Abstract
This study examined how timing (i.e., relative maturity) and rate (i.e., how quickly infants attain proficiency) of A-not-B performance were related to changes in brain activity from age 6 to 12 months. A-not-B performance and resting EEG (electroencephalography) were measured monthly from age 6 to 12 months in 28 infants and were modeled using logistic and linear growth curve models. Infants with faster performance rates reached performance milestones earlier. Infants with faster rates of increase in A-not-B performance had lower occipital power at 6 months and greater linear increases in occipital power. The results underscore the importance of considering nonlinear change processes for studying infants’ cognitive development, as well as how these changes are related to trajectories of EEG power.
Infants undergo substantial changes in cognitive functioning in the second half of the first year (Bell & Fox, 1992; Cuevas & Bell, 2010; Diamond, 1985). The A-not-B task, a classic measure of cognitive development in infancy, was developed by Jean Piaget (1954) to assess 8- to 12-month-old infants’ understanding of object permanence (i.e., knowledge that an object exists when it is no longer in sight). Although most infants are capable of completing the task by age 12 months, the timing and rate of progression toward successful performance differ among infants across the second half of the first year. Piaget’s model of qualitative stages in cognitive development implies nonlinearity, in which the development of children’s thinking is not additive, but undergoes substantial reorganization over time (van Geert, 1998). Previous work indicates that task performance is related to variation in brain structure and function. For example, rhesus monkeys with bilateral ablations of the dorsolateral prefrontal cortex make the A-not-B error at varying delays whereas monkeys without these lesions do not (Diamond, 1990). Prior research with human infants suggests positive associations between frontal electroencephalogram (EEG) power and object permanence task performance (Bell & Fox, 1992). These findings, among others, illustrate that advancements in children’s cognitive performance may parallel concurrent changes in the brain (Fischer & van Geert, 2014).
Although there are individual differences in infants’ development of object permanence, particularly when assessing the amount of delay necessary to evoke the A-not-B error (Bell & Fox, 1992; Diamond, 1985), we have not yet charted age-related changes in A-not-B task performance as a nonlinear developmental process. Moreover, questions still remain concerning whether or how changes in EEG power map onto children’s cognitive development. The current study used linear growth models with logistic model parameters as predictors to examine the relation between timing (i.e., relative maturity) and rate (i.e., how quickly infants attain proficiency) of A-not-B performance gains from 6 to 12 months of age. In turn, we mapped performance changes onto age-related changes in baseline EEG power.
The A-not-B Error
In 1954, Jean Piaget published work on the development of the object concept, or the understanding that objects exist as unique entities outside of one’s own actions (Piaget, 1954). This skill is thought to be obtained little by little across the six substages of sensorimotor development. It was argued that children do not conceptualize the object as having its own pattern of movement that is both distinct from the child and also predictable and logical until the second year. Piaget argued that children’s attainment of the object concept, or object permanence, is essential groundwork for building knowledge of the physical world. He believed that the intellectual structures of the child change through stages, and that each of these stages is qualitatively different from the stages that had come before (Flavell, Miller, & Miller, 1993).
In the 60-plus years since these observations took place, researchers have investigated the underlying skills necessary to correctly display mastery of object permanence. Arguably, one of the tasks most often used to measure object permanence is Piaget’s own A-not-B task (1954). In this task, an attractive object is hidden at one location (A) multiple times in front of the infant until it is switched to another, adjacent location (B). Further, the experimenter may impose a time delay between hiding the object at B and allowing the infant to search. The A-not-B error lies in the infant reaching toward A, despite having seen the object’s placement at B.
The skills necessary to perform the A-not-B task successfully may extend beyond the simple acquisition of object permanence. Diamond (1985; 1990) has demonstrated that as infants get older, the length of delay between hiding the object and allowing the child to search for the object must increase for infants to make the perseverative error. Thus, the A-not-B task is a marker of the infant’s ability to remember previous events, coupled with the ability to inhibit a prepotent motor response to form a correct response. The data also imply that the infant’s actions are governed by their intention, as opposed to a developed habit. Diamond (1990) later also proposed that both the integration of recall memory and the inhibition of a prepotent response are jointly necessary for solving the A-not-B task.
Current conceptualizations contend that the task can be interpreted as an early measure of children’s executive function (Diamond, 2006). The delays between hiding the object and allowing the infant to search can be taxing on infants’ memories, thus age-related change in A-not-B task performance may be indicative of improvements in the capacity and duration of working memory (Marcovitch & Zelazo, 1999). Smith and colleagues proposed that in order for the infant to correctly obtain the object at the second location, infants need to be able to discriminate locations that look similar as well be able to reach for the object (Smith, Thelen, Titzer, & McLin, 1999). Building on the large perceptual changes that co-occur with enhanced locomotor skills in the second half of the first year, infants are able to couple their looking and reaching abilities, such that each discrimination and reach emerges from memories of previous discriminations and reaches (Smith et al., 1999). The A-not-B error thus may not necessarily represent a failure to obtain object permanence, insomuch that it demonstrates the difficulty of keeping an object’s new location in mind when exerting control over a motor response. Building on the various interpretations of the constructs underlying the A-not-B task, we argue that the task taps the intersection of infants’ object permanence, executive functioning, and working memory. Additionally, we agree with the consensus that the task also requires flexibility in infant cognition and the deployment of motor behavior (e.g., Smith et al., 1999).
Given the number of skills required to successfully perform the A-not-B task at varying delays, it comes as no surprise that there are both interindividual and intraindividual variability in infant performance. Diamond (1985) found large between-subject differences in the delay at which same-age children made the A-not-B error through 12 months of age. Additionally, she demonstrated that infants persisted in making these errors until 12 months of age with increases in delay. Other work has found that performance on the A-not-B task is normally distributed by 8 months of age, such that there was a wide range of performance scores with a mode of 3 on a scale from 1 to 6 (Bell & Adams, 1999). Building on this foundation, we aimed to examine individual differences in A-not-B task performance from 6 to 12 months of age using logistic parameters to capture nonlinearities in this development.
