,857 | 6894 | 69.1 | 84.3 | 15.2 |
| First 5 years | 34 | 1,212,806 | 6304 | 68.3 | 84.4 | 14.1 |
| First 10 years | 26 | 990,645 | 4924 | 66.3 | 84.8 | 18.5 |
| First 15 years | 15 | 694,223 | 3088 | 63.2 | 85.1 | 21.9 |
| First 20 years | 10 | 393,192 | 1499 | 58.4 | 84.8 | 26.4 |
Abbreviation: BMI, body mass index.
Fig. 2.
Age-, sex-, and ethnicity-adjusted HR for incident dementia per 5-kg/m2 increase in BMI after progressive exclusion of the follow-up period in all studies (A)∗ and in studies with dementia ascertainment using dementia morbidity data (B)†. ∗39 studies, total N = 1,349,857. †5 studies, total N = 95,851. The figure shows that risk of bias due to preclinical dementia decreases with increasing exclusion of the follow-up period. Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio.
Table 2.
Association between BMI and dementia risk after serial adjustments for potential confounders at baseline and exclusion of the first 20 years of follow-up∗
| Adjustment in addition to age, sex, and ethnicity | Hazard ratio per 5 kg/m2 increase in BMI (95% confidence interval) | P value |
|---|---|---|
| None | 1.17 (1.07–1.22) | .0003 |
| Education/socioeconomic status | 1.15 (1.06–1.24) | .0005 |
| Smoking | 1.17 (1.08–1.27) | <.0001 |
| Cardiometabolic disease† | 1.17 (1.07–1.27) | .0003 |
| All the above | 1.16 (1.07–1.25) | .0005 |
Abbreviation: BMI, body mass index.
These analyses are based on eight studies with a follow-up longer than 20 years and data on covariates (total N = 147,581–147,893 depending on the model).
Prevalent coronary heart disease, stroke, or diabetes at baseline.
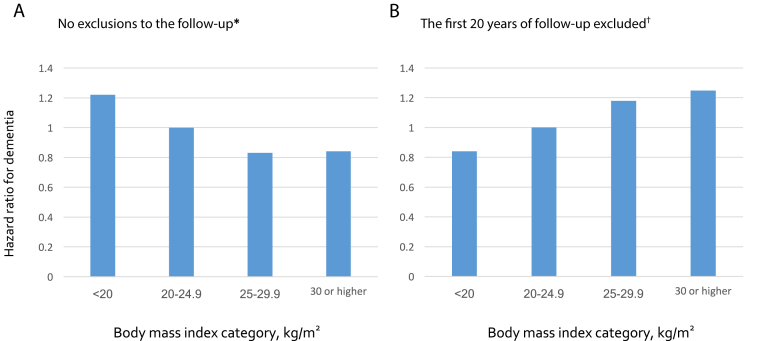
We repeated the age-, sex-, and ethnicity-adjusted analyses using BMI categories (Fig. 3). Without exclusions of the follow-up period, the highest hazard ratio was observed for underweight (BMI < 20 kg/m2; 1.22, 95% CI = 1.06–1.41, P = .005), the lowest for obesity (BMI ≥ 30 kg/m2; 0.84, 95% CI = 0.75–0.94, P = .002), and overweight (BMI = 25–29.9 kg/m2; 0.83, 95% CI = 0.77–0.90, P < .0001). This ordering was reversed when we sought to reduce reverse causation bias by excluding 20 years of the follow-up.
Fig. 3.
Shape of the association between BMI and dementia before (A) and after (B) exclusion of the first 20 years of follow-up. ∗39 studies, total N = 1,349,857. †10 studies, total N = 391,596. The figure shows that risk of bias due to preclinical dementia is smaller after exclusion of follow-up.
Assuming that less biased effect estimates are observed after the exclusion of cases with BMI assessment close to dementia diagnosis (i.e., during the preclinical phase), the opposite associations before and after exclusions support the hypothesis that the BMI-dementia association is attributable to two processes, a direct effect and reverse causation.
