Abstract
The development self-regulation has been called a primary task of childhood. One system of self-regulation, self-monitoring, is indexed at the level of neural activity as early as preschool as the error-related negativity (ERN). However, how context elicits developmental changes in neural processes of self-monitoring like the ERN is not well understood. Here, socioeconomic status (SES) and parenting were tested as environmental influences on ERN development between ages 3 and 4 (N = 119). Results showed the expected increases in ERN between ages 3 and 4 only when both maternal sensitivity and SES were high. This work demonstrates the importance of considering the early environment in order to understand the development of a neural process supporting self-regulation in young children.
Better self-regulation is linked to positive outcomes across domains of early function, leading some theorists to call understanding self-regulation “the single most crucial goal for advancing an understanding of development” (Posner & Rothbart, 2000, p. 427). One subcomponent of self-regulation, self-monitoring, emerges in the third year of life (Kopp, 1982; Rothbart, 2011) and is posited to precede more complex forms of control and regulation (Posner & Rothbart, 1998). Self-monitoring is indexed at the level of neural activity by the error-related negativity (ERN), which appears as a negative deflection in electroencephalograph (EEG) recordings following error commission. ERN has been linked to many aspects of self-regulation (Lewis & Stieben, 2004), but most likely reflects a general process of self-monitoring (Falkenstein, Hoormann, Christ, & Hohnsbein, 2000) that signals for enhanced cognitive control to promote adaptive behaviors (Moser, 2017; Van Veen & Carter, 2006). In children, atypically large ERNs may index risk for anxiety problems (Brooker, Buss, & Dennis, 2011; McDermott et al., 2009). Identifying critical periods of plasticity in neural processes like self-monitoring presents an opportunity to target interventions for high-risk children and disrupt trajectories toward disorder. However, such a goal necessitates that normative trajectories of ERN development first be delineated (Cicchetti & Cohen, 2006).
Developmental theory identifies the preschool age as a critical phase of development for identifying antecedents of self-regulation (Kopp, 1982). Importantly, ERN can be reliably detected in children as young as 3 (Grammer, Carrasco, Gehring, & Morrison, 2014; Canen & Brooker, 2017). ERN tends to be localized to frontocentral scalp sites in adults (Falkenstein et al., 1991), but is also visible in parietal regions in children (Brooker et al., 2011). ERN amplitudes become more negative (Ladouceur, Dahl, & Carter, 2007) and less variable with age (DuPuis et al., 2015). Previous work with young children, for whom neural structures are least mature, has found no (Grammer et al., 2014) or small (Meyer, Weinberg, Klein, & Hajcak, 2012; Torpey, Hajcak, Kim, Kujawa, & Klein, 2012) age differences in ERN amplitudes. Longitudinal studies of ERN in children have been reported in only two publications to date. This work suggests overall increases in ERN amplitudes across ages 6, 7, and 8 years in children at high risk for behavior problems (DuPuis et al., 2015). Work with slightly older children suggested moderate ERN stability between ages 8–13 and between ages 10–15 (Meyer, Bress, & Proudfit, 2014). Though important findings, further longitudinal research is needed earlier in development to fully delineate critical periods of organization in ERN.
The contextual factors that shape the development of neural processes of self-regulation and their biological underpinnings also need to be clarified (Bronfenbrenner & Morris, 2007). Some work has shown that more hostile parenting in early life predicts enhanced ERN at age 4 (Brooker & Buss, 2014a), and both greater ERN and more anxiety symptoms at age 6 (Meyer et al., 2012). This work suggests that parenting may be an important factor in the normative development of ERN. Parental sensitivity, which promotes self-regulation in early life (Leerkes, Blankson, & O’Brien, 2009), may be especially critical but has not been linked with ERN development. Moreover, longitudinal investigations of developing ERN in contexts broader than the maternal parenting environment have not been conducted. The absence of this information has led to a lack of knowledge predicted by ecological systems theory almost 30 years ago: despite evidence that ERN may be an important index of self-monitoring in both typical and atypical development, it is unclear whether the ERN changes across time and situation and how “ecological conditions… sustain, enhance, or impair the operation of [this] process” (Bronfenbrenner, 1979, p. 845). As a result, the degree to prior work may be applied in clinical or public health settings is limited.