The Role of Brain Development in A-not-B Performance
Differences in individual task performance may be associated with brain activity over this developmental window (Bell, 2001, 2012; Bell & Fox, 1992, 1997; Cuevas & Bell, 2011; Cuevas, Bell, Marcovitch, & Calkins, 2012). Fischer and van Geert (2014) argued that changes in the rapidly maturing brain underlie the quick bursts and discontinuities in behavior and the presumed knowledge base of the developing infant. Early in development, neurons that are relatively unused are pruned in order to increase neural efficiency (Huttenlocher & Dabholkar, 1997). With efficiency also comes the coordination of neural systems involved in newly emerging behaviors, as in mastering object permanence and the related skills needed to overcome the A-not-B error. Action systems, such as sustaining multiple actions, while also inhibiting the prepotent response, must be coordinated for successful performance (Fischer & van Geert, 2014). The prefrontal cortex plays a role in holding this type of information in the moment while the individual engages in other actions. Because the A-not-B task involves the ability to attend to visual stimuli and track moving objects in space, we may also expect to see links to the development of the occipital region. Occipital power has been positively related to cognitive performance in infants (Bell & Fox, 1997), and visual processes are more sensitive to the environment by the end of the first year (Fox et al., 1979).
Much of the research on early brain development has utilized electroencephalography (EEG). EEG has the temporal resolution to capture quick shifts in brain functioning that may parallel the rapid cognitive processes that unfold on the order of milliseconds (Bell, 1998). EEG power values characterize excitability in a cluster of neurons and are combined across individual 1-Hz frequency bins to indicate power measurement in a specific frequency band. The dominant frequency band during baseline recordings in infancy is 6- to 9-Hz (Bell & Fox, 1992; Marshall, Bar-Haim, & Fox, 2002), reflecting alpha activity in infancy (Stoganova & Orekhova, 2007). Increases in EEG power in the alpha frequency band may suggest extensive organization of neurons (Nunez, 1981) and more neural activity. This neural activity, in turn, has been associated with increased cognitive skill in infancy including inhibitory control (Orekhova, Stroganova, & Posikera, 2001) and working memory (Cuevas et al., 2012). Linear increases in baseline power over time are evident across the scalp at frontal, parietal, and occipital sites from 7 to 12 months of age in the 6- to 9-Hz frequency band, with the greatest increase between 9 and 10 months (Bell & Fox, 1994).
Performance on working memory tasks is associated with increases in baseline EEG power. Bell and Fox (1992) found that baseline EEG power increased monthly in the 6- to 9-Hz range from 7 to 12 months of age and changes in frontal power were associated with greater delay tolerance on the A-not-B task. Bell (2002) tested 8-month-old infants on a spatial working memory task and assessed EEG power in the 3- to 5-Hz, 6- to 9-Hz, and 10- to 12-Hz frequency bands both at baseline and during the task. She found that the 6- to 9-Hz band yielded differences in baseline and task power values, demonstrating increased power during the cognitive task. In addition, 8-month-old infants who successfully completed the looking version of the A-not-B task tolerating a 0-second delay or greater demonstrated increases in frontal, parietal, and occipital power in the 6- to 9-Hz frequency band from baseline to task performance. However, there was no increase in power for the children who did not complete the task correctly (Bell, 2001). Finally, eight-month-old infants who performed successfully on the A-not-B task with a 0-second delay or greater demonstrated greater medial frontal and occipital baseline EEG power in the 6- to 9-Hz frequency band than infants who did not successfully complete the task (Bell & Fox, 1997). These results underscore the evidence for scalp-wide increases in baseline power for infants who successfully performed the task compared to infants who did not.
To date, little research has longitudinally assessed both A-not-B task performance and parallel markers of neural development. Bell and Fox (1992) examined A-not-B performance from 7 to 12 months (N=13) and collected EEG at rest at each age. Infants who could tolerate a longer delay on the A-not-B task at 12 months of age showed a decrease between 7 and 8 months and an increase between 9 and 10 months in frontal EEG power. The group with a shorter delay period only showed an increase in frontal power between 10 and 11 months. Additionally, the two groups of infants first differed on task performance at 10 months of age, which was also the point in time when the long-delay group showed the largest increase in frontal EEG power. These findings suggest that toward the end of the first year, the relation between growth in cortical organization and object permanence ability becomes more established. The Bell and Fox (1992) paper was one of the first to establish that there is interindividual variability in A-not-B task performance. Although infants demonstrate remarkable progress on both brain activity and A-not-B task performance over the latter half of the first year, the literature has yet to chart the relations across trajectories over time at the level of the individual.
Timing and Rate of A-not-B Performance Gains
If observed behavior and the brain are part of a dynamic network of systems that co-occur and are responsible for each other’s change (e.g., Fischer & van Geert, 2014), we must model development in ways that best characterize how particular systems change over time and how the growth in these systems change in relation to one another. Adolph and Robinson (2008) argued that the field has been overly reliant on examining development through differences between a beginning time point and an end time point, often as a single difference score. They contended that developmental systems frameworks should aim to explain processes of change and the shape of the change across time. Additionally, development does not unfold in steady increments. Rather, it speeds up and slows down at different points in time (Fischer & Rose, 1997; Thatcher, 1994). Research on infant development is often characterized by spurts in behavior that could be due to distinct developmental processes, such as verbal or spatial skills and differences in brain activity. New behaviors can emerge from changes in clusters of discontinuities (Fischer & Rose, 1997). Around 8 months, for instance, multiple skills undergo sharp increases, such as crawling ability (Adolph, Bertenthal, Boker, Goldfield, & Gibson, 1997), vocal imitation (Petitto & Marentette, 1991), and object search performance (Bell & Fox, 1994). These dramatic shifts in behavior imply nonlinearities in infant development. Although many aspects of development are described as nonlinear and dynamic, the empirical literature has tended toward using methods that describe stability and linear change.
