Abstract
Background
Disruptions in redox balance lead to oxidative stress, a promoter of morbidity in critical illness. This study aimed to: 1) characterize the plasma and alveolar thiol/disulfide redox pools, 2) examine their associations with alveolar macrophage phagocytosis, and 3) determine the effect of high dose vitamin D3 on plasma thiol/disulfide redox.
Methods
Subjects were 30 critically ill, ventilated adults in a double-blind randomized trial of high-dose (250 000 or 500 000 IU) vitamin D3 or placebo. Baseline bronchoalveolar lavage fluid (BALF) samples were analyzed for determination of alveolar phagocytosis index (PI) and for concentrations of glutathione (GSH), glutathione disulfide (GSSG), cysteine (Cys), cystine (CySS), and their respective redox potentials (EhGSSG and EhCySS). Plasma redox outcomes were assessed at baseline and days 7 and 14.
Results
Baseline plasma Cys was inversely associated with alveolar PI (ρ = −0.69, P=0.003), and EhCySS was positively associated with PI (ρ = 0.61, P=0.01). Over time, among all subjects there was an increase in plasma GSH levels and a decrease in EhGSSG (P<0.01 for both), with no difference by treatment group. Vitamin D3 decreased oxidized plasma GSSG to a more normal state (P for group × time=0.009).
Conclusions
Oxidative stress indicators were positively associated with alveolar macrophage phagocytic function in acutely ill ventilated adults. High-dose vitamin D3 decreased plasma GSSG concentrations, which suggests that vitamin D can possibly improve the oxidative stress environment.
Background
In critically ill patients, oxidative stress is a key mechanism of injury that results from sepsis and multi-system organ failure and is associated with increased morbidity and mortality (1). The balance in complex biochemical oxidation-reduction (redox) states can be assessed by measuring thiol/disulfide couples, glutathione (GSH) and glutathione disulfide (GSSG)-a major intracellular thiol redox system- and cysteine (Cys) and cystine (CySS)- the predominant extracellular thiol redox system-(Figure 1) within biofluids or tissues (2).
Figure 1. Glutathione and Cysteine Redox Equipoise.
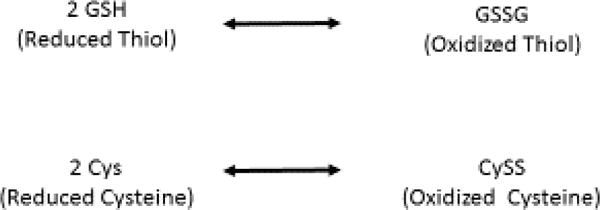
Both amino acids undergo reversible oxidation to form disulfides
Normally the thiol redox systems are tightly controlled; however extracellular redox imbalance of thiol pairs can impact key cellular functions, such as proliferation, differentiation and apoptosis (2). In acute critically ill pediatric patients, Grunwell et al (3) reported higher systemic oxidative stress compared to healthy controls, as indicated by a more oxidized plasma GSH/GSSG redox system. In adults with acute respiratory distress syndrome, GSH levels in the bronchoalveolar lavage fluid (BALF) have been reported to be depleted (4–6). However, the thiol/disulfide redox systems within the plasma or alveolar space of adults with critical illness have not been well-characterized.
The lung has an important role of providing effective barriers against oxidants and microorganisms, and GSH in extracellular lining fluid plays an important role in this host defense and are in high concentrations compared to other extracellular environments (2). Macrophages, key mediators in the response to critical illness, are highly influenced by oxidative stress. Oxidative stress controls a number of important macrophage signaling pathways (6) and a wide range of functions, including protein translation, wound healing (7), apoptosis (8), and phagocytosis (9). Liang et al have shown that GSH depletion and the resulting oxidative stress are associated with impaired alveolar macrophage phagocytosis, microbe clearance and increased risk for apoptosis (10, 11). The relationship between systemic and alveolar oxidative stress and macrophage phagocytosis has not previously been investigated in critically ill ventilated patients.
Experimental and some clinical studies in non-ICU clinical populations suggest that vitamin D can reduce oxidative stress (11–16). Approximately 60% of critically ill patients are vitamin D deficient (17–20). We have previously shown that high-dose vitamin D3 supplementation in ventilated, critically-ill patients’ decreases hospital length of stay (21). Amrein et al demonstrated a reduction in hospital mortality in a subgroup of patients with the highest degree of vitamin D deficiency (22). Also vitamin D status has been shown to correlate with plasma redox biomarkers in both healthy subjects (23) and in a pediatric intensive care unit population (24). Whether the improved outcome in our previous study with critically-ill patients treated with high-dose vitamin D3 was linked to improved reduced/oxidized redox states remains to be determined.
The aims of this study were to 1) examine the relationship between the plasma and alveolar thiol/disulfide redox pools, 2) determine if previous studies identifying a link between oxidative stress and impaired alveolar macrophage phagocytosis is true for mechanically ventilated critically ill patients, and 3) evaluate the effect of high-dose vitamin D3 on systemic oxidative stress. The primary and secondary clinical outcomes of this pilot, randomized control trial (e.g., plasma 25(OH) D concentrations, hospital length of stay, and mortality) have been previously published.(21) Here, we discuss secondary translational outcomes to provide more mechanistic data to supplement the clinical outcomes.