Socioeconomic status (SES) has long served as a proxy for a broad set of contextual factors that are important for child development, including geographic neighborhood, stress, access to resources, and exposure to hardships (Bradley & Corwyn, 2002). In this way, SES often serves as a starting point for identifying aspects of the developmental context that may be important (Leventhal & Brooks-Gunn, 2000). For instance, high SES parents appear to be more sensitive and report more diplomatic, child-centered parenting styles (Bradley & Corwyn, 2002; Hoffman, 1963). Low SES parents, in contrast, have been characterized as high in traditionally insensitive styles, including more controlling, restrictive, and punitive behaviors during infancy and early childhood (Bayley & Schaefer, 1960; Woodworth, Belsky, & Crnic, 1996). It is important to note that such effects do not necessarily denote a deficiency in low-SES parents. Indeed, additional factors remain “at play,” including systems of prejudice and injustice, inequalities in education, and parental stress (Hoff, Laursen, & Tardif, 2002). Nevertheless, SES does capture important differences in context that have implications for developing neural systems of regulation. It is therefore surprising that only one study has directly tested associations between SES and ERN. The study found that greater ERN in toddlers was associated with higher SES (Conejero, Guerra, Abundis-Gutiérrez, & Rueda, 2016), though ERN was assessed in response to observing rather than committing an error, resulting in potential differences in the degree to which neural processes reflect self-monitoring. Regardless, results were consistent with recent evidence that brain maturation is slowed in children from low-income families, potentially leading to decrements in self-monitoring (Hanson et al., 2013).
In the current study, I tested parenting and SES as proximal and distal factors, respectively, that may relate to developmental changes in ERN. I conducted this work in a typically-developing sample in order to begin charting typical and atypical developmental changes in a neural system of self-monitoring. This approach can identify a critical period of stability or change in ERN development and those factors that might be targeted for early intervention. Participants were assessed at ages 3 and 4, as this includes both the youngest age at which ERN has been observed and a critical period for the development of self-regulatory skills. I predicted that ERN would increase from age 3 to age 4 and that high SES and parental sensitivity would predict normative ERN development (i.e., greater ERN amplitudes over time).
Method
Participants
Preschoolers (N =119; 58% female) visited the laboratory across two waves of data collection as part of a larger longitudinal study. Families were recruited via mailings based on local birth records, fliers, media advertisements, in person, and via word-of-mouth. An initial laboratory visit occurred near the child’s 3.5-year birthday (n = 108; Mage = 3.59, SD = 0.15). Parents independently completed questionnaires packets. Physiological data were recorded from children during three episodes. The current report focuses on EEG data collected during a go/no-go paradigm and parents’ self reports of income, education, and parenting behaviors. Children returned at age 4 (N = 97; M = 4.56, SD = 0.15) for identical procedures.
All children were typically-developing. Most (96.2%) mothers and 94.9% of fathers identified as White; 1.9% of mothers and 1.3% of fathers identified as Asian, and 1.91 % of mothers and 3.8% of fathers identified as American Indian or Alaska Native. Roughly two percent (1.9%) of mothers and 5.6% and fathers identified as Hispanic or Latino.
Parent ratings of gross annual income represented the full range of possible values; maternal and paternal reports were composited. Of those who did report annual income, 3.03% reported earning less than $15,000, 4.04% reported $15,001 – $20,000, 7.07% reported $20,001 – $30,000, 7.07% reported $30,001 – $40,000, 8.08% reported $40,001 – $50,000, 16.16% reported $50,001 – $60,000, 7.07% reported $60,001 – $70,000, 11.11% reported $70,001 – $80,000, 9.09% reported $80,001 – $90,000, and 27.27% reported earning $90,000 or greater.
Measures
SES
Family SES was determined using the Four Factor Index of Social Status (Hollingshead, 1975). Education level was rated on a seven-point scale ranging from less than seventh grade (1) to graduate or professional training (7). Parent-reported occupation was ranked on a nine-step scale based on title and duties. Occupation and education scores were weighted and summed for each parent and then composited within family. SES scores reflected the full range of the scale (M = 32.10; SD = 17.46) suggesting that the average parents were skilled craftspeople, clerical, or sales workers (Hollingshead, 1975).
Parenting
Both parents-self-reported their typical parenting behaviors at ages 3 and 4 using the Coping With Children’s Negative Emotions questionnaire (CCNES; Eisenberg & Fabes, 1994; Eisenberg, Fabes, & Murphy, 1996), a 12-item assessment that asks parents to report on the likelihood that they would respond in specific ways to instances of challenging behavior in their children (e.g., “If my toddler spilled something and made a big mess on the carpet, and then gets upset and cries, I would… “). A principal components analysis suggested the presence of two factors (mothers: 66.27%; fathers: 64.09%), so separate composites reflecting sensitive and insensitive parenting were derived for mothers and fathers. For both mothers and fathers, sensitive parenting was quantified as the parent’s mean score on the expressive encouragement, emotion-focused, and problem focused reaction subscales (mean factor loading = 0.83). Insensitive parenting was quantified as the parent’s mean score on the distress reaction, punitive-minimizing, and wish granting reaction subscales (mean factor loading = 0.71). Given rolling recruitment, scores were composited across assessments.