3.2. Analysis stratified by follow-up time
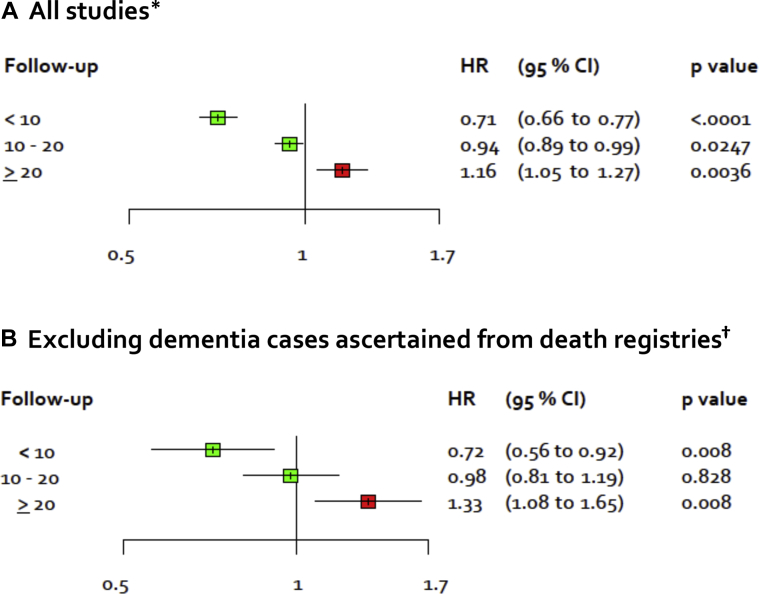
In the second set of analyses, we examined stratification by follow-up. Fig. 4A shows a forest plot for the association of BMI with dementia risk during the first 10 years, 10–20 years, and more than 20 years after baseline (study-specific results in Appendix 4). The corresponding age-, sex-, and ethnicity-adjusted summary hazard ratios were 0.71 (95% CI = 0.66–0.77, P < .0001), 0.94 (95% CI = 0.89–0.99, P = .02), and 1.16 (95% CI = 1.05–1.27, P = .004), respectively. The latter hazard ratio was 1.14 (95% CI = 1.04–1.25, P = .02) in studies with measured weight and height and 1.09 (95% CI = 0.99–1.19, P = .07) in studies with self-reported weight and height, suggesting that the result does not significantly vary by the method of BMI assessment. The increasing strength of the association between higher BMI and dementia as the length of follow-up increases and the diagnosis of dementia is increasingly distant from the assessment of BMI is consistent with the hypothesis that the harmful effect of a higher BMI becomes evident after removal of reverse causation bias.
Fig. 4.
Age-, sex-, and ethnicity-adjusted hazard ratio for dementia per 5-kg/m2 increase in BMI in analysis stratified by follow-up period in all studies (A) and in studies with dementia ascertainment using dementia morbidity data (B). ∗39 studies, total N = 1,349,857. †5 studies, total N = 95,851. The figure shows that risk of bias due to preclinical dementia is smaller at later follow-up periods. Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio.
3.3. Sensitivity analyses
We performed a series of sensitivity analyses with mortality as the outcome to examine potential ascertainment bias (Appendix 5). In most data sets included in this study, dementia cases included those ascertained using death certificates; thus, dementia could be ascertained only in participants who died. Higher baseline BMI was associated with higher overall mortality before the age of 65 years (when dementia is still rare) and lower mortality after 85 years (the median age of dementia diagnosis in this study). These findings suggest that survival bias, if anything, underestimates the status of high BMI as a risk factor.
