Abstract
Background
A panel of four kallikrein markers (total, free, and intact prostate-specific antigen [PSA] and human kallikrein-related peptidase 2 [hK2]) improves predictive accuracy for Gleason score≥7 (high-grade) prostate cancer among men biopsied for elevated PSA. A four-kallikrein panel model was originally developed and validated by the Dutch center of the European Randomized Study of Screening for Prostate Cancer (ERSPC). The kallikrein panel is now commercially available as 4Kscore.
Objective
To assess whether these findings could be replicated among participants in the Finnish section of ERSPC (FinRSPC) and whether β-microseminoprotein (MSP), a candidate prostate cancer biomarker, adds predictive value.
Design, setting, and participants
Among 4861 biopsied screening-positive participants in the first three screening rounds of FinRSPC, a case-control subset was selected that included 1632 biopsy-positive cases matched by age at biopsy to biopsy-negative controls.
Outcome measurements and statistical analysis
The predictive accuracy of prespecified prediction models was compared with biopsy outcomes.
Results and limitations
Among men with PSA of 4.0–25 ng/ml, 1111 had prostate cancer, 318 of whom had high-grade disease. Total PSA and age predicted high-grade cancer with an area under the curve of 0.648 (95% confidence interval [CI] 0.614–0.681) and the four-kallikrein panel increased discrimination to 0.746 (95% CI 0.717–0.774). Adding MSP to the four-kallikrein panel led to a significant (Wald test; p = 0.015) but small increase (0.003) in discrimination. Limitations include a risk of verification bias among men with PSA of 3.0–3.99 ng/ml and the absence of digital rectal examination results.
Conclusions
These findings provide additional evidence that kallikrein markers can be used to inform biopsy decision-making. Further studies are needed to define the role of MSP.
Patient summary
Four kallikrein markers and β-microseminoprotein in blood improve discrimination of high-grade prostate cancer at biopsy in men with elevated prostate-specific antigen.
Keywords: Prostate Cancer, Screening, Prediction models, Kallikriens, 4Kscore, Beta-microseminoprotein
1. Introduction
Prostate cancer (PC) screening with prostate-specific antigen (PSA) is controversial because of a questionable balance between harms (eg, overdiagnosis and overtreatment), and benefits (eg, reduced cancer mortality). The major drawback of PSA as a screening test is its low specificity for aggressive disease. While elevated PSA is associated with a higher risk of aggressive PC, it can also indicate benign prostatic disease or low risk PC [1]. As a result, many men with elevated PSA levels undergo unnecessary prostate biopsies, which can lead to complications such as infection and bleeding [2]. Prostate biopsy may detect indolent PC that would otherwise never become apparent to the patient during his lifetime (overdiagnosis). Better methods are needed to detect Gleason score ≥7 PC (high-grade) to reduce the negative consequences of screening by decreasing the number of men without aggressive PC who are unnecessarily referred for biopsy.
Measured PSA is a combination of a number of different molecular subforms: complexed PSA versus free PSA, intact versus nicked PSA, and human kallikrein-related peptidase 2 (hK2) [3–5]. It has been shown that measurement of subforms separately improves the prediction of biopsy results among men with elevated PSA [6,7]. We developed a prediction model based on a panel of four kallikrein markers (total, free, and intact PSA, and hK2) using serum samples from the Dutch and Swedish sections of the European Randomized Study of Screening for Prostate Cancer (ERSPC) and demonstrated better discrimination of high-grade PC (Gleason score ≥7) compared to clinical variables and total PSA alone [8–11]. In a large, representative, population-based cohort of unscreened men who gave blood at age 50 or 60 yr, the panel of four kallikrein markers significantly enhanced the accuracy of predicting future metastatic PC [12]. The markers are now commercially available as the 4Kscore test. It was shown that another abundant prostate-derived protein, β-microseminoprotein (MSP) [13] was lower in blood from men with PC, and a suggested association with high-grade PC was observed in two prospective studies, the STHLM3 trial [14] and the Multiethnic Cohort study [15]. We sought to determine whether our previous findings, as well as addition of MSP to the prediction model, could be replicated in men participating in the Finnish section of ERSPC (FinRSPC), the largest ERSPC section. This study is an independent external validation of prespecified predictive models based on a large, prospective, screening cohort, so the results of the study are generalizable to the Finnish and probably to other northern European populations.
