Abstract
Psoriasis is a common inflammatory cutaneous disease that affects approximately 120 million people worldwide. Systemic treatments have significantly improved disease burden, but concerns persist regarding their association with increased risk of malignancy. Patients with psoriasis have a slightly elevated baseline risk of lymphoproliferative diseases. Studies on methotrexate and cyclosporine and older biological agents such as tumor necrosis factor inhibitors have found no increased risk of non-cutaneous solid tumors. However, there are positive associations found between cutaneous squamous cell carcinomas and certain therapies. There is conflicting evidence regarding risk of lymphoma and melanoma. Further studies are needed to determine the long-term safety of newer psoriasis treatments (IL-12/23, IL-17, Janus Kinase 1/3, and phosphodiesterase-4 inhibitors) and, specifically, their safety in patients with a history of cancer. This review summarizes the most recent studies on malignancy risk from psoriasis and its treatments in patients and cancer survivors with highest available level of evidence.
1 Introduction
Psoriasis is a common inflammatory disease affecting 3.2% of the United States (US) adult population[1]. Systemic treatments such as methotrexate (MTX), cyclosporine (CsA), and biological agents: tumor necrosis factor alpha (TNFα) inhibitors, ustekinumab and newer biologics[2, 3] have significantly improved burden of disease in patients with moderate to severe psoriasis, improving quality of life[4–6]. A review on psoriasis treatment and malignancy risk was published in 2009[7]. Since then therapeutic options have expanded and additional safety data has become available. Herein, we summarize the most recent meta-analyses, randomized control trials (RCTs) and prospective cohort studies on malignancy risk from psoriasis and its treatments in patients and cancer survivors.
2 Baseline risk of malignancy in psoriasis patients
2.1 Systemic malignancies
Assessing baseline cancer risk in psoriasis is challenging as most studies include both treated and untreated patients. In 2001, Margolis et al. published a landmark study, comparing 17,000 psoriasis patients to patients with hypertension, revealing an increased risk ratio of overall malignancy in patients with severe psoriasis (1.78, 95% CI 1.3 to–2.40)[8]. Most cancers identified in this cohort were lymphoproliferative malignancies and nonmelanoma skin cancers (NMSC)[8], highlighting the increased risk of malignancy prior to the biologics era.
Gelfand et al. conducted a population-based cohort study revealing an increased relative risk (RR) for lymphoma (RR 2.95, 95% CI 1.83–4.76)[9] that persisted after adjusting for age, sex, MTX treatment, and development of mycosis fungoides. In a follow up study, they reported a positive association between psoriasis and lymphoma (adjusted hazard ratio, aHR 1.35, 95% CI 1.17–1.55) with strongest association between severe psoriasis with Hodgkin’s lymphoma (aHR 3.18, 95% CI 1.01–9.97) and cutaneous T-cell lymphoma (CTCL aHR 10.75, 95% CI 3.89–29.76)[10], though the latter association may be a result of misdiagnosing CTCL as psoriasis. Brauchli et al. confirmed an elevated risk of lymphohematopoietic cancer (odds ratio, OR 2.12, 95% CI 1.45–3.10) and in a nested case–control analysis, found overall cancer risk was increased in patients who did not receive oral treatment and had disease duration ≥2 years (adjusted OR 1.31, 95% CI 1.16–1.48)[11].
Most recently, a prospective, population-based cohort study of 200,000 psoriasis patients revealed minimally increased risk for all cancers excluding NMSC (aHR 1.06, 95% CI 1.02–1.09) and for lymphoma (aHR 1.34, 95%CI 1.18–1.51)[12]. In a meta-analysis conducted in 2013, the overall malignancy risk was elevated but consistent with previous studies (standard incidence ratio, SIR 1.16, 95% CI 1.07–1.25) with SIR 1.4 (95% CI 1.06–1.86) for non-Hodgkin lymphoma (NHL)[13]. A retrospective cohort study in biologic-naïve pediatric psoriasis patients found no significant increase in overall cancer risk compared to a matched pediatric population with no psoriasis, however increased lymphoma rate was observed when compared with the general population (SIR 5.42, 95% CI 1.62–12.94) [14]. Evidence for increase risk of solid tumors is inconsistent, but has been previously reported[11, 13, 12, 15] (Table 1).
Table 1.
Baseline risk of systemic malignancies stratified by organ systems
| Type of cancer | Study design | Malignancy risk (95% CI) | Comments |
|---|---|---|---|
| Any lymphoma | Population-based retrospective cohort study[10] | aHR 1.34 (1.16–1.54) | Mild psoriasis |
| Observational study with nested case-control analysis[11] | IRR 1.76 (1.19–2.58) | Moderate to severe psoriasis | |
| Population-based prospective cohort study[12] | aHR 1.86 (1.23–2.80) | ||
| Retrospective cohort study[14] | SIR 5.42 (1.62–12.94) | biologic-naïve pediatric patients | |
| Any lymphoma excluding CTCL | Observational study with nested case-control analysis[11] | IRR 1.55 (1.03–2.31) | Moderate to severe psoriasis |
| Population-based prospective cohort study[12] | aHR 1.62 (1.16–2.28) | ||
| Non Hodgkin’s lymphoma | Systematic review and meta-analysis of epidemiological studies[13] | SIR 1.40 (1.06–1.86) | |
| Population-based retrospective cohort study[10] | HR 1.14 (0.96–1.35) | Excluding CTCL, moderate to severe psoriasis | |
| Population-based cohort study[15] | HR 1.03 (0.59–1.75) | Mild to severe psoriasis | |
| Leukemia | Systematic review and meta-analysis of epidemiological studies[13] | SIR 1.84 (0.78–4.34) | High heterogeneity of included studies |
| Observational study with nested case-control analysis[11] | IRR 1.89 (1.21–2.94) | ||
| Population based prospective cohort study[12] | aHR 1.05 (0.67–1.65) | Moderate to severe Psoriasis | |
| Hodgkins Lymphoma | Population-based retrospective cohort study[10] | HR 1.42 (1.00–2.02) | Patient cohort without systemic treatments |
| Retrospective cohort study[102] | SIR 3.3 (1.4–6.4) | Hospitalized patients, did not control for systemic medications | |
| CTCL | Population based retrospective cohort study[10] | aHR 4.34 (2.89–6.52) | Moderate to severe psoriasis group |
| Population based prospective cohort study[12] | aHR 9.25 (3.69–23.22) | Possibly secondary to misclassification | |
| Lung | Systematic review and meta-analysis of epidemiological studies[13] | SIR 1.52 (1.35–1.71) | No adjustment for smoking |
| Population based prospective cohort study[12] | aHR 1.60 (1.14–2.24) | Persistent after adjustment for smoking | |
| Observational study with nested case-control analysis[11] | IRR 0.79 (0.6–1.06) | ||
| Population-based cohort study[15] | HR 1.05 (0.61–1.79) | Mild to severe psoriasis | |
| Upper aerodigestive Tract | Systematic review and meta-analysis of epidemiological studies[13] | SIR 3.05 ( 1.74–5.32) | No adjustment for smoking |
| Observational study with nested case-control analysis[11] | IRR 1.36 (0.72–2.54) | Mean follow up of 4.6 years, young age at diagnosis, adjusted for smoking | |
| Pancreas | Systematic review and meta-analysis of epidemiological studies[13] | SIR 1.46 (1.10–1.95) | |
| Observational study with nested case-control analysis[11] | IRR 2.20 (1.18–4.09) | Trend to increase with longer duration of disease | |
| Population based prospective cohort study[12] | aHR 1.29 (0.64–2.61) | Moderate to severe Psoriasis | |
| Liver | Systematic review and meta-analysis of epidemiological studies[13] | SIR 1.90 (1.48–2.44) | |
| Digestive tract | Population based retrospective cohort study[103] | RR 1.57 (1.41–1.74) | Taiwanese population |
| Population based retrospective cohort study[104] | HR 2.02 (1.33–3.07) | Taiwanese population | |
| Kidney/Uri nary Tract | Systematic review and meta-analysis of epidemiological studies[13] | SIR 1.31 (1.11–1.55) | |