Error-related Negativity
At ages 3 and 4, children completed a modified go-no-go task (Torpey et al., 2009), in which either a space ship (no-go stimulus) or an asteroid (go stimulus) was presented in the center of a 23” computer screen using Presentation stimulus delivery software (Neurobehavioral Systems, Inc.). Stimuli were always vertically aligned. The child was instructed to push the response button to “shoot the asteroids, but to be careful not to shoot other space ships”. The full task comprised 2 blocks of 40 trials for a maximum of 80 trials per child. Trials were presented in a pseudorandomized order such that roughly 60% of trials were go trials. Each trial began with a gray fixation cross in the center of the monitor for 200 ms followed by the stimulus for 1200 ms. Following this, the fixation cross was again presented for 300–800 ms prior to the beginning of the subsequent trial. Like other work with children (Stieben et al., 2007), an error rate of roughly 50% was maintained through an automated procedure that decreased stimulus presentation time by 50 ms following 2 consecutive correct responses and increased stimulus presentation time by 50 ms following 2 consecutive incorrect responses.
Prior to beginning the experimental blocks, children received instructions for the task using laminated pictures of go and no-go stimuli. When children could demonstrate, using the pictures, that they understood the directions, they completed two practice blocks of 10 trials each. Children were reminded to respond to the task as quickly and accurately as possible. All children received a sticker after each block (practice and experimental blocks) was completed.
EEG data were acquired during the go-no-go task using a BioSemi Active 2 recording system. Continuous EEG was recorded through a 64-channel cap using Ag-AgCl-tipped electrodes arranged according to the 10–20 labeling system. Electrodes were also placed at the outer canthi of the left and right eye to collect horizontal eye movements and at the supra and infra orbital sites of the left eye to collect vertical eye movement. Two electrodes were also placed on the mastoids for later re-referencing. Data were sampled at a rate of 2048 Hz. During recording, data were referenced to the Common Mode Sense and Driven Right Leg electrodes. Offline, all electrodes were re-referenced to the average of the right and left mastoids, high-pass filtered at 0.1 Hz, and corrected for eye movement or blinks (Gratton, Coles, & Donchin, 1983). Correct and incorrect trials were segmented (−200 to 600 ms), and baseline corrected for 200 milliseconds prior to the response. Artifacts were marked in segmented data when one of the following criteria were met: a voltage step of more than 75 μV between data points, a difference of 150 μV per within 200ms, amplitudes below 0.5 μV within a 50ms period, and activity that exceeded +100μV or −100 μV. Remaining segments were visually inspected for artifacts. Clean segments were averaged and low-pass filtered at 30 Hz. Correct trial amplitudes were subtracted from error trial amplitudes to isolate error-specific activity. For participants with at least six trials of usable data (Olvet & Hajcak, 2009b; Pontifex et al., 2010), peak negative amplitudes between 0 and 100 ms were marked as ERN at electrode FCz, which was selected based on previously-reported ERN in these data (Canen & Brooker, 2017). Split-half reliabilities for ERN were moderate at age 3 (r = 0.45; ICC = 0.64) and age 4 (r = 0.40; ICC = 0.42).
Additional information about recruitment procedures, the parent study, missing data, the data analysis plan, and study measures is available in the online supplement.
Results
Accuracy and response time data
Children performed slightly better on the task at age 4 relative to age 3, both responding correctly to a greater number of trials (t(75) = −7.46, p = 0.01) and responding incorrectly to fewer trials (t(75) = 7.46, p = 0.01) at age 4. Reaction times did not change with age. In general, children responded more quickly for error relative to correct trials (F(1, 49) = 57.08, p < 0.01), but this difference did not change over time (F(1, 49) = 0.02, p > 0.10). Children also did not show differences in post-error slowing at age 3 relative to age 4 (F(1, 45) = 2.94, p>0.05).
Preliminary analyses
Descriptive statistics and bivariate associations among study variables are reported in Table 1. Notably, ERN showed minimal stability across the two assessments. A small mean level increase in ERN from age 3 to age 4 was not significant (t(27) = −0.57, p = 0.58). There were no significant bivariate associations between ERN and parenting behaviors. Rather, sensitive and insensitive parenting were correlated across parents. Higher SES status was also linked to greater maternal sensitivity.
Table 1.