Trajectories of cognitive growth can be portrayed by timing, or how mature infants are in relation to their same-age peers, and rate, or how quickly or slowly infants gain proficiency in cognitive performance. Infants can be considered early, average, and late maturers contingent upon their performance relative to other infants. They are also considered as quick, average, or slow maturers, based upon the rate at which they progress from no proficiency to proficiency on the task. The current study focused on a time window (6 to 12 months) that would likely encompass the first emergence and competence of A-not-B performance.
To our knowledge, A-not-B task performance in infancy has not been examined using nonlinear growth models. Although we know that successful A-not-B performance increases over age (e.g., Bell & Fox, 1992; Cuevas & Bell, 2010; Diamond, 1985), it is likely that the shape of the trajectory is nonlinear and more representative of a sigmoid, or “S”-shaped, curve. There are known discontinuities in the development of cognitive processing, in that spurts of growth are clustered at specific points of time in the first year (Fischer & Rose, 1997). For example, Munakata (1998) traced infants’ reaches at both A and B. The percent of correct responses for reaching at A was flat, between 90% and 100%. The percent of correct responses for reaching at B, however, revealed a sigmoid shape with upper and lower asymptotes. Developmental models must acknowledge potential patterns of nonlinearity in infants’ early cognitive trajectories and ages at which cognitive performance levels off. The current study used nonlinear growth curve models, a relatively novel method in the field of infant cognition, to account for nonlinearities that other studies have implied but have not directly tested.
Growth models provide a framework for describing the timing and rate of this change (Grimm, Ram, & Hamagami, 2011; McArdle, 2009; Ram & Grimm, 2007). Following previous work using nonlinear growth models to examine between-person differences in timing and rate of pubertal development (Marceau, Ram, Houts, Grimm, & Susman, 2011), our work examines how between-person differences in the timing and rate of infants’ performance on the A-not-B task are related to changes in EEG power. Given that infants’ capabilities change rapidly over the latter half of the first year, and that there is within-person variability in performance on these tasks, the present study investigated the timing and rate of infants’ cognitive and brain growth within this important developmental time window.
We used linear and nonlinear growth models to relate timing and rate of A-not-B task performance to change in EEG power from 6 to 12 months of age. The age range was chosen based on Diamond’s (1985) work, which assessed A-not-B task development every two weeks from 6 to 12 months of age. She found that infants began reaching for the object at 7.5 months and noted increases in delay tolerance at the average rate of 2 seconds per month. However, there were large individual differences in the delay needed for the error at every age. Our objectives were to: (a) describe nonlinear growth trajectories in the timing and rate of performance gain in the A-not-B task, and (b) examine the associations between nonlinear performance change and linear age-related changes in infants’ EEG power across the second half of the first year. We hypothesized that earlier timing and faster rate of A-not-B task performance would be associated with higher initial level of, and linear change in, EEG power.
Method
Given page limitations, an abbreviated discussion of the methods is presented here with a full description in the on-line supplement.
Participants
Twenty-eight healthy 6-month-old infants (14 boys, 14 girls) and their parents were recruited through mailing lists to participate in a longitudinal study of infant development. The participants were Caucasian from generally middle to upper-middle class homes in the greater Washington D. C. metropolitan area. Infants were born within three weeks of their anticipated due date, weighed at least six pounds at birth, received no special medical intervention at birth, had no documented neurological problems, and both parents had at least a high school education.
Procedures
Families came to the laboratory every month from 6 to 12 months of age, each time within 5 days of their monthly birthday (total of 7 visits). At each visit, brain electrical activity (EEG) was recorded and the infant completed a series of cognitive tasks, including the A-not-B task.
Electrophysiological Recording
Brain electrical activity was recorded during a 2-minute pre-task baseline recording session at each visit. During the recording, the infant watched the experimenter spin balls in a bingo wheel, segmented into 10 seconds of spinning 1, 3, and 7 balls, each separated by a 10 second pause. The sequence was then repeated. Power values were calculated for each electrode site for each stimulus condition. A composite of EEG baseline power values were used in the current analyses.
EEG recordings were taken using an Electro-Cap stretch cap (10/20 electrode system) from scalp electrodes, left and right frontal, parietal, and occipital regions (F3, F4, P3, P4, O1, and O2), as well as channels A1 and A2, located at the ears. All sites were referenced to Cz. Impedances were kept below 5K Ohms. EOG was recorded from the external canthus to the supra-orbit of one eye. All leads were separately amplified by Grass Model 12A5 amplifiers as part of the Neuro-data acquisition system (Grass Model 12–32). Digitalization of data (512 Hz per channel) was completed online using a HEM A/D board mounted in an IBM PS2/80 computer with HEM customized software. The EEG data were re-referenced to an average reference configuration prior to editing.
The EEG data were edited for eye movement and gross movement artifact by trained coders. On average, 50% to 59% of the EEG data were artifact-free at each of the measurement occasions. All analyses used the artifact-free data only. Individual patterns of available EEG data were not associated with any study variables (p’s > .08). The EEG data were analyzed using a discrete Fourier transform with a Hanning window of a one-second width with 50% overlap. The mean voltage was subtracted from each data point to remove any power results due to DC offset before DFT computation. Power in single Hz bands was computed for frequencies between 1 and 12 Hz, and power was expressed in mean square microvolts. Plots of spectral power indicated that the dominant frequency in all leads at all ages was in the 6- to 9-Hz alpha band. Power values were log transformed (ln). Based on missing data patterns across sessions (see Supplement), data were handled using maximum likelihood estimation.
A-not-B Task with Delay
After the electrophysiological recording, infants were assessed on the A-not-B task with delay, which was modeled after the standard two-location task. The AB apparatus was a cardboard box with two wells, A and B (specifics in Supplement). The apparatus was positioned on the floor in front of the infant, such that the center of the box was at midline and the wells were within reach of the infant. While the parent sat behind the infant, the experimenter sat on the opposite side of the apparatus facing the infant and parent.