2. Patients and methods
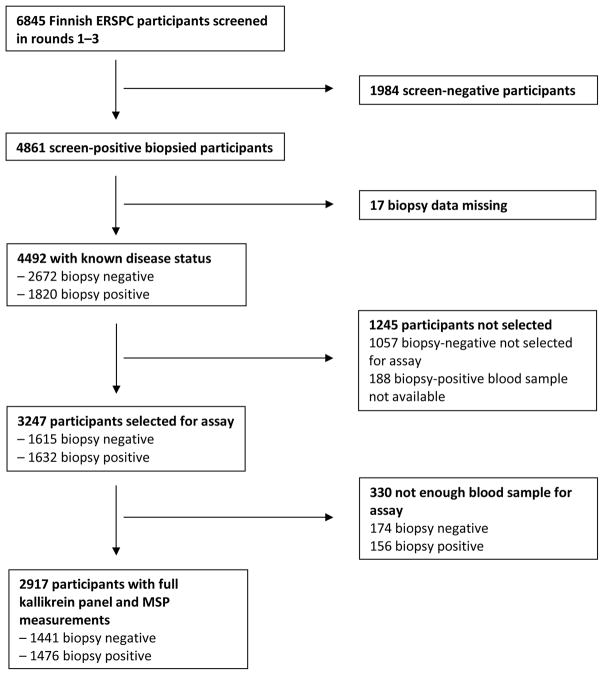
Our aim was to assess the predictive accuracy of prediction models comprising age, the four kallikreins, and MSP. A total of 4861 participants were biopsied during 1996–2008 as a result of elevated PSA either in the first round of screening (ie, first PSA on study) or in rounds 2 or 3 (ie, with 1 or 2 prior on-study PSA tests, respectively), as shown in Figure 1. Among screen-positive participants, a case-control subset was selected for evaluation of the four-kallikrein panel and MSP. This included 1632 biopsy-positive cases that were individually matched by age at biopsy to 1632 biopsy-negative controls. Biopsy data were missing for 17 of these subjects, leaving 1615 biopsy-negative controls. There were 156 cases and 174 controls with a missing or inadequate sample for assay of the four-kallikrein panel and MSP, leaving 1476 cases and 1441 controls for analysis.
Fig. 1.
Flow chart of participant inclusion. ERSPC = European Randomized Study of Prostate Cancer Screening; MSP = β-microseminoprotein.
Men randomly allocated to the screening arm in the FinRSPC trial with screening PSA of ≥4.0 ng/ml were referred to a urology clinic for prostate biopsy [16]. Men with PSA of 3.0–3.99 ng/ml were referred to undergo an additional test, which was digital rectal examination (DRE) during 1996–1998 and determination of the free/total PSA ratio with a cutoff point of <16% from 1999 onwards. Hence, among men with PSA of 3.0–3.99 ng/ml, only those with a suspicious DRE or free/total PSA ratio <16% were referred for biopsy [16,17].
Sextant biopsies were performed from the start of the trial in 1996, but a 10–12-core biopsy approach was adopted in 2002 [16]. Cryopreserved sample aliquots (serum or plasma) were shipped to the Wallenberg Research Laboratories at Lund University (Malmö, Sweden) for assay of the four kallikrein markers and MSP during 2014–2015. The analyses for total and free PSA [18], intact PSA [19], hK2 [20], and MSP [21] were previously reported [22,23]. The assay reagents used for measurements of intact PSA and hK2 were the same as those used in the 4Kscore test. Total and free PSA concentrations were measured in cryopreserved samples in Malmö. Our study excluded a small number (2%) of screening participants who had PSA of ≥3.0 ng/ml in the screening trial but whose total PSA was <3.0 ng/ml according to the World Health Organization–calibrated measurements performed in Malmö.
We compared the discrimination of a prespecified model including only total PSA and age, a prespecified model including age, total, and free PSA, and the prespecified kallikrein model based on age and the four kallikrein markers; nonlinear terms were included for free and total PSA. We did not include DRE in these models as it was inconsistent with clinical stage: 36% of patients with a positive DRE and cancer diagnosis with clinical stage T1C, and 17% with a negative DRE had clinical stage T2A or greater. These models were developed using serum sample measurements and biopsy data from the Rotterdam screening arm of ERSPC and were independently applied to this data set [8]. The model is calibrated to men undergoing sextant biopsy with pathologic grading according to 1990s’ criteria. By contrast, the proprietary 4Kscore model was developed [24] and validated [25] based on men aged 50–70 yr with PSA of ≥3.0 ng/ml undergoing prostate biopsy with ten or more cores and with pathology using contemporary Gleason grading approaches.