Descriptive statistics and bivariate correlations for variables.
| M(SD) | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| 1. ERN Age 3 | −10.25 (10.02) | ||||||
| 2. ERN Age 4 | −10.32 (12.66) | 0.15 | |||||
| 3. Maternal Sensitivity | 5.37 (0.82) | −0.03 | 0.04 | ||||
| 4. Maternal Insensitivity | 2.64 (0.62) | 0.10 | 0.08 | −0.03 | |||
| 5. Paternal Sensitivity | 4.96 (0.96) | 0.06 | 0.01 | 0.47** | −0.07 | ||
| 6. Paternal Insensitivity | 2.77 (0.59) | −0.03 | 0.02 | 0.07 | 0.32** | 0.23* | |
| 5. SES | −32.09 (17.39) | 0.02 | 0.05 | 0.27** | −0.00 | −0.02 | 0.09 |
p < 0.01
Maternal behaviors
The model that included maternal sensitivity revealed a significant three –way interaction among age 3 ERN, sensitivity, and SES (β = 0.49, SE(β) = 0.25, p = 0.05, R2 = 0.02) predicting age 4 ERN amplitude. This interaction was probed by recentering both SES and maternal sensitivity at high and low values. Probing in this manner revealed that greater age 3 ERN predicted greater age 4 ERN when both SES and maternal sensitivity was high (β = 1.12, SE(β) = 0.64, p = 0.08). Age 3 ERN was unrelated to age 4 ERN when SES was high but maternal sensitivity was low (β = −1.12, SE(β) = 0.73, p = 0.12) or when SES was low (high sensitivity: β = −0.64, SE(β) = 0.73, p = 0.38; low sensitivity: β = 0.38, SE(β) = 0.34, p = 0.26).
For the model including maternal insensitivity, the three-way interaction among age 3 ERN, insensitivity, and SES was not significant (β = 0.29, SE(β) = 0.37, p = 0.44), as were all two-way interactions (|β|s < 0.17, ps > 0.52) and simple main effects (βs < 0.19, ps > 0.30).
Paternal behaviors
For the model that included paternal sensitivity, the three-way interaction among age 3 ERN, sensitivity, and SES was not significant (β = 0.10, SE(β) = 0.26, p = 0.70). Similarly, there were no significant two-way interactions (|β|s < 0.22, ps > 0.35) or simple main effects (βs < 0.18, ps > 0.35).
For the model including paternal insensitivity, the three-way interaction among age 3 ERN, sensitivity, and SES was not significant (β = −0.03, SE(β) = 0.24, p = 0.86), as were all two-way interactions (|β|s < 0.10, ps > 0.61) and simple main effects (βs < 0.19, ps > 0.29).
Discussion
This work offers the first longitudinal description of ERN development in typically-developing preschoolers. Mean ERN amplitudes were similar at ages 3 and 4, though stability was limited between assessments. Apparent increases in variability in ERN amplitudes from age 3 to 4 may at least partially explain a lack of significant mean-level changes in amplitudes. Importantly, this pattern contrasts that of slightly older children, for whom variability in ERN amplitudes appears to decrease over time (DuPuis et al., 2015). Such differences in patterns of stability and change in ERN amplitude and ERN variability may reflect an inflection point near 4 years of age, as neural development shifts from comprising primarily the proliferation of synapses to competitive elimination of neural connections (Lenroot & Giedd, 2006), resulting in increased neural efficiency. Unique findings for early and middle childhood may also reflect the fallibility of age as a measure of developmental stage, as there are broad individual differences in the maturation of neural systems (Durston et al., 2002).
The current results are consistent with previous work showing a lack of developmental change in ERN amplitudes in very young children. Cross-sectional studies in groups of children between ages 3–7 (Grammer et al., 2014) and 4–8 (Brooker et al., 2011) reported no significant age differences in ERN amplitudes. Together, these studies suggest that the most prominent changes in ERN amplitudes, perhaps reflecting a second reorganizational period of neural underpinnings of self-monitoring, do not occur until middle childhood (DuPuis et al., 2015) and adolescence (Ladouceur et al., 2007). Such a possibility will be important to consider in the timing of data collection for subsequent studies that wish to capture developmental change in ERN. That is, to capture group-level changes in ERN, assessments may need to be less frequent at ages 3–4, when ERN is largely stable, than between ages 6–18 (Davies et al., 2004; DuPuis et al., 2015; Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006), when amplitudes are increasing.
