Abstract
High shyness during early adolescence is associated with impaired peer relationships and risk for psychiatric disorders. Little is known, however, about the relation between shyness and trajectories of brain development over early adolescence. The current study longitudinally examined trajectories of resting state functional connectivity (rs-fc) within 4 brain networks in 147 adolescents. Subjects underwent functional magnetic resonance imaging at 3 different timepoints, at average ages 10.5 (range 7.8–13.0), 11.7 (range 9.3–14.1), and 12.9 years (range 10.1–15.2). Multilevel linear modeling indicated that high shyness was associated with a less steep negative slope of default mode network (DMN) rs-fc over early adolescence relative to low shyness. Less steep decreases in DMN rs-fc may relate to increased self-focus in adolescents with high shyness.
Keywords: fMRI, child, anxiety, social anxiety, shyness, default mode network
Shyness is a temperament characterized by discomfort and reticence in novel social situations or in situations in which social evaluation is anticipated (Rubin, Coplan, & Bowker, 2009). During early adolescence (ages 10 to 14 years), excessive shyness is associated with a wide array of social impairments, including decreased number and quality of friendships (Schneider, 2009). Shyness during childhood and early adolescence is also predictive of future problems with anxiety and depression, and shyness is one of the most potent known risk factors for developing social phobia (Fox, Henderson, Marshall, Nichols, & Ghera, 2005). Notably, the median age of onset of social phobia occurs during early adolescence (Merikangas et al., 2010). Additionally, although levels of shyness can vary over development, shyness is highly to moderately stable over early adolescence (Fox et al., 2005; Rubin et al., 2009), and levels of shyness during early adolescence are more predictive of future problems with anxiety disorders than shyness during earlier developmental periods (Prior, Smart, Sanson, & Oberklaid, 2000). Together, these data suggest that early adolescence is a pivotal time period for individuals who are excessively shy. Early adolescence is also a time of marked developmental changes in brain structure and function, including widespread changes in brain structural and functional connectivity (Fair et al., 2012; Gu et al., 2015; Marek, Hwang, Foran, Hallquist, & Luna, 2015). The relation between levels of shyness and trajectories of brain development over early adolescence, however, is poorly understood. The current study begins to investigate this issue by relating individual differences in shyness to longitudinal measures of brain connectivity over early adolescence.
Shyness and closely related concepts such as behavioral inhibition (Fox et al., 2005; Kagan, Snidman, Kahn, & Towsley, 2007), social withdrawal (Rubin et al., 2009) and social reticence (Coplan, Rubin, Fox, Calkins, & Stewart, 1994) have an established developmental trajectory and clear associations with functional impairment; because these concepts are so closely related, we do not draw a sharp distinction in the discussion that follows. Although levels of shyness can vary somewhat in an individual over the course of development from infancy to adolescence, shyness tends to be moderately to highly stable over adolescence (Fox et al., 2005; Rubin et al., 2009). Children who are excessively shy are more likely to lack social competence, interact less with other children, have fewer friends, and have decreased quality of existing friendships (Schneider, 2009). Children who are shy are also more likely to be disliked by peers, rejected by peers (Chen 2006), and are more likely to be bullied (Perren & Alsaker, 2006).
In addition to impairments in peer relationships, high temperamental shyness, and the closely related construct behavioral inhibition, is one of the most potent known risk factors for future development of anxiety disorders in general, and social phobia in particular (Clauss & Blackford, 2012; Fox et al., 2005; Rubin et al., 2009). Shy children are also somewhat more likely to go on to develop depression (Bell-Dolan, Reaven, & Peterson, 1993). One possibility is that high temperamental shyness may interact with other individual characteristics, such as poor emotion regulation, increased error monitoring (Lahat et al., 2014) or impairments in attention allocation (White et al., 2017), to result in an anxiety disorder or depression. As such, excessive shyness may represent an early stage in the development of several psychiatric illnesses. Notably, the median age of onset of social phobia, the psychiatric disorder most closely associated with shyness, occurs during early adolescence (Merikangas et al., 2010). Because early adolescence is such a pivotal stage for individuals high in shyness, and because shyness may represent a first stage in developing psychopathology, examining relations between shyness and trajectories of brain development over early adolescence is expected to have broad relevance to both normative and pathological development.
In addition to a period in which shyness takes on increased functional importance, early adolescence is also a time of widespread developmental changes in brain structure and function (Grayson & Fair, 2017). Perhaps the most notable aspect of brain development over this period is the large-scale change in structural and functional connectivity. Developmental changes in connectivity are theorized to underlie improvements in information and emotional processing, and variation in these developmental changes is thought to confer risk for psychiatric disorders (Gu et al., 2015). Consistent with this hypothesis, variation in connectivity (at a single stage in development) has been related to a wide range of clinical syndromes, symptoms domains, and subject cognitive profiles (Di Martino et al., 2014). At present, however, very little is known about how shyness during early adolescence relates to these widespread changes in brain connectivity. Elucidating this relation is expected to have high impact by providing specific biomarkers of shyness, identifying specific brain systems in which information processing is likely to be disrupted, and providing targets for intervention (through, e.g., cognitive training of affected brain systems).
Resting state functional connectivity (rs-fc) provides an ideal tool to measure longitudinal changes in connectivity in individuals over early adolescence. rs-fc examines correlations in brain activity across different parts of the brain while the individual lies quietly at rest, often as measured by functional magnetic resonance imaging (fMRI). rs-fc is thought to reflect a past history of coordinated activity across brain regions and bears a close resemblance (but not one-to-one) to physical synaptic connections (Vincent et al., 2007). Regions that are consistently correlated with each other at rest, as measured with rs-fc, comprise ‘functional brain networks’, groups of regions that work together to implement a set of related processes. At least four functional brain networks are frequently implicated in the physiology of shyness, anxiety, and other psychiatric disorders. The default mode network (DMN) is involved in self-focused processes and disruptions may underlie excessive self-focus associated with problematic shyness. The salience network (SN) is involved in detecting external salient and emotionally evocative stimuli and shy individuals may have an overactive SN that places inappropriately high salience on social cues. The fronto-parietal network (FPN) is involved in executive function and has been implicated in deficits in cognitive control. Finally, the ventral attention network (VAN) is involved in the stimulus-driven, involuntary capture of attention and disruptions may underlie the involuntary capture of attention by mildly threatening social stimuli in shy individuals (Sylvester et al., 2012).