Our main analyses included men with total PSA of 4.0–25 ng/ml. The upper limit was chosen on the grounds that most urologists would hesitate not to biopsy men with PSA of >25 ng/ml, and the panel gives uniformly high risks to this group; therefore, a reflex test would not be required among these men [26]. Men with PSA of 3.0–3.99 ng/ml were biopsied depending on their free/total PSA ratio; we did not include these men in the main analysis because of the possibility of verification bias. We performed additional sensitivity analyses for other ranges of total PSA, including the range 3.0–10 ng/ml, often described as the diagnostic “grey zone” for biopsy. The rate of PSA testing before randomization was extremely low, at 1.4% [27]. Because it is likely that a prior PSA test or prior negative biopsy affects the accuracy of a predictive model, we assessed all participants who were biopsied as a result of screening round 1 and screening rounds 2–3 separately [9,11,26].
Analyses of calibration and clinical utility when reporting results for the four-kallikrein panel have typically included metrics such as the numbers of biopsies avoided and cancer diagnoses delayed, as well as decision analyses [28]. The current data set is complicated by the case-control design. Because we have data on the clinical utility of the panel, we did not see the value of such analyses here, as they would involve questions such as the number of men who would have high-grade PC, as defined in the 1990s, on sextant biopsy.
We investigated whether adding MSP to the prediction model with the four-kallikrein panel increased predictive value. To generate the MSP models for prediction of any PC and high-grade PC on biopsy separately, we used constrained logistic regression. Log-transformed MSP was entered into the prediction model along with cubic splines to allow for nonlinearity, while kallikrein model risk (estimated using the four-kallikrein model) was entered on the inverse-logit scale and the coefficient was constrained to be 1. Tenfold cross validation was used when assessing the performance of the model including MSP to adjust for statistical overfit.
Individual-level 5-α reductase inhibitor (5ARI) purchase data were obtained from a Finnish national prescription database and were available for all study participants. We investigated whether purchasing a 5ARI within 6 mo before screening affected the relationship between the four-kallikrein panel prediction model and the risk of high-grade PC. An interaction between 5ARI and the four-kallikrein panel model was tested using multivariable logistic regression. Previous studies suggest that total PSA values are reduced by approximately 50% in men who take 5ARIs [29]. We compared the accuracy defined as the Brier score for the four-kallikrein prediction model to a model that artificially doubles the values of total, free, and intact PSA among men who purchased a 5ARI within 6 mo before screening. All analyses were performed using Stata version 13.0 (StataCorp, College Station, TX, USA).
3. Results
The characteristics of participants with total PSA between 4.0 and 25 ng/ml included in the analyses are presented in Table 1. Within the first three screening rounds of FinRSPC, a total of 1111 cancers were diagnosed, of which 318 (29%) were identified as high-grade (Gleason ≥7). Table 1 shows that the concentrations of all four kallikrein markers and MSP differed significantly by biopsy status, including the nicked/total PSA ratio (nicked PSA is free PSA minus intact PSA). An exception is that intact PSA levels did not significantly differ between high-grade disease and low-grade or no cancer diagnosis. All prediction models demonstrated a significantly greater predicted risk among those with cancer versus no cancer and for high-grade cancer versus low-grade or no cancer (Table 1; Wilcoxon rank-sum test, all p < 0.0001).
Table 1.
Characteristics of participants with total PSA between 4 and 25 ng/ml by biopsy result a
| Variable | No cancer | Cancer | p value | No or low-grade cancer | High-grade cancer | p value |
|---|---|---|---|---|---|---|
| Patients, n (%) | 1019 (48) | 1111 (52) | 1810 (85) | 318 (15) | ||
| Age at biopsy (yr) | 67 (63–68) | 67 (63–68) | 0.2 | 67 (63–68) | 67 (63–70) | <0.0001 |
| Total PSA (ng/ml) | 5.2 (4.5–6.6) | 5.9 (4.7–8.2) | <0.0001 | 5.3 (4.6–7.0) | 6.5 (5.0–10.0) | <0.0001 |
| Free PSA (ng/ml) | 1.25 (0.94–1.71) | 1.02 (0.73–1.43) | <0.0001 | 1.16 (0.84–1.58) | 1.05 (0.78–1.42) | 0.005 |
| Free/total PSA ratio | 0.23 (0.18–0.29) | 0.16 (0.12–0.22) | <0.0001 | 0.20 (0.15–0.27) | 0.15 (0.11–0.21) | <0.0001 |
| Intact PSA (ng/ml) | 0.73 (0.55–0.99) | 0.64 (0.46–0.88) | <0.0001 | 0.69 (0.50–0.94) | 0.69 (0.51–0.94) | 0.9 |
| Nicked/total PSA ratio | 0.093 (0.071–0.127) | 0.058 (0.039–0.085) | <0.0001 | 0.081 (0.055–0.113) | 0.050 (0.033–0.073) | <0.0001 |
| hK2 (ng/ml) | 0.064 (0.041–0.097) | 0.075 (0.049–0.116) | <0.0001 | 0.067 (0.044–0.103) | 0.079 (0.053–0.117) | <0.0001 |
| MSP (ng/ml) | 28 (19–38) | 23 (15–31) | <0.0001 | 26 (18–36) | 23 (15–31) | 0.001 |
| Gleason score at biopsy, n (%) | ||||||
| ≤6 | 791 (71) | 791 (44) | ||||
| 7 | 231 (21) | 231 (73) | ||||
| ≥8 | 87 (7.8) | 87 (27) | ||||
| Unknown | 2 (0.2) |
PSA = prostate-specific antigen; hK2 = human kallikrein-related peptidase 2; MSP = β-microseminoprotein
p values are reported for comparison between participants with no diagnosis and participants with a cancer diagnosis, and between participants with no or a low-grade cancer diagnosis and participants with a high-grade cancer diagnosis, and were determined using a Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables. Results are presented as median (interquartile range) for continuous variables and as frequency (percentage) for categorical variables.