The experimenter signaled the beginning of a trial by holding up a toy to engage the infant’s attention. Subsequently, the experimenter lifted the cloth covering one of the wells (A or B), placed the toy in that well, and then covered the well again with the cloth, completely obscuring any sight of the toy. If attention was lost during the trial, the experimenter gained the infant’s attention and hid the toy again. A correct response was coded if the child recovered the toy from side A. Also included as a correct response was uncovering the correct well but failing to reach for the toy and uncovering both wells yet visually fixating on the correct well (see Supplement). Uncovering the empty well, not reaching for the toy, and not fixating on the well housing the toy were all deemed incorrect responses.
The A-not-B task was scored by delay on a scale from 0 to 4. A score of “0” represented a failure to complete the task at a 0-second delay. A score of “1” represented competence at completing the task with a 0-second delay, where the infant was allowed to immediately search for the toy once it was hidden. Scores of “2”, “3”, and “4” were given when the infant completed the task with a 2-second, 4-second, or 6-second or greater delay, respectively. During the delay period, the experimenter broke the infant’s attention from the task by clapping their hands together. The parent held the infant’s hands to prevent reaching during the hiding and delay periods. The delay period began once the experimenter observed the infant’s attention breaking from the second well. Two observers coded A-not-B performance by videotape. The principal investigator, the experimenter, and the observer in the session reviewed these codes independently to resolve any discrepancies.
Data Analysis
We viewed our analyses of this small and very unique sample as semi-exploratory – using linear and nonlinear growth models to extract meaningful parameters from the rich and unique repeated measures data, but not claiming or assessing model fits as confirmatory evidence for statistical inference.
Interindividual differences in the development of A-not-B task performance
Our first aim was to quantify interindividual differences in the development of infants’ A-not-B task performance from age 6 to 12 months. The data had some distinctive characteristics that suggested description using a logistic growth function (Ram & Grimm, 2007). By design, the A-not-B task imposes clear lower and upper bounds on performance. Theoretically, all children progress from a stage when they are not able to perform the task at a 0-second delay (performance = 0) to a stage when they perform the task at a 6-second or greater delay (performance = 4). As such, we modeled the seven repeated observations of each infant’s performance using formula 1, noted in the Supplement. The timing of change was the age at which an infant would reach a performance score = 2 (formally, the inflection point located halfway between the lower and upper asymptotes). The rate (i.e., rate governing change from the lower to the upper asymptote) was also noted. Higher rate values indicate that the infant is improving in A-not-B task performance more quickly over time at the inflection point, relative to the other infants. Analytically, the logistic growth model served as a “measurement model” to derive timing and rate scores (θ2i and ri) that could then be examined in relation to individual differences in the development of EEG power. Linearity was accommodated in the measurement scale (0 to 4). Nonlinearity was accommodated in development of cognitive performance, given the previous research revealing nonlinearities in infants’ cognitive development. Models were fit to each infant’s repeated measures data by looping the nls function in R (R Core Team, 2013) with a range of starting values that facilitated convergence for all N = 28 cases.
Associations between EEG power and A-not-B task performance over time
Interindividual differences in the development of EEG power were then modeled using a standard linear growth model with covariates (Grimm, Ram, & Estabrook, 2017). As noted in the Supplement with formulas 2 through 4, the seven repeated observations of EEG power were modeled, separately for each electrode location while modeling the person-specific intercept and linear slope coefficients. Of particular interest were the relations of EEG power development with the timing (γ01 and γ11) and rate (γ02 and γ12) of A-not-B development. Models were fit to the repeated measures data using the nlme package in R (Pinheiro, Bates, DebRoy, Sarkar, & Core Team, 2015) using maximum likelihood estimation, with incomplete data (5%) treated using missing at random assumptions.
Results
See supplement, Table 1 for descriptive statistics for the core measures. As expected, the mean level of A-not-B performance increased with age (from M = 0.00 at 6 months to M = 3.32 at 12 months), with peak interindividual differences in performance at 9-months (SD = 1.41). In parallel, average EEG power increased with age at all six electrodes, but with no clear pattern of change in the standard deviations.
Interindividual Differences in the Development of A-not-B Task Performance
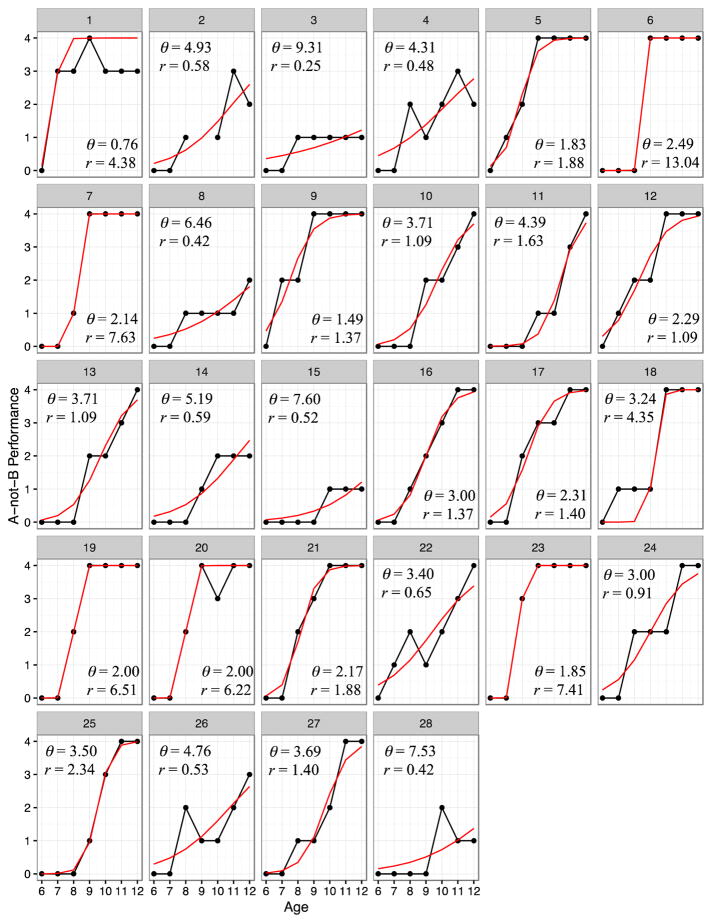
In the logistic growth trajectories derived from the logistic growth models (Figure 1), the model (solid lines) provided a good description of the repeated observations (dotted lines) of performance (residual standard errors ranged from 0.73 to 9.29, M = 3.68, SD = 2.04). The majority of infants were unsuccessful on A-not-B performance at age six months (score = 0), developed rapidly between age 7 and 11 months, and were able to complete the task at a 6-second delay or longer (score = 4) by age 12 months. The prototypical (average) infant obtained a score of 2 (i.e., halfway between lower and upper asymptotes) 3.68 months after the first observation (i.e., 6 + 3.68 = 9.68 months), at which point infants were developing at an average instantaneous rate of 2.55 (SD = 3.04). There were, of course, substantial individual differences both in timing and rate of development (SDθ2 = 2.04; SDr = 3.04). Notably, interindividual differences in timing and rate were correlated −0.49, indicating that infants growing faster reached the point of inflection earlier. A pseudo R-squared comparing the variance of all residuals to the collection of residuals from the individual models found substantial variability around parameter estimates (pseudo R2 = 0.842).