| Population based retrospective cohort study[104] | HR 3.18 (1.54–6.57) | Taiwanese population | |
| Observational study with nested case-control analysis[11] | IRR 1.25 (0.84–1.85) | Trend to increase with longer duration of disease | |
| Population-based cohort study[15] | HR 2.5 (1.27–4.92) | Mild to severe psoriasis | |
| Breast | Systematic review and meta-analysis of epidemiological studies[13] | SIR 1.15 (1.02–1.29) | |
| Observational study with nested case-control analysis[11] | IRR 1.04 (0.83–1.31) | Cohort with minimal systemic treatment | |
| Population based prospective cohort study[12] | aHR 0.96 (0.71–1.28) | Moderate to severe Psoriasis | |
| Population-based cohort study[15] | HR 0.89 (0.71–1.10) | Mild to severe psoriasis | |
| Colorectal | Systematic review and meta-analysis of epidemiological studies[13] | SIR 1.12 (0.95–1.32) | |
| Population based prospective cohort study[12] | aHR 1.20 (0.82–1.74) | Moderate to severe Psoriasis | |
| Observational study with nested case-control analysis[11] | IRR 1.35 (0.97–1.90) | Trend to increase with longer duration of disease | |
| Population-based cohort study[15] | HR 1.11 (0.71–1.73) | Mild to severe psoriasis | |
| CNS | Systematic review and meta-analysis of epidemiological studies[13] | SIR 1.24 (CI 0.98–1.59) | |
| Observational study with nested case-control analysis[11] | IRR 1.30 (0.69–2.45) | Cohort with minimal systemic treatment | |
| Prostate | Observational study with nested case-control analysis[11] | IRR 0.84 (0.63–1.12) | |
| Population based prospective cohort study[12] | aHR 1.16 (0.90–1.50)[12] | Moderate to severe Psoriasis | |
| Female genital organs | Observational study with nested case-control analysis[11] | IRR 1.38 (0.91–2.11)[11] | |
| Population-based cohort study [15] | HR 0.54 (0.26–1.15) endometrial; HR 1.28 (0.56–2.88) ovarian |
Mild to severe psoriasis | |
| Melanoma | Systematic review and meta-analysis of epidemiological studies[13] | aHR 1.07 (0.85–1.35) | |
| Observational study with nested case-control analysis[11] | IRR 0.83 (0.50–1.36) | ||
| Population based prospective cohort study[12] | aHR 1.28 (0.82–1.99) | Moderate to severe Psoriasis | |
| Population based retrospective cohort study[17] | IRR 1.19 (1.03–1.37); IRR 1.09 (0.75–1.58) |
Significant in mild psoriasis and not in severe psoriasis | |
| Population-based cohort study[15] | HR 1.95 (1.21–3.13) | Mild-to-severe psoriasis |
aHR = adjusted hazard ratio; CI = confidence interval; CNS = central nervous system; CTCL = cutaneous T-cell lymphoma; IRR = incidence rate ratio; HR = hazard ratio; SIR = standard incidence ratio.
2.2 Cutaneous malignancies
Baseline risk of skin cancer in psoriasis patients is difficult to assess due to confounding from phototherapy and immunosuppressive therapy. The rate of NMSC is increased[16], supported by a meta-analysis reporting SIR 5.3 for squamous cell carcinoma (SCC) (95% CI 2.63–10.71) and SIR 2.00 for basal cell carcinoma (BCC) (95% CI 1.83–2.20), whereas risk of melanoma was not increased[13]. A large population based cohort study confirmed these results, finding an aHR of 1.12 for NMSC (95% CI 1.07–1.16) in the overall psoriasis group, and 1.62 (95% CI 1.16–2.28) in patients with severe psoriasis[12]. A Danish cohort reported modestly increased melanoma risk in patients with mild psoriasis but not severe psoriasis[17].
3 Risk of cancer with psoriasis treatments
3.1 Phototherapy
Several studies found oral psoralen and ultraviolet A (PUVA) to be associated with increased risk of skin cancer in a dose dependent fashion[18–21] but not for non-cutaneous malignancies[22]. The risk of NMSC is greatest with >350 treatments, genital SCC risk is increased and persisted after cessation of treatment[23, 24]. Melanoma risk is increased with >250 treatments in the US[20], but not demonstrated in retrospective European studies with smaller numbers of patients enrolled or shorter follow up[24]. The risk of Merkel cell carcinoma is also increased[25].
No increase in skin cancer is noted with broadband or narrowband-UVB, especially in <100 treatments[26, 27, 24, 23, 28] or with bath PUVA[29]. However, in patients treated with broadband UVB with previous PUVA exposure, >300 UVB treatment exposure is associated with modest but significant increase in NMSC (SCC incidence rate ratio IRR 1.37, 95% CI 1.03–1.83; and BCC IRR 1.45, 95% CI 1.07–1.96)[30]. In summary, PUVA phototherapy increases risk for skin cancer in a dose dependent fashion.
3.2 Systemic nonbiologic therapies
MTX and CsA have been associated with an increased risk of malignancies. The association of these agents and lymphoproliferative disorders, including rare EBV-positive lymphoma, has been observed in small case reports and series[31–33]. While 30-year follow-up PUVA study found an elevated risk of lymphoma in MTX treated patients (≥ 36 months), the control was general population (IRR 4.39, 95% CI 1.59–12.06)[34]. Additionally, recent data shows that low-dose MTX treatment (≤30 mg/week oral or 17.5–22.5mg/week subcutaneously) monotherapy compared to placebo in patients with psoriasis and other diseases revealed no increased malignancy risk[35, 36]. Exposure to MTX in patients receiving PUVA was reported to be an independent risk factor for developing SCC (RR 2.1, CI 95% 1.4–2.8) with potential for metastatic disease[37]. Increased risk for NMSC was reported in rheumatoid arthritis (RA) and psoriatic arthritis patients taking MTX and concurrent CsA[38]. In a nationwide Swedish registry, a small risk for melanoma was observed in MTX-exposed patients, however diagnosis, length of exposure and dose were not reported (HR 1.17, 95% CI 1.08–1.26)[39]. The PSOLAR registry, a postmarketing cohort examining the safety of systemic treatments in psoriasis, found no increased risk of malignancy in patients treated with MTX (excluding NMSC).[40]
Paul et al. prospectively followed 1252 psoriasis patients treated with CsA for up to 5 years, and reported an increased overall malignancy risk compared to the general population (SIR 2.1). However, non-cutaneous malignancy incidence was not increased, and the risk was attributed to a 6-fold higher incidence of skin malignancies, mostly SCC, affected by longer duration of treatment (>2 years) and previous therapies (PUVA and MTX)[41], confirming the conclusions from a nested cohort showing high SCC risk with CsA treated patients, particularly after PUVA exposure[42].
In summary, MTX and CsA are generally considered safe. There is an elevated risk of SCC associated with CsA and MTX, increased by PUVA exposure.
3.3 TNF-alpha inhibitors
3.3.1 Systemic malignancies
In 2006, a meta-analysis suggested an increased risk of malignancy with infliximab and adalimumab (OR 3.7, 95% CI 1.0–13.2) analyzing RA RCTs[43]. However, subsequent data has not confirmed these findings. An updated meta-analysis including 64 RCTs of RA patients found no increased risk (OR 0.98, 95% CI 0.51–1.9)[44]. Additional meta-analyses pooling together malignancy cases in TNFα-inhibitors in rheumatologic, inflammatory bowel diseases (IBD) and psoriasis patients, failed to reveal increased cancer risk (see Table 2). In pooled clinical trial analyses of all indications, anti-TNFα treated RA patients were found to have a higher incidence of lymphoma[45, 10, 46, 47] compared to the overall population, however there is confounding from increased baseline lymphoma risk in RA patients[48, 49, 46, 47]. In a meta-analysis with Crohn’s disease patients, anti-TNFα treatments demonstrated an elevated NHL risk compared to the general population (SIR 3.23, 95% CI 1.5–6.9), but not when compared to immunomodulator treated patients[50], suggesting contributory roles of traditional immunosuppressive agents[51].
Table 2.