To further examine the robustness of our findings, we repeated the main analyses after excluding 34 studies where dementia was ascertained only using death certificates. In total, 437 dementia cases in five studies (total N = 95,851) were ascertained using data other than death certificates. The pattern of results in the main analyses was replicated. In fact, the hazard ratios per 5-unit increase in BMI were slightly higher: 0.92, 0.99, 1.11, 1.17 and 1.33 after excluding the first 0, 5, 10, 15 and 20 years of follow-up (Fig. 2B; study-specific findings in Appendix 6). Higher effect estimates in Fig. 2B, corresponding to approximately one 5-year exclusion, are expected given that dementia is recorded first in morbidity data and only later in mortality data (Fig. 2A). In analysis stratified by duration of follow-up, the hazard ratio per 5-unit increase in BMI was 0.72 during the first 10 years of follow-up, 0.98 between 10 and 20 years, and 1.33 more than 20 years after baseline, again replicating findings in the main analysis (Fig. 4B, Appendix 6).
4. Discussion
In this collaborative study of over 1.3 million adults from Europe, the United States, and Asia, higher BMI was associated with increased dementia risk when weight was measured >20 years before dementia diagnosis, but this association was reversed when BMI was assessed <10 years before dementia diagnosis.
The findings of this study are consistent with the hypothesis that the BMI-dementia association is attributable to two processes: a direct (causal) effect and reverse causation as a result of weight loss during the preclinical dementia phase. Analyses stratified by duration of follow-up offered an approach to study this because BMI assessment long before dementia onset is more likely to reflect a causal process, whereas BMI assessment near dementia onset is likely to be biased by preclinical dementia. Consistent with this hypothesis, higher BMI increased dementia risk when weight was measured >20 years before dementia diagnosis (typically in midlife) but was associated with reduced risk when BMI was assessed <10 years before dementia diagnosis (i.e., typically in old age). This pattern of results was replicated in analyses serially excluding the first years of follow-up.
Our findings are in agreement with the most recent systematic review and meta-analysis, published in 2016, which identified four cohort studies with BMI assessed in midlife and incident dementia ascertained at older ages by clinical examinations at follow-up [9]. Although this is not a universal observation [34], [35], the summary relative risk of dementia for obesity compared with normal weight in midlife was significantly elevated. In the Cardiovascular Health Study of 2800 US adults, an increased risk of dementia was found among those obese in midlife but was reversed for late-life BMI [5], a finding replicated in the CAIDE study of 1300 Finnish adults [7]. In the Honolulu-Asian Aging Study of 2000 men, midlife BMI was nonsignificantly higher in individuals who subsequently developed dementia, but at older ages, they lost weight and had a lower BMI than those who remained free of dementia [4]. In the Gothenburg study of 1500 Swedish women, those who developed dementia had less increase in BMI from age 38 to 70 years than those who remained dementia free [6], [36]. Our findings, based on data from 1.3 million individuals from different regions of the world, add to this evidence by demonstrating the reversion of the BMI-dementia association as a function of decreasing distance between BMI assessment and dementia onset (and increasing impact of preclinical dementia).
Our use of electronic health records to ascertain dementia, like all methods, has some advantages and disadvantages. An advantage of this method is that there is virtually no loss to follow-up and thus selection bias arising from differential sample retention is avoided. For comparison, in studies that use repeated cognitive testing as part of dementia ascertainment, dropout may be as high as 40% at examination, leading to a significant cumulated sample attrition and therefore potential bias [9]. A disadvantage of electronic health records, however, is that they are unlikely to capture all dementia cases [37], [38]. In particular, studies where dementia outcomes are based on mortality data are not complete as they are based only on deceased patients and, even for them, dementia recording is delayed by the 3- to 9-year median lag between the clinical onset of dementia and death [39], [40]. Other concerns include the possibility that dementia as a cause of death is listed only among those who have experienced weight loss (“wasting”) before death as this would contribute to overestimation of a reverse causation effect.