Figure 1.
Predicted trajectories of nonlinear intraindividual change of A-not-B performance for N = 28 children across seven assessments. Solid lines show the predicted trajectories for each individual; dotted lines show the raw data for each individual. θ = timing of change; r = rate of change.
Associations between EEG power and A-not-B task performance over time
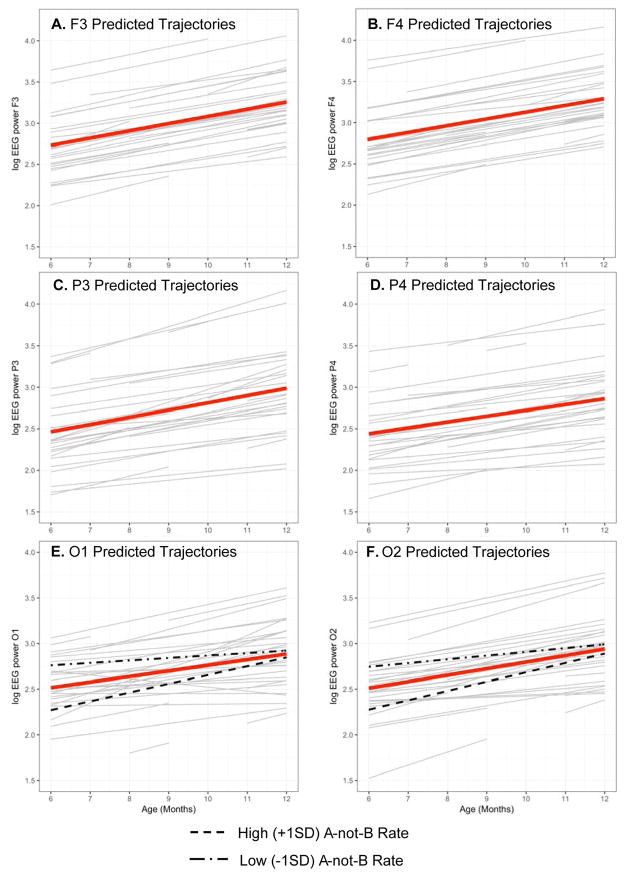
Interindividual differences in timing and rate of change in A-not-B task performance were examined in relation to concurrent development of EEG power at the six electrode locations using linear growth models (Supplement Equations 2 to 4). We used a stricter criterion of p ≤ .01 given the number of tests (Supplement, Table 2). As expected, all models indicated that EEG power increased, on average, across the second half of the first year. For example, for O2, the prototypical infant had (ln) power of γ00 = 2.51 at the first observation, with power increasing at γ10 = 0.07 (SE = 0.01, p < 0.001) units per month across the seven measurements.
Associations between the interindividual differences in A-not-B performance derived from the logistic growth model (timing and rate) and the interindividual differences in the trajectories of EEG power emerged for only occipital electrodes. Individuals with faster rates of increase in A-not-B performance (Panel E of Figure 2) had lower EEG (ln) power of O1 at age 6 months, γ02 = −0.08 (SE = .03, p = .01), and marginally greater increases in EEG power across the study, γ12 = 0.01 (p = .08). In parallel (Panel F of Figure 2), individuals with faster rates of increase in A-not-B performance had lower EEG (ln) power of O2 at age 6 months, γ02 = −0.08 (SE = .03, p = .01), and marginally greater increases in EEG (ln) power across the study, γ12 = 0.01 (p = .09).
Figure 2.
Predicted trajectories of linear intraindividual change of EEG power for N = 28 children across seven assessments. Bolded lines show the predicted trajectories for each individual; grey lines show the raw data for each individual.
Discussion
This study is the first to describe the timing and rate of development of A-not-B performance using a logistic growth function and to examine how differences in the behavioral development of cognitive performance are related to trajectories of brain activity. The first aim of the study was to identify the timing and rate of A-not-B task performance change, examining the sigmoid-shaped logistic curve that traversed performance from a score of 0 to 4. As predicted, the logistic growth model provided a good depiction of repeated A-not-B performance across the latter half of the first year, capturing the observation that the majority of infants were unsuccessful at the task at 6 months, rapidly increased in performance from 7 to 11 months, and succeeded at a 6-second or greater delay by 12 months. This description is consistent with Piaget’s theory of qualitative reorganization of children’s thinking.
Past research has shown nonlinearities in A-not-B performance, such as a surge in skill appearing around 8 months of age (Bell & Fox, 1994), and another between 10 and 12 months (Smith & Thelen, 2003). Other work has implied a sigmoid-shaped trajectory of reaching at location B across age in infancy (Munakata, 1998). However, previous research documented nonlinearities by revealing surges in performance at various ages, while often modeling change in behavior as linear (Fischer & Rose, 1997). The current study found that A-not-B performance is indeed nonlinear, with flat lower and upper bounds at 6 and 12 months respectively, and a spike in performance between 7 and 11 months of age.