Methods
Study Design and participants
This was a pilot double blind randomized control trial and was approved by Emory University Institutional Review Board and registered at www.clinicaltrials.gov (NCT01372995). The enrollment goal was 36 patients from two Atlanta, Georgia hospitals; Emory University Hospital (EUH) and Emory University Midtown (EUH-M). The study was conducted from July 2011–March 2014 and enrolled critically ill patients expected to remain ventilated for at least 72 hours after entry. Full details of trial design, inclusion and exclusion criteria, safety criteria and other methodological details are provided in the previous publication (21).
Participant Selection
In summary, major inclusion criteria were 1) age > 18 years; 2) respiratory failure requiring mechanical ventilation for at least 72 hours after study entry; and 3) anticipated stay in the ICU for at least 96 hours after entry. Major exclusion criteria were 1) use of high-dose vitamin D3 supplementation (≥ 50,000 IU a week) to treat vitamin D deficiency within the prior 6 months; 2) history of medical disorders associated with hypercalcemia, chronic renal failure requiring dialysis, cirrhosis or HIV infection; and 3) hypercalcemia (albumin-corrected serum calcium > 10.8 mg/dL or ionized calcium > 5.2 mg/dL).(21)
Intervention
Following informed consent, patients were assigned to either a daily dose of oral 50 000 IU vitamin D3 for five days [total of 250 000 IU] or daily dose of 100 000 IU vitamin D3 for five days [total 500 000 IU] or placebo in a 1:1:1 ratio using a blinded block randomization schedule, overseen by biostatisticians of the Atlanta Clinical and Translational Science Institute (ACTSI) biostatistics core.(21) Randomization was stratified based on clinical site and APACHE II score >15 or ≤ 15. The medications were dissolved in sterile water and administered through an enteral feeding tube. Cholecalciferol 50 000 IU tablets were manufactured from Tischon (Westbury, NY) and Biotech (Fayetteville, AR).(21) EUH and EUH-M Investigational Drug. With the exception of the pharmacists, all study staff were blinded to the group allocation. Full details of trial design, participant selection and intervention were published previously (21).
Data collection and laboratory analysis
Blood sampling
Twenty mL of venous blood was collected at baseline and every 7 days while the subject remained in the hospital.
Total 25(OH)D
Plasma total 25(OH)D was measured using a chemiluminescent-based automated machine (IDS- iSYS; Immunodiagnostic Systems, Scottsdale, AZ). The Emory Vitamin D laboratory participates in the Vitamin D External Quality Assessment Scheme and NIST/NIH Vitamin D Metabolites Quality Assurance Program. Insufficient vitamin D status was defined as plasma total 25(OH)D concentrations <30 ng/mL, with values ≥ 30 ng/mL considered sufficient.
BALF collection
A bronchoalveolar lavage was performed on study day 0 and day 7, if subjects were still intubated. If the subject was expected to be taken off the ventilator before day 7, the sample could be collected as early as day 5. The BALF procedure was performed by serial instillation of 30 mL aliquots of normal saline into a pulmonary sub segment and BALF was collected by suction.
Alveolar macrophage phagocytosis index and percent positive analysis
BALF was centrifuged at 1 200 rpm for 7 min for recovery of cell pellet. Manual cell counts were performed with a hemocytometer and differentials were obtained from 300 consecutive cells after Diff-Quik staining (Andwin Scientific, Addison, IL). The cell pellet was re-suspended in 10 ml of 1:1 Dulbecco’s modified Eagle’s medium/Ham’s F-12 solution containing 2% fetal bovine serum (FBS), L-glutamine, 15 mmol/L HEPES, penicillin (10 000 U), streptomycin (10 000 mg/mL), amphotericin (25 mg/mL), and gentamicin (4 μg/mL). Alveolar macrophages (100 000 cells) were added to glass-chamber slides containing 100 μl of medium and 20 μl of phosphate-buffered saline (PBS). Alveolar macrophages were incubated at 37° C with 10% CO2 for 15 h, after which 10 × 105 particles of pH-sensitive pHrodo fluorescein isothiocyanate-conjugated inactivated Staphylococcus aureus were added (10:1 ratio of S. aureus/alveolar macrophages) to the cell cultures. After a 2 h incubation, the cells were washed, fixed with 4% paraformaldehyde, and stored at 4°C until analysis. Fluorescence of phagocytized S. aureus was determined by quantitative computer analysis of images taken with a Zeiss inverted microscope using 10 fields per experimental condition with the same pinhole, detector gain, and amplifier offset. The basis of this assay is that S. aureus is internalized and the pHrodo component fluoresces once it reaches the lower pH (pH = 4) present in the phagolysosome. Macrophages with any internalized bacteria were considered positive for phagocytosis. Phagocytosis was quantified by phagocytic index, which was calculated as the percentage of cells positive for phagocytosis (percent positive) multiplied by the mean relative fluorescence units of S. aureus per cell (25).