Nonetheless, this work was the first to use a bioecological framework to show that proximal and distal factors in the early environment are associated with ERN development. Specifically, normative development of the ERN was observed only when both maternal sensitivity and SES were high. This finding is consistent with previous work suggesting that maternal sensitivity (Leerkes et al., 2009) and high SES (Howse, Lange, Farran, & Boyles, 2003) show independent positive associations with the development of children’s self-regulatory skills. Similarly, results are consistent with previous work suggesting that low SES may compromise early brain development (Hanson et al., 2013), and the normative development of the ERN in particular (Conejero et al., 2016). This study advances previous work by examining the development of a neural marker of self-monitoring in the context of multiple factors. As no main effect of ERN is visible, the contemporaneous consideration of multiple contextual factors may be critical for identifying conditions of plasticity and during the early maturation this neural process. Results further suggest that critical periods of ERN may not be uniform; that is, ERN may become organized earlier in development for children in optimal environments. Specifically, the presence of developmental increases in ERN amplitudes at high levels of sensitivity but not at low levels of insensitivity implies that environmental enrichment, rather than simply an absence of risk, plays a role in the early organization of ERN.
These results do not offer insight into whether an earlier-developing ERN is beneficial for long-term child outcomes. The ERN is generally an adaptive mechanism, offering a neural signal for enhancing cognitive support of self-control and regulation (Van Veen & Carter, 2006). Early skills of self-regulation are, in turn, associated with a host of positive outcomes later in life (Moffitt et al., 2011). However, anxious adolescents show a more adult-like ERN than do non-anxious controls (Ladouceur et al., 2006) and a more localized ERN is visible in 4-year olds who show high levels of fearfulness in low-threat contexts (Brooker & Buss, 2014b). Thus it will be important for future work to link differences in developmental trajectories of ERN to longitudinal outcomes during childhood. This work is currently underway in my laboratory.
Although not the focus of this investigation, response time differences offer potential insight into the meaningfulness of distinct trajectories of early ERN development. Specifically, children performed slightly better on the task at age 4 relative to age 3, though this effect size may be inflated by task constraints on performance. However, a lack of developmental changes in response times may indicate that increases of the ERN during this period reflect increases in ability to self-monitor rather than increases in efficiency. This is notable given that some work has suggested that a smaller – rather than enhanced - ERN is linked to greater risk for anxiety problems in younger children (Meyer et al., 2012). Thus, early increases in ERN may be more indicative of changes in ability to self-monitor, resulting in greater ERN being linked to greater ability to regulate, while later changes in ERN may reflect individual differences in neural efficiency and greater ERN indicating a lack of neural efficiency and propensity for risk.
This study is also the first to investigate putative differences in the influences of mothers and fathers on ERN development. Results suggest that in combination with SES, only maternal sensitivity affected ERN development between ages 3 and 4. It is possible that this difference reflects the high proportion of mothers in this sample (>90%) who identified as the primary caregiver. Although a growing number of fathers provide primary care for young children, roughly 80% of families still identify mothers as primary caregivers (U.S. Census Bureau, 2011). This may lead to mothers providing the early scaffolding for the development of processes of self-regulation to a greater degree than do fathers (Kopp, 1982). A comparative analysis in a sample of families for whom fathers act as the primary caregiver would help us to understand whether this is the case. There is also a possibility that methods play a role in the findings of differences in the influence of mothers and fathers. While some psychophysiological markers of regulation in young children have evidenced direct associations with paternal behaviors (Najjar & Brooker, 2017), the impact of paternal behaviors may be most apparent when observational data are used in place of self-report (Hastings et al., 2008). Notably, the current pattern of results are unchanged in a partial sample when observational data are substituted for self-report.
Perhaps unexpectedly, sensitive and insensitive parenting were modestly correlated for fathers. Similar associations have been reported in previous work (Kiel & Buss, 2010) and suggests that the domains of sensitive and insensitive parenting small percentage of variance. An absence of previous work with fathers using this measure makes it difficult to interpret the positive association. One possibility is that fathers are less likely to have a consistent parenting style with young children. At least one previous study has shown a lack of stability in paternal sensitivity between child ages 2 and 3 years (Brown, Mangelsdorf, & Neff, 2012). Thus, fathers may use early interactions to learn about parenting their child in the early years of life, trying a variety of approaches that include both sensitive and insensitive behaviors.