Consistent with known developmental changes in physical synapses that occur over early adolescence, several recent studies (Fair et al., 2012; Gu et al., 2015; Marek et al., 2015) have used cross-sectional data to argue that there are widespread changes in rs-fc both within and between many of these functional brain networks over early adolescence. Importantly, the direction of these effects and the networks involved vary substantially from study to study, likely because of methodological considerations including the specific age range studied, methods used to measure connectivity and manage motion-related artifacts, and use of cross-sectional designs (Grayson & Fair, 2017). In addition to the limitation of using cross-sectional data to approximate developmental change, it is unclear how variation in these changes in connectivity within these networks relates to variation in shyness.
Prior work has examined the neural correlates of shyness and behavioral inhibition from early infancy through adulthood. Much of this prior work has been cross-sectional in nature and focused on variation in localized brain activity in individuals with high versus low shyness. As such, this prior work provides candidate brain systems in which developmental trajectories of connectivity over early adolescence may vary based on shyness. One of the most consistently reported findings has been increased right lateralized brain activity, as measured with electroencephalography (EEG), in response to novelty in infants and older children with high versus low behavioral inhibition (Fox et al., 2005). Additional consistently described brain differences in children with high versus low shyness include increased activity in the amygdala to emotionally evocative faces (Pérez-Edgar et al., 2007; Schwartz, Wright, Shin, Kagan, & Rauch, 2003); alterations in activity in the nucleus accumbens, striatum, and other subcortical areas during reward processing (Guyer et al., 2014; Guyer et al., 2006); and structural and functional alterations in brain regions involved in directing attention and cognitive control, such as the FPN, SN, and VAN networks described above (Guyer et al., 2015; Jarcho, Fox, Pine, Etkin, et al., 2013; Jarcho, Fox, Pine, Leibenluft, et al., 2013; Sylvester et al., 2016). Cross-sectional studies using rs-fc have indicated that shyness or behavioral inhibition is associated with variation of connectivity of the amygdala as well as regions within the DMN, SN, FPN, VAN, and somatosensory networks (Clauss, Benningfield, Rao, & Blackford, 2016; Rogers et al., 2017; Roy et al., 2014; Sylvester et al., in press; Taber-Thomas, Morales, Hillary, & Pérez-Edgar, 2016). Together, these cross-sectional data suggest that shyness is marked by alterations in connectivity both in brain systems involved in self-directed processing (e.g. the DMN) and externally focused processing (e.g. the FPN).
The goal of this study was to test whether there are differences in developmental trajectories of connectivity within functional brain networks over early adolescence in individuals with high versus low shyness. As discussed above, previous cross-sectional work, although limited, is consistent with widespread changes in rs-fc within networks over early adolescence, and both over- and under- connectivity could affect information processing within affected networks. Understanding alterations of maturation in functional brain networks, therefore, is expected to have fundamental implications for understanding the biology of shyness. In order to examine this issue, we utilized data from an existing longitudinal dataset that includes a wealth of clinical data as well as neuroimaging data from 3 separate time intervals spaced approximately 1 year apart over early adolescence in each subject. We compared trajectories of four functional brain networks frequently implicated in shyness or anxiety disorders (the DMN, FPN, SN, and VAN) in relation to continuous measures of subject temperamental shyness.
METHOD
Participants
The Institutional Review Board at Washington University School of Medicine approved all procedures. Informed consent was obtained from parents and assent was obtained from child participants. This study used data from the ongoing longitudinal Validation of Preschool Depression Study (Luby, Belden, Pautsch, Si, & Spitznagel, 2009; Luby, Si, Belden, Tandon, & Spitznagel, 2009). Children ages 3 to 6 years were screened between 1992 and 1994 from pediatricians’ offices in the St. Louis, Missouri metropolitan area. Children were oversampled for symptoms of depression; non-depressed psychiatric comparison and healthy control groups were also obtained. The study sample was therefore enriched with children with preschool-onset depression but also included controls. Three waves of neuroimaging were collected, at mean ages 10.5 (range 7.8–13.0), 11.7 (range 9.3–14.1), and 12.9 years (range 10.1–15.2). Neuroimaging data were collected between November 2008 and December 2014. The current dataset uses rs-fc data from all 3 of these waves that met quality criteria in the subjects for whom at least one Early Adolescent Temperament Questionnaire – Revised (EATQ-R) had been obtained. From an initial pool of 212 subjects, N=147 subjects were included in the current study; the reasons for exclusion and a comparison of included versus excluded subjects is provided in the Supplementary Materials.
Shyness was measured using the shyness subscale of the parent-report version of the EATQ-R. The reporter for all parent-report measures was the primary caregiver, which was the biological mother for approximately 92% of participants (Luby, Si, et al., 2009). The initial description of the EATQ evaluated subjects 11 to 14 years old, and indicated that parent- and self-report measures of shyness with this scale were significantly correlated with each other over this age range (Capaldi & Rothbart, 1992). The shyness subscale of the EATQ-R has acceptable internal consistency (Cronbach’s alpha = 0.72) and consists of 5 questions rated on a 5-point Likert Scale (Ellis & Rothbart, 2001). The EATQ-R was administered at up to 3 different assessments in the longitudinal study, on the same day as scan 1 (n=4), scan 2 (n=85), and scan 3 (n=135). Subjects in the study therefore had one (n=72), two (n=73), or three (n=2) measures of shyness over the study. Because many subjects only had EATQ-R data from one assessment, we used the subject’s average score from all available data. The mean subject age for obtaining the EATQ-R across all measurements was 12.6 years, and there was no significant relation between age and shyness (r=−0.07, p=0.30). Annual Diagnostic and Statistical Manual (DSM) diagnoses were determined by parent report on the Preschool-Age Psychiatric Assessment (Egger, Ascher, & Angold, 2003) for children aged 8.0 years and younger and by combined parent and child report (from separate interviews) (Bird, Gould, & Staghezza, 1992) on the Child and Adolescent Psychiatric Assessment (Angold & Costello, 2000) for older children.