Determination of plasma and BALF oxidative stress
Details for redox determination have been previously described (26). Briefly, plasma and BALF samples were transferred to a microcentrifuge tube containing a preservative solution consisting of borate buffer, γ-glutamyl-glutamate (internal standard), serine, heparin, bathophenanthrolene disulfonate, and iodoacetic acid. Samples were centrifuged, the supernatant transferred to a second solution containing perchloric acid and boric acid, and immediately frozen at −80°C. For analysis, samples were thawed, protein precipitated, and supernatant treated with a dansyl chloride solution for derivatization. Plasma and BALF samples were subsequently analyzed using high-performance liquid chromatography (Waters 2690, Waters Corp., Milford, MA) with fluorescence detection (Waters 474 and Gilson 121 detectors). Plasma and BALF GSH, GSSG, Cys, and CySS were quantified based on the integral areas of the peak for each respective analyte relative to the integral area of the internal standard. A dilution factor for urea ([urea]plasma/[urea]BALF) was applied to BALF redox quantifications (26). The redox potential for the thiol pairs (Eh GSH/GSSG, Eh Cys/CySS) was calculated using the Nernst equation ([expressed in millivolts (mV)]; redox potential data for the two redox pools in BALF were corrected for pH. In this system, a less negative Eh redox potential value indicates a more oxidized state (26). The percentage of CySS in relation to the total Cys pool [%CySS = (CySS/(Cys + CySS)) *100] and percentage of GSSG in relation to the total GSH pool [%GSSG = (GSSG/(GSH + GSSG)] were also calculated.
Statistical analysis
Descriptive statistics were conducted on demographic data and study outcomes. Data are reported as mean (± SD) for normally distributed variables and median (IQR) for non-normally distributed variables. Variables deviating from a normal distribution were log-transformed for analyses, as necessary, or non-parametric methods were used. Baseline group differences (treatment group or vitamin D status group) were assessed with a t-test or Wilcoxon test. Paired t-tests were used to compare plasma and BALF redox status in subjects with both measurements available. Correlation analyses were assessed using a Pearson or Spearman method. Repeated measures were assessed with a linear mixed-effect method with Tukey’s post-hoc analyses. Study outcomes did not differ between the two vitamin D groups (250 000 IU vs 500 000 IU); therefore, these two groups were merged for reported analyses. This is secondary analysis from our previously published study (21) in which the primary outcome was the change in plasma 25(OH)D from baseline to 7 days;12 subjects in each group were required to test the hypothesis that high-dose vitamin D3 would achieve plasma 25(OH)D levels ≥ 30 ng/mL with power of 0.94 and α of 0.05. Data were analyzed using JMP statistical software (version 12, SAS Institute, Cary, NC). All analyses were two-sided with a significance level of α = 0.05.
Results
The study design, CONSORT diagram, enrollment and randomization are reported in a previous publication (21). Baseline demographic data by treatment group is shown in Table 1.
Table 1.
Baseline Demographic and Plasma Redox Variables by Treatment Group
| Variable | All (n = 30)a |
Placebo (n = 10) |
Vitamin D (n = 20) |
P |
|---|---|---|---|---|
| Age (yr) | 63.5 ± 17.3 | 64.8 ± 17.5 | 64.0 ± 17.7 | 0.87 |
| Male | 6 (60) | 13 (65) | 0.78 | |
| African American | 12 (40) | 4 (40) | 10 (50) | 0.10 |
| BMI (kg/m2) | 30.6 ± 7.59 | 28.2 ± 9.9 | 31.5 ± 6.4 | 0.39 |
| APACHE II | 21 ± 9 | 23.2 ± 8.8 | 20 ± 9 | 0.37 |
| Infection on Admission | 13 (43) | 6 (60) | 7 (35) | 0.17 |
| Plasma 25(OH)D (ng/mL) | 21.4 ± 9.1 | 21.5 ± 12.2 | 21.1 ± 7.9 | 0.75 |
| 25(OH)D <20 ng/mL | 13 (43) | 5 (50) | 8 (40) | 0.59 |
| 25(OH)D <30 ng/mL | 25 (83) | 8 (80) | 17 (85) | 0.73 |
| Cys (μM) | 1.11 (0.60, 2.61) | 1.36 (0.69, 8.34) | 0.98 (0.57, 1.80) | 0.40 |
| CySS (μM) | 7.05 (4.60, 9.07) | 6.62 (4.26, 12.34) | 7.25 (4.24, 8.87) | 0.77 |
| %CySS | 83.42 (73.70, 93.20) | 82.32 (50.42, 94.03) | 85.74 (72.47, 90.16) | 0.88 |
| EhCySS (mV) | −51.16 ± 31.21 | −54.79 ± 46.39 | −49.34 ± 21.59 | 0.68 |
| GSH (μM) | 0.21 (0.07, 0.35) | 0.21 (0.08, 0.72) | 0.21 (0.07, 0.32) | 0.63 |
| GSSG (μM) | 0.04 (0.008, 0.24) | 0.04 (0.007, 0.22) | 0.09 (0.009, 0.30) | 0.46 |
| %GSSG | 34.18 (3.19, 66.05) | 11.53 (2.00, 59.90) | 40.31 (7.84, 67.63) | 0.37 |
| EhGSSG (mV) | −75.71 ± 40.94 | −88.20 ± 42.13 | −69.47 ± 40.05 | 0.27 |
Data reported as N (%), mean ± SD for normal distributions, or median (IQR) for non-normal distributions.