The current study is not without limitations. Although this sample includes a notable percentage of Native American families, an often unrepresented group, it comprises largely White, Non-Hispanic families. Thus, it is unclear whether results would generalize to other racial and ethnic groups. This is not insignificant given the overrepresentation of racial and ethnic minorities in low-SES groups. In addition, although this is a sizeable sample for a longitudinal study of neural development in young children, a larger sample with less missing data would be ideal for retesting this moderated moderation model, as it may result in more precise parameter estimates. The current study also used a relatively broad index of family SES. Though the Hollingshead is beneficial as an established, commonly used index of SES that allows for comparison of findings across studies, its breadth makes it difficult to understand the mechanism by which SES comes to impact neural development. While possibilities such as nutrition, chronic stress, and exposure to environmental toxins (Bradley & Corwyn, 2002; Conejero et al., 2016; Hanson et al., 2013) have been proposed, additional work will be needed to associate the current findings with specific factors associated with early-poverty that may drive the current findings.
Finally, it is important to note that the internal consistency of the ERN in the present study is relatively low compared with published metrics, largely derived from adult samples. While typical development is associated with less behavioral variability, it is also linked to increases in variability in neural activity (McIntosh, Kovacevic, & Itier, 2008). Given that estimates derived from adult samples are variable across tasks and populations, one might expect even more variable reliability metrics in samples of preschoolers. Accordingly, the internal consistency estimates reported here are similar to some of those reported in the adult literature (Olvet & Hajcak, 2009a), but are notably smaller than others (Olvet & Hajcak, 2009b). To date, there are no examinations of internal consistency of ERN during preschool or early childhood. This work will be critical for determining whether a more reliable ERN is better able to discriminate inter-individual differences related to anxiety risk.
In sum, this work offers novel insights into the environmental factors that may contribute to changes in the normative development trajectory of a neural system of self-monitoring. Using an established developmental theory, I have shown that changes in the ERN between ages 3 and 4 is contingent upon environmental factors including the early parenting environment and family SES. This work provides a foundation for future longitudinal investigations identifying critical periods of development for self-regulation and those factors by which early contextual factors alter the course of developmental trajectories of neural systems in early life.
Supplementary Material
Figure 1.
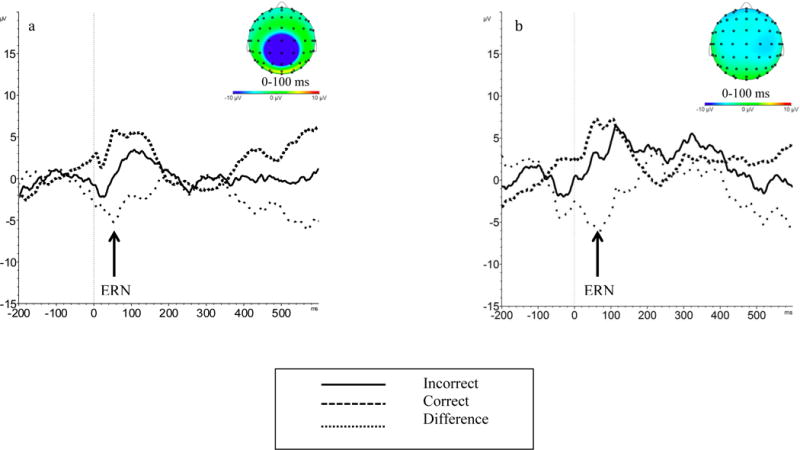
ERN at ages 3 (a) and 4 (b).
Acknowledgments
Author Notes
Data collection for this project was supported by K01 MH100240 (PI: Brooker) from the National Institute of Mental Health and P20 GM104417 (PI: Adams) from the National Institute of General Medical Sciences. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
I wish to thank the families that participated in this study and research assistants and staff members who helped with study procedures. I also wish to thank Dr. Matthew Vess for his feedback on an early version of this manuscript.