fMRI Scanning
Two resting state functional magnetic resonance imaging (fMRI) scans (164 frames, ~6.8 minutes each) were collected in each subject at each wave using a 3T TIM TRIO Scanner at Washington University School of Medicine. Subjects were instructed to lay awake quietly with their eyes closed. Pads were inserted around all sides of the head to minimize head motion. Data were acquired using an asymmetric spin-echo, echo-planar sequence, which was maximally sensitive to blood oxygenation level-dependent (BOLD) contrast (T2*) (repetition time [TR] = 2500 ms, echo time [TE] = 27 ms, field of view [FOV] = 256 mm, flip = 90°, voxel size = 4×4×4 mm, slices = 36). A T1 structural image was acquired for alignment purposes using a sagittal MP-RAGE three-dimensional sequence (TR=2400 ms, TE = 3.16 ms, flip = 8°, voxel size 1×1×1 mm). A T2 image was acquired in the same space as the functional scans to facilitate registration of the T1 image (TE=96 ms, TR = 5s, 189 × 256 acquisition matrix, 36 slices, voxel size = 1.0 × 1 × 3 mm).
fMRI Data Pre-Processing
Initial pre-processing included (1) removal of the first 5 frames of data from each run to allow for stabilization of the BOLD signal, (2) temporal realignment using sinc interpolation to correct odd versus even slice intensity differences attributable to interleaved acquisition, (3) realignment of data within and across runs to compensate for rigid body motion, (4) intensity normalization to a whole brain mode (across all TRs and voxels) of 1,000, (5) registration of the T1 to the atlas representative template in the Talairach coordinate system using a 12-parameter affine transform, (6) co-registration of the 3D fMRI volume to the T1 via the T2, and (7) transformation of the fMRI volumes to atlas space using a single affine 12-parameter transform that included re-sampling to a 3-mm cubic representation.
Following these initial pre-processing steps, data underwent functional connectivity pre-processing (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012), which included: (1) multiple regression of nuisance variables from the BOLD data, (2) a temporal band-pass filter (0.009 Hz < f < 0.08 Hz), and (3) spatial smoothing (6 mm full width at half maximum). Nuisance regressors were calculated using regions-of-interest (ROIs) including the average signals from the ventricles, white matter, whole brain, six head realignment parameters obtained by rigid body head motion correction, and the derivatives of each of these signals. Following these procedures, framewise displacement (FD) of each image acquisition was calculated (Power et al., 2012). Volumes with FD greater than 0.2 were censored in subsequent analyses. Only subjects with at least 110 remaining frames of data (4.6 minutes) were included in further analyses. Finally, the initial fcMRI pre-processing was redone (on the output of the initial pre-processing) using only the frames that had passed motion criteria. Out of the 147 subjects who had at least 110 frames of low-motion data available for at least one scan wave, 97 subjects had sufficient low-motion data at scan wave one, 109 had sufficient data at scan wave two, and 118 had sufficient data at scan wave three. After applying these motion criteria, 64 subjects (43.5%) had 3 waves of imaging data, 49 subjects (33.3%) had 2 waves of imaging data, and 34 subjects (23.1%) had 1 wave of imaging data available.
ROI definition
Regions-of-interest (ROIs) were selected from a commonly used set in which the network identities in adults are well-established (Power et al., 2011). We also independently verified network identifications of these ROIs in the study sample, as detailed in the Supplementary Methods. ROIs were spheres 6 mm in diameter, and the specific region centers are listed in Supplementary Table 1. These ROIs were selected on the basis of being distributed across the brain and covering 4 functional brain networks frequently implicated in neuropsychiatric illnesses: the VAN, FPN, the SN, and the DMN. For each network, the ROIs chosen were those that are consistently identified in both task-based and rs-fc neuroimaging studies and ROIs identified in past studies as relevant to behavior and pathology. We provide further rationale for the selection of each specific ROI in the Supplementary Materials.
Functional connectivity data analysis
We extracted the volume-censored time series from each ROI. Fisher-z transformed Pearson’s correlation coefficients were computed between the timeseries for every possible within-network ROI-ROI pair (3 possible pairs for networks with 3 ROIs and 6 pairs for networks with 4 ROIs). For initial analyses, we averaged connectivity values across all ROI pairs within a network in order to derive a single ‘network functional connectivity’ value for each functional network for each subject for each wave of neuroimaging.
Repeated data on many of the covariates, main predictors, and the outcome variables were available, therefore we analyzed change in each of the four networks across time using multilevel linear mixed models (MLM) in SPSS version 24 (Armonk, NY, USA) to account for the dependencies due to repeated measurements (Raudenbush & Bryk, 2002). A major advantage of mixed models is that they provide unbiased estimates in the presence of missing data, and so all subjects with any neuroimaging data can be included, without imputing or eliminating subjects with missing data. Bonferroni corrections were applied to correct for 4 tested models. Each model examined the ability of shyness as a predictor of both the main effect and slope (rate of change) in network functional connectivity over early adolescence. Fixed effects were included in each MLM for age (time), shyness, and the interaction between age and shyness. All models included sex, income-to-needs ratio, history of using a psychiatric medication, and lifetime history of depression as covariates. Follow-up analyses also included lifetime history of social phobia as a covariate. Random effects for each functional network connectivity intercept and slope for age were included to account for individual variability in mean levels of functional network connectivity and rate of change in functional network connectivity. We further explored networks in which there was a significant relation between trajectory over early adolescence and shyness (i.e., significant interaction between age and shyness). To do so, we tested whether slopes of change in connectivity over age were significantly different than zero for subjects with low shyness (one standard deviation beneath sample mean), medium (average) shyness, or high (one standard deviation above mean) shyness. Slopes were based on fits from the models described above that accounted for covariates. We additionally followed up significant network effects (interaction between shyness and age) with a post-hoc analysis in which we examined the trajectory of each individual ROI-to-ROI connection. Post-hoc MLMs for individual connections were set up in the same manner as above. Additional models, presented in the Supplementary Materials, explored non-linear (quadratic) relations between age and connectivity; as well as potential sex differences in relations between shyness and trajectories of connectivity over early adolescence.