Meta-analyses studies reporting on the malignancy risk of anti-TNFα therapy
| Studied agents and population | All malignancy risk (95% CI) | Malignancy risk excluding NMSC (95% CI) | Specific malignancy risk (95% CI) |
|---|---|---|---|
| Adalimumab infliximab in RA[43] | OR 3.3 (1.20–9.10) | OR 3.7 (1.00–13.20) | |
| Adalimumab infliximab etanercept in RA[105] | RR 1.5 (0.80–3.00) | ||
| Adalimumab infliximab etanercept in RA[60] | OR 1.34 (0.75–2.39) | OR 1.31 (0.69–2.48) | NMSC OR 1.27 (0.67–2.42) Lymphoma OR 1.26 (0.52–3.06) |
| Etanercept in RA[106] | HR 1.84 (0.79–4.28) | HR 1.86 (0.62–5.59) | |
| Adalimumab infliximab etanercept in RA[107] | Adalimumab risk ratio 0.55 (0.14–2.11) Etanercept risk ratio 0.98 (0.32–3.02) Infliximab risk ratio 1.64 (0.30–8.89) |
||
| Adalimumab certolizumab etanercept golimumab infliximab in Pso, PsoA[52] | OR 1.48 (0.71–3.09) | OR 1.26 (0.39–4.15) | NMSC OR 1.33 (0.58–3.04) |
| TNF inhibitors in AS, PsoA, RA[58] | RR 0.95 (0.85–1.05) | NMSC RR 1.45 (1.15–1.76) Melanoma RR 1.79 (0.92–2.67) Lymphoma RR 1.11 (0.70–1.51) |
|
| Adalimumab infliximab etanercept in AS, CD, Pso, PsoA, RA[59] | RR 1.30 (0.89–1.95) | RR 0.99 (0.61–1.68) | NMSC RR 2.02 (1.11–3.95) |
| Adalimumab certolizumab etanercept golimumab infliximab in RA[108] | OR 1.08 (0.50–2.32) | ||
| Adalimumab infliximab etanercept in RA[109] | Lymphoma adjusted rate difference 1.29/1,000 person-years (−0.21–2.79) | ||
| Adalimumab certolizumab etanercept golimumab infliximab in RA[44] | OR 0.98 (0.51–1.90) | NMSC OR 1.37 (0.59–3.19) Melanoma OR 1.08 (0.11–10.21) Solid tumors OR 1.31 (0.78–2.20) Lymphoma OR 2.14 (0.55–8.38) Other hematologic malignancies OR 5.30 (0.80–34.99) Unspecified malignancies OR 0.39 (0.07–2.16) |
|
| Adalimumab certolizumab etanercept golimumab infliximab in RA[61] | OR 0.93 (0.59–1.44) | NMSC OR 1.37 (0.71–2.66) Solid tumors OR 0.90 (0.57–1.42) Hematologic malignancies OR 0.62 (0.31–1.24) |
|
| Certolizumab golimumab in RA[62] | OR 1.06 (0.39–2.85) | NMSC OR 0.69 (0.23–2.11) |
|
| Adalimumab certolizumab golimumab infliximab in IBD[110] | RR 0.77 (0.37–1.59) | RR 0.90 (0.40–2.02) | |
| Adalimumab certolizumab etanercept golimumab infliximab in AS, PsA, AR[111] | OR 1.31 (0.89 – 1.95) | ||
| TNF inhibitors in RA[68] | Melanoma Pooled effect estimate 1.60 (1.16–2.19) |
CD = Crohn’s disease; CI = confidence interval; AS = ankylosis spondylitis; IBD = inflammatory bowel disease; NMSC = non-melanoma skin cancer; OR = odds ratio; Pso = psoriasis; RA = rheumatoid arthritis; PsA = psoriatic arthritis; RR = risk ratio.
The risk of malignancy in anti-TNFα exposed psoriasis patients examined in a meta-analysis did not find any increased risk (OR 1.26, 95% CI 0.39–4.15)[52]. Pariser et al. studied the risk of malignancy and etanercept therapy in psoriasis patients by an integrated analysis of short-term placebo-controlled clinical trials and long-term uncontrolled open-label trials, revealing no increase of cancer incidence for etanercept compared to the control group and the overall population (RR 1.11, 95% CI 0.16–12.23 and SIR 1.2, 95% CI 0.8–1.6, respectively). The risk did not increase with increasing dosage or exposures[53]. The PSOLAR registry examined the safety of anti–TNFα agents and ustekinumab and reported that long-term (>=12 months) anti–TNFα therapy, but not shorter-term treatment, may increase malignancy risk, excluding NMSC (OR 1.54, 95% CI 1.10–2.15), however analyses performed for individual anti–TNFα agents were not statistically significant and a monotherapy analysis that excluded cases with multiple exposures to study agents, observed no elevated risk[40]. The OBSERVE-5, a 5-year surveillance registry, studied “real-world” etanercept use in psoriasis patients and found cumulative incidences of 3.2% for malignancies excluding NMSC (95% CI 2.3%–4.1%) and 0.1% for lymphoma (95% CI 0.0%–0.3%), which were not higher than expected (SIR <1)[54]. The safety of adalimumab in psoriasis patients was demonstrated by the initial 7-year results of the ESPRIT registry[55]. Studying a large healthcare delivery system database, systemic malignancy and lymphoma rates were not increased for biologics in psoriasis patients[56].
3.3.2 Non-melanoma skin cancer
Association between biologics and NMSC has been reported in RA and IBD patients [57–59]; however, several meta-analyses found no increased risk for NMSC[60, 52, 53, 44, 61, 62]. Additional studies have shown that anti-TNFα treated patients were at increased risk for SCC but not BCC[45, 63]. Asgari et al. recently reported that NMSC rates were 42% higher among psoriasis patients ever exposed to biologics (HR 1.42, 95% CI 1.12–1.80), largely driven by SCC risk (HR 1.81, 95% CI, 1.23–2.67) and not BCC[56]. A comparison of NMSC risk in anti-TNFα exposed psoriasis versus RA patients showed a significantly higher risk in psoriasis patients (HR 6.0, 95% CI 1.6–22.4)[64] highlighting contributory roles of prior psoriasis therapies such as phototherapy. Prior or concurrent MTX treatment was reported to increase the risk of NMSC in RA [65, 66].
3.3.3 Melanoma
A meta-analysis investigating the risk of malignant melanoma and TNFα inhibitors found a non-significantly elevated risk (RR 1.79, 95% CI 0.92–2.67)[58]. A recent pooled-analysis of 11 European registries that included over 48,000 anti-TNFα treated RA patients did not reveal any elevated risk[67]. However, a meta-analysis of four studies found an increased risk of melanoma in RA patients treated with anti-TNFα compared to non-biologics (pooled effect estimate 1.60, 95% CI 1.16–2.19)[68]. Raaschou et al. found an increased risk of invasive melanoma in RA patients treated with anti-TNFα therapy (HR 1.5, 95% CI 1.0 to 2.2), but not melanoma in-situ[69]. Elevated risk of melanoma was also reported in IBD patients treated with TNFα inhibitors (OR 1.88, 95% CI 1.08–3.29)[70]. A retrospective single center study on anti-TNFα treated patients for any indication reported an increased risk of developing melanoma (RR 1.75, 95% CI 1.25–2.43)[71]. Safety data from clinical studies showed increased melanoma incidence in adalimumab treated psoriasis patients compared to the overall population (SIR 3.67[72]–4.37[47], 95% CI 1.47–7.57; 1.89–8.61), however in a large cohort, melanoma risk was similar for psoriasis patients treated with biologic and non-biologic therapies[56].
In summary, numerous recent meta-analyses, observational and cohort studies found no significantly increased risk of systemic malignancies, including lymphoma for anti-TNFα in psoriasis, providing reassurance regarding the widespread use of these agents. An increased risk of SCC has been confirmed by several studies, but there is conflicting evidence regarding risk for melanoma.