Because there was little difference in discrimination between serum and plasma samples, we included both in the primary analysis. The discriminative accuracy increased with the addition of each marker (Table 2). Discrimination based on age and total PSA was low (0.595 and 0.648 in predicting any- and high-grade PC, respectively; Table 2). The highest increase in discrimination occurred on addition of free PSA to the model with age and total PSA for prediction of both any- and high-grade PC, with area under the curve (AUC) gains of 0.126 and 0.051, respectively. Although MSP was predictive of any- and high-grade PC after adjusting for the kallikrein panel (Wald test; p < 0.0001 and 0.015, respectively), it represented the smallest increase in AUC, with gains of 0.012 and 0.003, respectively. The prespecified four-kallikrein model showed moderately strong discriminative ability, with AUCs of 0.743 and 0.746 in predicting any- and high-grade PC, respectively.
Table 2.
Prediction performance for prostate cancer status among participants with total PSA of 4–25 ng/ml
| Outcome | AUC (95% CI) | ΔAUC | AUC (95% CI) | ΔAUC | ΔAUC | AUC (95% CI) | ΔAUC | |
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| PSAAM | PSAAM + fPSA | for fPSA | KM | for KM vs PSAM | for KM vs PSAAM + fPSA | KM + MSP | for MSP | |
| Any PC 1111 cases 1019 controls |
0.595 (0.571–0.619) | 0.721 (0.700–0.743) | 0.126 | 0.743 (0.722–0.763) | 0.148 | 0.022 | 0.755 (0.734–0.775) | 0.012 |
| High-grade PC 318 cases 1810 controls |
0.648 (0.614–0.681) | 0.699 (0.668–0.731) | 0.051 | 0.746 (0.717–0.774) | 0.098 | 0.047 | 0.749 (0.721, 0.778) | 0.003 |
AUC = area under the curve; CI = confidence interval; ΔAUC = increase in AUC on addition of a variable to the prediction model; PSA = prostate-specific antigen; fPSA = free PSA; PSAAM = PSA + age model; KM = kallikrein model; MSP = β-microseminoprotein; PC = prostate cancer.
Although the discriminative ability of all the prediction models varied slightly across the PSA ranges, the increment in predictive accuracy associated with the markers was similar in all analyses. The exceptions were for discrimination by screening round and when MSP was added to the four-kallikrein model: discrimination improved for men without prior screening but not for those with a previous PSA test. Conversely, intact PSA and hK2 added discrimination for previously screened men (Table 3). The lowest increase in discrimination from the model based on age and total PSA compared to the four-kallikrein model occurred when we analyzed measurements on serum samples only (0.071; Ttable 3), which could suggest degradation of the decay-prone components, free and intact PSA, in serum.
Table 3.