RESULTS
Sample
Table 1 describes demographic, diagnostic, symptom, and data quality measures for the study sample. Supplementary Table 2 depicts statistical relations between shyness and potential confounding factors. These tests revealed that girls were significantly shyer than boys (p=0.004), and shyness scores were significantly higher in children with a current or prior history of any anxiety disorder (p=0.001) or a specific diagnosis of social phobia (p<0.001). Subjects with usable data at Scan 2 had significantly higher shyness scores relative to subjects without usable data at Scan 2 (p=0.04) and shyness was non-significantly positively correlated with number of included MRI frames (after motion-based frame censoring) in Scan 1 (p=0.06). All relations in this study were unchanged when additionally controlling for usable data at Scan 2 and number of included frames at Scan 1.
Table 1.
Demographic, diagnostic, symptom, and data quality measures for subjects included in the current study. Income to needs ratio is derived from Wave 1. Dx = diagnosis; ADHD = attention deficit hyperactivity disorder; CD = conduct disorder; ODD = oppositional defiant disorder.
| Sex | |
| Female, n (%) | 71 (48.3%) |
| Male, n (%) | 76 (51.7%) |
|
| |
| Ethnicity | |
| White, n (%) | 74 (50.3%) |
| Black, n (%) | 57 (38.8%) |
| Other, n (%) | 16 (10.9%) |
|
| |
| Income to Needs Ratio, Mean (SD) | 1.7 (0.91) |
|
| |
| IQ, Mean (SD) | 106.9 (14.2) |
|
| |
| EAT-QR Shyness, Mean (SD) | 2.6 (0.95) |
|
| |
| Age at Wave 1, years (SD) | 10.5 (1.3) |
|
| |
| Usable Wave 1 data, n (%) | |
| Yes, n (%) | 97 (66.0%) |
| No, n (%) | 50 (34.0%) |
|
| |
| Usable Frames Wave 1, mean (range) | 222 (110 – 315) |
|
| |
| Age at Wave 2, years (SD) | 11.7 (1.2) |
|
| |
| Usable Wave 2 data, n (%) | |
| Yes, n (%) | 109 (74.1%) |
| No, n (%) | 38 (25.9%) |
|
| |
| Usable Frames Wave 2, mean (range) | 225 (113 – 312) |
|
| |
| Age at Wave 3, years (SD) | 12.9 (1.1) |
|
| |
| Usable Wave 3 data, n (%) | |
| Yes, n (%) | 118 (80.3%) |
| No, n (%) | 29 (19.7%) |
|
| |
| Usable Frames Wave 3, mean (range) | 231 (112 – 318) |
|
| |
| Lifetime Dx. Anxiety | |
| Yes, n (%) | 80 (54.4%) |
| No, n (%) | 67 (45.6%) |
|
| |
| Lifetime Dx. Social Phobia | |
| Yes, n (%) | 40 (27.2%) |
| No, n (%) | 107 (72.8%) |
|
| |
| Lifetime Dx. Depression | |
| Yes, n (%) | 67 (45.6%) |
| No, n (%) | 80 (54.4%) |
|
| |
| Lifetime Dx. ADHD/CD/ODD | |
| Yes, n (%) | 61 (41.5%) |
| No, n (%) | 86 (58.5%) |
Network Development
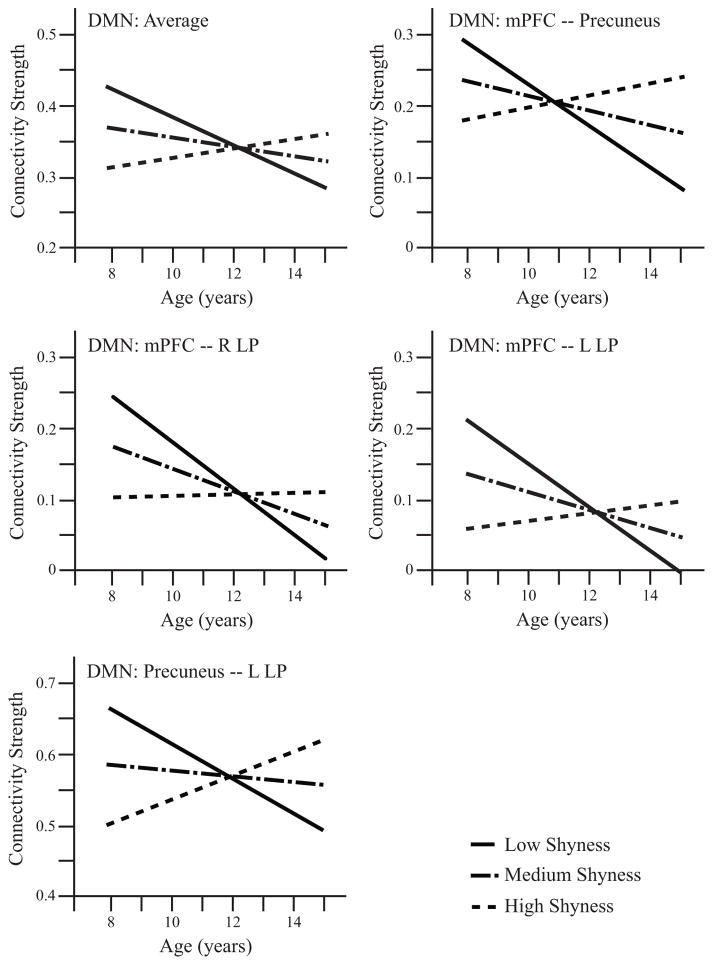
We examined shyness as a predictor of the level and change in functional network connectivity across early adolescence in four separate models, one for each network of interest. Statistics for each model are fully detailed in Supplementary Table 3. There was a main effect of shyness on DMN rs-fc, such that adolescents with high shyness had higher rs-fc within the DMN compared to adolescents with low shyness (p=0.052, Bonferroni corrected). This main effect was qualified by an interaction between shyness and age (p=0.04, corrected). This interaction between shyness and age suggests that adolescents lower in shyness evidenced declines in default mode network functional connectivity over time (upper left panel, Figure 1). Follow-up significance tests of the simple slopes revealed that default mode network connectivity declined over early adolescence for subjects with low but not high shyness: the slope for subjects with shyness one standard deviation beneath the mean was significantly negative (t(82.7)=−2.9, p=0.005), while the slope was not significant for subjects with average shyness (p=0.17) or shyness one standard deviation above the mean (p=0.33). Results for the DMN remained significant when lifetime history of social phobia was included as a predictor. Functional connectivity of the other 3 networks tested did not vary with shyness or the interaction between age and shyness. Functional connectivity of the VAN was significantly negatively related to age (p=0.044, corrected); the main effect of age was not significant for the other 3 networks.