N = 27 for plasma redox outcomes (n = 9 for placebo group, n = 18 for vitamin D group).
Baseline Systemic and Alveolar Oxidative Stress
Baseline systemic and alveolar macrophage oxidative stress data is shown in Table 2. A comparison of baseline plasma thiol/disulfide redox measures revealed significantly higher Cys, CySS, %CYSS and GSSG and more reduced EhCYSS compared to BALF-derived redox measures.
Table 2.
Comparison of Baseline Plasma and Alveolar Oxidative Stressa
| Plasma | BALF | p-value | |
|---|---|---|---|
| Cys (μM) | 0.94 (0.66, 2.95) | 0.06 (0.01, 0.11) | <0.001 |
| CySS (μM) | 7.05 (3.86, 10.12) | 0.05 (0.01, 0.21) | <0.001 |
| %CySS | 83.42 (60.68, 93.21) | 56.03 (33.44, 62.21) | 0.007 |
| EhCySS (mV) | −42.77 (−77.85, −34.00) | −23.96 (−38.38, −8.05) | 0.01 |
| GSH (μM) | 0.26 (0.08, 0.51) | 0.08 (0.02, 0.19) | 0.22 |
| GSSG (μM) | 0.13 (0.02, 0.64) | 0.03 (0.02, 0.05) | 0.006 |
| %GSSG | 60.89 (3.76, 77.73) | 37.07 (20.30, 69.58) | 0.85 |
| EhGSSG (mV) | −62.80 (−116.44, −32.99) | −39.07 (−70.05, −13.76) | 0.10 |
N= 17 subjects with paired plasma and bronchoalveolar lavage fluid (BALF) samples
Correlation of Systemic Oxidative Stress and Phagocytosis Index (PI) and Percent of Cells Positive for Phagocytosis (Percent Positive)
Correlation analyses of plasma baseline redox measures against phagocytosis outcomes are shown in Table 3. Plasma Cys was inversely associated with both PI (ρ = −0.69, p = 0.003) and percent positive (ρ = −0.51, p = 0.04). Both plasma %CySS and EhCySS were strongly positively correlated with PI (ρ = 0.56 and 0.61, respectively; p ≤ 0.02 for both). There were no significant correlations between the alveolar Cys/CySS or GSH/GSSG redox indexes and the macrophage phagocytosis indexes.
Table 3.
Spearman Correlations Between Systemic Oxidation Stress and Alveolar Macrophage Phagocytosis
| Phagocytosis Index | Percent of cells phagocytosis-positive | |
|---|---|---|
| Plasma Cys | 0.69 (0.003) | −0.51 (0.04) |
| Plasma CySS | 0.23 (0.39) | 0.16 (0.55) |
| Plasma %CySS | 0.56 (0.02) | 0.44 (0.09) |
| Plasma EhCySS | 0.61 (0.01) | 0.46 (0.07) |
| Plasma GSH | −0.06 (0.81) | −0.30 (0.25) |
| Plasma GSSG | −0.26 (0.32) | −0.39 (0.13) |
| Plasma %GSSG | −0.06 (0.83) | −0.13 (0.63) |
| Plasma EhGSSG | 0.13 (0.64) | 0.11 (0.70) |
Spearman Correlations. N=17
Effect of Vitamin D on Systemic Oxidative Stress
There were no differences in oxidative stress indices at baseline between placebo and vitamin D-treated subjects (data not shown). Over time, both groups demonstrated an increase in plasma GSH levels (P for time = 0.001) but the difference between treatment groups and treatment-by-time effect were not statistically significant (Figure 2a). Plasma GSSG increased in the placebo group, while there was a decrease in plasma GSSG in the vitamin D group (P for treatment × time =0.009, Figure 2b). Plasma EhGSSG significantly decreased (i.e., became more reduced) over time (P for time = 0.009), although there was no significance between treatment group effect or treatment-by-time effect (Figure 2c). There were no statistically significant changes in the plasma Cys/CySS redox system (P for time, group, and group × time > 0.05 for all).
Figure 2. Plasma GSH/GSSG redox outcomes over time in critically ill adults.