References
- Bayley N, Schaefer ES. Relationships between socioeconomic variables and the behavior of mothers toward young children. The Journal of Genetic Psychology. 1960;96(1):61–77. doi: 10.1080/00221325.1960.10534275. https://doi.org/10.1080/00221325.1960.10534275. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annual Review of Psychology. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. https://doi.org/10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U. The ecology of human development: Experiments by nature and design. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- Bronfenbrenner U, Morris PA. Handbook of Child Psychology. John Wiley & Sons, Inc; 2007. The bioecological model of human development. https://doi.org/10.1002/9780470147658.chpsy0114. [Google Scholar]
- Brooker RJ, Buss KA. Harsh parenting and fearfulness in toddlerhood interact to predict amplitudes of preschool error-related negativity. Developmental Cognitive Neuroscience. 2014a;9:148–159. doi: 10.1016/j.dcn.2014.03.001. https://doi.org/10.1016/j.dcn.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA. Toddler fearfulness is linked to individual differences in error-related negativity during preschool. Developmental Neuropsychology. 2014b;39(1):1–8. doi: 10.1080/87565641.2013.826661. https://doi.org/10.1080/87565641.2013.826661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA, Dennis TA. Error-monitoring brain activity is associated with affective behaviors in young children. Developmental Cognitive Neuroscience. 2011;1(2):141–152. doi: 10.1016/j.dcn.2010.12.002. https://doi.org/10.1016/j.dcn.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GL, Mangelsdorf SC, Neff C. Father involvement, paternal sensitivity, and father-child attachment security in the first 3 years. Journal of Family Psychology. 2012;26(3):421–430. doi: 10.1037/a0027836. https://doi.org/10.1037/a0027836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canen MJ, Brooker RJ. ERN, theta power, and risk for anxiety problems in preschoolers. Biological Psychiatry. 2017;123:103–110. doi: 10.1016/j.biopsycho.2016.12.003. https://doi.org/10.1016/j.biopsycho.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Cohen DJ. Developmental Psychopathology, Developmental Neuroscience. John Wiley & Sons; 2006. [Google Scholar]
- Conejero Á, Guerra S, Abundis-Gutiérrez A, Rueda MR. Frontal theta activation associated with error detection in toddlers: influence of familial socioeconomic status. Developmental Science. 2016:n/a–n/a. doi: 10.1111/desc.12494. https://doi.org/10.1111/desc.12494. [DOI] [PubMed]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology. 2004;25(3):355–376. doi: 10.1207/s15326942dn2503_6. https://doi.org/10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- DuPuis D, Ram N, Willner CJ, Karalunas S, Segalowitz SJ, Gatzke-Kopp LM. Implications of ongoing neural development for the measurement of the error-related negativity in childhood. Developmental Science. 2015;18(3):452–468. doi: 10.1111/desc.12229. https://doi.org/10.1111/desc.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Uluğ AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5(4):F9–F16. https://doi.org/10.1111/1467-7687.00235. [Google Scholar]
- Eisenberg N, Fabes RA. Mothers’ reactions to children’s negative emotions: Relations to children’s temperament and anger behavior. Merrill-Palmer Quarterly. 1994;40(1):138–156. [Google Scholar]
- Eisenberg N, Fabes RA, Murphy B. Parents’ reactions to children’s negative emotions: Relations to children’s social competence and comforting behavior. Child Development. 1996;67(5):2227–2247. [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78(6):447–455. doi: 10.1016/0013-4694(91)90062-9. https://doi.org/10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology. 2000;51(2–3):87–107. doi: 10.1016/s0301-0511(99)00031-9. https://doi.org/10.1016/S0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Grammer JK, Carrasco M, Gehring WJ, Morrison FJ. Age-related changes in error processing in young children: A school-based investigation. Developmental Cognitive Neuroscience. 2014;9:93–105. doi: 10.1016/j.dcn.2014.02.001. https://doi.org/10.1016/j.dcn.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. https://doi.org/10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Hair N, Shen DG, Shi F, Gilmore JH, Wolfe BL, Pollak SD. Family poverty affects the rate of human infant brain growth. PLoS ONE. 2013;8(12):e80954. doi: 10.1371/journal.pone.0080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PD, Sullivan C, McShane KE, Coplan RJ, Utendale WT, Vyncke JD. Parental socialization, vagal regulation, and preschoolers’ anxious difficulties: Direct mothers and moderated fathers. Child Development. 2008;79(1):45–64. doi: 10.1111/j.1467-8624.2007.01110.x. https://doi.org/10.1111/j.1467-8624.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- Hoff E, Laursen B, Tardif T. Socioeconomic status and parenting. In: Bornstein MH, editor. Handbook of Parenting. Second. Vol. 2. Mahwah, NJ: Lawrence Erlbaum Associates; 2002. pp. 231–252. (Biology and ecology of parenting). [Google Scholar]
- Hoffman ML. Personality, family structure, and social class as antecedents of parental power assertion. Child Development. 1963;34(4):869–884. doi: 10.1111/j.1467-8624.1963.tb05160.x. https://doi.org/10.2307/1126532. [DOI] [PubMed] [Google Scholar]
- Howse RB, Lange G, Farran DC, Boyles CD. Motivation and self-regulation as predictors of achievement in economically disadvantaged young children. The Journal of Experimental Education. 2003;71(2):151–174. https://doi.org/10.1080/00220970309602061. [Google Scholar]