Figure 1.
Functional connectivity trajectories over early adolescence that vary with shyness. Lines represent model fits of the impact of shyness on trajectories of functional connectivity, accounting for covariates described in the main text. Lines representing low shyness are model fits for subjects one standard deviation beneath the sample mean, medium shyness represents subjects at the sample mean, and high shyness represents subjects with shyness one standard deviation above sample mean. The upper left panel indicates average DMN resting state functional connectivity, and the other four panels depict individual connections within the DMN in which trajectories over early adolescence varied with shyness.
Development of Individual Functional Connections
In post-hoc analyses, we examined shyness as a predictor of the level and change of individual functional connections within the default mode network (statistics are fully described in Supplementary Table 4). There were main effects of shyness in predicting average rs-fc between the medial prefrontal cortex (mPFC) and precuneus; between the mPFC and right lateral parietal cortex, between the mPFC and left lateral parietal cortex; and between the precuneus and left lateral parietal cortex (in all cases, high shyness was associated with higher rs-fc). These main effects were qualified by interactions between shyness and age in all four of the individual connections above; results remained significant when also including lifetime history of social phobia in the models except for the functional connection between mPFC and precuneus, which became non-significant. These significant interactions indicate that adolescents lower in shyness evidenced significant declines in rs-fc in the individual functional connections over adolescence within the default mode network. For each of these connections, follow-up analyses of the simple slopes revealed that connectivity declined significantly (p<0.05) over early adolescence for subjects with low (one standard deviation beneath the mean) shyness but not high (one standard deviation above the mean) shyness; for the precuneus to left lateral parietal connection, however, this effect did not reach significance (p=0.069). Subjects at the mean level of shyness had significant decline in connectivity with age for the functional connection between mPFC and right lateral parietal region (p=0.04), but no significant changes for the other DMN connections. Figure 1 depicts the trajectories of DMN resting state functional connectivity over early adolescence for these four connections. The strength of the other two connections examined was unrelated to shyness or the interaction between shyness and age. Figure 2 provides an illustration of the DMN, highlighting the specific connections in which the trajectory of development of individual connections was related to shyness.
Figure 2.
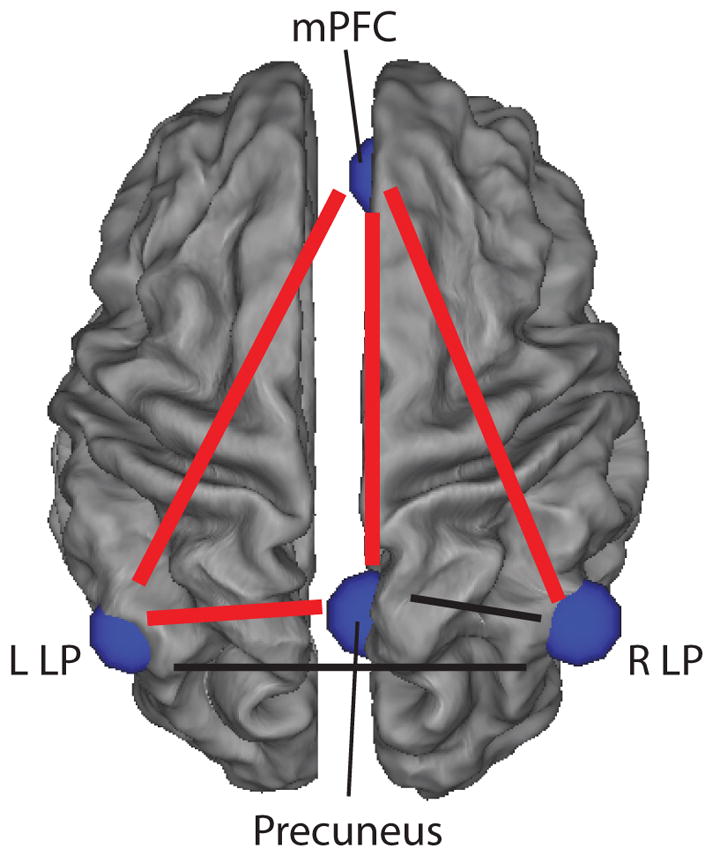
The development of specific default mode network functional connections over early adolescence varies with shyness. The 6 functional connections among 4 default mode network regions are depicted; the change in strength over development of thicker red connections varies with shyness while the strength of thinner black connections does not vary over time. mPFC: medial prefrontal cortex; L LP: left lateral parietal cortex; R LP: right lateral parietal cortex.
DISCUSSION
The current study used longitudinal neuroimaging data to test whether variation in the levels and trajectories of functional brain network connectivity over early adolescence (measured between 7.8 and 15.2 years) is related to variation in temperamental shyness. As expected, temperamental shyness was strongly related to lifetime history of a diagnosis of social phobia. Of the four functional brain networks tested, only the default mode network exhibited significant differences in the trajectory of functional connectivity over early adolescence based on temperamental shyness. Shyness remained significantly related to the trajectory of DMN rs-fc even after controlling for lifetime history of social phobia. Subjects with low temperamental shyness demonstrated a more negative slope compared to the subjects with high temperamental shyness, indicating that subjects with high shyness had less of a decrease in connectivity within the DMN over early adolescence. Post-hoc analyses of the 6 connections studied within the DMN revealed significantly more negative slopes for subjects with low shyness in 4 connections: between the mPFC and precuneus, between the mPFC and right LP, between the mPFC and left LP, and between the precuneus and the left LP. Consistent with prior work demonstrating stability of shyness in early adolescence (Prior et al., 2000), we did not detect any relations between age and levels of shyness in the current study sample.