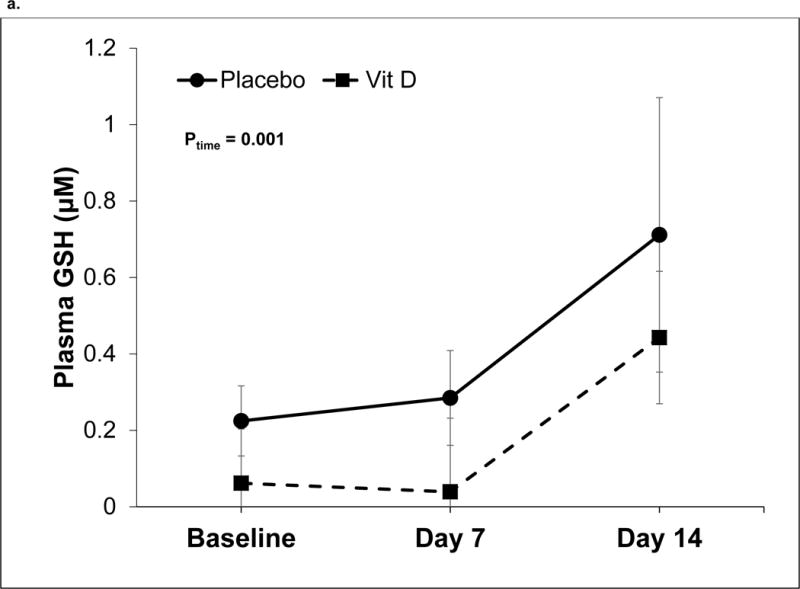
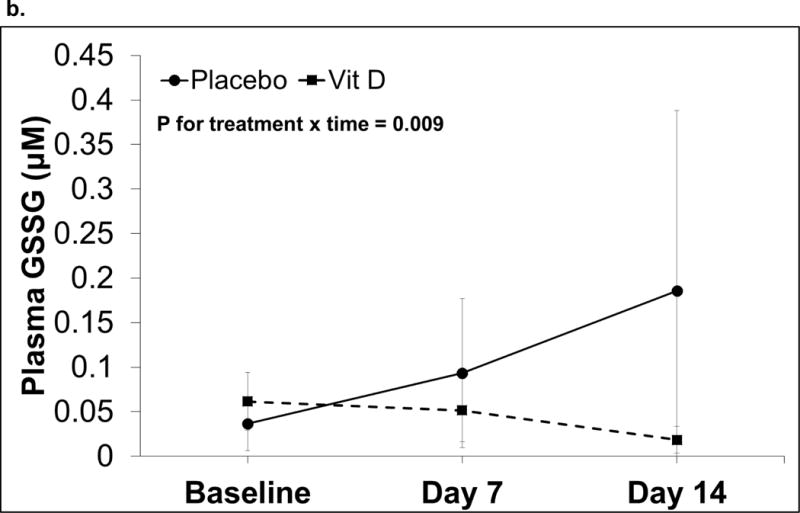
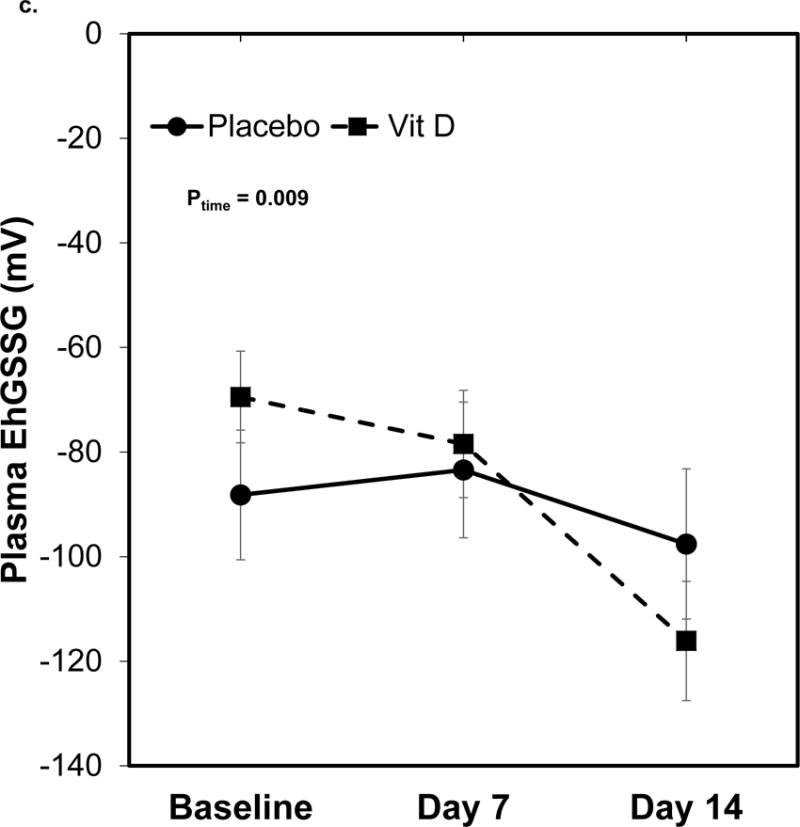
Data are shown as least squares (LS) mean ± SEM on back-transformed data. Data were analyzed using linear mixed-effect models with Tukey’s post-hoc analyses. Points not connected by the same letter indicate statistically significant differences. N=20 on day 0 and 7, n=15 on day 14. a) Plasma GSH concentrations. The time effect was statistically significant (P= 0.001). The treatment and treatment-by-time effects were not statistically significant (P = 0.22 and 0.98, respectively). b) Plasma GSSG concentrations. The treatment-by-time effect was statistically significant (P = 0.009). The treatment and time effects were not statistically significant (P = 0.18 and 0.74, respectively). c) Plasma EhGSSG. The time effect was statistically significant (P= 0.009). The treatment and treatment-by-time effects were not statistically significant (P = 0.18 and 0.74, respectively).