3.4 New biologics and small molecule inhibitors
The emergence of targeted biologics for psoriasis in recent years has greatly expanded the spectrum of therapeutic options. Current biologic targets of interest in psoriasis include IL-12, IL-23, IL-17, Janus Kinase, and Phosphodiesterase-4. IL-12 has demonstrated anti-tumor effects in mouse models via enhancement of both innate resistance and adaptive immunity [73]. IL-23 has predominantly anti-tumor effects in mouse models through IFN gamma and CD8+ T cell dependent pathways[74]. IL-17 is a pro-inflammatory cytokine that produces anti-tumor effects in immune-competent mice, but pro-tumor effects in immune-deficient mice[74]. The JAK-STAT pathway is responsible for the transduction of a myriad of extracellular signals, and dysregulation of this pathway has been linked to a variety of hematological malignancies[75]. Phosphodiesterase-4 has been shown to promote angiogenesis in both lung cancer and lymphoma models through elevation of cyclic-AMP[76, 77].
As with all new drugs, the robustness of safety profiles obtained from clinical trials data should not be broadly extrapolated as they have yet to be validated in a real-world setting. Table 3 summarizes incidence rates of malignancy for these newer treatments including biologics targeting IL 12/23 (ustekinumab)[78–83], IL-23 (guselkumab)[84, 85], IL-17 (ixekizumab, secukinumab)[86–88], and small molecule inhibitors of Janus kinase (tofacitinib)[89] and phosphodiesterase-4 (apremilast)[90–92]. Malignancy incidence rates for these therapies are less than or comparable to those seen in the psoriasis population (1.14 per 100 person-years) and general population (0.95 per 100 person-years) [16], but currently available evidence is not sufficient to draw conclusions on their safety. Long-term studies focused on safety surveillance are still lacking for these agents, especially ixekizumab, secukinumab, and apremilast, as well as data for their use in cancer patients and survivors.
Table 3.
Malignancy incidence rates of new biologics and small molecule inhibitors
| Study agent (mechanism of action) | Study design | Number of patients receiving drug of interest | Follow up time | Malignancy Risk (excluding NMSC) IR per 100 PY (95% CI) | NMSC Risk IR per 100 PY (95% CI) | Pro-tumor or anti-tumor effects of agent target in preclinical studies |
|---|---|---|---|---|---|---|
| Ustekinumab (IL-12/23 inhibitor) | Pooled analyses of clinical trials and LTEs[82] | 3117 | Up to 5 years | 0.60 (0.45–0.78) | 0.52 (0.39–0.70) | IL-12 has anti-tumorigenic functions[73] IL-23 has mostly anti-tumorigenic functions[74] |
| Postmarketing cohort study[83] | 4364 | Up to 7 years | 0.48 (95% CI not given) | Not given | ||
| Guselkumab (IL 23 inhibitor) | Pooled Phase 3 trial[84, 85] | 825 | Up to 48 weeks | Not given (3 cases of malignancy) | Not given (3 cases of NMSC) | |
| Ixekizumab (IL-17 inhibitor) | Pooled analyses of phase 3 trials[86] | 3736 | Up to 60 weeks | 0.40 (0.20–0.70) | 0.60 (0.40–0.90) | IL-17 has both anti- and pro-tumorigenic effects[74] |
| Pooled analyses of clinical trials[88] | 4209 | Up to 264 weeks | 0.50 (95% CI not given) | 0.40 (95% CI not given) | ||
| Secukinumab (IL-17 inhibitor) | Pooled analyses of phase 2 and phase 3 trials[87] | 3430 | Up to 52 weeks | 0.48 (0.25–0.82) | 0.48 (0.25–0.82) | |
| Tofacitinib (JAK 1/3 inhibitor) | Pooled analyses of phase 3 trials and LTEs[89] | 1861 | Up to 33 months | 1.15 (0.78–1.63) | 0.71 (0.43–1.10) | Dysregulation of JAK pathway is associated with hematologic malignancies [75] |
| Apremilast (PDE-4 inhibitor) | Pooled analyses of phase 3 trials[92, 91] | 1184 | Up to 52 weeks | Not given (0 cases of malignancy) | Not given (2 cases of NMSC) | Mostly pro-tumorigenic effects [76, 77] |
| Single phase 3 trial[90] | 83 | Up to 52 weeks | Not given (0 cases of malignancy) | Not given (0 cases of NMSC) |
CI = confidence interval; IL = interluken; IR = incidence rates; JAK = Janus kinase; LTEs = long-term extension studies; NMSC = non-melanoma skin cancer; PDE-4 = phosphodiesterase-4; PY = patient years
4 Psoriasis treatments and malignancy risk in cancer patients and survivors
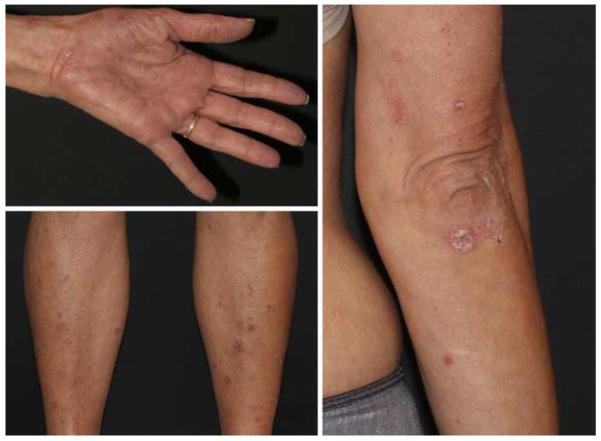
Evidence based data on the association between psoriasis systemic treatments and cancer recurrence is limited since patients with history of malignancies are usually excluded from participating in clinical trials and dermatologists are hesitant to initiate immunosuppressive agents in cancer survivors. Moreover, psoriasis flares have been noted with the advent of the new immune checkpoint inhibitors for malignant melanoma, such as ipilimumab[93], nivolumab and pembrolizumab[94], making treatment decisions challenging (Figure 1).
Figure 1. Immunotherapy induced psoriasis.
54 year-old female with no prior history of psoriasis, developed numerous scaly erythematous papules and plaques on her limbs including the palms and the trunk. The rash appeared after starting therapy with nivolumab (anti-PD-1) and varlilumab (anti-CD27) therapy for recurrent glioblostoma. Histology of the scaly plaque on the elbow confirmed the diagnosis of psoriasis.
4.1 Systemic malignancies
A recent meta-analysis of cohort, case-control and case-series studies including 11,702 cancer survivors with RA, IBD and psoriasis treated with anti-TNFα agents, immunomodulator therapies (MTX or thiopurines) or no immunosuppression therapy, aimed to investigate cancer incidences[95]. Follow-up was 21–102 months with a median of 6 years between the index cancer and immunosuppression initiation. Cancer recurrence incidences were similar for anti-TNFs, immunosuppression therapy and no immunosuppression (33.8, 36.2 and 37.5 cancer recurrences or new primary cancer cases per 1000 patient years, PY). A non-significant higher incidence rate was revealed for combination immunosuppression therapy (54.5/1000 PY, P=0.47). Similar non-significant differences were identified when excluding skin cancers. Additional sub-analyses were performed: new cancer and recurrent cancer incidences, analyses by inflammatory disease and comparing thiopurines versus MTX, and none showed statistically significant differences between the groups[95]. A recent study of the British RA registry confirmed no increased risk of malignancy in cancer survivors with RA treated with TNFα inhibitors compared to non-biologics after a median follow-up of 6.8 years[96].
4.2 Cutaneous malignancies
A retrospective study of RA and IBD patients with NMSC history showed MTX use was associated with an increased risk of a second NMSC in RA (HR 1.60, 95% CI 1.08–2.37). The addition of an anti-TNFα agent increased the risk, as did longer MTX exposure[66]. No increased risk for a consecutive primary melanoma was found in patients with MTX-exposure[97]. A non-significant increased rate of a second melanoma was reported in RA patients treated with anti-TNFα agents compared to non-biologics (HR 3.2, 95% CI 0.8–13.1)[69],[98]. Notably, use of infliximab and adalimumab for ipilimumab-induced colitis in advanced staged melanoma patients has been reported to have no effect on worsening prognosis and survival[99].