The DMN is a functional brain network that was originally defined as a set of brain regions with activity decreases over a wide range of externally-focused cognitive tasks (Raichle et al., 2001). Based largely on this finding, the DMN is theorized to implement a variety of internally focused processes including emotion processing and regulation, self-referential mental activity, and specific aspects of memory (Raichle, 2015). The current study indicates that, while individuals with low shyness exhibit a steady decrease in connectivity within the DMN over early adolescence, individuals with high shyness have less of a decrease in connectivity over development. By the end of early adolescence, this trajectory results in hyper-connectivity among DMN regions in individuals with high versus low shyness. One possibility is that the resulting hyper-connectivity may be associated with more of an internal focus, self-preoccupation, and self-referential thinking.
It is notable that while the negative slope of DMN connectivity was flatter in adolescents with high versus low shyness, we did not detect differences in the trajectories of other functional brain networks over adolescence. Since these other functional brain networks tend to be involved in externally focused tasks, the hyper-connectivity that is specific to the DMN may be associated with a relative increase in internal focus relative to external focus. Previous authors have described selective increases in DMN rs-fc as reflecting an ‘internal shift’ that may be a feature of highly shy individuals (Taber-Thomas et al., 2016). In contrast to the current results, other studies have reported shyness-related variation in functional connectivity (Roy et al., 2014; Taber-Thomas et al., 2016), task-related activity (Clauss et al., 2016; Guyer et al., 2014; Guyer et al., 2006), and structure (Schwartz et al., 2010; Sylvester et al., 2016) in networks beyond the DMN. The most likely explanation for this discrepancy is the specific developmental period of subjects in the different studies. While the current results suggest that the trajectory of DMN connectivity varies with shyness over early adolescence, such that individuals with high shyness have increased DMN functional connectivity by the end of early adolescence, other shyness-related connectivity variation may be more evident at other periods of development.
The current results are consistent with and extend prior cross-sectional studies examining resting state functional connectivity in children with high shyness or behavioral inhibition. In a sample of children ages 9 to 12, Taber-Thomas et al. (2016) reported increased rs-fc of the precuneus (a DMN region) to several regions in the frontal and temporal cortices in children with high behavioral inhibition. Notably, this increased rs-fc of the DMN region was in contrast to widespread decreases reported in rs-fc of regions within salience, fronto-parietal, and sensory networks. Similarly, Clauss et al. (2016) reported increased rs-fc between the mPFC and precuneus (both potentially within the DMN) during viewing of fearful faces in children ages 8 to 10 years with high versus low behavioral inhibition; again this result was in contrast to decreases in rs-fc in other, non-DMN regions. The current results replicate these prior findings, as there was a main effect of increased DMN rs-fc in adolescents with high versus low shyness. The current results extend these past studies in two ways. One is by providing longitudinal rs-fc DMN data and a second is by linking trajectories of connectivity over development to shyness. This developmental data therefore provides a plausible and face valid explanation for DMN hyper-connectivity: is less of a decrease in the normative decline of DMN connectivity over early adolescence in individuals with high shyness.
The current results also extend prior work comparing within-network rs-fc in subjects of different ages. As detailed in a recent review (Grayson & Fair, 2017), reports of connectivity change over development have been highly variable in terms of the direction of changes and the specific networks affected. Recent studies examining connectivity changes over development, for example, have variously reported widespread decreases in within-network connectivity (Marek et al., 2015), widespread increases in within-network connectivity (Fair et al., 2012), or increases versus decreases that depend on the specific network (Gu et al., 2015). This variability is likely a result of methodological factors such as variable control of motion-related artifact and measurement of connectivity, variability in the age range studied, the use of mostly cross-sectional data, and variable control of important factors that may affect connectivity such as sex or temperament. The current study builds on this prior work by using very stringent methods to control motion-related artifact, examining connectivity among core regions for each network, using a longitudinal design, and controlling for many confounding variables. Importantly, we found very little evidence for developmental changes in within-network connectivity at the whole-group level, as only the ventral attention network had a modest but significant negative slope of connectivity over early adolescence in the whole sample. Potential explanations for the lack of age-related change in within-network connectivity in most networks include the relatively tight age range studied, the careful control of confounding factors such as motion-related artifact, and the use of a sample with a high rate of psychiatric disorders. This large-scale lack of developmental effects was qualified by a significant interaction between age and shyness, such that individuals with low shyness exhibited a greater decline in DMN connectivity over early adolescence relative to individuals with high shyness. Critically, trajectories of DMN resting state functional connectivity in the current study were based on longitudinal data, with subjects contributing up to 3 datapoints over approximately 3 years. The current study is one of the first to collect repeated measurements of rs-fc in the same individuals, describe longitudinal patterns of connectivity, and relate variations in trajectories to temperament. As in behavioral data, by accounting for within-subject variability, analyses of longitudinal rather than cross-sectional data provides a more sensitive and accurate measure of developmental changes in brain-behavior relations.
The current study also underscores the importance of interpreting group differences in the context of the developmental stage of the study sample. The variability of developmental studies discussed above notwithstanding, there is reasonable support for the hypothesis that connectivity changes over development are non-linear, with increases at some stages of development and decreases at other stages (Marek et al., 2015; Smyser et al., 2010). The number of physical synaptic connections similarly follows a non-linear developmental trajectory, with a rapid increase in early childhood, followed by pruning throughout much of adolescence (Stiles & Jernigan, 2010). Group differences must be considered in the context of these developmental changes, and deviations from normal may be in one direction during one phase of development and in the opposite direction during another phase of development. Along these lines, Rogers et al. (2017) related rs-fc of the amygdala at birth to symptoms of behavioral inhibition at age 2 years, while Roy et al. (2014) examined amygdala rs-fc in adults who had been classified as inhibited or non-inhibited during early childhood. Rogers et al. (2017) reported a positive relation between behavioral inhibition and rs-fc between the amygdala and part of the mPFC (which may have been within the DMN), while Roy et al. (2014) reported a negative association between behavioral inhibition and a similar amygdala-mPFC connection. These seemingly opposite results are likely attributed to the different ages of studied samples, and the current study provides a framework for how to use longitudinal data to clarify connectivity differences over different developmental periods.