Sensitivity analyses: discrimination performance for high-grade prostate cancer
| Analysis | AUC (95% CI) | ΔAUC | AUC (95% CI) | ΔAUC | ΔAUC | AUC (95% CI) | ΔAUC | |
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| PSAAM | PSAAM + fPSA | for fPSA | KM | for KM vs PSAM | for KM vs PSAAM + fPSA | KM + MSP | for MSP | |
| Main analysis: PSA 4–25 mg/ml 318 cases, 1810 controls |
0.648 (0.614–0.681) | 0.699 (0.668–0.731) | 0.051 | 0.746 (0.717–0.774) | 0.098 | 0.047 | 0.749 (0.721–0.778) | 0.003 |
| PSA ≥4 ng/ml 349 cases, 1843 controls |
0.668 (0.635–0.700) | 0.715 (0.685–0.745) | 0.047 | 0.756 (0.729–0.783) | 0.088 | 0.041 | 0.759 (0.733–0.786) | 0.003 |
| PSA 3–10 ng/ml 287 cases, 2234 controls |
0.615 (0.579–0.651) | 0.698 (0.666–0.729) | 0.083 | 0.744 (0.715–0.772) | 0.129 | 0.046 | 0.746 (0.718–0.775) | 0.002 |
| PSA 3–25 ng/ml 365 cases, 2417 controls |
0.655 (0.623–0.687) | 0.709 (0.680–0.738) | 0.054 | 0.758 (0.733–0.783) | 0.103 | 0.049 | 0.761 (0.736–0.786) | 0.003 |
| Serum only 160 cases, 1273 controls |
0.671 (0.624–0.718) | 0.699 (0.655–0.744) | 0.028 | 0.742 (0.703–0.782) | 0.071 | 0.043 | 0.749 (0.711–0.788) | 0.007 |
| Screening round 1 43 cases, 516 controls |
0.682 (0.593–0.770) | 0.779 (0.709–0.850) | 0.097 | 0.774 (0.710–0.838) | 0.092 | −0.005 | 0.787 (0.726–0.848) | 0.013 |
| Screening rounds 2–3 275 cases, 1294 controls |
0.644 (0.606–0.681) | 0.706 (0.671–0.741) | 0.062 | 0.759 (0.728–0.790) | 0.115 | 0.053 | 0.757 (0.725–0.788) | −0.002 |
AUC = area under the curve; CI = confidence interval; ΔAUC = change in AUC on addition of a variable to the prediction model; PSA = prostate-specific antigen; fPSA = free PSA; PSAAM = PSA + age model; KM = kallikrein model; MSP = β-microseminoprotein.
We did not find evidence of an interaction between 5ARI status and the four-kallikrein model (p = 0.4). In addition, we found no evidence that predictive accuracy improved when the levels of PSA isoforms were doubled in patients on 5ARIs, with a poorer Brier score (0.199) for the adjusted marker levels than for the unadjusted levels (0.170).
4. Discussion
In this study of FinRSPC participants with an elevated PSA result, we found that a prespecified model based on the four-kallikrein panel improved predictive discrimination for any- and high-grade (Gleason ≥7) PC compared to a prediction model based on age and total PSA, and compared to a model that also included free PSA. These results support the earlier findings that the 4Kscore is strongly predictive of the risk of high-grade PC at biopsy such that use of the model could guide biopsy decision-making, which would reduce the harms of PSA screening. Further empirical research is necessary to ascertain how the four-kallikrein panel should be amended for patients taking 5ARIs.
We found that addition of MSP to the kallikrein panel yields a small improvement in diagnostic performance. In one study, MSP provided no added discriminatory value to age and the four-kallikrein panel. However, this was observed among men in a community-based setting with an indication for biopsy because of elevated PSA (≥3.0 ng/ml) and a free/total PSA ratio <20% or suspicious DRE [23]. In another large prospective screening trial (STHLM3), MSP improved discrimination when added to a model including PSA, age, family history, prior biopsy, and a genomic risk score. However, these authors did not report whether MSP was added to a model already incorporating free PSA, intact PSA, and hK2 [14]. In the Multiethnic Cohort, MSP levels in blood were inversely correlated with the risk of subsequent PC, but sis not add to PSA in terms of risk prediction [15]. Further well-defined studies on MSP are warranted to determine its role in PC prediction.
Our findings add to the growing body of evidence that the four-kallikrein panel can be used as a reflex test among those with an elevated PSA result to aid in biopsy decision-making. Many studies have shown that the kallikrein panel improves discrimination compared to a model with only age and total PSA, including three other sections of the ERSPC: Tarn, Göteborg, and Rotterdam [8–11,23–26,30]. We previously developed a prediction model including age and the four-kallikrein panel using data for previously unscreened men in the Göteborg arm and showed that it dramatically increased discrimination on top of age and total PSA from 0.68 to 0.87 among men with total PSA of ≥3 ng/ml [10]. Among Rotterdam participants there was a statistically significant increase from 0.776 to 0.825 on addition of free PSA, intact PSA, and hK2 to a model comprising age and total PSA for detection of high-grade PC [8]. The four-kallikrein panel exhibited high discriminative capability (AUC 0.82) in a large prospective setting among US men and a superior net benefit compared to the widely used Prostate Cancer Prevention Trial Risk Calculator 2.0, which incorporates standard clinical variables (AUC 0.74) [25]. The AUC for high-grade PC we report is slightly lower than in these prior studies, but the added benefit of the markers was very similar. For instance, in the US validation study, the kallikrein panel had an AUC of 0.08 units greater than a model including PSA only, compared to an increase of 0.10 in the present study.