In the PSOLAR study, 3.8% of the psoriasis patients had history of systemic malignancy while 6.2% had skin cancer history. History of malignancy was reported to be associated with higher cancer rates in this cohort (HR 2.64, 95% CI 1.88–3.69)[83], however, malignancy recurrence rates were comparable in patients who were treated with biologic (anti–TNFα agents or ustekinumab) or non-biologic therapies (2.48 versus 3.78 per 100 PY, respectively)[100].
In summary, risk of new or recurrent systemic malignancies is similar between biologics and non-biologic treatments. Risk of additional NMSC in patients with history of NMSC may be increased; however data regarding additional primary melanomas and melanoma recurrence is inconclusive in melanoma survivors.
5 Conclusion
Based on the recent studies with a high level of evidence, we conclude that there is little evidence of an association between non-cutaneous malignancies and the reviewed psoriasis treatments, regardless of a history of prior cancer. There is conflicting data regarding risk of melanoma in patients treated with anti-TNFα agents. There is an elevated risk of SCC for both anti–TNFα agents and non-biologic therapies that increased with PUVA exposure and/or immunosuppressive treatments. Our conclusions are based on meta-analyses from the rheumatology, gastroenterology and dermatology literature that compared treated patients to control patients rather than general population, thereby eliminating possible confounding from baseline malignancy risk of the inflammatory disease[101]. Results from meta-analyses are limited by relatively short exposure and follow-up periods, but large observational and cohort studies confirm these findings. New or recurrent cancer incidence rates are modest and should be weighed against the advantage of controlling disease in psoriasis patients. The newer biologic and non-biologic agents seem promising and effective, however, additional studies are needed to evaluate malignancy risk in these agents.
Key points.
Psoriasis and some of its treatments have been associated with increased malignancy risk.
Summarizing the most recent studies with highest available level of evidence on malignancy risk from psoriasis and its treatments in patients and cancer survivors, we found little evidence suggesting increased risk of non-cutaneous solid tumors with psoriasis treatments or lymphoma from TNF inhibitors.
Based on high-level evidence, psoriasis therapies appear safe. Additional long-term data is warranted for newer treatments and for their use in cancer survivors.
Acknowledgments
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
IRB Statement: This manuscript was exempt from IRB review.
Conflicts of Interests: Dr Lebwohl is an employee of Mount Sinai which receives research funds from: Abbvie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Kadmon, Medimmune/Astra Zeneca, Novartis, and Pfizer. Dr Lacouture has consulting agreements with Quintiles, Boehringer Ingelheim, AstraZeneca pharmaceuticals, Legacy Healthcare, Foamix, Adgero Bio Pharmaceuticals, Janssen R&D, Novartis and Novocure. He receives research grants from Berg and Bristol-Myers Squibb. Dr Geller, Mr Xu, Dr Nardone and Dr Kheterpal have no conflicts of interest to declare.
References
- 1.Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–6. doi: 10.1016/j.jaad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Brezinski EA, Armstrong AW. An evidence-based review of the mechanism of action, efficacy, and safety of biologic therapies in the treatment of psoriasis and psoriatic arthritis. Curr Med Chem. 2015;22(16):1930–42. doi: 10.2174/0929867322666150429111804. [DOI] [PubMed] [Google Scholar]
- 3.Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082. doi: 10.1038/nrdp.2016.82. [DOI] [PubMed] [Google Scholar]
- 4.Mattei PL, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol. 2014;28(3):333–7. doi: 10.1111/jdv.12106. [DOI] [PubMed] [Google Scholar]
- 5.Hugh J, Van Voorhees AS, Nijhawan RI, Bagel J, Lebwohl M, Blauvelt A, et al. From the Medical Board of the National Psoriasis Foundation: The risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70(1):168–77. doi: 10.1016/j.jaad.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. Jama. 2006;296(14):1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 7.Patel RV, Clark LN, Lebwohl M, Weinberg JM. Treatments for psoriasis and the risk of malignancy. J Am Acad Dermatol. 2009;60(6):1001–17. doi: 10.1016/j.jaad.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 8.Margolis DBW, Hennessy S, Vittorio C, Santanna J, Strom BL. The risk of malignancy associated with psoriasis. Arch Dermatol. 2003 Nov;137(6):778–83. 2001 Jun. [PubMed] [Google Scholar]
- 9.Gelfand JM, Berlin J, Van Voorhees A, Margolis DJ. Lymphoma rates are low but increased in patients with psoriasis: results from a population-based cohort study in the United Kingdom. Arch Dermatol. 2003;139(11):1425–9. doi: 10.1001/archderm.139.11.1425. [DOI] [PubMed] [Google Scholar]
- 10.Gelfand JMSD, Neimann AL, Wang X, Margolis DJ, Troxel AB. The risk of lymphoma in patients with psoriasis. J Invest Dermatol. 2006 Oct;126(10):2194–201. doi: 10.1038/sj.jid.5700410. [DOI] [PubMed] [Google Scholar]
- 11.Brauchli YB, JS, Miret M, Meier CR. Psoriasis and risk of incident cancer: an inception cohort study with a nested case-control analysis. J Invest Dermatol. 2009 Nov;129(11):2604–12. doi: 10.1038/jid.2009.113. [DOI] [PubMed] [Google Scholar]
- 12.Chiesa Fuxench ZC, SD, Ogdie Beatty A, Gelfand JM. The Risk of Cancer in Patients With Psoriasis: A Population-Based Cohort Study in the Health Improvement Network. JAMA Dermatol. 2016 Mar;152(3):282–90. doi: 10.1001/jamadermatol.2015.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pouplard CBE, Horreau C, Barnetche T, Misery L, Richard MA, Aractingi S, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013 Aug;27(suppl3):36–46. doi: 10.1111/jdv.12165. [DOI] [PubMed] [Google Scholar]
- 14.Gu Y, Nordstrom BL. The risk of malignancy among biologic-naive pediatric psoriasis patients: A retrospective cohort study in a US claims database. J Am Acad Dermatol. 2017;77(2):293–301e1. doi: 10.1016/j.jaad.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 15.Li WQ, Han J, Cho E, Wu S, Dai H, Weinstock MA, et al. Personal history of psoriasis and risk of incident cancer among women: a population-based cohort study. Br J Dermatol. 2016;174(5):1108–11. doi: 10.1111/bjd.14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimball AB, Schenfeld J, Accortt NA, Anthony MS, Rothman KJ, Pariser D. Incidence rates of malignancies and hospitalized infectious events in patients with psoriasis with or without treatment and a general population in the U.S.A.: 2005–09. Br J Dermatol. 2014;170(2):366–73. doi: 10.1111/bjd.12744. [DOI] [PubMed] [Google Scholar]
- 17.Egeberg A, Thyssen JP, Gislason GH, Skov L. Skin cancer in patients with psoriasis. J Eur Acad Dermatol Venereol. 2016;30(8):1349–53. doi: 10.1111/jdv.13619. [DOI] [PubMed] [Google Scholar]
- 18.McKenna KE, Patterson CC, Handley J, McGinn S, Allen G. Cutaneous neoplasia following PUVA therapy for psoriasis. Br J Dermatol. 1996;134(4):639–42. doi: 10.1111/j.1365-2133.1996.tb06962.x. [DOI] [PubMed] [Google Scholar]
- 19.Bruynzeel I, Bergman W, Hartevelt HM, Kenter CC, Van de Velde EA, Schothorst AA, et al. ‘High single-dose’ European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol. 1991;124(1):49–55. doi: 10.1111/j.1365-2133.1991.tb03281.x. [DOI] [PubMed] [Google Scholar]
- 20.Stern RS, Nichols KT, Vakeva LH. Malignant melanoma in patients treated for psoriasis with methoxsalen (psoralen) and ultraviolet A radiation (PUVA). The PUVA Follow-Up Study. N Engl J Med. 1997;336(15):1041–5. doi: 10.1056/nejm199704103361501. [DOI] [PubMed] [Google Scholar]