The current work should be considered in the light of a few limitations. Many of the subjects in this study had preschool-onset depression when they were recruited between the ages of 3 to 6 years, and the sample is therefore enriched for children at high risk for having depressive and anxiety problems. Although excessive temperamental shyness generally precedes development of anxiety disorders, it is possible that in the current study shyness followed the onset of psychiatric disorders for a subset of subjects. Although we controlled for psychiatric disorders in all analyses, it is nevertheless possible that current results may therefore partially reflect shyness-related properties of clinical samples. Future studies should extend these results by examining whether results apply to normative samples. A second factor to consider is that the neuroimaging portion of the study occurred over early adolescence and future studies are required to examine trajectories over broader developmental periods beginning earlier in childhood and continuing through late adolescence. This study also focused on relations between concurrent symptoms and connectivity of functional brain networks, and future longitudinal studies are required to test whether variability in trajectories of functional network connectivity are predictive of future symptoms.
This study demonstrates that variation in the trajectory of DMN resting state functional connectivity over early adolescence is related to temperamental shyness. Given the impairment associated with high shyness during adolescence and the relation to psychiatric disorders such as social phobia, these results are relevant to both normative and pathological development. This study is also one of the first to examine longitudinal changes in rs-fc, and results highlight the increased power of utilizing longitudinal as opposed to cross-sectional data. An exciting opportunity in the future will be to extend these results across a wider age range and to test whether trajectories of brain network development can be used to predict future problematic symptoms and impairment.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (K23MH109983, T32MH100019, R01MH090786), the McDonnell Center for Systems Neuroscience, the Taylor Family Institute, the Parker Fund for Young Investigators in Psychiatry, and Blueprint. Dr. Barch has served as a consultant for Takeda, Roche, Pfizer, and Amgen for work outside the scope of the current manuscript. Dr. Luby receives book royalties from Guilford Press for work outside the scope of the current manuscript.
References
- Angold A, Costello EJ. The Child and Adolescent Psychiatric Assessment (CAPA) J Am Acad Child Adolesc Psychiatry. 2000;39(1):39–48. doi: 10.1097/00004583-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Bell-Dolan DJ, Reaven N, Peterson L. Child depression and social functioning: a multidimensional study of linkages. J Child Psychol Psychiatry. 1993;22:306–315. [Google Scholar]
- Bird HR, Gould MS, Staghezza B. Aggregating data from multiple informants in child psychiatry epidemiological research. J Am Acad Child Adolesc Psychiatry. 1992;31(1):78–85. doi: 10.1097/00004583-199201000-00012. [DOI] [PubMed] [Google Scholar]
- Capaldi DM, Rothbart MK. Development and Validation of an Early Adolescent Temperament Measure. Journal of Early Adolescence. 1992;12(2):153–173. [Google Scholar]
- Clauss JA, Benningfield MM, Rao U, Blackford JU. Altered Prefrontal Cortex Function Marks Heightened Anxiety Risk in Children. Journal of the American Academy of Child and Adolescent Psychiatry. 2016;55(9):809–816. doi: 10.1016/j.jaac.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(10):1066–1075. e1061. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan RJ, Rubin KH, Fox NA, Calkins SD, Stewart SL. Being alone, playing alone, and acting alone: distinguishing among reticence and passive and active solitude in young children. Child Dev. 1994;65(1):129–137. [PubMed] [Google Scholar]
- Di Martino A, Fair DA, Kelly C, Satterthwaite TD, Castellanos FX, Thomason ME, … Milham MP. Unraveling the miswired connectome: a developmental perspective. Neuron. 2014;83(6):1335–1353. doi: 10.1016/j.neuron.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, Ascher B, Angold A. The preschool age psychiatric assessment: Version 1.4. Durham, NC: Duke University Medical Center; 2003. [Google Scholar]
- Ellis LK, Rothbart MK. Revision of the Early Adolescent Temperament Questionnaire. Minneapolis, MN. Poster presented at the bienniel meeting of the Society for Research in Child Development.2001. [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, … Milham MP. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front Syst Neurosci. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox Nathan A, Henderson Heather A, Marshall Peter J, Nichols Kate E, Ghera Melissa M. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56(1):235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Grayson DS, Fair DA. Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. Neuro Image. 2017 doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Satterthwaite TD, Medaglia JD, Yang M, Gur RE, Gur RC, Bassett DS. Emergence of system roles in normative neurodevelopment. Proc Natl Acad Sci U S A. 2015;112(44):13681–13686. doi: 10.1073/pnas.1502829112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer Amanda E, Benson Brenda, Choate Victoria R, Bar-Haim Yair, Perez-Edgar Koraly, Jarcho Johanna M, … Nelson Eric E. Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Development and Psychopathology. 2014;26(1):229–243. doi: 10.1017/S0954579413000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer Amanda E, Jarcho Johanna M, Pérez-Edgar Koraly, Degnan Kathryn A, Pine Daniel S, Fox Nathan A, Nelson Eric E. Temperament and Parenting Styles in Early Childhood Differentially Influence Neural Response to Peer Evaluation in Adolescence. Journal of abnormal child psychology. 2015 doi: 10.1007/s10802-015-9973-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer Amanda E, Nelson Eric E, Perez-Edgar Koraly, Hardin Michael G, Roberson-Nay Roxann, Monk Christopher S, … Ernst Monique. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(24):6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho Johanna M, Fox Nathan A, Pine Daniel S, Etkin Amit, Leibenluft Ellen, Shechner Tomer, Ernst Monique. The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biological Psychology. 2013;92(2):306–314. doi: 10.1016/j.biopsycho.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho Johanna M, Fox Nathan A, Pine Daniel S, Leibenluft Ellen, Shechner Tomer, Degnan Kathryn A, … Ernst Monique. ENDURING INFLUENCE OF EARLY TEMPERAMENT ON NEURAL MECHANISMS MEDIATING ATTENTION-EMOTION CONFLICT IN ADULTS. Depression and Anxiety. 2013;31(1):53–62. doi: 10.1002/da.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Snidman N, Kahn V, Towsley S. The preservation of two infant temperaments into adolescence. Monogr Soc Res Child Dev. 2007;72(2):1–75. vii. doi: 10.1111/j.1540-5834.2007.00436.x. discussion 76–91. [DOI] [PubMed] [Google Scholar]