As the present meta-analysis is based on a series of studies in which investigators ascertained dementia in different ways, we had the possibility to undertake a validation exercise. Thus, we repeated the main analyses excluding dementia status drawn from death certificates. The same pattern of results was evident as in the main analyses: higher BMI was associated with greater risk of dementia when BMI was measured many years before dementia onset, whereas an inverse relationship was apparent when BMI was measured closer to dementia ascertainment. In analyses exploring survival bias, we found that higher baseline BMI was associated with an increased risk of all-cause mortality before the age of 65 years but lower mortality risk after the age of 85 years (the median age of dementia diagnosis). These findings suggest that, compared with their normal weight counterparts, obese individuals were less likely to live long enough to develop dementia and more likely to die from conditions that are known to be related to increased dementia risk, such as diabetes and cardiovascular diseases [30], [31], [33], [41]. Given these findings, differences in survival may have contributed, if anything, to an underestimation of the strength of the association between BMI and dementia.
Stratification and progressive exclusion to the follow-up period to examine direct effect and reverse causation are, in effect, subgroup analyses, raising the question as to whether our findings could be an artifact of random variability as a result of reduced sample size. However, the consistent stepwise change in the BMI-dementia association at each additional exclusion of 5 years of the follow-up period in the main and supplementary analyses suggests that random variability is an unlikely explanation of our findings.
Taken together, these findings provide new evidence for the hypothesis that the association between BMI and dementia is attributable to two distinct processes; one of which is a harmful effect of higher BMI and the other reverse causation bias contributing to an inverse association between BMI and dementia. By dissecting these processes in stratified analyses, our study provides a plausible explanation for the inconsistencies in some of the prior studies on BMI and dementia. Further research is needed to examine underlying mechanisms for weight loss during the preclinical stage, including cognitive impairment leading to impaired self-care, reduced appetite due to decreased olfactory perception or changes in the regulation of satiety, and disturbed energy homeostasis. Future studies should also examine whether the role of BMI in dementia etiology varies between dementia subtypes, such as Alzheimer's disease, vascular dementia, frontotemporal dementia, and Lewy body dementia.
Research in Context.
-
1.
Systematic review: High body mass index (BMI) is suggested to increase the risk of dementia, but evidence is inconsistent. One hypothesis to explain this inconsistency is that weight loss during the preclinical phase of dementia biases the association, such that studies with a short follow-up, which assess BMI in late life, show that BMI has a protective association with dementia. However, few studies have examined the importance of length of follow-up and whether high BMI is more strongly related to dementia when assessed before preclinical dementia stage, that is, decades before dementia onset, than when assessed near dementia onset.
-
2.
Interpretation: We explored the BMI-dementia association using raw, unpublished data from 1.3 million adults from Europe, the United States, and Asia. Our findings from analyses stratified by duration of follow-up lend support for a direct association between BMI and dementia and also provide some support for reverse causation. Higher BMI was associated with increased dementia risk when BMI was assessed more than 20 years before dementia diagnosis, but lower BMI predicted dementia when BMI was assessed less than 10 years before diagnosis.
-
3.
Future directions: To avoid bias, the etiologic phase should be taken into account in studies examining dementia risk factors.
Acknowledgments
The IPD-Work Consortium was supported by NordForsk, the Nordic Programme on Health and Welfare, the UK Medical Research Council (K013351), the Academy of Finland (311492), and the Finnish Work Environment Fund, Finland. Funding bodies for each participating cohort study are listed on their websites.
Footnotes
The authors have declared that no conflict of interest exists.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jalz.2017.09.016.