- Kiel EJ, Buss KA. Maternal accuracy and behavior in anticipating children’s responses to novelty: Relations to fearful temperament and implications for anxiety development. Social Development. 2010;19(2):304–325. doi: 10.1111/j.1467-9507.2009.00538.x. https://doi.org/10.1111/j.1467-9507.2009.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18(2):199–214. [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error‐related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47(10):1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. https://doi.org/10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental Science. 2007;10(6):874–891. doi: 10.1111/j.1467-7687.2007.00639.x. https://doi.org/10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Leerkes EM, Blankson AN, O’Brien M. Differential effects of maternal sensitivity to infant distress and nondistress on social-emotional functioning. Child Development. 2009;80(3):762–775. doi: 10.1111/j.1467-8624.2009.01296.x. https://doi.org/10.1111/j.1467-8624.2009.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Methodological and Conceptual Advances in the Study of Brain-Behavior Dynamics: A Multivariate Lifespan Perspective. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. https://doi.org/10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Leventhal T, Brooks-Gunn J. The neighborhoods they live in: The effects of neighborhood residence on child and adolescent outcomes. Psychological Bulletin. 2000;126(2):309–337. doi: 10.1037/0033-2909.126.2.309. https://doi.org/10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Stieben J. Emotion regulation in the brain: Conceptual issues and directions for developmental research. Child Development. 2004;75(2):371–376. doi: 10.1111/j.1467-8624.2004.00680.x. https://doi.org/10.1111/j.1467-8624.2004.00680.x. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65(5):445–448. doi: 10.1016/j.biopsych.2008.10.043. https://doi.org/10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Kovacevic N, Itier RJ. Increased brain signal variability accompanies lower behavioral variability in development. PLOS Computational Biology. 2008;4(7):e1000106. doi: 10.1371/journal.pcbi.1000106. https://doi.org/10.1371/journal.pcbi.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Bress JN, Proudfit GH. Psychometric properties of the error-related negativity in children and adolescents. Psychophysiology. 2014;51(7):602–610. doi: 10.1111/psyp.12208. https://doi.org/10.1111/psyp.12208. [DOI] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience. 2012;2(1):152–161. doi: 10.1016/j.dcn.2011.09.005. https://doi.org/10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, … Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences. 2011;108(7):2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS. The nature of the relationship between anxiety and the error-related negativity across development. Current Behavioral Neuroscience Reports. 2017 doi: 10.1007/s40473-017-0132-7. https://doi.org/10.1007/s40473-017-0132-7. [DOI] [PMC free article] [PubMed]
- Olvet DM, Hajcak G. Reliability of error-related brain activity. Brain Research. 2009a;1284(Supplement C):89–99. doi: 10.1016/j.brainres.2009.05.079. https://doi.org/10.1016/j.brainres.2009.05.079. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The stability of error‐related brain activity with increasing trials. Psychophysiology. 2009b;46(5):957–961. doi: 10.1111/j.1469-8986.2009.00848.x. https://doi.org/10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Scudder MR, Brown ML, O’Leary KC, Wu C, Themanson JR, Hillman CH. On the number of trials necessary for stabilization of error‐related brain activity across the life span. Psychophysiology. 2010;47(4):767–773. doi: 10.1111/j.1469-8986.2010.00974.x. https://doi.org/10.1111/j.1469-8986.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philosopical Transactions of the Royal Society of London B. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. https://doi.org/10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Developing mechanisms of self-regulation. Development and Psychopathology. 2000;(3):427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Rothbart MK. Becoming who we are: Temperament and personality development. New York, New York: The Guilford Press; 2011. [Google Scholar]
- Stieben J, Lewis MD, Granic I, Zelazo PD, Segalowitz S, Pepler D. Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Development and Psychopathology. 2007;19(02):455–480. doi: 10.1017/S0954579407070228. https://doi.org/10.1017/S0954579407070228. [DOI] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Kim J, Kujawa A, Klein DN. Electrocortical and behavioral measures of response monitoring in young children during a Go/No‐Go task. Developmental Psychobiology. 2012;54(2):139–150. doi: 10.1002/dev.20590. https://doi.org/10.1002/dev.20590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Klein DN. An examination of error-related brain activity and its modulation by error value in young children. Developmental Neuropsychology. 2009;34(6):749–761. doi: 10.1080/87565640903265103. https://doi.org/10.1080/87565640903265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. Conflict and cognitive control in the brain. Current Directions in Psychological Science. 2006;15(5):237–240. https://doi.org/10.1111/j.1467-8721.2006.00443.x. [Google Scholar]
- Woodworth S, Belsky J, Crnic K. The determinants of fathering during the child’s second and third years of life: A developmental analysis. Journal of Marriage and Family. 1996;58(3):679–692. https://doi.org/10.2307/353728. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