Regarding timing, infants reached the point of inflection on the A-not-B task on average at age 9.68 months. Nine months of age may be an important time for coordinating multiple systems involved in the A-not-B task, such as motor control, attending to and tracking visual stimuli, inhibitory control, and working memory. Previous work found a sharp increase in performance at 8 months of age (Bell & Fox, 1994) and that task performance at 8 months is normally distributed (Bell & Adams, 1999). A second study with eight infants (Fox et al., 1979) found that all 9-month-old infants were able to complete the A-not-B task at a 3-second delay, while by 10 months, all infants completed the task at a 7-second delay. These findings indicate that between 9 and 10 months infants experience a rapid increase in performance on the A-not-B task. It is, however, important to note that the 7-second delay in Fox and colleagues’ (1979) study is larger than the 2-second delay at the point of inflection in the current study. This difference could be due to the disparity in sample size. While the greatest shift in behavior occurred at around 9 months of age for both studies, this was also the age when individual differences were greatest, and many infants were tolerating delays of six seconds or greater.
The substantial interindividual differences in the timing of task proficiency gains are consistent with previous work. Diamond (1985) and Bell and Fox (1992) found large variability at 8 and 7 months of age, respectively, in delay tolerance. Whereas previous work has found that A-not-B performance increases monthly for at least six months (Bell & Fox, 1992; Cuevas & Bell, 2010; Diamond, 1985; Fox et al., 1979), the present study is the first to quantify those increases with respect to a logistic function that accommodates the nonlinear age-related changes in behavior across this time window. Indeed, while the relative rate at the point of inflection was 2.55 on average, the range was from 0.25 to 13.04.
This study is the first to identify significant individual differences in how quickly or slowly infants attain proficiency relative to their peers. Because there was variation in both timing and rate A-not-B task performance, we can assume that while the sigmoid-shaped curve describes the infants’ performance gains across 6 to 12 months, the trajectories for the 28 infants are all somewhat unique (see Figure 1). Further, the negative correlation of timing and rate illustrates that earlier timing on the task is related to faster rates of acquisition, such that children who reach the halfway point in task performance at younger ages are mostly the same individuals who are making successful task gains more quickly. These findings suggest that although the latter half of the first year is a time during which there is substantial normative change, there is already substantial idiosyncrasy in individual development.
Our second aim was to examine the associations between linear change in baseline EEG power and nonlinear change in A-not-B performance. First, the results demonstrated that for all electrode sites, there was a linear increase in baseline EEG power values across 6 to 12 months of age. These findings are consistent with previous work that has described an overall linear increase in power at frontal, parietal, and occipital sites across the first year (Bell & Fox, 1992; Cuevas & Bell, 2011). The fact that the 6- to 9-Hz alpha band captured increases in power validates the existing literature that this is an appropriate frequency band for measuring brain electrical activity during infancy (Marshall et al., 2002). Previous work has suggested that increases in baseline EEG power in the infancy are indicative of emerging neural organization (Nunez, 1981). The increase in baseline power at all six electrode sites over time is suggestive of neural maturation and myelination that support the coordination of higher-order systems (Fischer & van Geert, 2014). In order to capture the functional consequences of brain activity, it is necessary to investigate how changes in baseline EEG power are related to changes in cognitive processing.
Interestingly, the current study found that infants with a faster rate of increase in A-not-B performance had lower baseline EEG power of O1 and O2 at 6 months of age, and they had larger increases in baseline power at these electrode locations across the 7-month period. Infants with higher power at the first time point (6 months of age) tended to advance on the A-not-B task more slowly over time. It may be that these infants were already performing the A-not-B task more successfully with longer delays and were thus ahead of infants with lower levels of initial power. Infants with lower initial levels of baseline EEG power had relatively more catching up to do, which required steeper (or faster) rates of performance over time.
Specific to occipital power, previous work has found that infants who could tolerate longer delays in A-not-B task performance also demonstrated greater baseline left hemisphere occipital power over age (Bell & Fox, 1992). Additionally, infants who did not complete the A-not-B task at 8 months had significantly lower baseline occipital power values than children who could complete the task at 0- or 2-second delays (Bell & Fox, 1997). The A-not-B task is a spatial working memory task, which requires attending to objects in space and tracking moving objects. Infants show an increase in general attentiveness to their environment by the end of the first year (Fox et al., 1979), which may provide further support for the relations noted here. In other words, infants’ ability to advance quickly on cognitive tasks may be in part linked to their increasing capability to attend to nonsocial visual events. Timing of A-not-B performance, on the other hand, was not related to initial levels or change in baseline occipital power. This suggests that the average point of change in the curvature of the A-not-B trajectory is not dependent on levels or changes in occipital power.
Substantial changes in children’s cognitive ability can be attributed to the coordination of multiple brain and behavior systems (Fischer & van Geert, 2014). It is somewhat surprising, then, that the frontal sites were not significantly associated with rates of increase in A-not-B performance for our sample, given previous literature suggesting that the prefrontal cortex plays an important role in infants’ development of object permanence (Bell, 2001, 2012; Bell & Fox, 1992; Bell & Fox, 1997; Cuevas et al., 2012). Occipital development may be more robust than frontal development in the first year. Past research suggests that the occipital lobe typically develops earlier in infancy, whereas frontal development is particularly variable and develops at differing rates in the latter half of the first year (Johnson, 2001). The stability of occipital development may contribute to the significant associations between A-not-B task performance and occipital power changes. However, we are hesitant to suggest that changes in baseline occipital power are the only neural correlates of performance on the A-not-B task. Future research should replicate these analyses with a larger sample size to generalize these findings and isolate the mechanism of A-not-B performance associated with baseline occipital power.
In sum, the current findings demonstrate that faster improvement in A-not-B performance could reflect changes in the organization and excitability of neurons in the occipital region. The use of associations between nonlinear cognitive performance change and linear baseline EEG power change is an innovative way of examining interrelated developmental processes. However, increases in occipital power need not cause increased cognitive performance or vice versa. Additionally, it is only assumed that scalp electrodes are indicative of brain activity from specific cortical regions underlying the electrodes, and we have not directly tested whether changes in baseline EEG power are reflective of brain maturation. Although this work does not measure brain maturation directly or EEG power in-task, knowledge of these patterns of brain activity in infants, in conjunction with cognitive performance, opens up the possibility to predict, and eventually understand, individual differences in executive function over time.