Supplementary data
References
- 1.Wimo A., Guerchet M., Ali G.C., Wu Y.T., Prina A.M., Winblad B. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13:1–7. doi: 10.1016/j.jalz.2016.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NICE National Institute for Health and Care Excellence . 2015. Disability, Dementia and Frailty in Later Life–Mid-life Approaches to Prevent or Delay the Onset of these Conditions (NG16)https://www.nice.org.uk/guidance/ng16/resources/dementia-disability-and-frailty-in-later-life-midlife-approaches-to-delay-or-prevent-onset-pdf-1837274790085 Available at: [Google Scholar]
- 3.Winblad B., Amouyel P., Andrieu S., Ballard C., Brayne C., Brodaty H. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15:455–532. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- 4.Stewart R., Masaki K., Xue Q.L., Peila R., Petrovitch H., White L.R. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 5.Fitzpatrick A.L., Kuller L.H., Lopez O.L., Diehr P., O'Meara E.S., Longstreth W.T., Jr. Midlife and late-life obesity and the risk of dementia: Cardiovascular Health Study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustafson D.R., Backman K., Joas E., Waern M., Östling S., Guo X. 37 years of body mass index and dementia: observations from the prospective population study of women in Gothenburg. Sweden J Alzheimers Dis. 2012;28:163–171. doi: 10.3233/JAD-2011-110917. [DOI] [PubMed] [Google Scholar]
- 7.Tolppanen A.M., Ngandu T., Kareholt I., Laatikainen T., Rusanen M., Soininen H. Midlife and late-life body mass index and late-life dementia: results from a prospective population-based cohort. J Alzheimers Dis. 2014;38:201–209. doi: 10.3233/JAD-130698. [DOI] [PubMed] [Google Scholar]
- 8.Anstey K.J., Cherbuin N., Budge M., Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12:e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 9.Pedditizi E., Peters R., Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing. 2016;45:14–21. doi: 10.1093/ageing/afv151. [DOI] [PubMed] [Google Scholar]
- 10.Qiu C. Preventing Alzheimer's disease by targeting vascular risk factors: hope and gap. J Alzheimers Dis. 2012;32:721–731. doi: 10.3233/JAD-2012-120922. [DOI] [PubMed] [Google Scholar]
- 11.Beydoun M.A., Beydoun H.A., Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev. 2008;9:204–218. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Profenno L.A., Porsteinsson A.P., Faraone S.V. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Qizilbash N., Gregson J., Johnson M.E., Pearce N., Douglas I., Wing K. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diab Endocrinol. 2015;3:431–436. doi: 10.1016/S2213-8587(15)00033-9. [DOI] [PubMed] [Google Scholar]
- 14.Bateman R.J., Xiong C., Benzinger T.L., Fagan A.M., Goate A., Fox N.C. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casanova R., Varma S., Simpson B., Kim M., An Y., Saldana S. Blood metabolite markers of preclinical Alzheimer's disease in two longitudinally followed cohorts of older individuals. Alzheimers Dement. 2016;12:815–822. doi: 10.1016/j.jalz.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu X., Hicks C.W., He W., Wong P., Macklin W.B., Trapp B.D. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 17.Jansen W.J., Ossenkoppele R., Knol D.L., Tijms B.M., Scheltens P., Verhey F.R. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alhurani R.E., Vassilaki M., Aakre J.A., Mielke M.M., Kremers W.K., Machulda M.M. Decline in weight and incident mild cognitive impairment: Mayo Clinic Study of Aging. JAMA Neurol. 2016;73:439–446. doi: 10.1001/jamaneurol.2015.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knopman D.S., Edland S.D., Cha R.H., Petersen R.C., Rocca W.A. Incident dementia in women is preceded by weight loss by at least a decade. Neurol. 2007;69:739–746. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 20.Kivimaki M., Jokela M., Nyberg S.T., Singh-Manoux A., Fransson E.I., Alfredsson L. Long working hours and risk of coronary heart disease and stroke: a systematic review and meta-analysis of published and unpublished data for 603,838 individuals. Lancet. 2015;386:1739–1746. doi: 10.1016/S0140-6736(15)60295-1. [DOI] [PubMed] [Google Scholar]