We found that addition of intact PSA and hK2 improved discrimination in all sensitivity analyses except among screening round 1 participants, where most men had not had a prior PSA test, whereas other studies demonstrate the added value of intact PSA and hK2 is independent of screening history. In particular, the statistical model used in the 4Kscore test was built using unscreened participants in the ProtecT trial [24], but had excellent discrimination and calibration when applied to a US clinical cohort [25] in which almost all patients had prior screening. We believe that our finding was probably a chance result attributable to multiple testing: just as an ineffective marker might by chance show predictiveness in at least one of multiple subgroups tested, an effective marker might by chance fail to show predictiveness in at least one subgroup.
This study is an independent external validation of prespecified predictive models based on a large, prospective, screening cohort, and thus the results of the study are generalizable to the Finnish and probably to other northern European populations. A notable aspect of our study is that participants were biopsied using a scheme unique to FinRSPC. PSA of ≥4.0 ng/ml was the cutoff for biopsy, and men with PSA of 3.0–3.99 ng/ml underwent follow-up testing to determine if biopsy should be performed, as previously described. Since the panel incorporates free PSA, there is a risk of verification bias when assessing the value of the panel among men with PSA of 3.0–3.99 ng/ml. Therefore, our primary analysis was performed for men with PSA of 4.0–25 ng/ml. Another limitation is that we did not incorporate DRE results into our prediction model. Although this may slightly underestimate the value of the panel, several other studies, including those by Braun et al [23] and Bryant et al [24], demonstrated that inclusion of intact PSA and hK2 increases the prediction of any- and high-grade PC detection when DRE is not incorporated. We did not assess clinical utility or calibration as we were limited by the fact that some participants underwent sextant biopsy and biopsies were graded using pre-International Society of Urological Pathology (ISUP) criteria. However, we do not believe it likely that this would importantly affect discrimination, as it is unlikely that participants who would have been upgraded according to ISUP criteria or with a 10–12-core biopsy versus sextant biopsy would have low panel risk scores.
5. Conclusions
In FinRSPC, a large, prospective independent cohort, we replicated the finding that a panel of four kallikreins can improve predictive accuracy for PC and high-grade PC. We also found that addition of MSP marginally improves prediction, although further studies are needed to define the role of this marker. Our data provide further evidence that kallikrein models can be used as a reflex test to determine which men with elevated PSA can avoid biopsy, reducing unnecessary biopsies and overdiagnosis of low-grade disease.
Take Home Message.
Four kallikrein markers and β-microseminoprotein (MSP) in blood improve discrimination of high-grade cancer at biopsy in men with elevated prostate-specific antigen. These kallikrein markers can be used to inform biopsy decision-making. Further studies are needed to define the role of MSP.
Acknowledgments
Funding/Support and role of the sponsor: The work was supported in parts by grants from the Finnish Funding Agency for Technology and the Innovation Finland Distinguished Professor program, the Academy of Finland, the Cancer Society of Finland, the Sigrid Juselius Foundation, the Medical Research Fund of Tampere University Hospital, the National Cancer Institute [R01CA160816, R01 CA175491, P50-CA92629, and P30-CA008748], the Sidney Kimmel Center for Prostate and Urologic Cancers, and David H. Koch through the Prostate Cancer Foundation, the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Program in UK, the Swedish Cancer Society (Cancerfonden project no. 14-0722) and the Swedish Research Council (VR-MH project no. 2016-02974). The Finnish screening trial was supported by grants from the Academy of Finland (grant #260931), the Cancer Society of Finland, and Competitive Research Funding (Pirkanmaa Hospital District). The sponsors played no direct role in the study.
We thank Riina Kylätie for technical assistance and Mona Hassan Al-Battat, and AnnaPeri Erlandsson for expert assistance with immunoassay measurements of blood samples at the Wallenberg Research Laboratories at Lund University in Malmö, Sweden.
Footnotes
Author contributions: Hans Lilja had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Vickers, Lilja.