This study is the first to use growth curve modeling to delineate trajectories of infants’ cognitive growth in relation to their brain development. Bell and Fox’s (1992) study was pivotal in longitudinally assessing change in baseline EEG power over time for two groups of infants varying in A-not-B task performance, but the longitudinal sample was smaller (N = 13), and sensitive analytic techniques could not be applied. Conversely, Cuevas and colleagues (2012) used a much larger sample (N = 290) but fewer time points (5 and 10 months) and traditional regression analyses. We used nonlinear growth curves of task performance to more accurately describe the monthly change occurring for infants (N = 28) from 6 to 12 months of age. In this way, we illustrate how contemporary analytic tools can be used to assess, and reassess, classic developmental phenomena. Using nonlinear mixed-effects models allowed us to most effectively describe trends in A-not-B development that support the discontinuities central to Piaget’s theory of cognitive development, while also revealing how brain development maps onto the rates at which children advance in task attainment. Additionally, individual differences in timing and rate of A-not-B performance were shown in the sigmoid shape of the trajectories, permitting a closer and more comprehensive understanding of how variable cognitive development is in the first year. It is important to mention that the sigmoid shape of the A-not-B performance trajectory is dependent on the sampling method (Adolph, Robinson, Young, & Gill-Alvarez, 2008), and more frequent assessments within the age window could have demonstrated linear development. However, we found little change between 6 and 7 months and noted that between 11 and 12 months performance levels off. Without the addition of these beginning and end time points, one might assume linear increases between these missing time points. Moreover, individuals vastly differed in their A-not-B performance (Figure 1), and the inclusion of initial emergence through to competence therefore allowed us to capture the full developmental progression for most of the infants in the study.
The current results should be viewed in the context of study limitations. Primarily, the sample was small for detecting interindividual differences in intraindividual change. More participants will be needed to generalize these findings. Second, the A-not-B task involved a well that was uncovered then recovered again once the toy was placed, rather than having both wells covered simultaneously. The procedure we used may have primed the infant to maintain attention on the correct well (Diamond, Cruttenden, & Neiderman, 1994). Third, infant behavior was not coded during the EEG baseline session. Had there been information regarding the time the infant spent attending to the spinning wheel or amount of infant motor movement, we may have been able to more closely tie individual differences in occipital power to individual differences in visual attention. Fourth, EEG was not recorded during the A-not-B task so we could not capture any task-related variations in power. Fifth, the mathematical equation used in the analysis relied on the inflection point to measure interindividual differences at a score of 2. As this score is in the middle of the continuous scale from 0 to 4, its meaning on the scale is somewhat unclear. Moving the inflection point to different locations on the growth curve would likely reveal how rate changes across the logistic function. The selection of the score of 2 was simply to have a common point, or a parameterized feature of the trajectory, on which to compare individuals parsimoniously using the entire measurement scale. Moreover, we assumed that the measurement scale was linear and that cognitive development was nonlinear. An alternative approach would be to accommodate a nonlinear measurement scale and linear development. Lastly, the current study did not assess outcome behaviors beyond the age of 12 months, so the implications of these varying trajectories on later developmental processes and outcomes remain unknown. Future research should assess larger samples and extend measurement of these constructs beyond the age of 12 months so that we may better understand the role of early brain maturation in the long-term development of cognitive skills.
Nevertheless, this work provides evidence for the relations between brain activity and cognitive performance trajectories across the second half of the first year. By capturing the initial emergence of a skill and tracing attainment from absence through to advanced performance, this approach has the potential to not only inform our understanding of cognitive processing in infancy, but also provide theoretical and methodological foundations for future investigations of emerging developmental pathways. The contemporary analytic techniques illustrated here provide an exciting opportunity to empirically reassess our theoretical understanding of core developmental functions and processes.
Supplementary Material
Acknowledgments
This study was supported by a grant from the National Institutes of Health (1R01HD026768) to Nathan Fox. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the many individuals who contributed to data collection and processing. We especially thank the families who participated in the study.
References
- Adolph KE, Bertenthal BI, Boker SM, Goldfield EC, Gibson EJ. Learning in the Development of Infant Locomotion. Monographs of the Society for Research in Child Development. 1997;62:i–162. doi: 10.2307/1166199. [DOI] [PubMed] [Google Scholar]
- Adolph KE, Robinson SR. In defense of change processes. Child Development. 2008;79:1648–1653. doi: 10.1111/j.1467-8624.2008.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Robinson SR, Young JW, Gill-Alvarez F. What is the shape of developmental change? Psychological Review. 2008;115:527–543. doi: 10.1037/0033-295X.115.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA. The ontogeny of the EEG during infancy and childhood: Implications for cognitive development. In: Garreau B, editor. Neuroimaging in child neuropsychiatric disorders. Berlin: Springer-Verlag; 1998. pp. 97–111. [Google Scholar]
- Bell MA. Brain electrical activity associated with cognitive processing during a looking version of the A-not-B task. Infancy. 2001;2:311–330. doi: 10.1207/S15327078IN0203_2. [DOI] [PubMed] [Google Scholar]
- Bell MA. Power changes in infant EEG frequency bands during a spatial working memory task. Psychophysiology. 2002;39:450–458. doi: 10.1017/S0048577201393174. [DOI] [PubMed] [Google Scholar]