- 21.Kivimaki M., Nyberg S.T., Batty G.D., Fransson E.I., Heikkilä K., Alfredsson L. Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet. 2012;380:1491–1497. doi: 10.1016/S0140-6736(12)60994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Health Services (NHS) London strategic clinical network; London: 2014. “Coding Clean-up” Exercise Guidance to GPs to Improve Dementia Coding, and Raise Diagnosis Rates–A Step by Step Approach. [Google Scholar]
- 23.National Health Services (NHS) England . 2015. Dementia Diagnosis and Management: A Brief Pragmatic Resource for General Practitioners.https://www.england.nhs.uk/wp-content/uploads/2015/01/dementia-diag-mng-ab-pt.pdf London. Available at: [Google Scholar]
- 24.Juster F.T., Suzman R. An overview of the Health Retirement Study. J Hum Resour. 1995;30:S7–S56. [Google Scholar]
- 25.Dawson D.A. Ethnic differences in female overweight: data from the 1985 National Health Interview Survey. Am J Public Health. 1988;78:1326–1329. doi: 10.2105/ajph.78.10.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flegal K.M., Carroll M.D., Kit B.K., Ogden C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 27.Madans J.H., Cox C.S., Kleinman J.C., Makuc D., Feldman J.J., Finucane F.F. 10 years after NHANES I: mortality experience at initial followup, 1982-84. Public Health Rep. 1986;101:474–481. [PMC free article] [PubMed] [Google Scholar]
- 28.Kaprio J., Koskenvuo M. Genetic and environmental factors in complex diseases: the older Finnish Twin Cohort. Twin Res. 2002;5:358–365. doi: 10.1375/136905202320906093. [DOI] [PubMed] [Google Scholar]
- 29.Whitlock G., Lewington S., Sherliker P., Clarke R., Emberson J., Halsey J. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berrington de Gonzalez A., Hartge P., Cerhan J.R., Flint A.J., Hannan L., MacInnis R.J. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Global Burden of Metabolic Risk Factors for Chronic Diseases C. Lu Y., Hajifathalian K., Ezzati M., Woodward M., Rimm E.B. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–983. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willett W.C., Dietz W.H., Colditz G.A. Guidelines for healthy weight. N Engl J Med. 1999;341:427. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- 33.Gustafson D. BMI and dementia: feast or famine for the brain? Lancet Diab Endocrinol. 2015;3:397–398. doi: 10.1016/S2213-8587(15)00085-6. [DOI] [PubMed] [Google Scholar]
- 34.Batty G.D., Galobardes B., Starr J.M., Jeffreys M., Davey Smith G., Russ T.C. Examining if being overweight really confers protection against dementia: sixty-four year follow-up of participants in the Glasgow University alumni cohort study. J Negat Results Biomed. 2016;15:19. doi: 10.1186/s12952-016-0062-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kivimaki M., Singh-Manoux A., Shipley M.J., Elbaz A. Does midlife obesity really lower dementia risk? Lancet Diab Endocrinol. 2015;3:498. doi: 10.1016/S2213-8587(15)00216-8. [DOI] [PubMed] [Google Scholar]
- 36.Gustafson D.R., Backman K., Waern M. Adiposity indicators and dementia over 32 years in Sweden. Neurol. 2009;73:1559–1566. doi: 10.1212/WNL.0b013e3181c0d4b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prince M., Bryce R., Ferri C.P. Alzheimer's Disease International; London: 2011. World Alzheimer Report 2011: The Benefits of Early Diagnosis and Intervention. [Google Scholar]
- 38.Jin Y.P., Gatz M., Johansson B., Pedersen N.L. Sensitivity and specificity of dementia coding in two Swedish disease registries. Neurol. 2004;63:739–741. doi: 10.1212/01.wnl.0000134604.48018.97. [DOI] [PubMed] [Google Scholar]
- 39.Larson E.B., Shadlen M.F., Wang L., McCormick W.C., Bowen J.D., Teri L. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004;140:501–509. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- 40.Xie J., Brayne C., Matthews F.E., Medical Research Council Cognitive Function and Ageing Study Collaborators Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. BMJ. 2008;336:258–262. doi: 10.1136/bmj.39433.616678.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J., Yang D.L., Chen Z.Z., Gou B.F. Associations of body mass index with cancer incidence among populations, genders, and menopausal status: A systematic review and meta-analysis. Cancer Epidemiol. 2016;42:1–8. doi: 10.1016/j.canep.2016.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.