Discussion
GSH and GSSG represent a major intracellular thiol/disulfide redox system, while Cys and CySS represent a major extracellular redox system (27). Assessment of these redox systems can provide useful data on oxidative stress and redox balance within critically ill patient populations. In this pilot study of mechanically-ventilated adults in the ICU, we showed that oxidative stress, as assessed by thiol/disulfide redox systems, is higher in the alveolar space compared to the plasma and is associated with phagocytosis. Furthermore, reductions in plasma oxidative stress are apparent over time and can be influenced by high-dose vitamin D3 supplementation.
We evaluated the baseline thiol/disulfide redox environment in both the plasma and the lung of adult ICU patients. The lower EhCySS within the plasma compartment indicates a more reduced (less oxidized) environment relative to the alveolar compartment. A previous study showed that, in patients with acute respiratory distress syndrome the alveolar environment is more oxidized than healthy controls (4). The alveolar space in our patient population is far more oxidized compared to BALF of previous critically ill patients (4, 5). One possible explanation is that mechanical ventilation contributed to a more oxidized environment in the alveolar space. A reduction in oxidative stress during the course of recovery in critically-ill populations is expected. However, the novel evaluation of plasma thiol/disulfide redox measures over time in our population showed a significant increase in plasma GSH and a more reduced EhGSSG, with no changes in the plasma Cys/CySS redox system. Our data are, therefore, consistent with pediatric cross-sectional data by Grunwell (3) indicating that plasma Cys/CySS redox pools are preserved in critical illness and possibly maintained by changes in the supply of GSH from tissue to plasma.
We further investigated the relationship between macrophage phagocytosis function and systemic and alveolar oxidative stress. Experiments with alcohol-fed rodents by Liang et al (11) showed that GSH depletion within alveolar macrophages and related chronic oxidative stress impairs alveolar macrophage function. We found that alveolar macrophage phagocytosis of Staph. Aureus was strongly positively correlated with the plasma Cys/CySS redox potential (EhCySS) and %CySS, both indicative of higher oxidative stress. This data suggest that the extracellular redox environment is closely linked to innate immune functions of alveolar macrophages. It is possible that increased phagocytosis may create more reactive oxygen species and apoptosis resulting in a more oxidized environment. Increased oxidation may lead to further dysregulation of immune function contributing to more deleterious clinical effects over time in this patient population. Alternatively, a more oxidized plasma Cys/CySS redox pool may be a homeostatic response to critical illness required to promote phagocytosis by macrophages (9). Additional detailed studies are required to establish causal relationships between the plasma redox environment and alveolar macrophage function in critical illness. Furthermore, additional studies are needed to determine if phagocytosis is upregulated in the resident alveolar macrophage pool or reflective of macrophages newly recruited from the oxidized systemic environment.
Cross-sectional studies by members of our group have previously demonstrated significant associations between vitamin D status and plasma thiol/disulfide redox biomarkers in both a generally healthy adult population (23) and in critically ill pediatric patients (24). In healthy adults, plasma 25(OH)D was positively associated with plasma GSH and inversely associated with EhGSSG (23); whereas in critically ill children, a more sufficient vitamin D status was associated with lower plasma GSH and GSSG and a more reduced plasma EhCySS (24). In the current study in mechanically ventilated adults, we have measured, for the first time, the effect of a high-dose vitamin D3 intervention on plasma thiol/disulfide redox. Our data show that vitamin D3 supplementation decreases GSSG, the oxidized disulfide form of GSH, relative to the placebo group, although there were no effects on other measured redox outcomes. Experiments performed in vitro support our clinical finding in that vitamin D, in its active hormonal form (calcitriol), upregulates glutathione reductase, which catalyzes the reduction of GSSG to GSH (28). Administration of vitamin D3 in patients with type 2 diabetes and in vitamin D insufficient women revealed an improvement in oxidative stress as measured by increased GSH, total antioxidant capacity, and malondialdehyde (15, 16). Our primary study found a decrease in hospital length of stay (21); this secondary analysis provides a potential mechanism by which vitamin D may impact clinical outcomes.