- Bell MA. A psychobiological perspective on working memory performance at 8 months of age. Child Development. 2012;83:251–265. doi: 10.1111/j.1467-8624.2011.01684.x. [DOI] [PubMed] [Google Scholar]
- Bell MA, Adams SE. Comparable performance on looking and reaching versions of the A-not-B task at 8 months of age. Infant Behavior and Development. 1999;22:221–235. doi: 10.1016/S0163-6383(99)00010-7. [DOI] [Google Scholar]
- Bell MA, Fox NA. The relations between frontal brain electrical activity and cognitive development during infancy. Child Development. 1992;63:1142–1163. doi: 10.1111/j.1467-8624.1992.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Bell MA, Fox NA. Brain development over the first year of life. In: Dawson G, Fischer KW, editors. Human behavior and the developing brain. New York, NY: The Guilford Press; 1994. pp. 314–345. [Google Scholar]
- Bell MA, Fox NA. Individual differences in object permanence performance at 8 months: Locomotor experience and brain electrical activity. Developmental Psychobiology. 1997;31(4):287–297. [PubMed] [Google Scholar]
- Cuevas K, Bell MA. Developmental progression of looking and reaching performance on the A-not-B task. Developmental Psychology. 2010;46:1363–1371. doi: 10.1037/a0020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Bell MA. EEG and ECG from 5 to 10 months of age: Developmental changes in baseline activation and cognitive processing during a working memory task. International Journal of Psychophysiology. 2011;80:119–128. doi: 10.1016/j.ijpsycho.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Bell MA, Marcovitch S, Calkins SD. Electroencephalogram and heart rate measures of working memory at 5 and 10 months. Developmental Psychology. 2012;48:907–917. doi: 10.1037/a0026448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. The Development and Neural Bases of Memory Functions as Indexed by the AB and Delayed Response Tasks in Human Infants and Infant Monkeys. Annals of the New York Academy of Sciences. 1990;608:267–317. doi: 10.1111/j.1749-6632.1990.tb48900.x. [DOI] [PubMed] [Google Scholar]
- Diamond A. The early development of executive functions. In: Bialystok E, Craik FIM, editors. Lifespan cognition: Mechanisms of change. New York: Oxford University Press; 2006. pp. 70–95. [Google Scholar]
- Diamond A, Cruttenden L, Neiderman D. AB with multiple wells: I. Why are multiple wells sometimes easier than two wells? II. Memory or memory + inhibition? Developmental Psychology. 1994;30:192–205. doi: 10.1037/0012-1649.30.2.192. [DOI] [Google Scholar]
- Fischer KW, Rose SP. Dynamic growth cycles of brain and cognitive development. In: Thatcher RW, Lyon GR, Rumsey J, Krasnegor N, editors. Developmental neuroimaging: Mapping the development of brain and behavior. San Diego, CA: Academic Press; 1997. pp. 263–279. [Google Scholar]
- Fischer KW, Van Geert P. Dynamic development of brain and behavior. In: Molenaar PCM, Lerner RM, Newell KM, editors. Handbook of developmental systems theory and methodology. New York, NY: The Guilford Press; 2014. pp. 287–315. [Google Scholar]
- Flavell JH, Miller PH, Miller SA. Cognitive development. Upper Saddle River, NJ: Prentice-Hall, Inc; 1993. [Google Scholar]
- Fox N, Kagan J, Weiskopf S. The growth of memory during infancy. Genetic Psychology Monographs. 1979;99:91–130. [PubMed] [Google Scholar]
- Grimm KJ, Ram N, Hamagami F. Nonlinear Growth Curves in Developmental Research. Child Development. 2011;82:1357–1371. doi: 10.1111/j.1467-8624.2011.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm KJ, Ram N, Estabrook R. Growth Modeling: Structural Equation and Multilevel Modeling Approaches. New York: Guilford; 2017. [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in humans. Nature Reviews Neuroscience. 2001;2(7):475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Marceau K, Ram N, Houts RM, Grimm KJ, Susman EJ. Individual differences in boys’ and girls’ timing and rate of puberty: Modeling development with nonlinear growth models. Developmental Psychology. 2011;47:1389–1409. doi: 10.1037/a0023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcovitch S, Zelazo PD. The A-Not-B error: Results from a logistic meta-analysis. Child Development. 1999;70:1297–1313. doi: 10.1111/1467-8624.00095. [DOI] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/S1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annual Review of Psychology. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- Munakata Y. Infant perseveration and implications for object permanence theories: A PDP model of the AB task. Developmental Science. 1998;1:161–184. https://doi.org/10.1111/1467-7687.00021. [Google Scholar]
- Nunez P. Electrical Fields of the Brain. New York: Oxford University Press; 1981. [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN. Alpha activity as an index of cortical inhibition during sustained internally controlled attention in infants. Clinical Neurophysiology. 2001;112:740–749. doi: 10.1016/S1388-2457(01)00502-8. [DOI] [PubMed] [Google Scholar]
- Petitto LA, Marentette PF. Babbling in the manual mode: Evidence for the ontogeny of language. Science. 1991;251(5000):1493. doi: 10.1126/science.2006424. [DOI] [PubMed] [Google Scholar]
- Piaget J. The construction of reality in the child. New York, NY: Basic Books; 1954. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, Core Team R. nlme: Linear and Nonlinear Mixed Effects Models. R Package version. 2015;3:1–120. http://CRAN.R-project.org/package=nlme. [Google Scholar]
- Ram N, Grimm K. Using simple and complex growth models to articulate developmental change: Matching theory to method. International Journal of Behavioral Development. 2007;31:303–316. doi: 10.1177/0165025407077751. [DOI] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. Retrieved from http://www.R-project.org/ [Google Scholar]
- Smith LB, Thelen E. Development as a dynamic system. Trends in Cognitive Sciences. 2003;7:343–348. doi: 10.1016/S1364-6613(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Smith LB, Thelen E, Titzer R, McLin D. Knowing in the context of acting: the task dynamics of the A-not-B error. Psychological Review. 1999;106:235–260. doi: 10.1037/0033-295X.106.2.235. [DOI] [PubMed] [Google Scholar]
- Stroganova TA, Orekhova EV. EEG and infant states. In: de Haan M, editor. Infant EEG and Event-Related Potentials. Psychology Press; New York: 2007. pp. 251–287. [Google Scholar]
- van Geert P. A dynamic systems model of basic developmental mechanisms: Piaget, Vygotsky, and beyond. Psychological Review. 1998;105:634–677. doi: 10.1037/0033-295X.105.4.634-677. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.