- 21.Stern RS. The risk of melanoma in association with long-term exposure to PUVA. J Am Acad Dermatol. 2001;44(5):755–61. doi: 10.1067/mjd.2001.114576. [DOI] [PubMed] [Google Scholar]
- 22.Stern RS, Vakeva LH. Noncutaneous malignant tumors in the PUVA follow-up study: 1975–1996. J Invest Dermatol. 1997;108(6):897–900. doi: 10.1111/1523-1747.ep12292698. [DOI] [PubMed] [Google Scholar]
- 23.Stern RS. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: a 30-year prospective study. J Am Acad Dermatol. 2012;66(4):553–62. doi: 10.1016/j.jaad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Archier EDS, Castela E, Gallini A, Aubin F, Le Maître M, Aractingi S, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012 May;26(Suppl 3):22–31. doi: 10.1111/j.1468-3083.2012.04520.x. [DOI] [PubMed] [Google Scholar]
- 25.Lunder EJ, Stern RS. Merkel-cell carcinomas in patients treated with methoxsalen and ultraviolet A radiation. N Engl J Med. 1998;339(17):1247–8. doi: 10.1056/nejm199810223391715. [DOI] [PubMed] [Google Scholar]
- 26.Hearn RM, Kerr AC, Rahim KF, Ferguson J, Dawe RS. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol. 2008;159(4):931–5. doi: 10.1111/j.1365-2133.2008.08776.x. [DOI] [PubMed] [Google Scholar]
- 27.Weischer M, Blum A, Eberhard F, Rocken M, Berneburg M. No evidence for increased skin cancer risk in psoriasis patients treated with broadband or narrowband UVB phototherapy: a first retrospective study. Acta Derm Venereol. 2004;84(5):370–4. doi: 10.1080/00015550410026948. [DOI] [PubMed] [Google Scholar]
- 28.Pittelkow MR, Perry HO, Muller SA, Maughan WZ, O’Brien PC. Skin cancer in patients with psoriasis treated with coal tar. A 25-year follow-up study. Arch Dermatol. 1981;117(8):465–8. [PubMed] [Google Scholar]
- 29.Hannuksela-Svahn A, Pukkala E, Koulu L, Jansen CT, Karvonen J. Cancer incidence among Finnish psoriasis patients treated with 8-methoxypsoralen bath PUVA. J Am Acad Dermatol. 1999;40(5 Pt 1):694–6. doi: 10.1016/s0190-9622(99)70148-9. [DOI] [PubMed] [Google Scholar]
- 30.Lim JL, Stern RS. High levels of ultraviolet B exposure increase the risk of non-melanoma skin cancer in psoralen and ultraviolet A-treated patients. J Invest Dermatol. 2005;124(3):505–13. doi: 10.1111/j.0022-202X.2005.23618.x. [DOI] [PubMed] [Google Scholar]
- 31.Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009;68(7):1100–4. doi: 10.1136/ard.2008.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bewtra M, Lewis JD. Update on the risk of lymphoma following immunosuppressive therapy for inflammatory bowel disease. Expert Rev Clin Immunol. 2010;6(4):621–31. doi: 10.1586/eci.10.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nalesnik MA, Jaffe R, Starzl TE, Demetris AJ, Porter K, Burnham JA, et al. The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988;133(1):173–92. [PMC free article] [PubMed] [Google Scholar]
- 34.Stern RS. Lymphoma risk in psoriasis: results of the PUVA follow-up study. Arch Dermatol. 2006;142(9):1132–5. doi: 10.1001/archderm.142.9.1132. [DOI] [PubMed] [Google Scholar]
- 35.Mazaud C, Fardet L. Relative Risk of and Determinants for Adverse Events of Methotrexate Prescribed at a Low Dose: A Systematic Review and Meta-Analysis of Randomized, Placebo-Controlled Trials. Br J Dermatol. 2017 doi: 10.1111/bjd.15377. [DOI] [PubMed] [Google Scholar]
- 36.Warren RB, Mrowietz U, von Kiedrowski R, Niesmann J, Wilsmann-Theis D, Ghoreschi K, et al. An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque-type psoriasis (METOP): a 52 week, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10068):528–37. doi: 10.1016/s0140-6736(16)32127-4. [DOI] [PubMed] [Google Scholar]
- 37.Stern RSLN. The carcinogenic risk of treatments for severe psoriasis. Photochemotherapy Follow-up Study. Cancer. 1994 Jun;73(11):2759–64. doi: 10.1002/1097-0142(19940601)73:11<2759::aid-cncr2820731118>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 38.Lange E, Blizzard L, Venn A, Francis H, Jones G. Disease-modifying anti-rheumatic drugs and non-melanoma skin cancer in inflammatory arthritis patients: a retrospective cohort study. Rheumatology (Oxford) 2016;55(9):1594–600. doi: 10.1093/rheumatology/kew214. [DOI] [PubMed] [Google Scholar]
- 39.Polesie S, Gillstedt M, Sonnergren HH, Osmancevic A, Paoli J. Methotrexate treatment and risk for cutaneous malignant melanoma: a retrospective comparative registry-based cohort study. Br J Dermatol. 2017;176(6):1492–9. doi: 10.1111/bjd.15170. [DOI] [PubMed] [Google Scholar]
- 40.Fiorentino D, Ho V, Lebwohl MG, Leite L, Hopkins L, Galindo C, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77(5):845–54e5. doi: 10.1016/j.jaad.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Paul CF, Ho VC, McGeown C, Christophers E, Schmidtmann B, Guillaume JC, et al. Risk of malignancies in psoriasis patients treated with cyclosporine: a 5 y cohort study. J Invest Dermatol. 2003;120(2):211–6. doi: 10.1046/j.1523-1747.2003.12040.x. [DOI] [PubMed] [Google Scholar]
- 42.Marcil I, Stern RS. Squamous-cell cancer of the skin in patients given PUVA and ciclosporin: nested cohort crossover study. Lancet. 2001;358(9287):1042–5. doi: 10.1016/s0140-6736(01)06179-7. [DOI] [PubMed] [Google Scholar]
- 43.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. Jama. 2006;295(19):2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Olivo MA, Tayar JH, Martinez-Lopez JA, Pollono EN, Cueto JP, Gonzales-Crespo MR, et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: a meta-analysis. Jama. 2012;308(9):898–908. doi: 10.1001/2012.jama.10857. [DOI] [PubMed] [Google Scholar]
- 45.Gottlieb AB, Gordon K, Giannini EH, Mease P, Li J, Chon Y, et al. Clinical trial safety and mortality analyses in patients receiving etanercept across approved indications. J Drugs Dermatol. 2011;10(3):289–300. [PubMed] [Google Scholar]
- 46.Burmester GR, Mease P, Dijkmans BA, Gordon K, Lovell D, Panaccione R, et al. Adalimumab safety and mortality rates from global clinical trials of six immune-mediated inflammatory diseases. Ann Rheum Dis. 2009;68(12):1863–9. doi: 10.1136/ard.2008.102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72(4):517–24. doi: 10.1136/annrheumdis-2011-201244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allison L, Smitten TAS, Hochberg Marc C, Suissa Samy. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res Ther. 2008;10(2):R45. doi: 10.1186/ar2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teresa A, Simon AT, Gandhi Kunal K, Hochberg Marc C, Suissa Samy. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17(1):212. doi: 10.1186/s13075-015-0728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7(8):874–81. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deepak P, Sifuentes H, Sherid M, Stobaugh D, Sadozai Y, Ehrenpreis ED. T-cell non-Hodgkin’s lymphomas reported to the FDA AERS with tumor necrosis factor-alpha (TNF-alpha) inhibitors: results of the REFURBISH study. Am J Gastroenterol. 2013;108(1):99–105. doi: 10.1038/ajg.2012.334. [DOI] [PubMed] [Google Scholar]