Acquisition of data: Visakorpi, Sjöblom, Talala, Kujala, Murtola.
Analysis and interpretation of data: Assel, Vickers, Lilja.
Drafting of the manuscript: Assel, Vickers, Lilja.
Critical revision of the manuscript for important intellectual content: Stenman, Taari, Auvinen, Tammela, Visakorpi, Murtola.
Statistical analysis: Assel.
Obtaining funding: Vickers, Lilja.
Administrative, technical, or material support: None.
Supervision: Vickers, Lilja.
Other: None.
Financial disclosures: Hans Lilja certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Hans Lilja holds patents for free PSA, hK2, and intact PSA assays, and is named, along with Andrew Vickers, on a patent for a statistical method to detect prostate cancer. The marker assay patents and the patent for the statistical model have been licensed and commercialized as 4Kscore by OPKO Diagnostics. Andrew Vickers and Hans Lilja receive royalties from sales of this test. Hans Lilja owns stock and Andrew Vickers owns stock options in OPKO. The remaining authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bozeman CB, Carver BS, Eastham JA, Venable DD. Treatment of chronic prostatitis lowers serum prostate specific antigen. J Urol. 2002;167:1723–6. [PubMed] [Google Scholar]
- 2.Mkinen T, Auvinen A, Hakama M, Stenman UH, Tammela TL. Acceptability and complications of prostate biopsy in population-based PSA screening versus routine clinical practice: a prospective, controlled study. Urology. 2002;60:846–50. doi: 10.1016/s0090-4295(02)01864-2. [DOI] [PubMed] [Google Scholar]
- 3.Lilja H, Christensson A, Dahlen U, et al. Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin Chem. 1991;37:1618–25. [PubMed] [Google Scholar]
- 4.Nurmikko P, Pettersson K, Piironen T, Hugosson J, Lilja H. Discrimination of prostate cancer from benign disease by plasma measurement of intact, free prostate-specific antigen lacking an internal cleavage site at Lys145–Lys146. Clin Chem. 2001;47:1415–23. [PubMed] [Google Scholar]
- 5.Piironen T, Lovgren J, Karp M, et al. Immunofluorometric assay for sensitive and specific measurement of human prostatic glandular kallikrein (hK2) in serum. Clin Chem. 1996;42:1034–41. [PubMed] [Google Scholar]
- 6.Christensson A, Bjork T, Nilsson O, et al. Serum prostate specific antigen complexed to alpha 1-antichymotrypsin as an indicator of prostate cancer. J Urol. 1993;150:100–5. doi: 10.1016/s0022-5347(17)35408-3. [DOI] [PubMed] [Google Scholar]
- 7.Stenman UH, Leinonen J, Alfthan H, Rannikko S, Tuhkanen K, Alfthan O. A complex between prostate-specific antigen and alpha 1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: assay of the complex improves clinical sensitivity for cancer. Cancer Res. 1991;51:222–6. [PubMed] [Google Scholar]
- 8.Vickers A, Cronin A, Roobol M, et al. Reducing unnecessary biopsy during prostate cancer screening using a four-kallikrein panel: an independent replication. J Clin Oncol. 2010;28:2493–8. doi: 10.1200/JCO.2009.24.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A, Roobol MJ, Savage CJ, et al. A four-kallikrein panel for the prediction of repeat prostate biopsy: data from the European Randomized Study of Prostate Cancer screening in Rotterdam, Netherlands. Br J Cancer. 2010;103:708–14. doi: 10.1038/sj.bjc.6605815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vickers AJ, Cronin AM, Aus G, et al. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Goteborg, Sweden. BMC Med. 2008;6:19. doi: 10.1186/1741-7015-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vickers AJ, Cronin AM, Roobol MJ, et al. A four-kallikrein panel predicts prostate cancer in men with recent screening: data from the European Randomized Study of Screening for Prostate Cancer, Rotterdam. Clin Cancer Res. 2010;16:3232–9. doi: 10.1158/1078-0432.CCR-10-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stattin P, Vickers AJ, Sjoberg DD, et al. Improving the specificity of screening for lethal prostate cancer using prostate-specific antigen and a panel of kallikrein markers: a nested case-control study. Eur Urol. 2015;68:207–13. doi: 10.1016/j.eururo.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lilja H, Abrahamsson PA. Three predominant proteins secreted by the human prostate gland. Prostate. 1988;12:29–38. doi: 10.1002/pros.2990120105. [DOI] [PubMed] [Google Scholar]