The major strength of this study was the use of a randomized, placebo-controlled design in a well-characterized cohort. A limitation of this pilot study was the small sample size, particularly in the number of subjects undergoing bronchoscopy. The primary outcome of our primary study was change in plasma total 25(OH)D (21), therefore this study may not have been adequately powered to detect changes in the secondary outcomes reported here. Despite our small sample size, we were able to determine that systemic oxidative stress improved over time with high-dose vitamin D3 administration. Additional markers of oxidative stress or antioxidants were not measured. An additional limitation is that we only assessed macrophage phagocytosis with Staph. Aureus and no other pathogens, which could have different responses. We chose a bacterial model since that is still the most common pathogen in the lungs of hospitalized critically ill patients. Finally, many subjects were liberated from the ventilator before repeat bronchoscopy on day 7, thus limiting our ability to conduct longitudinal BALF analyses.
Conclusion
We showed that a more oxidized plasma Cys/CySS redox pool was associated with alveolar macrophage phagocytic function, while systemic GSH-related oxidative stress decreased during clinical recovery. Finally, in a randomized, placebo-controlled trial, high-dose vitamin D3 decreased plasma GSSG concentrations, which suggests that vitamin D can possibly improve the oxidative stress environment, which may provide potential mechanism by which vitamin D may impact clinical outcomes.
Acknowledgments
We would like to acknowledge Mona Brown, Guatam Hebbar, Jennifer Jones, Frank Harris, and Janine Ward for their contribution to the study.
Funding: This project was supported, in part, by National Institutes of Health grants: NIH R21 HL110044 (GSM, TRZ, LAB, VT, JEH), K24 DK096574 (TRZ), UL1 TR000454 (JEH, GSM, TRZ, VT), K01 DK102851 (JAA), T32 HL076118 (BS), and T32 AA013528 (JEH).
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- APACHE II
Acute Physiology and Chronic Health Evaluation II score
- BALF
bronchoalveolar lavage fluid
- Cys
cysteine
- CySS
cystine
- GSH
glutathione
- GSSG
glutathione disulfide
- IQR
inter-quartile range
- PI
Phagocytosis Index
- Percent Positive
Percent of Cells Positive for Phagocytosis
- SD
Standard Deviation
Footnotes
Authors have no conflict of interest.
References
- 1.Gunaydin B, Sancak B, Candan S, Sariahmetoglu M, Ozcagli G, Tunctan B, et al. Temporal variation of oxidant stress in critically ill patients. Minerva anestesiologica. 2007;73(5):261–6. [PubMed] [Google Scholar]
- 2.Moriarty-Craige SE, Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annual review of nutrition. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- 3.Grunwell JR, Gillespie SE, Ward JM, Fitzpatrick AM, Brown LA, Gauthier TW, et al. Comparison of Glutathione, Cysteine, and Their Redox Potentials in the Plasma of Critically Ill and Healthy Children. Frontiers in pediatrics. 2015;3:46. doi: 10.3389/fped.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunnell E, Pacht ER. Oxidized glutathione is increased in the alveolar fluid of patients with the adult respiratory distress syndrome. The American review of respiratory disease. 1993;148(5):1174–8. doi: 10.1164/ajrccm/148.5.1174. [DOI] [PubMed] [Google Scholar]
- 5.Pacht ER, Timerman AP, Lykens MG, Merola AJ. Deficiency of alveolar fluid glutathione in patients with sepsis and the adult respiratory distress syndrome. Chest. 1991;100(5):1397–403. doi: 10.1378/chest.100.5.1397. [DOI] [PubMed] [Google Scholar]
- 6.Brune B, Dehne N, Grossmann N, Jung M, Namgaladze D, Schmid T, et al. Redox control of inflammation in macrophages. Antioxidants & redox signaling. 2013;19(6):595–637. doi: 10.1089/ars.2012.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metukuri MR, Namas R, Gladstone C, Clermont T, Jefferson B, Barclay D, et al. Activation of latent transforming growth factor-beta1 by nitric oxide in macrophages: role of soluble guanylate cyclase and MAP kinases. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17(4):578–88. doi: 10.1111/j.1524-475X.2009.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinloth A, Brune B, Fischer B, Galle J. Nitric oxide prevents oxidised LDL-induced p53 accumulation, cytochrome c translocation, and apoptosis in macrophages via guanylate cyclase stimulation. Atherosclerosis. 2002;162(1):93–101. doi: 10.1016/s0021-9150(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 9.Duan J, Kodali VK, Gaffrey MJ, Guo J, Chu RK, Camp DG, et al. Quantitative Profiling of Protein S-Glutathionylation Reveals Redox-Dependent Regulation of Macrophage Function during Nanoparticle-Induced Oxidative Stress. ACS nano. 2016;10(1):524–38. doi: 10.1021/acsnano.5b05524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang Y, Yeligar SM, Brown LA. Chronic-alcohol-abuse-induced oxidative stress in the development of acute respiratory distress syndrome. TheScientificWorldJournal. 