- 52.Dommasch ED, Abuabara K, Shin DB, Nguyen J, Troxel AB, Gelfand JM. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64(6):1035–50. doi: 10.1016/j.jaad.2010.09.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pariser DM, Leonardi CL, Gordon K, Gottlieb AB, Tyring S, Papp KA, et al. Integrated safety analysis: short- and long-term safety profiles of etanercept in patients with psoriasis. J Am Acad Dermatol. 2012;67(2):245–56. doi: 10.1016/j.jaad.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 54.Kimball AB, Rothman KJ, Kricorian G, Pariser D, Yamauchi PS, Menter A, et al. OBSERVE-5: observational postmarketing safety surveillance registry of etanercept for the treatment of psoriasis final 5-year results. J Am Acad Dermatol. 2015;72(1):115–22. doi: 10.1016/j.jaad.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menter A, Thaci D, Wu JJ, Abramovits W, Kerdel F, Arikan D, et al. Long-Term Safety and Effectiveness of Adalimumab for Moderate to Severe Psoriasis: Results from 7-Year Interim Analysis of the ESPRIT Registry. Dermatol Ther (Heidelb) 2017;7(3):365–81. doi: 10.1007/s13555-017-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asgari MM, Ray GT, Geier JL, Quesenberry CP. Malignancy rates in a large cohort of patients with systemically treated psoriasis in a managed care population. J Am Acad Dermatol. 2017 doi: 10.1016/j.jaad.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 57.Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56(9):2886–95. doi: 10.1002/art.22864. [DOI] [PubMed] [Google Scholar]
- 58.Mariette X, Matucci-Cerinic M, Pavelka K, Taylor P, van Vollenhoven R, Heatley R, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70(11):1895–904. doi: 10.1136/ard.2010.149419. [DOI] [PubMed] [Google Scholar]
- 59.Askling J, Fahrbach K, Nordstrom B, Ross S, Schmid CH, Symmons D. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20(2):119–30. doi: 10.1002/pds.2046. [DOI] [PubMed] [Google Scholar]
- 60.Leombruno JP, Einarson TR, Keystone EC. The safety of anti-tumour necrosis factor treatments in rheumatoid arthritis: meta and exposure-adjusted pooled analyses of serious adverse events. Ann Rheum Dis. 2009;68(7):1136–45. doi: 10.1136/ard.2008.091025. [DOI] [PubMed] [Google Scholar]
- 61.Moulis G, Sommet A, Bene J, Montastruc F, Sailler L, Montastruc JL, et al. Cancer risk of anti-TNF-alpha at recommended doses in adult rheumatoid arthritis: a meta-analysis with intention to treat and per protocol analyses. PLoS One. 2012;7(11):e48991. doi: 10.1371/journal.pone.0048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.PLEB, Mouterde G, Barnetche T, Morel J, Combe B. Short-term risk of total malignancy and nonmelanoma skin cancers with certolizumab and golimumab in patients with rheumatoid arthritis: metaanalysis of randomized controlled trials. J Rheumatol. 2012;39(4):712–5. doi: 10.3899/jrheum.110982. [DOI] [PubMed] [Google Scholar]
- 63.Raaschou P, Simard JF, Asker Hagelberg C, Askling J. Rheumatoid arthritis, anti-tumour necrosis factor treatment, and risk of squamous cell and basal cell skin cancer: cohort study based on nationwide prospectively recorded data from Sweden. Bmj. 2016;352:i262. doi: 10.1136/bmj.i262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Lumig PP, Menting SP, van den Reek JM, Spuls PI, van Riel PL, van de Kerkhof PC, et al. An increased risk of non-melanoma skin cancer during TNF-inhibitor treatment in psoriasis patients compared to rheumatoid arthritis patients probably relates to disease-related factors. J Eur Acad Dermatol Venereol. 2015;29(4):752–60. doi: 10.1111/jdv.12675. [DOI] [PubMed] [Google Scholar]
- 65.Chakravarty EF, Michaud K, Wolfe F. Skin cancer, rheumatoid arthritis, and tumor necrosis factor inhibitors. J Rheumatol. 2005;32(11):2130–5. [PubMed] [Google Scholar]
- 66.Scott FI, Mamtani R, Brensinger CM, Haynes K, Chiesa-Fuxench ZC, Zhang J, et al. Risk of Nonmelanoma Skin Cancer Associated With the Use of Immunosuppressant and Biologic Agents in Patients With a History of Autoimmune Disease and Nonmelanoma Skin Cancer. JAMA Dermatol. 2016;152(2):164–72. doi: 10.1001/jamadermatol.2015.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mercer LK, Askling J, Raaschou P, Dixon WG. Risk of invasive melanoma in patients with rheumatoid arthritis treated with biologics: results from a collaborative project of 11. European biologic registers. 2017;76(2):386–91. doi: 10.1136/annrheumdis-2016-209285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olsen CM, Hyrich KL, Knight LL, Green AC. Melanoma risk in patients with rheumatoid arthritis treated with tumour necrosis factor alpha inhibitors: a systematic review and meta-analysis. Melanoma Res. 2016;26(5):517–23. doi: 10.1097/cmr.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 69.Raaschou P, Simard JF, Holmqvist M, Askling J. Rheumatoid arthritis, anti-tumour necrosis factor therapy, and risk of malignant melanoma: nationwide population based prospective cohort study from Sweden. Bmj. 2013;346:f1939. doi: 10.1136/bmj.f1939. [DOI] [PubMed] [Google Scholar]
- 70.Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143(2):390–9e1. doi: 10.1053/j.gastro.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nardone B, Hammel JA, Raisch DW, Weaver LL, Schneider D, West DP. Melanoma associated with tumour necrosis factor-alpha inhibitors: a Research on Adverse Drug events And Reports (RADAR) project. Br J Dermatol. 2014;170(5):1170–2. doi: 10.1111/bjd.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Craig Leonardi KP, Strober Bruce, Thaçi Diamant, Warren Richard B, Tyring Stephen, Arikan Dilek, Karunaratne Mahinda, Valdecantos Wendell. Long-Term Safety of Adalimumab in Clinical Trials for Adult Patients With Moderate to Severe Plaque Psoriasis. POSTER 3822. J Am Acad Dermatol; American Academy of Dermatology 74th Annual Meeting; March 4–8, 2016; 2016. p. AB259. [DOI] [PubMed] [Google Scholar]
- 73.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 74.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10(4):248–56. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Furqan M, Mukhi N, Lee B, Liu D. Dysregulation of JAK-STAT pathway in hematological malignancies and JAK inhibitors for clinical application. Biomark Res. 2013;1(1):5. doi: 10.1186/2050-7771-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suhasini AN, Wang L, Holder KN, Lin AP, Bhatnagar H, Kim SW, et al. A phosphodiesterase 4B-dependent interplay between tumor cells and the microenvironment regulates angiogenesis in B-cell lymphoma. Leukemia. 2016;30(3):617–26. doi: 10.1038/leu.2015.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pullamsetti SS, Banat GA, Schmall A, Szibor M, Pomagruk D, Hanze J, et al. Phosphodiesterase-4 promotes proliferation and angiogenesis of lung cancer by crosstalk with HIF. Oncogene. 2013;32(9):1121–34. doi: 10.1038/onc.2012.136. [DOI] [PubMed] [Google Scholar]
- 78.Lebwohl M, Leonardi C, Griffiths CE, Prinz JC, Szapary PO, Yeilding N, et al. Long-term safety experience of ustekinumab in patients with moderate-to-severe psoriasis (Part I of II): results from analyses of general safety parameters from pooled Phase 2 and 3 clinical trials. J Am Acad Dermatol. 2012;66(5):731–41. doi: 10.1016/j.jaad.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 79.Gordon KB, Papp KA, Langley RG, Ho V, Kimball AB, Guzzo C, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (Part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66(5):742–51. doi: 10.1016/j.jaad.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 80.Reich K, Papp KA, Griffiths CE, Szapary PO, Yeilding N, Wasfi Y, et al. An update on the long-term safety experience of ustekinumab: results from the psoriasis clinical development program with up to four years of follow-up. J Drugs Dermatol. 2012;11(3):300–12. [PubMed] [Google Scholar]
- 81.Langley RG, Lebwohl M, Krueger GG, Szapary PO, Wasfi Y, Chan D, et al. Long-term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate-to-severe psoriasis: results from the PHOENIX 2 study through 5 years of follow-up. Br J Dermatol. 2015;172(5):1371–83. doi: 10.1111/bjd.13469. [DOI] [PubMed] [Google Scholar]