- 14.Gronberg H, Adolfsson J, Aly M, et al. Prostate cancer screening in men aged 50–69 years (STHLM3): a prospective population-based diagnostic study. Lancet Oncol. 2015;16:1667–76. doi: 10.1016/S1470-2045(15)00361-7. [DOI] [PubMed] [Google Scholar]
- 15.Haiman CA, Stram DO, Vickers AJ, et al. Levels of beta-microseminoprotein in blood and risk of prostate cancer in multiple populations. J Natl Cancer Inst. 2013;105:237–43. doi: 10.1093/jnci/djs486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilpelainen TP, Tammela TL, Malila N, et al. Prostate cancer mortality in the Finnish randomized screening trial. J Natl Cancer Inst. 2013;105:719–25. doi: 10.1093/jnci/djt038. [DOI] [PubMed] [Google Scholar]
- 17.Kilpelainen TP, Tammela TL, Malila N, et al. The Finnish prostate cancer screening trial: analyses on the screening failures. Int J Cancer. 2015;136:2437–43. doi: 10.1002/ijc.29300. [DOI] [PubMed] [Google Scholar]
- 18.Mitrunen K, Pettersson K, Piironen T, Bjork T, Lilja H, Lovgren T. Dual-label one-step immunoassay for simultaneous measurement of free and total prostate-specific antigen concentrations and ratios in serum. Clin Chem. 1995;41:1115–20. [PubMed] [Google Scholar]
- 19.Peltola MT, Niemela P, Alanen K, Nurmi M, Lilja H, Pettersson K. Immunoassay for the discrimination of free prostate-specific antigen (fPSA) forms with internal cleavages at Lys145 or Lys146 from fPSA without internal cleavages at Lys145 or Lys146. J Immunol Methods. 2011;369:74–80. doi: 10.1016/j.jim.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaisanen V, Eriksson S, Ivaska KK, Lilja H, Nurmi M, Pettersson K. Development of sensitive immunoassays for free and total human glandular kallikrein 2. Clin Chem. 2004;50:1607–17. doi: 10.1373/clinchem.2004.035253. [DOI] [PubMed] [Google Scholar]
- 21.Valtonén-Andre C, Sävblom C, Fernlund P, Lilja H, Giwercman A, Lundwall A. Beta-microseminoprotein in serum correlates with the levels in seminal plasma of young, healthy males. J Androl. 2008;29:330–7. doi: 10.2164/jandrol.107.003616. [DOI] [PubMed] [Google Scholar]
- 22.Kim EH, Andriole GL, Crawford ED, et al. Detection of high-grade prostate cancer among PLCO participants using a prespecified four kallikrein marker panel. J Urol. 2017;197:1041–7. doi: 10.1016/j.juro.2016.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun K, Sjoberg DD, Vickers AJ, Lilja H, Bjartell AS. A four-kallikrein panel predicts high-grade cancer on biopsy: independent validation in a community cohort. Eur Urol. 2016;69:505–11. doi: 10.1016/j.eururo.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryant RJ, Sjoberg DD, Vickers AJ, et al. Predicting high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured in blood in the ProtecT study. J Natl Cancer Inst. 2015;107:djv095. doi: 10.1093/jnci/djv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parekh DJ, Punnen S, Sjoberg DD, et al. A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. Eur Urol. 2015;68:464–70. doi: 10.1016/j.eururo.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Vickers AJ, Cronin AM, Aus G, et al. Impact of recent screening on predicting the outcome of prostate cancer biopsy in men with elevated prostate-specific antigen: data from the European Randomized Study of Prostate Cancer Screening in Gothenburg, Sweden. Cancer. 2010;116:2612–20. doi: 10.1002/cncr.25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilpeläinen TP, Pogodin-Hannolainen D, Kemppainen K, et al. Estimate of opportunistic prostate specific antigen testing in the Finnish Randomized Study of Screening for Prostate Cancer. J Urol. 2017;198:50–7. doi: 10.1016/j.juro.2017.01.048. [DOI] [PubMed] [Google Scholar]
- 28.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decision Making. 2006;26:565–74. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guess HA, Gormley GJ, Stoner E, Oesterling JE. The effect of finasteride on prostate specific antigen: review of available data. J Urol. 1996;155:3–9. [PubMed] [Google Scholar]
- 30.Benchikh A, Savage C, Cronin A, et al. A panel of kallikrein markers can predict outcome of prostate biopsy following clinical work-up: an independent validation study from the European Randomized Study of Prostate Cancer screening, France. BMC Cancer. 2010;10:1–7. doi: 10.1186/1471-2407-10-635. [DOI] [PMC free article] [PubMed] [Google Scholar]