2012;2012:740308. doi: 10.1100/2012/740308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang Y, Harris FL, Brown LA. Alcohol induced mitochondrial oxidative stress and alveolar macrophage dysfunction. BioMed research international. 2014;2014:371593. doi: 10.1155/2014/371593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ke CY, Yang FL, Wu WT, Chung CH, Lee RP, Yang WT, et al. Vitamin D3 Reduces Tissue Damage and Oxidative Stress Caused by Exhaustive Exercise. International journal of medical sciences. 2016;13(2):147–53. doi: 10.7150/ijms.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sales de Almeida JP, Liberatti LS, Nascimento Barros FE, Kallaur AP, Batisti Lozovoy MA, Scavuzzi BM, et al. Profile of oxidative stress markers is dependent on vitamin D levels in patients with chronic hepatitis C. Nutrition (Burbank, Los Angeles County, Calif) 2016;32(3):362–7. doi: 10.1016/j.nut.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Kanikarla-Marie P, Jain SK. 1,25(OH)2D3 inhibits oxidative stress and monocyte adhesion by mediating the upregulation of GCLC and GSH in endothelial cells treated with acetoacetate (ketosis) The Journal of steroid biochemistry and molecular biology. 2016;159:94–101. doi: 10.1016/j.jsbmb.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Medeiros Cavalcante IG, Silva AS, Costa MJ, Persuhn DC, Issa CI, de Luna Freire TL, et al. Effect of vitamin D3 supplementation and influence of BsmI polymorphism of the VDR gene of the inflammatory profile and oxidative stress in elderly women with vitamin D insufficiency: Vitamin D3 megadose reduces inflammatory markers. Experimental gerontology. 2015;66:10–6. doi: 10.1016/j.exger.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Shab-Bidar S, Neyestani TR, Djazayery A. The interactive effect of improvement of vitamin D status and VDR FokI variants on oxidative stress in type 2 diabetic subjects: a randomized controlled trial. European journal of clinical nutrition. 2015;69(2):216–22. doi: 10.1038/ejcn.2014.240. [DOI] [PubMed] [Google Scholar]
- 17.Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. Journal of translational medicine. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mata-Granados JM, Vargas-Vasserot J, Ferreiro-Vera C, Luque de Castro MD, Pavon RG, Quesada Gomez JM. Evaluation of vitamin D endocrine system (VDES) status and response to treatment of patients in intensive care units (ICUs) using an on-line SPE-LC-MS/MS method. The Journal of steroid biochemistry and molecular biology. 2010;121(1–2):452–5. doi: 10.1016/j.jsbmb.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 19.Higgins DM, Wischmeyer PE, Queensland KM, Sillau SH, Sufit AJ, Heyland DK. Relationship of vitamin D deficiency to clinical outcomes in critically ill patients. JPEN Journal of parenteral and enteral nutrition. 2012;36(6):713–20. doi: 10.1177/0148607112444449. [DOI] [PubMed] [Google Scholar]
- 20.Nair P, Lee P, Reynolds C, Nguyen ND, Myburgh J, Eisman JA, et al. Significant perturbation of vitamin D-parathyroid-calcium axis and adverse clinical outcomes in critically ill patients. Intensive care medicine. 2013;39(2):267–74. doi: 10.1007/s00134-012-2713-y. [DOI] [PubMed] [Google Scholar]
- 21.Han JE, Jones JL, Tangpricha V, Brown MA, Brown LA, Hao L, et al. High Dose Vitamin D Administration in Ventilated Intensive Care Unit Patients: A Pilot Double Blind Randomized Controlled Trial. Journal of clinical & translational endocrinology. 2016;4:59–65. doi: 10.1016/j.jcte.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. Jama. 2014;312(15):1520–30. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez JA, Chowdhury R, Jones DP, Martin GS, Brigham KL, Binongo JN, et al. Vitamin D status is independently associated with plasma glutathione and cysteine thiol/disulphide redox status in adults. Clinical endocrinology. 2014;81(3):458–66. doi: 10.1111/cen.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvarez JA, Grunwell JR, Gillespie SE, Tangpricha V, Hebbar KB. Vitamin D deficiency is associated with an oxidized plasma cysteine redox potential in critically Ill children. The Journal of steroid biochemistry and molecular biology. 2016 doi: 10.1016/j.jsbmb.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauthier TW, Ping XD, Harris FL, Wong M, Elbahesh H, Brown LA. Fetal alcohol exposure impairs alveolar macrophage function via decreased glutathione availability. Pediatric research. 2005;57(1):76–81. doi: 10.1203/01.PDR.0000149108.44152.D3. [DOI] [PubMed] [Google Scholar]
- 26.Yeh MY, Burnham EL, Moss M, Brown LA. Chronic alcoholism alters systemic and pulmonary glutathione redox status. American journal of respiratory and critical care medicine. 2007;176(3):270–6. doi: 10.1164/rccm.200611-1722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free radical biology & medicine. 2009;47(10):1329–38. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain SK, Micinski D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochemical and biophysical research communications. 2013;437(1):7–11. doi: 10.1016/j.bbrc.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