- 82.Papp KA, Griffiths CE, Gordon K, Lebwohl M, Szapary PO, Wasfi Y, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168(4):844–54. doi: 10.1111/bjd.12214. [DOI] [PubMed] [Google Scholar]
- 83.Papp K, Gottlieb AB, Naldi L, Pariser D, Ho V, Goyal K, et al. Safety Surveillance for Ustekinumab and Other Psoriasis Treatments From the Psoriasis Longitudinal Assessment and Registry (PSOLAR) J Drugs Dermatol. 2015;14(7):706–14. [PubMed] [Google Scholar]
- 84.Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–31. doi: 10.1016/j.jaad.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 85.Blauvelt A, Papp KA, Griffiths CE, Randazzo B, Wasfi Y, Shen YK, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–17. doi: 10.1016/j.jaad.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 86.Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N Engl J Med. 2016;375(4):345–56. doi: 10.1056/NEJMoa1512711. [DOI] [PubMed] [Google Scholar]
- 87.van de Kerkhof PC, Griffiths CE, Reich K, Leonardi CL, Blauvelt A, Tsai TF, et al. Secukinumab long-term safety experience: A pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75(1):83–98e4. doi: 10.1016/j.jaad.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 88.Strober B, Leonardi C, Papp KA, Mrowietz U, Ohtsuki M, Bissonnette R, et al. Short- and long-term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: Etanercept comparisons and integrated data. J Am Acad Dermatol. 2017;76(3):432–40e17. doi: 10.1016/j.jaad.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 89.Papp KA, Krueger JG, Feldman SR, Langley RG, Thaci D, Torii H, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: Long-term efficacy and safety results from 2 randomized phase-III studies and 1 open-label long-term extension study. J Am Acad Dermatol. 2016;74(5):841–50. doi: 10.1016/j.jaad.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 90.Reich K, Gooderham M, Green L, Bewley A, Zhang Z, Khanskaya I, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE) J Eur Acad Dermatol Venereol. 2016 doi: 10.1111/jdv.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2) Br J Dermatol. 2015;173(6):1387–99. doi: 10.1111/bjd.14164. [DOI] [PubMed] [Google Scholar]
- 92.Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73(1):37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 93.Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, Ye F, et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2016;2(2):234–40. doi: 10.1001/jamaoncol.2015.4368. [DOI] [PubMed] [Google Scholar]
- 94.Bonigen J, Raynaud-Donzel C, Hureaux J, Kramkimel N, Blom A, Jeudy G, et al. Anti-PD1-induced psoriasis. A study of 21 patients. J Eur Acad Dermatol Venereol. 2016 doi: 10.1111/jdv.14011. [DOI] [PubMed] [Google Scholar]
- 95.Shelton E, Laharie D, Scott FI, Mamtani R, Lewis JD, Colombel JF, et al. Cancer Recurrence Following Immune-Suppressive Therapies in Patients With Immune-Mediated Diseases: A Systematic Review and Meta-analysis. Gastroenterology. 2016;151(1):97–109e4. doi: 10.1053/j.gastro.2016.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Silva-Fernandez L, Lunt M, Kearsley-Fleet L, Watson KD, Dixon WG, Symmons DP, et al. The incidence of cancer in patients with rheumatoid arthritis and a prior malignancy who receive TNF inhibitors or rituximab: results from the British Society for Rheumatology Biologics Register-Rheumatoid Arthritis. Rheumatology (Oxford) 2016;55(11):2033–9. doi: 10.1093/rheumatology/kew314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Polesie S, Gillstedt M, Paoli J, Osmancevic A. Methotrexate treatment in patients with a history of cutaneous melanoma and the risk of a consecutive primary melanoma: A national retrospective registry-based cohort study. J Am Acad Dermatol. 2017;77(1):161–3. doi: 10.1016/j.jaad.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 98.Dixon WG, Watson KD, Lunt M, Mercer LK, Hyrich KL, Symmons DP. Influence of anti-tumor necrosis factor therapy on cancer incidence in patients with rheumatoid arthritis who have had a prior malignancy: results from the British Society for Rheumatology Biologics Register. Arthritis Care Res (Hoboken) 2010;62(6):755–63. doi: 10.1002/acr.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–8. doi: 10.1200/jco.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Langley RGGK, Fiorentino D, Bagel J, Lebwohl M, Strober B, Ho V, Langholff W, Calabro S, Fakharzadeh S. Experience in Patients With a History of Malignancy in the Psoriasis Longitudinal Assessment and Registry (PSOLAR) Study. Abstract 1777. 24th European Academy of Dermatology and Venereology (EADV) Congress; October 7–11, 2015. [Google Scholar]
- 101.Garcia-Doval I, Hernandez MV, Vanaclocha F, Sellas A, de la Cueva P, Montero D. Should tumour necrosis factor antagonist safety information be applied from patients with rheumatoid arthritis to psoriasis? Rates of serious adverse events in the prospective rheumatoid arthritis BIOBADASER and psoriasis BIOBADADERM cohorts. Br J Dermatol. 2017;176(3):643–9. doi: 10.1111/bjd.14776. [DOI] [PubMed] [Google Scholar]
- 102.Hannuksela-Svahn APE, Läärä E, Poikolainen K, Karvonen J. Psoriasis, its treatment, and cancer in a cohort of Finnish patients. J Invest Dermatol. 2000 Mar;114(3):587–90. doi: 10.1046/j.1523-1747.2000.00898.x. [DOI] [PubMed] [Google Scholar]
- 103.Tsai TFWT, Hung ST, Tsai PI, Schenkel B, Zhang M, Tang CH. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci. 2011 Jul;63(1):40–6. doi: 10.1016/j.jdermsci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 104.Chen YJWC, Chen TJ, Shen JL, Chu SY, Wang CB, Chang YT. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011 Jul;65(1):84–91. doi: 10.1016/j.jaad.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 105.Alonso-Ruiz A, Pijoan JI, Ansuategui E, Urkaregi A, Calabozo M, Quintana A. Tumor necrosis factor alpha drugs in rheumatoid arthritis: systematic review and metaanalysis of efficacy and safety. BMC Musculoskelet Disord. 2008;9:52. doi: 10.1186/1471-2474-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bongartz T, Warren FC, Mines D, Matteson EL, Abrams KR, Sutton AJ. Etanercept therapy in rheumatoid arthritis and the risk of malignancies: a systematic review and individual patient data meta-analysis of randomised controlled trials. Ann Rheum Dis. 2009;68(7):1177–83. doi: 10.1136/ard.2008.094904. [DOI] [PubMed] [Google Scholar]
- 107.Wiens A, Venson R, Correr CJ, Otuki MF, Pontarolo R. Meta-analysis of the efficacy and safety of adalimumab, etanercept, and infliximab for the treatment of rheumatoid arthritis. Pharmacotherapy. 2010;30(4):339–53. doi: 10.1592/phco.30.4.339. [DOI] [PubMed] [Google Scholar]
- 108.Thompson AE, Rieder SW, Pope JE. Tumor necrosis factor therapy and the risk of serious infection and malignancy in patients with early rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum. 2011;63(6):1479–85. doi: 10.1002/art.30310. [DOI] [PubMed] [Google Scholar]
- 109.Wong AK, Kerkoutian S, Said J, Rashidi H, Pullarkat ST. Risk of lymphoma in patients receiving antitumor necrosis factor therapy: a meta-analysis of published randomized controlled studies. Clin Rheumatol. 2012;31(4):631–6. doi: 10.1007/s10067-011-1895-y. [DOI] [PubMed] [Google Scholar]
- 110.Williams CJ, Peyrin-Biroulet L, Ford AC. Systematic review with meta-analysis: malignancies with anti-tumour necrosis factor-alpha therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39(5):447–58. doi: 10.1111/apt.12624. [DOI] [PubMed] [Google Scholar]
- 111.Bonovas S, Minozzi S, Lytras T, Gonzalez-Lorenzo M, Pecoraro V, Colombo S, et al. Risk of malignancies using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf. 2016;15(sup1):35–54. doi: 10.1080/14740338.2016.1238458. [DOI] [PubMed] [Google Scholar]