- Lahat Ayelet, Lamm Connie, Chronis-Tuscano Andrea, Pine Daniel S, Henderson Heather A, Fox Nathan A. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(4):447–455. doi: 10.1016/j.jaac.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby Joan L, Belden Andy C, Pautsch Jennifer, Si Xuemei, Spitznagel Edward. The clinical significance of preschool depression: impairment in functioning and clinical markers of the disorder. Journal of Affective Disorders. 2009;112(1–3):111–119. doi: 10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby Joan L, Si Xuemei, Belden Andy C, Tandon Mini, Spitznagel Ed. Preschool depression: homotypic continuity and course over 24 months. Archives of general psychiatry. 2009;66(8):897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek Scott, Hwang Kai, Foran William, Hallquist Michael N, Luna Beatriz. The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLOS Biology. 2015;13(12):e1002328. doi: 10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas Kathleen Ries, He Jian-Ping, Burstein Marcy, Swanson Sonja A, Avenevoli Shelli, Cui Lihong, … Swendsen Joel. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar Koraly, Roberson-Nay Roxann, Hardin Michael G, Poeth Kaitlin, Guyer Amanda E, Nelson Eric E, … Ernst Monique. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35(4):1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perren Sonja, Alsaker Francoise D. Social behavior and peer relationships of victims, bully-victims, and bullies in kindergarten. Journal of Child Psychology and Psychiatry. 2006;47(1):45–57. doi: 10.1111/j.1469-7610.2005.01445.x. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes Kelly A, Snyder Abraham Z, Schlaggar Bradley L, Petersen Steven E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen Alexander L, Nelson Steven M, Wig Gagan S, Barnes Kelly Anne, Church Jessica A, … Petersen Steven E. Functional Network Organization of the Human Brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior M, Smart D, Sanson A, Oberklaid F. Does shy-inhibited temperament in childhood lead to anxiety problems in adolescence? J Am Acad Child Adolesc Psychiatry. 2000;39(4):461–468. doi: 10.1097/00004583-200004000-00015. [DOI] [PubMed] [Google Scholar]
- Raichle ME. The brain’s default mode network. Annual review of neuroscience. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Application and data analysis methods. Vol. 1. Sage; 2002. [Google Scholar]
- Rogers Cynthia E, Sylvester Chad M, Mintz Carrie, Kenley Jeanette K, Shimony Joshua S, Barch Deanna M, Smyser Christopher D. Neonatal Amygdala Functional Connectivity at Rest in Healthy and Preterm Infants and Early Internalizing Symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2017;56(2):157–166. doi: 10.1016/j.jaac.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Amy Krain, Benson Brenda E, Degnan Kathryn A, Perez-Edgar Koraly, Pine Daniel S, Fox Nathan A, Ernst Monique. Alterations in amygdala functional connectivity reflect early temperament. Biological Psychology. 2014;103:248–254. doi: 10.1016/j.biopsycho.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin Kenneth H, Coplan Robert J, Bowker Julie C. Social Withdrawal in Childhood. Annual Review of Psychology. 2009;60(1):141–171. doi: 10.1146/annurev.psych.60.110707.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BH. An observational study of the interactions of socially withdrawn/anxious early adolescents and their friends. J Child Psychol Psychiatry. 2009;50(7):799–806. doi: 10.1111/j.1469-7610.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- Schwartz Carl E, Kunwar Pratap S, Greve Douglas N, Moran Lyndsey R, Viner Jane C, Covino Jennifer M, … Wallace Stuart R. Structural differences in adult orbital and ventromedial prefrontal cortex predicted by infant temperament at 4 months of age. Archives of general psychiatry. 2010;67(1):78–84. doi: 10.1001/archgenpsychiatry.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz Carl E, Wright Christopher I, Shin Lisa M, Kagan Jerome, Rauch Scott L. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300(5627):1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20(4):327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester Chad M, Barch Deanna M, Harms Michael P, Belden Andy C, Oakberg Timothy J, Gold Andrea L, … Pine Daniel S. Early Childhood Behavioral Inhibition Predicts Cortical Thickness in Adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 2016;55(2):122–129. e121. doi: 10.1016/j.jaac.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester Chad M, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, … Lenze EJ. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35(9):527–535. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester Chad M, Smyser CD, Smyser T, Kenley J, Ackerman JJ, Jr, Shimony J, … Rogers CE. Cortical functional connectivity evident after birth and behavioral inhibition at age two years. Am J Psych. doi: 10.1176/appi.ajp.2017.17010018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber-Thomas Bradley C, Morales Santiago, Hillary Frank G, Pérez-Edgar Koraly E. ALTERED TOPOGRAPHY OF INTRINSIC FUNCTIONAL CONNECTIVITY IN CHILDHOOD RISK FOR SOCIAL ANXIETY. Depression and Anxiety. 2016;33(11):995–1004. doi: 10.1002/da.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, … Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- White Lauren K, Degnan Kathryn A, Henderson Heather A, Pérez-Edgar Koraly, Walker Olga L, Shechner Tomer, … Fox Nathan A. Developmental Relations Among Behavioral Inhibition, Anxiety, and Attention Biases to Threat and Positive Information. Child development. 2017;88(1):141–155. doi: 10.1111/cdev.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.